Abstract
High levels of reactive oxygen species such as hydrogen peroxide (H2O2) cause oxidative stress in the lens and lead to cataractogenesis. The present investigation was undertaken to find out whether the mammalian lens aquaporins (AQPs) 0, 1, and 5 perform H2O2 transport across the plasma membrane to reduce oxidative stress. Our in vitro cell culture and ex vivo lens experiments demonstrated that in addition to the established water transport role, mouse AQP0, AQP1 and AQP5 facilitate transmembrane H2O2 transport and function as peroxiporins. Human lens epithelial cells expressing AQP1, AQP5 and AQP8, when treated with 50μM HgCl2 water channel inhibitor showed a significant reduction in H2O2 transport. Data obtained from the experiments involving H2O2-degrading enzyme glutathione peroxidase 1 (GPX1) knockout lenses showed H2O2 accumulation suggesting H2O2 transport level by AQPs in the lens is regulated by GPX1. Under hyperglycemic conditions, there was an increased loss of transparency, and enhanced production and retention of H2O2 in AQP5−/− lenses compared to similarly-treated WT lenses. Overall, the results show that lens AQPs function as peroxiporins and cooperate with GPX1 to maintain lens H2O2 homeostasis to prevent oxidative stress, highlighting AQPs and GPX1 as promising therapeutic drug targets to delay/treat/prevent age-related lens cataracts.
Keywords: Lens AQP0, AQP1, AQP5, AQP8, Peroxiporin, H2O2
1. Introduction
Aquaporins (AQPs) are transmembrane proteins that are assembled in a homotetrameric manner in the plasma membrane. Each monomer functions as a channel that facilitates the influx and efflux of water. In mammals, thirteen AQPs (AQP0-AQP12) are expressed in various tissues. The ocular lens, which has a monolayer of anterior epithelial cells and multiple layers of fiber cells, expresses three AQPs, namely, AQP0, AQP1 and AQP5. These AQPs are classified as classical AQPs, permeable selectively to water. AQP1 and AQP5 are expressed in the epithelial cells, and AQP0 and AQP5 are expressed in the fiber cells. The avascular lens generates a circulating flux of ions that cause water to flow through AQPs. This microcirculation [1,2] carries anti-oxidants, glucose and other nutrients into the lens and removes metabolic byproducts. Thus, AQPs play a major role in the lens microcirculation, transparency and homeostasis [3–14].
In the lens, AQPs play diverse roles such as volume regulation [1–3], cell-to-cell adhesion [6,15], and establishment of biomechanics (16) and refractive index gradient (7). Recent studies report that some mammalian AQPs, such as AQP3, AQP8, and AQP9 transport hydrogen peroxide (H2O2) across the membrane and function as peroxiporins [17–21]. H2O2 is a major reactive oxygen species (ROS) that are produced in various mammalian systems due to environmental radiation, aerobic metabolism and endogenous biochemical processes. Accumulation of H2O2 oxidant causes oxidative stress and leads to cataractogenesis [22–24]. Even though the underlying mechanism by which AQPs permeate H2O2 remains unclear, structural analyses favor the notion that the classical AQPs should be able to permeate H2O2 [19,20,24] owing to the structural similarities of H2O and H2O2 molecules.
The present study was undertaken to determine whether the lens AQPs, AQP0, AQP1, and AQP5 permeate H2O2 bidirectionally across the plasma membrane. We used MDCK cells stably expressing mouse AQP0, AQP1, AQP5 and human lens epithelial cells to determine the role of the specific AQPs in H2O2 transport. Lenses of WT and AQP knockout mouse models were also tested ex vivo for H2O2 transport and hyperglycemia-induced H2O2 production and transport.
2. Materials and methods
2.1. Mice
The wild type (WT), and knockout mouse models used in this investigation were in the C57BL/6J strain. AQP0 original knockout (in a mixed strain) was from Dr. Shiels (Washington University, MO) and we strain-transferred to C57BL/6J [8]. AQP1−/− was originally developed by Dr. Verkman Lab [25]. AQP5 knockout was from Dr. Menon Lab. (University of Cincinnati, OH;[26]). Glutathione peroxidase 1 (GPX1) knockout was developed by Dr. Ho Lab. (Wayne State University, MI; [27–28]). For animal procedures, the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, the National Institutes of Health’s (NIH; Bethesda, MD, USA) “Guide for the Care and Use of Laboratory Animals” and protocols approved by Stony Brook University Animal Care and Use Committee, were followed.
2.2. Western blotting and immunofluorescence analyses
Previously established MDCK cells that stably-express mouse WT AQP0, AQP1 or AQP5 [6,29,30] were used for both analyses. HLE lysates from ScienCell Research Laboratories (Carlsbad, CA) were also used for Western blotting. Details are given in the Supplementary Section as Appendix I).
2.3. In vitro analysis of H2O2 transport by lens AQPs
2.3.1. Using a cell culture expression model
MDCK cells stably-expressing mouse WT AQP0, AQP1 or AQP5 were cultured in 24-well plates in serum-free medium 199 (Gibco). Quantitative H2O2 assay was done using the H2O2-specific AbGreen indicator (H2O2 Assay Kit; Abcam, Cambridge, MA), according to the manufacturer’s instructions. The cell-permeable AbGreen reacts with H2O2 and produces a green fluorescence. The AbGreen probe was added to MDCK control (parental) cells, and AQP0, AQP1 or AQP5 stably-expressing cells, incubated for 30 min and washed three times 5 min each in 1XPBS. AbGreen-loaded cells were exposed to 10 μM H2O2 and imaged simultaneously for 15 min using a Zeiss confocal epifluorescent microscope with a FITC filter. Fluorescence intensity was quantified using the SigmaScan Pro, Version 5 (Systat Software, Inc., San Jose, CA). H2O2 transported through each AQP was calculated by subtracting the background fluorescence value for MDCK control cells from the fluorescence intensity value of AQP0, AQP1 or AQP5-expressing cells.
2.3.2. Using human lens epithelial (HLE) cells
Two groups of HLE cells (HLEpiC, ScienCell Research Laboratories, Carlsbad, CA) were cultured in 8-well plates in triplicates using ScienCell epithelial medium. At ~70% confluence, experimental cells were treated with 50 μM mercuric chloride (HgCl2) for 30 min to inhibit H2O2 uptake by AQPs. H2O2 assay was done as described above (section 2.3.1). HLE cells not treated with HgCl2 served as control. Cells were imaged and AbGreen fluorescence due to H2O2 uptake was quantified as above.
2.4. Ex vivo investigation of H2O2 transport by mouse lens AQPs
2.4.1. Fluorometric Assay using WT and AQP0+/− or WT and AQP5−/− mouse lenses, and AbGreen probe
Ex vivo quantitative measurement of H2O2 in 1-month-old lenses of WT and AQP0+/−, and 4-month old lenses of WT and AQP5−/− mice was done using the H2O2 Assay Kit (Abcam). WT, AQP0+/− and AQP5−/− lenses were dissected out gently, placed on a BSA-coated culture dish containing lens culture medium (in mM: NaCl 150, KCl 4.7, MgCl2 1, glucose 5, HEPES 5, pH 7.4), incubated at 37°C for 24 hours and exposed to AbGreen probe for 1 hour. The lenses were washed three times 5 min each using lens culture medium to remove the probe in the extracellular spaces of the lens. Exposure of the lenses to 50μM H2O2 and fluorescence intensity imaging were done simultaneously as described in section 2.3.1; fluorescence intensity was quantified using the SigmaScan Pro software. H2O2 uptake was calculated using the pixel brightness intensity, which is proportional to H2O2-specific AbGreen uptake.
2.4.2. Colorimetric Assay using WT, AQP0+/−, AQP1−/−, AQP5−/− and GPX1−/− mouse lenses, and OxiRed probe
Three-month-old transparent lenses of WT, AQP0+/−, AQP1−/− AQP5−/− and GPX1−/− were used for experiments using the OxiRed probe as given in the Supplementary section, Appendix I.
2.5. Effect of Hyperglycemia in AQP5−/− mouse lens transparency and H2O2 transport
The hyperglycemic effect on WT and AQP5−/− mouse lenses was tested as described previously with modifications [31]. H2O2 was quantified by means of the Fluorometric Assay using the AbGreen probe as described on section 2.3.1. Details are provided in the Supplementary section as Appendix I.
2.6. Statistical analysis
Statistical analysis was performed using the two-tailed Student’s t-test.
3. Results and Discussion
We sought to find out whether lens AQP0, AQP1 and AQP5 permeate H2O2 in the cultured cells in vitro. As a first step, MDCK cells stably-expressing AQP0, AQP1 or AQP5 [6,29–31] were immunostained with the corresponding antibody to verify the expression of the respective protein; Figure 1 shows the expression AQP0 (A), AQP1 (B) and AQP5 (C). To investigate H2O2 transport by these AQPs, the MDCK cells expressing AQP0, AQP1 or AQP5 were exposed to AbGreen for 30 minutes. The AbGreen-loaded cells were exposed to 10 μM H2O2 and instantaneously imaged for 15 minutes to record the progression in the green fluorescence due to H2O2 uptake (Fig. 1D). Fluorescence intensity was quantified and expressed in pixel brightness intensity (Fig. 1E) which is proportional to the H2O2 transport into the cells. Compared to the MDCK control cells, the fluorescence intensity values for AQP0, AQP1 or AQP5-expressing cells were higher indicating the presence of significantly (P<0.01) more intracellular H2O2 (Fig. 1E). This experiment demonstrates that each of these AQPs can transport H2O2 in vitro; the transport efficiency can be represented as AQP5>AQP1>AQP0.
Fig.1.

Expression of lens aquaporins and H2O2 transport. MDCK cells expressing (A) AQP0, (B) AQP1 or (C) AQP5 immunostained with anti-AQP0, anti-AQP1 and anti-AQP5. Arrows - Texas-Red tagged secondary antibody binding to the respective antibody. N- Nucleus. D. AbGreen fluorescence due to H2O2 transport by control MDCK cells or MDCK cells expressing transfected AQP0, AQP1 or AQP5. E. Quantification of H2O2 levels. AQP-expressing cells transported H2O2 significantly more compared to the Control cells, P< 0.05.
Next, we tested in vitro H2O2 transport by the AQPs expressed in the human lens epithelial (HLE) cells. The expression of the different AQPs in these cells was explored by Western blotting using the appropriate antibodies. We found that in addition to the already reported AQP1 and AQP5, HLE cells also express AQP8 (Fig. 2A, lane 3). H2O2 transport by AQPs in HLE cells was verified by conducting an inhibition experiment. Experimental HLE cells were treated with 50μM HgCl2 for 30 minutes, to inhibit H2O2 transport; Figure 2B shows inhibition of H2O2 in HgCl2-treated cells (right panel) compared to untreated control cells (left panel). Quantification of the fluorescence intensity data (Fig. 2C) showed that the reduction in H2O2 transport was statistically significant (P< 0.01).
Fig. 2.
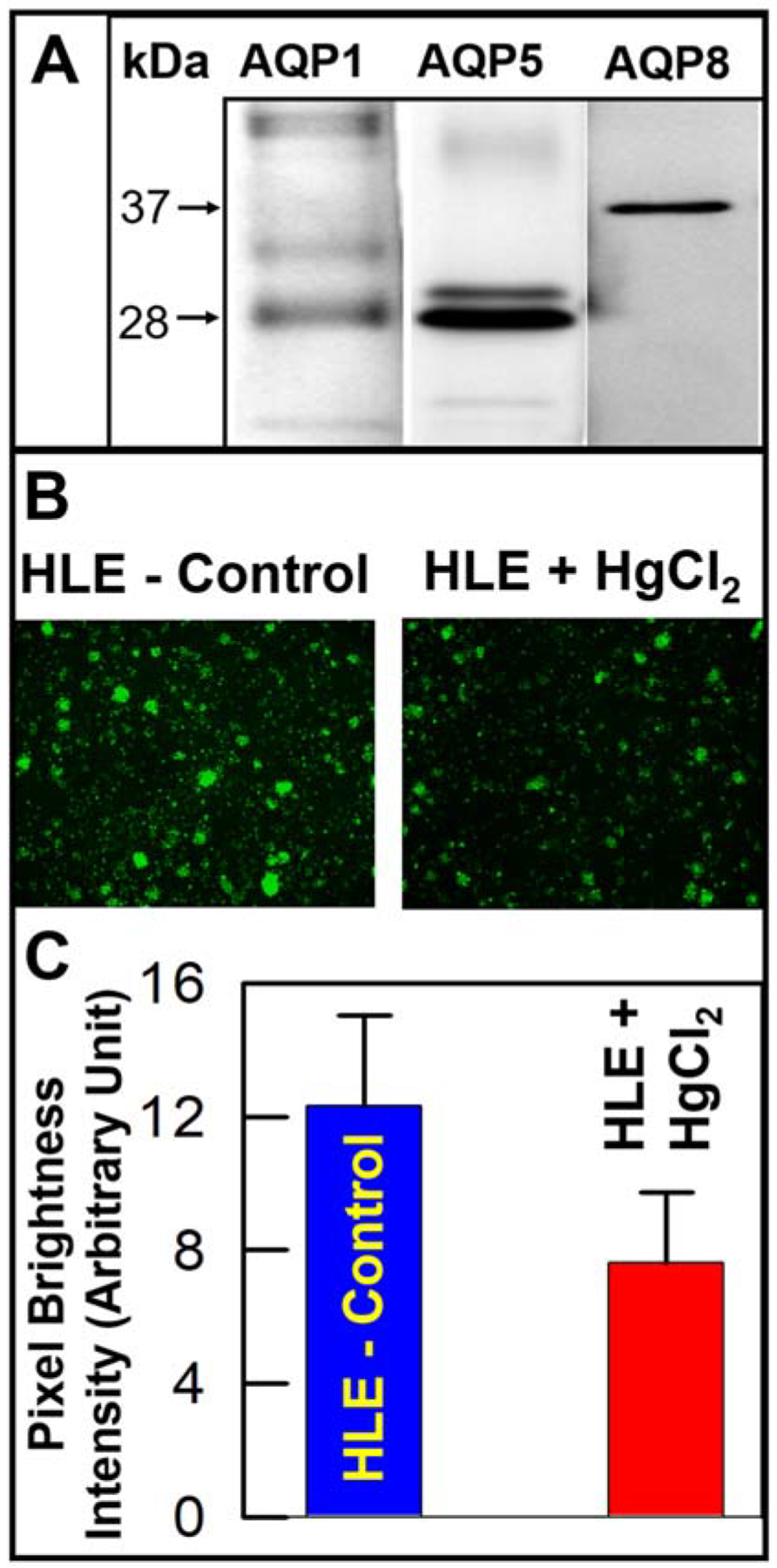
Expression of AQPs in human lens epithelial (HLE) cells and H2O2 transport. A. Western blotting showing AQP1, AQP5 and AQP8 expression in human HLE cells, using AQP-specific antibodies. AQP1 and AQP5 antibodies each bound to a ~28 kDa band. Antibody to AQP5 also bound to a slightly larger molecular size band, most likely a product of posttranslational modification. AQP8 antibody immunoreacted with a ~37 kDa protein. B. AbGreen fluorescence in HLE cells due to H2O2 transport by the AQPs expressed (left panel) and that in HLE cells treated with HgCl2 to inhibit H2O2 transport (right panel). C. Quantification of H2O2 transport level in control HLE cells and those treated with HgCl2.
Ex vivo experiments for H2O2 transport were conducted in one-month-old WT and AQP0+/−, and 4-month-old WT and AQP5−/− mouse lenses using the AbGreen probe. Figure 3A shows higher levels of intracellular H2O2 in the WT than in the other lenses. Quantification of H2O2 uptake showed that loss of 50% AQP0 or 100% AQP5 significantly (P< 0.001) reduced H2O2 transport into the lens by ~44 and ~50% respectively (Fig. 3B). Ex vivo quantification studies were pursued using another probe OxiRed combined with a Colorimetric method. The experiments were conducted in 3-month-old AQP0+/−, AQP1−/−, AQP5−/− and GPX1−/− mouse lenses. GPX1 is an H2O2 scavenging enzyme; lenses lacking GPX1 were tested to find out the role of GPX1 in lenticular H2O2 homeostasis. Monitoring and quantifying the H2O2 transport for 30 minutes showed that compared to the WT lenses, there was significant (P<0.01) reduction in the transport in the lenses of the mouse models (Fig. 3C). The WT lenses, with AQP0, AQP1, AQP5 and GPX1, transported H2O2 at a rate of 90 ± 5 nM/s. Loss of 50% AQP0 reduced the rate to ~73 ± 5 nM/s. AQP0−/− lenses were not included in the study because they develop cataracts even at embryonic stages. Knockout of epithelial cell AQP1, reduced the H2O2 transport to a rate of 76 ± 5 nM/s; loss of AQP5, which is present both in the lens epithelial and fiber cells and accounts for ~0.5% of the membrane protein in the lens, reduced the H2O2 transport to 67 ± 5 nM/s. Based on the data obtained from our experiments, the degree of ex vivo H2O2 transport can be represented as AQP5>AQP0>AQP1. However, based on the quantity of each type of AQP protein expressed in the lens, the extent of H2O2 transport could be AQP0>AQP5>AQP1. AQP0 contributes about 45% of the total membrane protein in the lens, justifying the possibility of being the most significant H2O2 transporter in the lens, a noncanonical role for a classical water pore. By functioning as peroxiporins, AQPs could reduce H2O2-induced oxidative damage to the lens. The H2O2 transported into the cells by AQPs are scavenged as necessary by GPX1 to maintain homeostasis. The loss of GPX1 reduced the H2O2 transport to 77 ± 4 nM/s (Fig. 3C). Absence of GPX1 causes accumulation of H2O2 in the lens, which eventually reaches an equilibrium with the extracellular H2O2 and prevents the further influx of H2O2 through AQPs. During aging, a reduction in GPX1 protein level or activity could cause H2O2 accumulation in the lens and lead to oxidative stress-induced cataractogenesis [28,32,33].
Fig. 3.
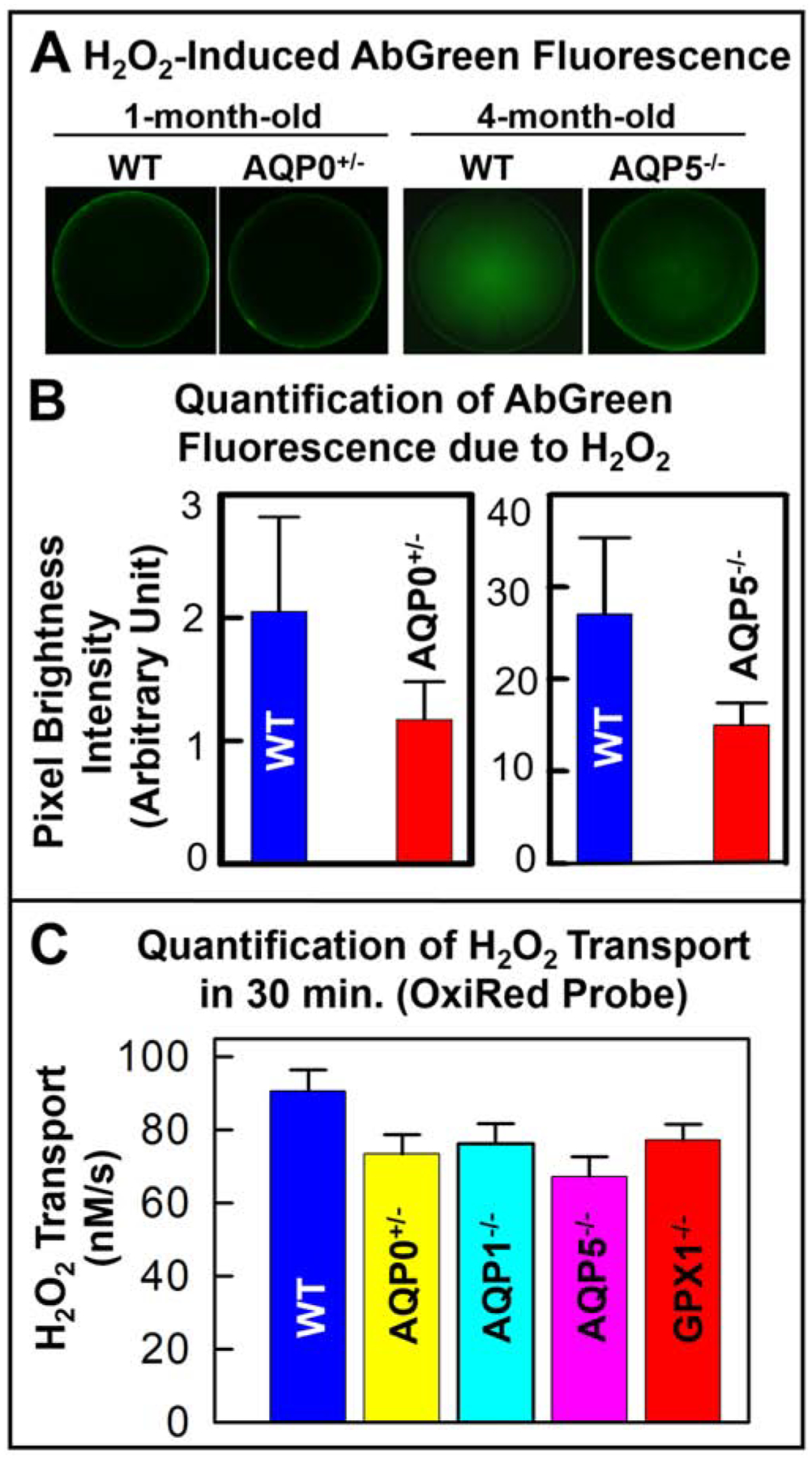
H2O2 transport in the lenses of mouse models. A. H2O2 uptake by 1-month-old lenses of WT and AQP0+/− or 4-month-old lenses of WT and AQP5−/− exposed to 50μM H2O2 for 30 min., using a Fluorometric assay; B. Quantification of the H2O2 levels. C. Quantification of H2O2 transport (in 30 min.) in 4-month-old lenses of WT, AQP0+/−, AQP1−/−, AQP5−/− and GPX1−/−, using OxiRed probe and a Colorimetric Assay.
Hyperglycemia induces increased production of H2O2 in the mitochondria and leads to oxidative stress [34,35]. We tested hyperglycemia-induced intracellular H2O2 levels in the lenses of WT and AQP5−/− mouse lenses, which were cultured in 3 mM (normal, control) or 30 mM (hyperglycemic) glucose for 48 hours. At the end of the experiments, transparency was reduced in the lenses cultured under hyperglycemic conditions (Fig. 4A, right column). Lenses of AQP5−/− showed a significant decrease in transparency (P< 0.01) at high glucose compared to those of WT (Fig. 4B); in the Figure, the pixel brightness intensity is inversely proportional to transparency. WT and AQP5−/− lenses were exposed to AbGreen for one hour and imaged for the green fluorescence due to the level of H2O2 present. The intracellular H2O2 levels were quantified. Figure 4 panels C and D show that the high glucose in the culture medium causes an increase in the intracellular H2O2 levels; in panel D, the pixel brightness intensity is proportional to the H2O2 level. When the levels of H2O2 were compared, AQP5−/− lenses subjected to hyperglycemia showed significantly (P<0.01) higher H2O2 level than the WT control, the WT lenses exposed to the same hyperglycemic conditions or the untreated AQP5−/− lenses. In the WT, when there is a higher level of intracellulaH2O2, AQP5-facilitated-efflux from the cells helps to maintain H2O2 balance and homeostasis, as there is no significant build-up of H2O2 (Fig. 4D). In AQP5−/− lenses exposed to high glucose, the AQP5-facilitated efflux route is not present and there is a significant build-up of H2O2 causing lens opacity, possibly due to oxidative damage.
Fig. 4.

Effect of hyperglycemia on lens transparency. A. Lens transparency in 4-month- old WT and AQP5−/− mouse lenses incubated in normal (3mM glucose) or hyperglycemic (30mM glucose) culture medium for 48 hrs. B. Quantification of the lens transparency due to glucose treatment. Pixel brightness intensity is inversely proportional to lens transparency. C. AbGreen fluorescence due to H2O2 transport in 4-month-old AQP5−/− lenses incubated in normal glucose (3 mM) and hyperglycemic glucose (Hyp-Gly; 30 mM) conditions. WT lenses not shown. D. Quantification of H2O2 level in 4-month-old lenses of WT and AQP5−/− subjected to normal glucose (blue and black bars) and hyperglycemic (red bars; Hyp-Gly) conditions. Pixel brightness intensity is directly proportional to the H2O2 level.
H2O2 plays a paradoxical dual role. At low concentrations, H2O2 functions as a signaling molecule [36,37], while at high concentrations it leads to oxidative damage that alters cellular functions and causes apoptosis [38,39]. High concentrations of H2O2 are cytotoxic to a wide range of animal, plant and bacterial species depending upon the physiological state and duration of exposure of the tissue [22, 40–42]. In the lens, H2O2 is mainly produced in the mitochondria and endoplasmic reticulum of epithelial and peripheral fiber cells. Acute H2O2-induced oxidative stress in human lens epithelial cells showed significant up- and down-regulation involving 1171 genes [43]. High production of ROS during aging or a significant decrease in the ROS scavenging ability of the lens, causes oxidative stress and leads to cataracts [44–48]. H2O2 concentration in the aqueous humor is ~30–70 μM; higher concentrations (>600 μM) are seen in certain cataract patients [47–49].
Maintaining a balance of H2O2 is critical for lens transparency since H2O2 in excess causes oxidative damage to lens proteins, lipids, and DNA that results in opacity and cataract [50]. The current investigation demonstrates that lens AQPs, AQP0, AQP1 and AQP5 can transport extracellular and intracellular H2O2 into and out of cells, respectively, with varying degrees of efficiency. In vitro expression of AQP0, AQP1 or AQP5 into MDCK cells increased the uptake of exogenous H2O2. Treatment with AQP-inhibitor mercury significantly reduced the uptake of H2O2 by HLE cells. Absence of AQP0 (partial), AQP1, AQP5 or GPX1 significantly inhibited the entry of H2O2 into the lens. Reduction in H2O2 transport by the reduction or loss of lens AQPs or GPX1 further suggests that lens AQPs and GPX1 coordinate in maintaining optimum levels of H2O2 to prevent oxidative damage to the long-lasting proteins in the lens; age-related loss or reduction of these proteins could lead to lens cataract. Hyperglycemic condition induced increased H2O2, and loss of AQP5 increased H2O2 accumulation. Our results support the involvement of lens AQPs in H2O2 transport in both mouse and human lens cells.
Among the three AQPs tested, the AQP5 channel appears to be the most efficient H2O2 transporter in the lens. AQP5 is expressed in both epithelial and fiber cells. Previous studies have shown that after the synthesis, AQP5 is stored in the cytoplasmic vesicles and upon demand, traffics to the plasma membrane. It is a regulatory AQP in the lens [30,51]. Therefore, AQP5 could be acting as the predominant regulator of H2O2 to prevent oxidative stress in the lens. Under normal conditions, AQP0 in the fiber cells and AQP1 in the epithelial cells function as H2O2 transporters for housekeeping; however, under stressful conditions, AQP5 could be trafficking to the membrane to reduce H2O2 accumulation.
In conclusion, our in vitro and ex vivo investigations demonstrate the noncanonical function of lens AQPs as H2O2 transporters/ peroxiporins. In coordination with GPX1, AQPs play a significant role in maintaining H2O2 homeostasis in the lens, thereby reducing H2O2-induced oxidative damage. Further studies involving lens AQPs and H2O2 detoxifying enzymes will help to understand their coordinated roles in modulating H2O2 transport and managing oxidative stress to prevent lens age-related cataracts.
Supplementary Material
Highlights.
Mammalian lens aquaporins transport hydrogen peroxide
AQP0, AQP1 and AQP5 function as peroxiporins
Normal human lens epithelial cells express AQP1, AQP5 and AQP8
Hyperglycemia enhances H2O2 production and accumulation in the lens
Knockout of AQP5 causes significant increase in H2O2 accumulation in the lens
Acknowledgments
This work was supported by NIH-NEI grant R01 EY026155.
Footnotes
Publisher's Disclaimer: This is a PDF File of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its Final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Mathias RT, Rae JL, Baldo GJ, Physiological properties of the normal lens, Physiol. Rev 77 (1997) 21–50. [DOI] [PubMed] [Google Scholar]
- [2].Mathias RT, Kistler J, Donaldson P, The lens circulation J Membr. Biol 216 (2007) 1–16. [DOI] [PubMed] [Google Scholar]
- [3].Varadaraj K, Kumari SS, Shiels A, et al. , Regulation of aquaporin water permeability in the lens, Invest. Ophthalmol. Vis. Sci 46 (2005)1393–1402. [DOI] [PubMed] [Google Scholar]
- [4].Varadaraj K, Kumari S, Deletion of seventeen amino acids at the C-terminal end of Aquaporin 0 causes distortion aberration and cataract in the lenses of AQP0ΔC/ΔC mice, Invest. Ophthalmol. Vis. Sci 60 (2019) 858–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Varadaraj K, Gao J, Mathias RT, et al. , C-terminal end of Aquaporin 0 regulates lens gap junction channel function, Invest. Ophthalmol. Vis. Sci 60 (2019) 2525–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kumari SS, Varadaraj K, Intact AQP0 performs cell-to-cell adhesion, Biochem. Biophys. Res. Commun 390 (2009) 1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kumari SS, Varadaraj K, Aquaporin 0 plays a pivotal role in refractive index gradient development in mammalian eye lens to prevent spherical aberration, Biochem. Biophys. Res. Commun 452 (2014) 986–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kumari S, Gao J, Mathias RT, et al. , Aquaporin 0 Modulates Lens Gap Junctions in the Presence of Lens-Specific Beaded Filament Proteins, Invest. Ophthalmol. Vis. Sci 58 (2017) 6006–6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kumari SS, Varadaraj K, A predominant form of C-terminally end-cleaved AQP0 functions as an open water channel and an adhesion protein in AQP0ΔC/ΔC mouse lens, Biochem. Biophys. Res. Commun 511 (2019) 626–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shiels A, Bassnett S, Varadaraj K, et al. , Optical dysfunction of the crystalline lens in aquaporin-0-deficient mice, Physiol. Genomics, 7 (2001) 179–186. [DOI] [PubMed] [Google Scholar]
- [11].Schey KL, Wang ZL, Wenke J, et al. , Aquaporins in the eye: expression, function, and roles in ocular disease, Biochim. Biophys. Acta 1840 (2014) 1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schey KL, Petrova RS, Gletten RB, et al. , 2017. The Role of Aquaporins in ocular lens homeostasis. Int. J. Mol. Sci 18, e2693. doi: 10.3390/ijms18122693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gu S, Biswas S, Rodriguez L, et al. , Connexin 50 and AQP0 are essential in maintaining organization and integrity of lens fibers, Invest. Ophthalmol. Vis. Sci 60 (2019) 4021–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tapodi A, Clemens DM, Uwineza A, et al. , 2019. BFSP1 C-terminal domains released by post-translational processing events can alter significantly the calcium regulation of AQP0 water permeability. Exp. Eye Res 185, 107585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Varadaraj K, Kumari SS, Molecular mechanism of Aquaporin 0-induced fiber cell to fiber cell adhesion in the eye lens, Biochem. Biophys. Res. Commun 506 (2018) 284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sindhu Kumari S, Gupta N, Shiels A, et al. , Role of Aquaporin 0 in lens biomechanics, Biochem. Biophys. Res. Commun 462 (2015) 339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bienert GP, Moller AL, Kristiansen KA, et al. , Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes, J. Biol. Chem 282 (2007) 1183–1192. [DOI] [PubMed] [Google Scholar]
- [18].Miller EW, Dickinson BC, Chang CJ, Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling, Proc. Natl. Acad. Sci. USA 107 (2010) 15681–15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bienert GP, Chaumont F, Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide, Biochim. Biophys. Acta, 1840 (2014) 1596–1604. [DOI] [PubMed] [Google Scholar]
- [20].Almasalmeh A, Krenc D, Wu B, et al. , Structural determinants of the hydrogen peroxide permeability of aquaporins, FEBS J. 281 (2014) 647–656. [DOI] [PubMed] [Google Scholar]
- [21].Watanabe SS, Moniaga CS, Nielsen S, et al. , Aquaporin-9 facilitates membrane transport of hydrogen peroxide in mammalian cells, Biochem. Biophys. Res. Commun 471 (2016) 191–197. [DOI] [PubMed] [Google Scholar]
- [22].Smith AJ, Ball SS, Manzar K, et al. , Ku80 counters oxidative stress-induced DNA damage and cataract formation in the human lens, Invest. Ophthalmol. Vis. Sci 56 (2015) 7868–7874. [DOI] [PubMed] [Google Scholar]
- [23].Ji Y, Cai L, Zheng T, et al. , The mechanism of UVB irradiation induced-apoptosis in cataract, Mol. Cell Biochem 401 (2015) 87–95. [DOI] [PubMed] [Google Scholar]
- [24].Cordeiro RM, Molecular dynamics simulations of the transport of reactive oxygen species by mammalian and plant aquaporins, Biochim. Biophys. Acta 1850 (2015) 1786–1794. [DOI] [PubMed] [Google Scholar]
- [25].Ma TT, Yang B, Gillespie A, et al. , Severely impaired urinary concentrating ability in transgenic mice lacking aquaporin-1 water channels, J. Biol. Chem 273 (1998) 4296–4299. [DOI] [PubMed] [Google Scholar]
- [26].Krane CM, Melvin JE, Nguyen HV, et al. , Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation, J. Biol. Chem 276 (2001) 23413–23420. [DOI] [PubMed] [Google Scholar]
- [27].Ho Y-S, Magnenat JL, Bronson RT, et al. , Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia, J. Biol. Chem 272 (1997)16644–16651. [DOI] [PubMed] [Google Scholar]
- [28].Reddy VN, Lin L-R, Ho Y-S, et al. , Peroxide-induced damage in lenses of transgenic mice with deficient and elevated levels of glutathione peroxidase, Ophthalmologica. 211 (1997) 192–200. [DOI] [PubMed] [Google Scholar]
- [29].Sindhu Kumari S, Varadaraj K, Intact and N- or C-terminal end truncated AQP0 function as open water channels and cell-to-cell adhesion proteins: end truncation could be a prelude for adjusting the refractive index of the lens to prevent spherical aberration, Biochim. Biophys. Acta 1840 (2014) 2862–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kumari SS, Varadaraj M, Yerramilli VS, Spatial expression of aquaporin 5 in mammalian cornea and lens, and regulation of its localization by phosphokinase A, Mol. Vis 18 (2012) 957–967. [PMC free article] [PubMed] [Google Scholar]
- [31].Kumari SS, Varadaraj K, Aquaporin 5 knockout mouse lens develops hyperglycemic cataract, Biochem. Biophys. Res. Commun 441 (2013) 333–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang H, Gao J, Sun X, The effects of GPX-1 knockout on membrane transport and intracellular homeostasis in the lens, J. Membr. Biol 227 (2009) 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Spector A, Kuszak JR, Ma W, The effect of aging on glutathione peroxidase-1 knockout mice-resistance of the lens to oxidative stress, Exp. Eye Res 72 (2001) 533–545. [DOI] [PubMed] [Google Scholar]
- [34].Wu X, Zhu L, Zilbering A, et al. , Hyperglycemia potentiates H(2)O(2) production in adipocytes and enhances insulin signal transduction: potential role for oxidative inhibition of thiol-sensitive protein-tyrosine phosphatases, Antioxid. Redox. Signal 7 (2005) 526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Saygili EI, Aksoy SN, Gurler B, et al. , Oxidant/Antioxidant Status of Patients with Diabetic and Senile Cataract. Biotechnology & Biotechnological Equipment, 24 (2010) 1648–1652. [Google Scholar]
- [36].Dickinson BC, Chang CJ, Chemistry and biology of reactive oxygen species in signaling or stress responses, Nat. Chem. Biol 7 (2011) 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lismont C, Revenco I, Fransen M, 2019. Peroxisomal hydrogen peroxide metabolism and signaling in health and disease. Int. J. Mol. Sci 20, E3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pizzino G, Irrera N, Cucinotta M, 2017. Oxidative Stress: harms and benefits for human health. Oxid. Med. Cell Longev 2017, 8416763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hampton MB, Orrenius S, Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis, FEBS Lett. 414 (1997) 552–556. [DOI] [PubMed] [Google Scholar]
- [40].Uwineza A, Kalligeraki AA, Hamada N, Cataractogenic load - A concept to study the contribution of ionizing radiation to accelerated aging in the eye lens, Mutat. Res 779 (2019) 68–81. [DOI] [PubMed] [Google Scholar]
- [41].Taylor A, Davies KJ, Protein oxidation and loss of protease activity may lead to cataract formation in the aged lens, Free Radic. Biol. Med 3 (1987) 371–377. [DOI] [PubMed] [Google Scholar]
- [42].Halliwell B., Gutteridge JMC, Free Radicals in Biology and Medicine, third ed., Clarendon Press, Oxford, 1999. [Google Scholar]
- [43].Goswami S, Sheets NL, Zavadil J, Spectrum and range of oxidative stress responses of human lens epithelial cells to H2O2 insult, Invest. Ophthalmol. Vis. Sci 44 (2003) 2084–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Spector A A, Oxidative stress-induced cataract: mechanism of action, FASEB J. 9 (1995) 1173–1182. [PubMed] [Google Scholar]
- [45].Truscott RJ, Age-related nuclear cataract-oxidation is the key, Exp. Eye Res 80 (2005) 701–725. [DOI] [PubMed] [Google Scholar]
- [46].Lofgren S, Solar ultraviolet radiation cataract, Exp Eye Res. 156 (2016) 112–116. [DOI] [PubMed] [Google Scholar]
- [47].Spector A, Garner WH, Hydrogen peroxide and human cataract. Exp Eye Res. 33 (1981) 673–681. [DOI] [PubMed] [Google Scholar]
- [48].Ramachandran S, Morris SM, Devamanoharan P, et al. , Radio-isotope determination of hydrogen peroxide in aqueous humor and urine, Exp. Eye Res 53 (1991) 503–506. [DOI] [PubMed] [Google Scholar]
- [49].Sharma Y, Druger R, Mataic D, et al. , 1997. Aqueous humor hydrogen peroxide and cataract. Invest. Ophthalmol. Vis. Sci 38, S1149. [Google Scholar]
- [50].Berthoud VM, Beyer EC, Oxidative stress, lens gap junctions, and cataracts, Antioxid. Redox. Signal 11 (2009) 339–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Petrova RS, Webb KF, Vaghefi E, Dynamic functional contribution of the water channel AQP5 to the water permeability of peripheral lens fiber cells, Am. J. Physiol. Cell Physiol 314 (2018) C191–C201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


