Abstract
Inheritance of apolipoprotein E4 (APOE4) is a major risk factor for development of Alzheimer disease (AD). This lipoprotein, in contrast to apoE2, has arginine residues at positions 112 and 158 in place of cysteines in the latter isoform. In apoE3, the Cys at residue 158 is replaced by an arginine residue. This differential amino acid composition of the three genotypes of APOE have profound influence on the structure, binding properties, and multiple functions of this lipoprotein. Moreover, AD brain is under a high degree of oxidative stress, including that associated with amyloid β-peptide (Aβ) oligomers. Lipid peroxidation produces the highly reactive and neurotoxic molecule, 4-hydroxynonenal (HNE) that forms covalent bonds with cysteine residues (Cys) [as well as with Lys and His residues]. Covalently modified Cys significantly alter structure and function of modified proteins. HNE bound to Cys residue(s) on apoE2 and apoE3 lessens the chance of HNE damage other proteins. apoE4, lacking Cys residues, is unable to scavenge HNE, permitting this latter neurotoxic molecule to lead to oxidative modification of neuronal proteins and eventual cell death. We posit that this lack of HNE scavenging activity in apoE4 significantly contributes to the association of APOE4 inheritance and increased risk of developing AD. Apoe knock-out mice provide insights into the role of this lipoprotein in oxidative stress. Targeted replacement mice in which the mouse gene of Apoe is separately replaced by the human APOE2, APOE3, or APOE4 genes, while keeping the mouse promoter assures the correct location and amount of the human protein isoform. Human APOE targeted replacement mice have been used to investigate the notion that oxidative damage to and death of neurons in AD and its earlier stages is related to APOE genotype. This current paper reviews the intersection of human APOE genotype, oxidative stress, and diminished function of this lipoprotein as a major contributing risk factor for development of AD. Discussion of potential therapeutic strategies to mitigate against the elevated risk of developing AD with inheritance of the APOE4 allele also is presented.
Keywords: Apolipoprotein E, oxidative stress, Alzheimer disease, key cysteine residues, targeted replacement mice
1. Introduction
Apolipoprotein E (apoE) has several functions in the brain, among which are cholesterol transport and efflux of key moieties from brain to blood, for example efflux of amyloid β-peptide [Aβ] in cooperation with the low density lipoprotein receptor-like protein 1 [LRP-1] (Munoz et al., 2019; Mahley, 1988; Herz and Beffert, 2000). apoE exists in three isoforms, encoded by three different gene alleles, APOE2, APOE3, and APOE4. In humans, the levels of isoforms of apoE occur in the order, apoE3 > apoE4 > apoE2. In brain, apoE is localized in astrocytes, but can be transported to neurons.
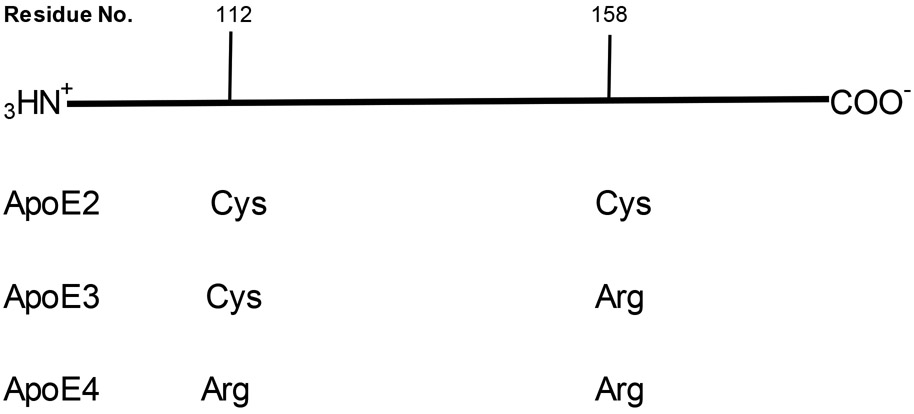
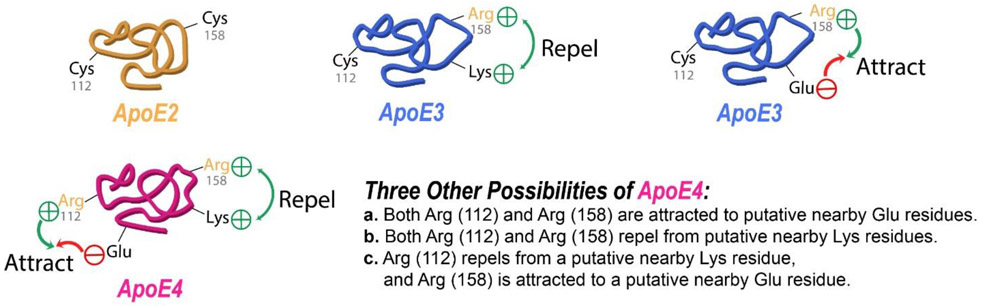
There are key conformational and structural differences among the three isoforms of apoE (Figure 1): apoE2 has two cysteine residues (residues 112 and 158), while apoE3 has residue 158 replaced by a positively charged arginine residue, and apoE4 has both cysteine residues replaced by positively charged arginine residues (Munoz et al., 2019; Yamazaki et al., 2019). This differential amino acid sequence among the three isoforms of apoE leads to differential conformations (Figure 2), which lead to differential interactions among these isoforms with amyloid β-peptide and hyperphosphorylated tau protein resulting in neurotoxicity (Strittmatter et al., 1993; Winkler et al., 1999; Liu et al., 2017; Richey et al., 1995; Shi et al., 2017). These differences in conformation are easily understood in terms of electrostatics. Namely, either one or two positively charged arginines will be forced away from or toward nearby positively charged Lys or negatively charged glutamate or aspartate residues, respectively, thereby changing the 3-dimensional structure of the apoE3 or apoE4 relative to apoE2.
Figure 1.
Schematic representation of the amino acid sequence of apoE2, apoE3, and apoE4, showing the differential cysteine content among the three isoforms at residues 112 and 158. Cys residues can covalently bind HNE to prevent this highly reactive and neurotoxic molecule from binding to cellular proteins, thereby protecting these proteins, while Arg cannot perform this function. See text.
Figure 2.
Schematic representation of idealized conformations in apoE2, apoE3, and apoE4 to illustrate different putative conformations possible by replacement of Cys residues by positively charged Arg and the resultant repulsion or attraction between these Arg residues and positively charged or negatively charged amino acids, respectively. In the case of apoE4 there exist four possibilities.
These same principles affect how apoE3 or apoE4 interact with other proteins or peptides. Indeed, interaction of apoE isoforms with Aβ is critical to the increased risk of developing Alzheimer disease (AD), with persons inheriting the APOE4 allele having a significantly different interaction between apoE4 and Aβ and also a greater risk of age-dependent development of this dementing disorder (Strittmatter et al., 1993; Liu et al., 2017; Richey et al., 1995; Winkler et al., 1999). In persons with AD, the presence of apoE4 appears to strengthen the pathology of this disorder, including Aβ fibril deposition and Aβ oligomer production, neurofibrillary tangle formation, neuronal death, decreased synaptic plasticity associated with learning and memory, loss of lipid bilayer compositional asymmetry and lipid homeostasis, and oxidative stress (Markesbery, 2010; Bader-Lange et al., 2008; Cedazo-Minguez and Cowburn, 2001; Tudorache et al., 2017; Mahley et al., 2006; Cedazo-Minguez, 2007). Oxidative damage can induce or is associated with each of these pathological alterations in AD brain (Butterfield et al., 2001; Butterfield and Halliwell, 2019).
As discussed further below, another factor that can influence the role of apoE isoforms on development of AD may be related to oxidative stress that varies with apoE isoforms. Brains from persons with AD and its earlier stage, amnestic mild cognitive impairment (MCI), are under significant oxidative and nitrosative stress (Hensley et al., 1995; Keller et al., 2005; Butterfield et al., 2001; 2006a; 2012; Butterfield and Halliwell, 2019; Butterfield and Boyd-Kimball, 2019).
The current paper reviews the relationships of among oxidative stress, APOE allele status, and AD.
2. Oxidative and Nitrosative Stress in Brain
Due to the high levels of polyunsaturated fatty acid content, the high exposure to inspired oxygen, and the presence of redox-active transition metal ions, i.e., Fe2+, Cu+), oxidative damage in brain is a prominent risk. The principal types of oxidative damage are protein oxidation [indexed by protein carbonyls; 3-nitrotyrosine], lipid peroxidation [indexed by 4-hydroxy-2-trans-nonenal (HNE), among other indices] and DNA oxidation [indexed by 8-hydroxy-2-deoxyguanosine] (Halliwell and Gutteridge, 2015).
Protein carbonyls arise due to: a) free radical-mediated scission of the primary amino acid sequence of proteins, with subsequent covalent binding to both cleaved ends by paramagnetic oxygen, forming aldehyde or ketone moieties; b) Oxidation of side chains of many amino acids, i.e., 4-oxohistidine; c) Covalent binding by Michael addition to cysteine, histidine, and lysine residues of target proteins by HNE, thereby bringing one or more aldehyde moieties to the protein; and 4) formation of advance glycation end products, resulting from lysine residues reacting with reducing sugars and subsequent Amadori chemistry to form aldehyde or ketone moieties (Butterfield and Stadtman, 1997).
Nitrosative stress is most often indexed by elevated levels of 3-nitrotyrosine [3-NT] (Estevez et al., 1998; Ferrer-Sueta et al., 2018; Butterfield, 2014). In the presence of nitric oxide [NO], produced from arginine in a reaction catalyzed by nitric oxide synthase [NOS]. Cytosolic-resident NOS II, also known as inducible NOS [i-NOS], is released from microglia upon activation of this brain cell. Mitochondria and certain enzymatic reactions lead to elevated superoxide free radicals. Reaction of NO with O2− in an extremely fast radical-radical recombination reaction forms the non-radical peroxynitrite [ONOO−], which through a series of reactions leads to formation of nitrogen dioxide [NO2], also a free radical. In the presence of a free radical within 0.4 nanometers of a tyrosine residue, the H-atom of the OH-group in the 4-position of the aromatic group of tyrosine is abstracted, leaving a free radical on the O-atom in this position, which translocates to the 3-position due to chemical principles. Then, the NO2 free radical rapidly reacts with the free radical on the 3-postion to form 3-nitrotyrosine. One important implication of 3-NT is that the steric interference of the NO2 group makes tyrosine kinase-catalyzed reactions more difficult if not impossible to accomplish, since the active site of the enzyme cannot easily access the OH group on the 4-position of tyrosine. Inhibition of soluble- or receptor- tyrosine kinases severely hinders required cellular signaling processes (Butterfield and Stadtman, 1997; Butterfield, 2014).
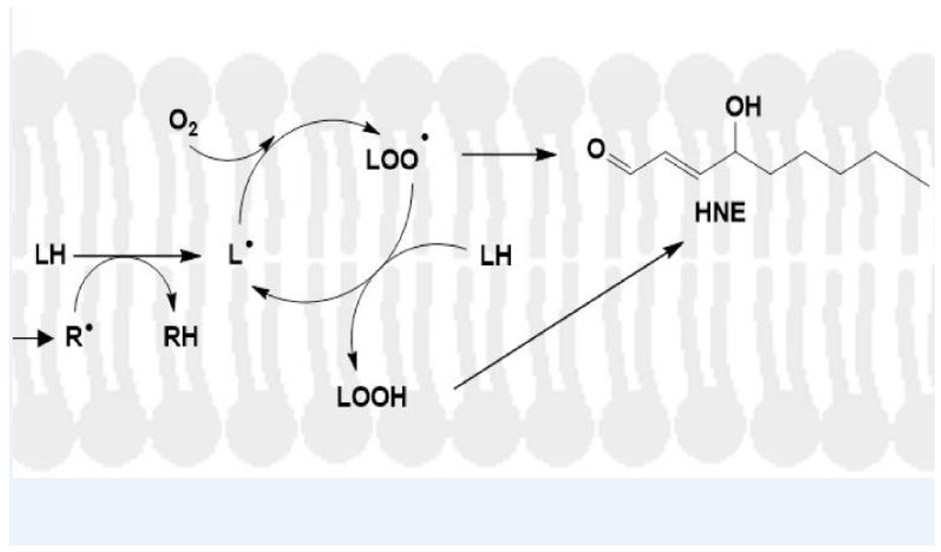
Lipid peroxidation occurs by the following mechanism (Figure 3) (Esterbauer et al., 1991; Butterfield and Halliwell, 2019: a) A free radical (R.) attack within a Van der Waals distance of labile H-atoms on acyl chains of phospholipids leads to H-atom abstraction to form RH and a carbon-centered radical on the acyl chain of the lipid; b) Binding of paramagnetic molecular oxygen [zero dipole moment, therefore highly soluble in the hydrophobic environment of the lipid bilayer] to the fatty acid-resident, C-centered free radical by radical-radical recombination forms a lipid peroxyl free radical; c) Abstraction of another allylic H-atom from the acyl chains of phospholipids by the lipid peroxyl free radical leads to formation of the lipid hydroperoxide and another acyl chain-resident C-centered free radical. That is, this is a chain reaction that can continue as long as oxygen and nearby allylic H-atoms near lipid peroxyl free radicals are present. The lipid hydroperoxide, through a series of organic reactions, leads to formation of highly reactive HNE. This is why it only takes a small concentration of initiating free radicals to greatly amplify both the free radical levels in the lipid bilayer and subsequent formation of highly reactive HNE.
Figure 3.
Mechanism of lipid peroxidation. A free radical, for example associated with Aβ42 oligomers inserted into a lipid bilayer (Butterfield and Halliwell, 2019), attacks a lipid acyl chain-resident, labile allylic hydrogen atom to form the RH compound and a carbon-centered lipid free radical, L.. Oxygen, which is paramagnetic with two unpaired electrons and has zero dipole moment, meaning it is highly soluble in the lipid bilayer, binds to the carbon-centered radical on the acyl chain to form the lipid peroxyl free radical, LOO.. This free radical, in turn, abstracts another allylic hydrogen on a fatty acid chain of the lipid to from LOOH, the lipid hydroperoxide, and another lipid-resident, carbon-centered free radical, i.e., propagating the chain reaction. LOOH, through a series of chemical reactions, spontaneously will form HNE that can covalently bind to and change the conformation and function of membrane-resident proteins (Subramaniam et al., 1997; Sultana et al., 2013). In addition, HNE can diffuse from the lipid bilayer to covalently modify cytosolic proteins (Butterfield and Stadtman, 1997). Modified from Butterfield and Boyd-Kimball, 2019.
3. Oxidative Damage in Brains from Persons with Alzheimer Disease and Mild Cognitive Impairment and Down Syndrome and Down Syndrome with Alzheimer-like Neuropathology and Dementia
Many researchers reported long ago and more recently that Aβ oligomers led to oxidative damage to neurons in culture, in animal models of AD, and in human brains (Butterfield et al., 2001; Pedersen et al., 2000; Nunomura et al., 2001; Smith et al., 1996; Bowling and Beal, 1995; Montine et al., 2005; Reddy, 2006; Cardoso et al., 2016; Verdile et al., 2015; Martins et al., 2018; Di Domenico et al., 2017b; Sultana et al., 2013). Basically, small hydrophobic Aβ oligomers insert into lipid bilayers of neurons where lipid oxidation ensues, producing HNE that modifies key proteins by covalent Michael addition as noted above (Sultana et al., 2013; Di Domenico et al., 2017b; Mattson, 2009). As reviewed recently, AD and MCI brains demonstrate elevated indices of protein carbonyls, protein-bound HNE, and 3-NT (Butterfield and Halliwell, 2019; Butterfield and Boyd-Kimball, 2019; Keller et al., 2005). Others showed oxidative stress is elevated and synaptic protein levels are lowered in elderly cognitively intact individuals who display Alzheimer disease pathology (Scheff et al., 2016), demonstrating that a very early preclinical stage of AD, oxidative damage is already present (Aluise et al., 2010). Redox proteomics, developed in the Butterfield laboratory (Butterfield et al., 2012), leads to identification of oxidatively modified and almost always dysfunctional proteins. Table 1 summarizes the results of many redox proteomics studies that identified cellular functions damaged in brains from persons with MCI, early-stage AD, late-stage AD, Down syndrome (DS), and Down syndrome with AD-like neuropathology and dementia following oxidative dysfunction of proteins that perform these functions (Butterfield and Halliwell, 2019; Butterfield and Boyd-Kimball, 2019). In persons with DS, often in the decade between 40-50 years of age, transition to AD-like neuropathology and dementia occurs (Di Domenico et al., 2018; Di Domenico et al., 2019; Cenini et al., 2012; Head et al., 2018).
Table 1.
Altered Biochemical Pathways Assessed from Redox Proteomics-Identified, Oxidatively Modifieda Brain Proteins in Amnestic Mild Cognitive Impairment, Early-Stage AD, Late-Stage AD, Down Syndrome, and Down Syndrome with AD Neuropathology and Dementiab
| Glucose Metabolism |
| Synaptic Plasticity, Vesicle-mediated Transport and Cell Structure |
| Cellular Redox Homeostasis and Detoxification |
| Excitotoxicity |
| Protein Synthesis |
| Protein Folding and Degradation |
| Cell Signaling |
| Neuroinflammation |
Proteins modified by protein carbonyls, bound HNE, or resident 3-nitrotyrosine
A prominent dysfunction in brain from persons with MCI, AD and of DS and DS with AD is glucose dysmetabolism. This diminution of glucose metabolism leads to decreased levels of ATP, which would cause loss of cell potential and the opening of voltage-gated ion channels, including Ca2+ channels and decreased function of ion-motive ATPases that would otherwise normally pump Ca2+ back out of neurons and maintain cell potentials (Mark et al. 1995). Once large amounts of Ca2+ accumulate inside neurons, numerous biochemical pathways are disrupted, as are mitochondria, leading to neuronal death by both apoptosis and necrosis (Butterfield and Halliwell, 2019). When enough neurons die, major brain areas involved in cognition, such as hippocampus and frontal cortex, would decrease in thickness and loss of cognition would become apparent.
This above scenario, proposed decades ago by both authors of this current paper, was recently validated by studies involving persons carrying the mutated genes that lead to autosomal dominantly inherited, familial AD, i e., persons in the dominantly inherited Alzheimer network, DIAN and other large population studies of AD (Gordon et al., 2018; Martins et al., 2018). Researchers involved in the DIAN network, located across several continents, used various PET and MRI imaging modalities to monitor three imaging-detected pathological brain alterations of thousands of such persons regularly for more than two decades before onset of symptoms to determine the order of events that occurred: a) Aβ deposition outside neurons [and since fibrillar Aβ arises from and is in equilibrium with Aβ oligomers, fibrillar Aβ provides a surrogate marker for Aβ oligomers, some of the latter cansolubilize in neuronal lipid bilayers,]; glucose metabolism; and thickness of frontal cortical and hippocampal regions of the brains. These authors reported that the first imaging-detected pathological alteration observed was Aβ fibrillar deposition [and as noted above Aβ fibrils are formed in stages originating from Aβ oligomers, which would lead to Aβ oligomer-associated oxidative damage to neurons], followed at a later time by the second PET-detected pathological alteration, glucose dysmetabolism [which would lead to diminution of ATP and the subsequent Ca2+-related events leading to neuronal death as noted above], and the last imaging-detected pathological alteration, MRI revelation of thinning of frontal cortical and hippocampal regions of the brain, occurred when enough neurons had died.
In contrast to what is observed in familial AD as described above, persons carrying the APOE4 allele demonstrate glucose dysmetabolism as young adults well before deposition of Aβ (Reiman et al., 2004). Even though it is widely asserted that the most robust correlation of apoE4 in brain is with Aβ oligomers (Yamazaka et al., 2019), glucose dysmetabolism in young adults carrying the APOE4 allele suggests at least two tentative hypotheses: a) There may be multiple mechanisms that lead to glucose dysmetabolism, i e., mechanisms other than through Aβ-mediated HNE formation; and b) apoE4, noted below to be associated with oxidative damage (Ramaswamy et al., 1999;2000), may, in ways independent of Aβ, lead to HNE formation and subsequent HNE formation. More experimentation will be required to determine if these hypotheses have merit.
Other cellular functions that are dysfunctional as a result of oxidative modification noted in Table 1 also contribute to neuronal death in AD and MCI brains (Butterfield et al., 2006; Sultana et al., 2013; Di Domenico et al., 2017b; Butterfield and Halliwell, 2019; Butterfield and Boyd-Kimball, 2019;).
4. Oxidative Stress and ApoE in Alzheimer Disease-Relevant Settings
4.1. Human Studies.
As discussed below, the degree of oxidative damage in AD brain reportedly is directly associated with APOE allele in the order APOE2 < APOE3 < APOE4 (Dose et al., 2016). Ramassamy and Poirier and colleagues used the thiobarbituric acid reactive substances [TBARS] method to assess elevated lipid peroxidation in hippocampus of AD subjects and noted the greatest elevation of TBARS signal in AD brain with ApoE4 (Ramassamy et al., 2000). These researchers also reported lower activities of catalase and glutathione peroxidase [GPx] and lower levels of glutathione [GSH] in brains of APOE4 subjects compared to those parameters in brain of subjects homozyogous for APOE3. In an earlier study, these researchers used frontal cortex of AD subjects to investigate similar endpoints (Ramaswamy et al., 1999). However, while increased TBARS signals were observed as previously, in contrast to their later study, the authors reported elevated activities of catalase GPx). Two potential concerns of these studies are: a) TBARS signals are not good measures of lipid peroxidation, since several other moieties beyond dialdehyde units yield TBARS signals as well; however, partially mollifying this concern and as discussed below, lipid peroxidation-specific signals also evince elevated lipid peroxidation in brain of APOE4 AD subjects and targeted-replacement mice. b)The length of time between patient death and acquisition of brain [the post-mortem interval, PMI] was excessive. Generally, higher confidence in measures of oxidative stress or other pathological indices arise with short [fewer than 4 h] PMIs (Butterfield and Hallliwell, 2019).
Others used 3-nitrotyrosine and 8-OHdG to demonstrate elevated oxidative stress early in the progression of AD, but less oxidative stress late in the disorder when Aβ deposition was greater, and that these effects were greater in subjects with APOE4 (Numomura et al., 2001). These authors contributed to AD understanding by noting that oxidative stress is important early in the disease, an observation that was reinforced by demonstration that oxidative stress occurs in brain of subjects with amnestic MCI, i.e., no dementia (Keller et al., 2005; Butterfield et al., 2006a,b; Butterfield et al., 2007a,b).
Increased risk for development of AD may be dependent on several, potentially interacting variables, including APOE4 geneotype, gender, body mass index, inflammation, insulin resistance, and in some cases exposure to particulates in air pollution (Calderon-Garciduenas and de la Monte, 2017). Each of these risk factors also are associated with elevated oxidative damage. Others showed that APOE4 was associated with elevated risk of developing AD in persons with mutations in glutathione-S-transferase [GST] compared to the risk of APOE4 alone, and since GST is dependent on GSH, this study suggests oxidative damage also is associated with these mutations in this transferase (Bernardini et al., 2005; Pinhel et al., 2008). Similarly, mutations in the gene for uncoupling protein-4 (UCP4) interact with APOE4 to affect risk of development of AD (Montesanto et al., 2016), and a C47T mutation in manganese superoxide dismutase [MnSOD] interact with APOE4 to increase risk of developing AD (Gamarra et al., 2015. Since UCP proteins are related to mitochondrial metabolism, MnSOD is resident in mitochondria, and mitochondrial routinely leak superoxide free radicals especially from Complex I, then these studies are consistent with the notion that elevated oxidative stress is a key factor in AD pathogenesis.
As noted elsewhere in this paper, the apoE4 isoform is linked to both senile plaque and neurofibrillary tangle elevation as well as to elevated oxidative damage in brain. apoE of molecular mass 35 kDa undergoes proteolytic fragmentation in brain in patterns that are apoE-isoform dependent (Munoz et al., 2019). The 25 kDa, N-terminal fragment of apoE is more prevalent in APOE3 individuals and is neuroprotective, while full-length ApoE4 and the N-terminal residues 1-271 and 1-272 are neurotoxic but by different mechanisms (Huang et al., 2001; Chang et al., 2005), including Ca2+ accumulation and mitochondrial dysfunction that can lead to oxidative damage (Butterfield and Halliwell, 2019).
Integration of transciptomic and genomic analyses suggested several potential mechanisms for apoE4-mediated damage in AD, prominent among which were mechanisms related to oxidative stress (Caberlotto et al., 2016). The researchers reported that homozygous APOE4/4 carriers with AD demonstrated an intersection point of pathways at Notch signaling, and the latter is related to presenilin-mediated contribution to gamma-secretase cleavage of APP to form Aβ42. As noted above, this peptide is associated with oxidative damage in AD and MCI brains. Consistent with this assertion, examination of CSF of individuals as a function of age and APOE genotype showed that levels of phosphorylated tau and isoprostanes, the latter a marker of lipid peroxidation, were statistically correlated in ApoE4 (Glodzik-Sobanska et al., 2009). Cerebrospinal fluid in AD and MCI demonstrates a specific oxidative signature (Di Domenico et al., 2016).
4.2. Apoe Knockout Mice Studies.
A few studies of oxidative stress in Apoe knockout mice have been performed general under the principles that apoE4 is associated with elevated oxidative stress in AD brain as noted above, and the extent of oxidative damage in AD brain correlated to the presence of apoE4, consistent with the notion that of an association of between APOE4 allele and free radical damage in AD brain. Consequently, in one study, Apoe4 null mice were subjected to oxidative stress by folate deprivation and/or elevated iron in the diet (Shea et al. 2002). Interestingly, glutathione and other antioxidant levels in brain were elevated, suggested by the authors to be responses to the elevated oxidative stress. A combination of folate deprivation and excess dietary iron led to oxidative damage not rescued by the elevated glutathione, supporting the idea that Apoe deficiency is associated with oxidative damage in brain. A second study by the same group (Tchantchou et al., 2005) showed protection to brain from Apoe knockout mice that had been pretreated with N-acetylcysteine, a glutathione precursor. A similar approach was taken by others, who also found elevated oxidative damage in brain of Apoe-deficient mice, but in contrast to the two studies noted above, these authors found a decrease in small antioxidants, i.e., vitamin E in such mice (Ramasammy et al. 2001).
We showed that synaptosomal membrane preparations isolated from Apoe knockout mice subsequently treated with amyloid beta-peptide (1-40) had elevated indices of protein oxidation and lipid peroxidation (Lauderback et al., 2001) and displayed mitochondrial dysfunction and caspase activation (Keller et al., 2000) compared to similarly treated synaptosomes from wild-type mice. These results are consistent with a role for apoE in maintaining homeostasis by modulation of synaptotomal oxidative stress, caspase inactivation, and mitochondrial dysfunction. As noted below, the APOE4 allele is unable to provide this modulation relative to the other APOE alleles.
4.3. Human APOE Targeted-replacement Mice Studies.
Apoe knock-out [null] mice or targeted replacement mice, in which the mouse gene for Apoe is knocked out and replaced by the human gene for APOE2, APOE3, or APOE4, while keeping the mouse promoter [thereby ensuring that the human apoE isoform is directed to the correct locations and in the usual amounts], are excellent resources with which insights into the role of human apoE in brain can be investigated in vivo. Indeed, as described further below, such mice have been used to validate most human studies.
As noted previously, apoE is a multifunctional protein with potential functions in microtubule assembly and stability, intracellular signaling, glucose metabolism, Aβ deposition and oxidative stress, among other functions (Saunders, 2000). With respect to oxidative stress and apoE, Gandy and co-workers (Yao et al., 2004) reported that factors such as aging and gender couple with apoE isoform to modulate metabolism of Aβ and lipid peroxidation [assessed by F2-isoprostanes that derive mostly from arachidonic acid]. Specifically, APOE3 and APOE4 human targeted replacement mice or the corresponding Apoe knockout mice with subsequently added transgenes for APOE3 or APOE4 revealed that the transgenic APOE4 mice led to elevated F2-isoprostanes in brain not observed in APOE3 mice. Moreover, the changes in APOE4 mice correlated with levels of Aβ42 in likely an oligomeric form.
The antioxidant nature of apoE depends on its allele, with apoE4 being the least functional antioxidant . Nitrosative stress was observed in greater level in brains of APOE4 mice than those of APOE3 mice (Colton et al., 2002; Brown et al. 2002). Mattson and co-workers demonstrated that apoE4 bound less of the lipid peroxidation product HNE than did apoE3 (Pedersen et al., 2000). These researchers suggested that the Cys residue at 158 bound HNE, thereby keeping this neurotoxic agent from damaging neuronal proteins. In contrast, since apoE4 is devoid of Cys, no HNE was bound, permitting HNE to bind to neuronal proteins. The researchers opine that this may be a prominent mechanism by which APOE4 is a major risk factor for AD. Presaging this work by Mattson and co-workers, Montine and colleagues demonstrated that apoE3 was more crosslinked by HNE than was apoE4 in CSF from adult subjects with or without dementia (Montine et al., 1996).
Consistent with these above studies, and consonant with the notion that inheritance of the APOE4 gene is a major risk factor for AD, the Butterfield laboratory studied the role of different human APOE alleles in modulating Aβ42-induced protein oxidation [protein carbonyls; 3-nitrotyrosine] and lipid peroxidation [protein-bound HNE] in synaptosomes isolated from brains of human APOE2, APOE3, and APOE4 targeted-replacement mice (Lauderback et al., 2002). After addition of Aβ42 to synaptosomes from these mice, increased reactive oxygen species formation and elevated levels of protein carbonyls and 3-nitrotyrosine and protein bound HNE were observed in synaptic membranes in the order apoE4 > apoE3 > apoE2. These results reflect the function of apoE as a modulator of oxidative stress, and the order by which this occurs is related to the number of Cys residues in the protein, i e., apoE2 > apoE3 > apoE4, and we opine these results have mechanistic implications for the intersection of the oxidative stress associated with Aβ42 oligomers (Butterfield et al., 2001; Butterfield and Halliwell, 2019) and apoE4 in the elevated risk for neurodegeneration and development of AD (Lauderback et al., 2002; Pedersen et al., 2000).
apoE is involved in efflux of Aβ peptide from brain employing LRP-1 as described above. This transport protein is oxidatively modified, and therefore likely dysfunctional, in brains of AD subjects, which could contribute to the accumulation of Aβ in the brain parenchyma (Owen et al., 2010). Consistent with this notion, others reported that in LRP-1 knockout mice, there is increased susceptibility to Aβ-induced neurotoxicity (de Oliveira et al., 2014) and that following intracerebroventricular injection of aggregated Aβ42, apoE protein levels lost their temporal regulation in brain that was accompanied by loss of GSH and elevation of lipid peroxidation (Navigatore-Fonzo et al., 2017).
Thioredoxin-1, an endogenous antioxidant protein, is involved in regulation of apoptosis though inhibition of apoptosis signal-regulating kinase (Ask-1). Compared to APOE3 targeted replacement mice, APOE4 mice have decreased levels of thioredoxin-1, which was associated with disrupted lysosomal integrity evinced by relocalization of the key lysosomal cathepsin D from this organelle to cytosol, making degradation of autophagic mediation of lysosomal degradation of proteins less efficient (Persson et al., 2017). These researchers opine that down-regulation of thioredoxin-1 plays a role in the neurotoxicity associated with the presence of the APOE4 allele.
Ozone exposure has been suggested to be a potential risk factor for late-onset AD, but the mechanisms for this potential risk factor remain unknown. To address this gap, Jiang and co-workers (Jiang et al., 2019) reported ozone exposure to old APOE3 mice led to impaired memory, which was not observed in old APOE4 or young APOE3 or APOE4 mice. Further, APOE4 mice had elevated expression of antioxidant enzymes and diminished oxidative stress [also reported by Ramasammy et al., as noted above]. No explanation of these results were provided, but it is conceivable, as suggested elsewhere in this current review, that the decreased cysteine content of apoE4 provides fewer opportunities for HNE modification of this protein [i.e., antioxidant scavenging] than is the case for apoE3 [Figure 1] (Pedersen et al., 2000; Lauderback et al., 2002).
Alzate’s group has investigated the role of mortalin in AD (Londono et al., 2012). This protein is a chaperone that is transported from the cytosol to the matrix of mitochondria by the TOM and TIM transporters, and in the matrix, motralin reportedly serves as an antioxidant protein, protecting mitochondria. This group used proteomics to identify that mortalin is regulated by apoE in hippocampi of APOE targeted replacement mice, with APOE4 mice showing increased levels compared to APOE3 mice, and mortalin in APOE4 mice appeared to be highly phosphorylated or oxidized (Osorio et al., 2007). In AD hippocampi decreased expression in subjects with an APOE4 genotype was observed. The authors propose that the differential regulation of the normally protective mortalin in AD by the APOE genotype is a cellular defense against oxidative stress, and consistent with this finding, APOE knockout mice brains were reported to have oxidatively modified mortalin (Choi et al., 2004).
Studies of human targeted replacement mice taken together demonstrate that the presence of APOE4 genotype increases susceptibility to Aβ-associated oxidative damage, likely due to its lack of Cys residues that are known to bind to the neurotoxic lipid peroxidation product, HNE. Such consideration provides one highly plausible explanation for the increased risk of developing AD with persons carrying the APOE4 allele. Consistent with this notion, examination of medical records showed carriers of APOE2 genotype, with two key Cys residues (Figure 1), is associated with less cognitive decline with aging than persons with APOE3 (1 key Cys residue) or APOE4 (no key Cys residues) genotypes (Shinohara et al., 2016).
5. Conclusions and Future Directions
Brains from persons with Alzheimer disease and its earlier stage, amnestic mild cognitive impairment, are characterized by elevated indices of oxidative damage to proteins, lipids, and DNA (Butterfield et al., 2001). Cognitive dysfunction in AD is correlated with the levels of oligomeric Aβ42 (Cleary et al., 2005), the latter associated with elevated markers of oxidative damage (Butterfield and Halliwell, 2019). Similarly, brains from persons with Down syndrome, in which there is elevated levels of Aβ oligomers, often transition to AD-like neuropathology and dementia between 40-50 years of age (Di Domenico et al., 2018; Di Domenico et al., 2019; Cenini et al., 2012; Head et al., 2018).
Inheritance of APOE4 leads to elevated risk of developing AD. This current review assesses the hypothesis that a significant factor that leads to this elevated risk of developing AD by inheritance of APOE4 is the inability of this isoform to scavenge the neurotoxic lipid peroxidation product, HNE, due to the absence of the two critical Cys residues in the protein (Pedersen et al., 2000; Lauderback et al., 2002). Consistent with this hypothesis, and as shown in Table II, elevated oxidative stress in brain occurs in persons who inherit the ApoE4 isoform. Moreover, in human targeted replacement mice, lipid peroxidation and tyrosine nitration, emanating from oxidative stress and inflammatory processes, brains from APOE4 mice demonstrate elevated indices of these oxidative and nitrosative damages compared to brains from APOE3 mice. As stated above, the presence of the two key Cys residues, or residue, in apoE 2 and apoE3, respectively, permit scavenging the highly reactive and neurotoxic lipid peroxidation product, HNE that is produced in membranes by oligomeric Aβ42. These scavenging processes not available to APOE4, thereby, we opine, resulting in lipid peroxidation and nitration of tyrosine residues, oxidative damage that leads to neuronal death and AD. We hypothesize that these considerations make significant contributions to the known risk of developing AD by those persons who inherit the APOE4 genotype.
TABLE II.
| Human CNS Studies | APOE KO Mice Brain | APOE TR Mice Brain | |||
|---|---|---|---|---|---|
| Observation | Comment and/or Ref. |
Observation | Comment and/or Ref. |
Observation | Comment and/or Ref. |
| Oxidative stress varies apoE2<apoE3<apoE4 | Dose et al., 2016. | Elevated oxidative stress compared to WT mice. | Rescued by NACd. Tchantchou et al. 2005 | Elevated F2-isoprostanes, greater in brains of APOE4 mice than APOE3 mice, and results correlated with oligomeric Aβ | Yao et al., 2004 |
| Increased LPO; decreased GSH; | Ramasammy et al., 2000 | Decreased in small antioxidants such as vitamin E. | Ramasammy et al. 2001. | Elevated nitrosative stress in APOE4 mice compared to ApoE3 mice | Colton et al., 2002; Brown et al., 2002 |
| Elevated risk of AD with mutations in GST, UCP4, or MnSOD | All related to decreased function with consequent elevated oxidative stress. Bernadini et al., 2005; Pinhel et al., 2008; Montesanto et al., 2016; Gamarra et al., 2015 | Synaptosomal membranes showed elevated oxidative stress and elevated dysfunction of mito. compared to WT following Aβ addition. | Lauderback et al. 2001; Keller et al., 2000. | apoE4 had less HNE bound than did apoE3, consistent with idea that apoE2 and apoE3 have antioxidant properties, and increased risk of AD with APOE4 could be related to this characteristic | Pedersen et al., 2000; Montine et. al., 1996 |
| ApoE undergoes proteolysis, with protective NTF fragment of 25kD more prevalent in ApoE3; full-length ApoE is neurotoxic | Munoz et al., 2019; Huang et al., 2001; Chang et al., 2005 | Aβ added to synaptic membranes showed elevated oxidative stress in order apoE4>apoE 3>apoE2, i.e., inverse of number of key Cys residues in apoE isoforms | Lauderback et al., 2002 | ||
| Brain-resident presenilin component of γ-secretase-mediated APP cleavage to form Aβ42 highly present in ApoE4/4 individuals | Predicted to lead to more oxidative stress and neurotoxicity. Caberlotto et al., 2016 | ICV injection of Aβ led to loss of temporal regulation of apoE levels along with loss of GSH and increased lipid peroxidation | Navigatore-Fonzo et al., 2017 | ||
| CSF-resident p-Tau and F2-isoprostanes elevated in in ApoE4 individuals | Glodzik-Sobanska et al., 2009 | APOE4 brain has decreased levels of Thx-1, affecting lysosomal integrity | Persson et al., 2017 | ||
Oxidative stress indices cited: LPO = lipid peroxidation indexed by HNE = 4-hydroxynonenal or F2-isoprostanes; 3-NT = 3-nitrotyrosine; PC = protein carbonyls. 8-OHdG = 8-Hydroxy-2-deoxyguanosine; GSH = glutathione
KO = Mouse Apoe gene deleted
TR = Human Targeted Replacement ApoE gene
NAC = N-acetylcysteine
Diagnosing AD before symptoms appear arguably is critical to slowing or stopping the progression of this dementing disorder. A very recent study (Schindler et al., 2019) demonstrated that using sophisticated analyses of plasma-resident Aβ42 and Aβ40 in more than 150 asymptomatic people allowed a model to be developed that predicted reasonably accurately who had deposition of Aβ in their brains using PET scanning. Relevant to this review article, addition of age and APOE genetic status to their model greatly improved the prediction accuracy of who would eventually deposit Aβ in their brains.
So, even if this or other models that might arise can predict pathology of AD and who may be of great risk of developing this dementing disorder, what can be done to mitigate against an elevated risk of developing AD with inheritance of the ApoE4 genotype? The response to this question is confounded by the differential interactions of many therapeutic approaches in AD by APOE genotype and by gender (Hanson et al., 2015). It is unethical to change one’s genetic makeup, except in rare circumstances where the gene involved directly causes the devastating disorder, i.e., Huntington disease; Duchenne muscular dystrophy). Moreover, apoE is multifunctional; therefore, targeting knockout of the APOE4 gene likely would lead to unintended serious consequences.
Recent studies suggest that apoE4 leads to microglial dysfunction, resulting in accumulation of phosphotau and neuronal death (Shi et al., 2014; Shi et al., 2019). This exciting result may lead to pharmacological approaches to protect microglia in APOE4 individuals.
Alternatively, and in agreement with preclinical studies that suggest that a high antioxidant diet, continuous lifelong stimulation of synapse formation by learning new tasks, and significant exercise regimens increase cognition and lower brain indices of AD (Opii et al., 2008), persons who inherit one or more copies of the APOE4 allele would be strongly suggested to follow these programs to lower the risk of developing AD. Clearly, no promise that development of AD can be prevented is implied here; rather, that the risk for AD development likely can be materially lowered.
In the meantime, APOE4 targeted-replacement mice offer preclinical models with which to test promising therapeutic strategies of the future.
6. Acknowledgements
This work was supported in part by a grant from the National Institutes of Health to D.A.B. [1R01 AG060056]. We thank Dr. Xiaojia Ren with assistance with preparation of Figure 2.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aluise CD, Robinson RA, Beckett TL, Murphy MP, Cai J, Pierce WM, Markesbery WR, Butterfield DA, 2010. Preclinical Alzheimer disease: brain oxidative stress, Abeta peptide, and proteomics. Neurobiol. Dis 39, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader-Lange ML, Cenini G, Piroddi M, Abdul HM, Sultana R, Galli F, Memo M, Butterfield DA, 2008. Loss of phospholipid asymmetry and elevated brain apoptotic protein levels in subjects with amnestic mild cognitive impairment and Alzheimer disease. Neurobiol. Dis 29, 456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardini S, Bellincampi L, Ballerini S, Federici G, lori R, Trequattrini A, Ciappi F, Baldinetti F, Bossu P, Caltagirone C, Spaletta G, 2005. Glutathione S-transferase P1 *C allelic variant increases susceptibility for late-onset Alzheimer disease: association study and relationship with apolipoprotein E epsilon4 allele. Clin. Chem 51, 944–951. [DOI] [PubMed] [Google Scholar]
- Bowling AC, Beal MF 1995. Bioenergetic and oxidative stress in neurodegenerative diseases. Life Sci. 56, 1151–1171. [DOI] [PubMed] [Google Scholar]
- Brown CM, Wright E, Colton CA, Sullivan PM, Laskowitz DT, Vitek MP, 2002. Apolipoprotein E isoform mediated regulation of nitric oxide release. Free Radic. Biol. Med 32, 1071–1075. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, 2014. The 2013 SFRBM Discovery Award: selected discoveries from the Butterfield laboratory of oxidative stress and its sequela in brain in cognitive disorders exemplified by Alzheimer disease and chemotherapy induced cognitive mpairment. Free Radic. Biol. Med 74, 157–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA and Boyd-Kimball D, Redox proteomics and amyloid β-peptide: insights into Alzheimer disease, 2019. J. Neurochem In press. doi: 10.1111/jnc.14589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Drake J, Pocernich C, Castegna A 2001. Evidence of oxidative damage in Alzheimer’s disease brain: central role of amyloid β-peptide. Trends Mol. Med 7, 548–554. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Halliwell B, 2019. Oxidative stress, gluocose dysmetabolism, and Alzheimer disease. Nat. Rev. Neurosci 20, 148–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Perluigi M, Reed T, Muharib T, Hughes CP, Robinson RA, Sultana R, 2012. Redox proteomics in selected neurodegenerative disorders: from its infancy to future applications. Antioxidant Redox Signal. 17, 1610–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF, St. Clair D. Keller JN., Pierce WM., Klein JB., Markesbery WR., 2006a. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer's disease. Neurobiol. Dis 22, 223–232. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Perluigi M, De Marco C, Cini C, Sultana R, 2006b. Elevated protein-bound levels of the lipid peroxidation product, 4-hydroxy-2-nonenal, in brain from persons with mild cognitive impairment. Neurosci. Lett 397, 170–173. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Reed T, Newman SF, Sultana R, 2007a. Roles of amyloid beta-peptide-associated oxidative stress and brain protein modifications in the pathogenesis of Alzheimer's disease and mild cognitive impairment. Free Radic. Biol. Med 43, 658–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Reed TT, Perluigi M, De Marco C, Coccia R, Keller JN, Markesbery WR, Sultana R, 2007b. Elevated levels of 3-nitrotyrosine in brain from subjects with amnestic mild cognitive impairment: implications for the role of nitration in the progression of Alzheimer's disease. Brain Res 1148, 243–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, Reed TT, Sultana R, 2011. Roles of 3-nitrotyrosine- and 4-hydroxynonenal-modified brain proteins in the progression and pathogenesis of Alzheimer’s disease. Free Radic. Res 45, 59–72. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Stadtman ER, 1997. Protein Oxidation Processes in Aging Brain. Adv. Cell Aging Gerontol. 2, 161–191. [Google Scholar]
- Caberlotto L, Marchetti L, Lauria M, Scotti M, Parolo S., 2016. Integration of transcriptomic and genomic data suggests candidate mechanisms for ApoE4-mediated pathogenic action in Alzheimer’s disease. Sci. Rep 6, 32583. doi: 10.1038/srep32583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, de la Monte SM, 2017. Apolipoprotein E4, gender, body mass index, inflammation, insulin resistance, and air pollution interactions: recipe for Alzheimer’s disease development in Mexico City young females. J. Alzheimers Dis 58, 613–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso S, Carvalho C, Correia SC, Seica RM, Moreira PI 201. Brain Pathol. 26, 632–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedazo-Minguez A, 2007. Apolipoprotein E and Alzheimer’s disease: molecular mechanisms and therapeutic opportunities. J. Cell. Mol. Med 11, 1227–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedazo-Minguez A, Cowburn RF, 2001. Apolipoprotein E: a major piece in the Alzheimer disease puzzle. J. Cell. Mol. Med 5, 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenini G, Fiorini A, Sultana R, Perluigi M, Cai J, Klein JB, Head E, Butterfield DA, 2012. An investigation of the molecular mechaisms engaged before and after the development of Alzheimer disease neuropathology in Down syndrome: a proteomics approach. Free Radic. Biol. Med 76, 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, ran Ma T, Miranda RR, Balestra ME, Huang Y, 2005. Lipid-and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc. Nat. Acad. Sci. USA 102, 18694–18699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Forster MJ, McDonald SR, Weintraub ST, Carroll CA, Gracy RW, 2004. Proteomic identification of specific oxidized proteins in ApoE-knockout mice: relevance to Alzheimer’s disease. Free Radic. Biol. Med 36, 1155–1162. [DOI] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH, 2005. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci 8, 79–84. [DOI] [PubMed] [Google Scholar]
- Colton CA, Brown CM, Cook D, Needham LK, Xu Q, Czapiga M, Saunders AM, Schmechel DE, Rasheed K, Vitek MP, 2002. APOE and the regulation of microglial nitric oxide production: a link between genetic risk and oxidative stress. Neurobiol. Aging 23, 777–785. [DOI] [PubMed] [Google Scholar]
- De Oliveira J, Moreira EL, dos Santos DB, Piermartiri TC, Dutra RC, Tasca CI, Farina M, Prediger RD, de Bem AF, 2014. Increased susceptibility to amyloid-β-induced neurotoxicity in mice lacking the low-density lipoprotein receptor. J. Alzheimers Dis 41, 43–60. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Barone E, Perluigi M, Butterfield DA, 2017a. The triangle of death in Alzheimer disease brain: the aberrant cross talk among energy metabolism, mTOR signaling and protein homeostasis revealed by redox proteomics. Antioxidant Redox Signal. 26, 364–387. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Pupo G, Giraldo E, Badìa MC, Monllor P, Lloret A, Schininà ME, Giorgi A, Cini C, Tramutola A, Butterfield DA, Viña J, Perluigi M, 2016. Oxidative signature of cerebrospinal fluid from mild cognitive impairment and Alzheimer disease patients. Free Radic. Biol. Med 64, 1–9. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Tramutola A, Butterfield DA, 2017b. Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of Alzheimer disease and other selected age-related neurodegenerative disorders. Free Radic. Biol. Med 111, 253–261. [DOI] [PubMed] [Google Scholar]
- Di Domenico F, Tramutola A, Foppoli C, Head E, Perluigi M, Butterfield DA, 2018. mTOR in Down syndrome: role in Aβ and tau neuropathology and transition to Alzheimer disease-like dementia. Free Radic. Biol. Med 114, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico F, Tramutola A, Barone E, Lanzillotta C, Defever O, Arena A, Zuliani I, Foppoli C, Iavarone F, Vincenzoni F, Castagnola M, Butterfield DA, Perluigi M, 2019. Restoration of aberrant mTOR signaling by intranasal rapamycin reduces oxidative damage: focus on HNE-modified proteins in a mouse model of Down syndrome. Redox Biol., in press. doi: 20.1016/j.redox.2019.101162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dose J, Huebbe P, Nebel A, Rimbach G, 2016. APOE genotype and stress response – a mini review. Lipids Health Dis. 15, 121. doi: 10.1186/s12944-016-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H, 1991. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med 11, 81–128. [DOI] [PubMed] [Google Scholar]
- Estevez AG, Spear N, Manuel SM, Barbeito L, Radi R, Beckman JS, 1998. Role of endogenous nitric oxide and peroxynitrite formation in the survival and death of motor neurons in culture. Prog. Brain Res 118, 269–280. [DOI] [PubMed] [Google Scholar]
- Ferrer-Sueta G, Campolo N, Trujillo M, Bartesaghi S, Carballal S Romero N., Alvarez B., Radi R., 2018. Biochemistry of peroxynitrite and protein tyrosine nitration. Chem. Rev 118, 1338–1408. [DOI] [PubMed] [Google Scholar]
- Gamarra D, Elcoroaristizabal X, Fernandez-Martinez M, de Panorbo MM, 2015. Association of the C47T polymorphism in SOD2 with amnestic mild cognitive impairment and Alzheimer’s disease in carriers of the ApoEε4 allele. Dis. Markers 2015, 746329. doi: 10.1155/2015/746329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodzik-Sobanska L, Pirraglila E, Brys M, de Santi S, Mosconi L, Rich KE, Switalski R, Saint Louis L, Sadowski MJ, Martiniuk F, Mehta P, Pratico D, Zinkowski RP, Blennow K, de Leon MJ, 2009. The effects of normal aging and ApoE genotype on the levels of CSF biomarkers for Alzheimer’s disease. Neurobiol. Aging 30, 672–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golabek AA, Soto C, Vogel T, Wisienski T, 1996. The interaction between apolipoprotein E and Alzheimer’s amyloid beta-peptide is dependent on beta-peptide conformation. J. Biol. Chem 271, 10602–10606. [DOI] [PubMed] [Google Scholar]
- Gordon BA, Blazey TM, Su Y, Hari-Raj A, Dincer A, Flores S, Christensen J, McDade E, Wang G, Xiong C, Cairns NJ, Hassenstab J, Marcus DS, Fagan AM, Jack CR Jr., Hornbeck RC, Paumier KL, Ances BM, Berman SB, Brickman AM, Cash DM, Chhatwal JP, Correia S, Förster S, Fox NC, Graff-Radford NR, la Fougère C, Levin J, Masters CL, Rossor MN, Salloway S, Saykin AJ, Schofield PR, Thompson PM, Weiner MM, Holtzman DM, Raichle ME, Morris JC, Bateman RJ, Benzinger TLS, 2018. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer's disease: a longitudinal study. Lancet Neurol. 17, 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC, 2015. Free Radicals in Biology and Medicine, 5th Edition, Oxford University Press, Oxford, UK. [Google Scholar]
- Hanson AJ, Craft S, Banks WA, 2015. The APOE genotype: modification of therapeutic responses in Alzheimer’s disease. Curr. Pharm. Des 21, 114–120. [DOI] [PubMed] [Google Scholar]
- Head E, Helman AM, Powell D, Schmitt FA, 2018. Down syndrome, beta-amyloid and neuroimaging. Free Radic. Biol. Med 114, 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley K, Hall N, Subramaniam R, Cole P, Harris M, Aksenov M, Aksenova M, Gabbita SP, Wu JF, Carney JM., Lovell M., Markesbery WR., Butterfield DA., 1995. Brain regional correspondence between Alzheimer's disease histopathology and biomarkers of protein oxidation. J. Neurochem. 65, 2146–2156. [DOI] [PubMed] [Google Scholar]
- Herz J, Beffert U., 2000. Apolipoprotein E receptors: linking brain development and Alzheimer’s disease. Nat. Rev. Neurosci 1, 51–58. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW, 2001. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc. Nat. Acad. Sci. USA 98, 8838–8843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Stewart LT, Kuo HC, McGilberry W, Wall SB, Liang B, van Groen T, Bailey SM, Kim YI, Tipple TE, Jones DP, McMahon LL, Liu RM, 2019. Cyclic O3 exposure synergizes with aging leading to memory impairment in male APOE ε3, but not APOE ε4, targeted replacement mice. Neurobiol. Aging 81, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Lauderback CM, Butterfield DA, Kindy MS, Markesbery WR, 2000. Amyloid beta-peptide effects on synaptosomes from apolipoprotein E-deficient mice. J. Neurochem 74, 1579–1586. [DOI] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR, 2005. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology 64, 1152–1156. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Hackett JM, Keller JN, Varadarajan S, Szweda L, Kindy M, Markesbery WR, Butterfield DA, 2001. Vulnerability of synaptosomes from apoE knock-out mice to structureal and oxidative modifications induced by Abeta(1-40): implications for Alzheimer’s disease. Biochem. 40, 2548–2554. [DOI] [PubMed] [Google Scholar]
- Lauderback CM, Kanski J, Hackett JM, Maeda N, Kindy MS, Butterfield DA, 2002. Apolipoprotein E modulates Alzheimer’s Abeta(1 -42)-induced oxidative damage to synaptosomes in an allele-specific manner. Brain Res. 924, 90–97. [DOI] [PubMed] [Google Scholar]
- Liu S, Park S, Allington G, Prelli F, Sun Y, Marta-Ariza M, Scholtzova H, Biswas G, Brown B, Verghese PB, Mehta PD, Kwon YU, Wisniewski T, 2017. Targeting apolipoprotein E/amyloid β binding by peptoid CPO_Aβ17-21 amerliorates Alzheimer’s disease related pathology and cognitive decline. Sci. Rep 7, 8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londono C, Osorio C, Gama V, Alzate O, 2012. Mortalin, apoptosis, and neurodegeneration. Biomolecules 2, 143–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, 1988. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science 240, 622–630. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y, 2006. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc. Nat. Acad. Sci. USA 103, 5644–5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark RJ, Hensley K, Butterfield DA, Mattson MP, 1995. Amyloid beta-peptide imparies ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J. Neurosci 15, 6239–6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markesbery WR, 2010. Neuropathological alterations in mild cognitive impairment: a review. J. Alzheimers Dis 19, 221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins RN, Villemagne V, Sohrabi HR, Chatterjee P, Shah TM, Verdile G, Fraser P, Taddei K, gupta VV, Rainey-Smith SR, Hone E, Pedrini S, Lim WL, Martins I, Frost S, Gupta S, O’Bryant S, Remback A, Amers D, Ellis K, Fuller SJ, Brown B, Gardener SL, Fernando B, Bharadwaj P, Burnham S, Laws SM, Barron AM, Goozee K, Wahjoepramono EJ, Asih PR, Doecke JD, Salvado o., Bush AI., Rowe CC., Gandy SE., Masters CL., 2018. Alzheimer’s disease: a journey from amyloid peptides and oxidative stress, to biomarker technologies and disease prevention strategies – Gains from AIBL and DIAN cohort studies. J. Alzheimers Dis. 62, 965–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, 2009. Roles of the lipid peroxidation product 4-hydroxynonenal in obesity, the metabolic syndrome, and associated vascular and neurodegenerative disorders. Exp. Gerontol 44, 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, Boccardi V, Cecchetti R, Bastiani P, Scamosci M, Ruggiero C, Baroni M 2018. A Long Journey into Aging, Brain Aging, and Alzheimer's Disease Following the Oxidative Stress Tracks. J. Alzheimers Dis 62, 1399–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montine TJ, Huang DY, Valentine WM, Amarnath V, Saunders A, Weisgraber KH, Graham DG, Strittmatter WJ, 1996. Crosslinking of apolipoprotein E by products of lipid peroxidation. J. Neuropathol. Exp. Neurol 55, 201–210. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Montine KS, McMahan W, Markesbery WR, Quinn JF, Morrow JD 2005. F2-isoprostanes in Alzheimer and other neurodegenerative diseases. Antioxid. Redox Signal 7, 269–275. [DOI] [PubMed] [Google Scholar]
- Montsanto A, Crocco P, Anfossi M, Smime N, Puccio G, Colao R, Maletta R, Passarino G, Bruni AC, Rose G, 2016. The genetic variability of UCP4 affects the individual susceptibility to late-onset Alzheimer’s disease and modifies the disease’s risk in APOE-ε4 carriers. J. Alzheimers Dis 51, 1265–1274. [DOI] [PubMed] [Google Scholar]
- Munoz SS, Garner B, Ooi L, 2019. Understanding the role of ApoE fragments in Alzheimer’s disease. Neurochem. Res 44, 1297–1305. [DOI] [PubMed] [Google Scholar]
- Navigatore-Fonzo L, Castro A, Pignataro V, Garraza M, Casais M, Anzulovich AC, 2017. Daily rhythms of cognition-related factors are modified in an experimental model of Alzheimer’s disease. Brain Res. 1660: 27–35. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR, 2009. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J. Neuropath. Exp. Neurol 18, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS., Petersen RB., Smith MA., 2001. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol 60, 759–767. [DOI] [PubMed] [Google Scholar]
- Osorio C, Sullivan PM, He DN, Mace BE, Ervin JF, Strittmatter WJ, Alzate O., 2007. Mortalin is regulated by APOE in hippocampus of AD patients and by human APOE in TR mice. Neurobiol. Aging 28, 1853–1862. [DOI] [PubMed] [Google Scholar]
- Owen JB, Sultana R, Aluise CD, Erickson MA, Price TO, Bu G, Banks WA, Butterfield DA, 2010. Oxidative modification to LDL receptor-related protein 1 in hippocampus from subjects with Alzheimer's disease: implications for Aβ accumulation in AD brain. Free Radic. Biol. Med 49, 1798–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen WA, Chan SL, Mattson MP, 2000. A mechanism for the neuroprotective effect of Apolipoprotein E: isoform-specific modification by the lipid peroxidation product 4-hydroxynonenal. J. Neurochem 74, 1426–1433. [DOI] [PubMed] [Google Scholar]
- Persson T, Lattanzio F, Calvo-Garrido J, Rimondini R, Rubio-Rodrigo M, Sunstrom E, Maioli S, Sandebring-Matton A, Cedazo-Minguez A, 2017. Apolipoprotein E4 elicits lysosomal cathepsin D release, decreased thioredoxin-1 levels, and apoptosis. J. Alzheimers Dis 56, 601–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinhel MA, Nakazone MA, Cacao JC, Piteri RC, Godoy MF, Tognola WA, Conforti-Froes ND, Souza D, 2008. Glutathione S-transferase variants increase susceptibility for late-onset Alzheimer disease: associated study and relationship with apolipoprotein E epsilon4 allele. Clin. Chem. Lab. Med 46, 439–435. [DOI] [PubMed] [Google Scholar]
- Ramassamy C, Averill D, Beffert U, Bastianetto S, Theroux L, Lussier-Cacan S, Cohn JS, Christen Y, Davignon J, Quirion R, Poirer J, 1999. Oxidative damage and protection by antioxidants in the frontal cortex of Alzheimer’s disease is related to apolipoprotein E genotype. Free Radic. Biol. Med 27, 544–553. [DOI] [PubMed] [Google Scholar]
- Ramassamy C, Averill D, Beffert U, Theroux L, Lussier-Cacan S, Cohn JS, Christen Y, Schoofs A, Davignon J, Poirer J, 2000. Oxidative insults are associated with apolipoprotein E geneotype in Alzheimer’s disease brain. Neurobiol. Dis 7, 23–37. [DOI] [PubMed] [Google Scholar]
- Ramassamy C, Krzywkowski P., Averill D., Lussier-Cacan S., Theroux L., Christen Y, Davignon J. Poirer J., 2001. Impact of apoE deficiency on oxidative insults and antioxidants in the brain. Brain Res. Mol. Brain Res 86, 76–83. [DOI] [PubMed] [Google Scholar]
- Reddy PH 2006. Amyloid precursor protein-mediated free radicals and oxidative damage: implications for the development and progression of Alzheimer disease. J. Neurochem 96, 1–13. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J 2004. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc. Natl. Acad. Sci. U.S.A 101, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey PL, Siedlak SL, Smith MA, Perry G 1995. Apolipoprotein E interaction with the neurofibrillary tangles and senile plaques in Alzheimer disease. Biochem. Biophys. Res. Commun 208, 657–663. [DOI] [PubMed] [Google Scholar]
- Saunders AM, 2000. Apolipoprotein E and Alzheimer disease: an update on genetic and functional analyses. J. Neuropathol. Exp. Neurol 59, 751–758. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Ansari MA, Mufson EJ, 2016. Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer’s disease pathology. Neurobiol. Aging 42, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, Holtzman DM, Morris JC, Benzzinger TLS, Xiong C, Fagan AM, Bateman RJ, 2019. High-precision plasma b-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93, in press. doi: 10.1212/WNL.0000000000008081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea TB, Rogers E, Ashline D, Ortiz D, Sheu MS, Apolipoprotein E deficiency promotes increased oxidative stress and compensatory increases in antioxidants in brain tissue. Free Radic. Biol. Med 33, 1115–1120. [DOI] [PubMed] [Google Scholar]
- Shi Y, Manis M., Long J., Wang K., Sullivan PM., Ramolina-Serrano J., Hoyle R., Holtzman DM., 2019. Microglia drive APOE-dependent neurodegeneration in a tauopathy mouse model. J. Exp. Med, in press. doi: 10.1084/jem.20190980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Yamada K, Liddellow SA, Smith ST, Zhao L, Luo W, Tsai RM, Spina S, Grinberg LT, Rojas JC, Gallardo G, Wang K, Roh J, Robinson G, Finn MB, Jiang H, Sullivan PM, Baufeld C, Wood MW, Sutphen C, McCue L, Xiong C, Del-Aguila JL, Morris JC, Cruchaga C; Alzheimer’s Disease Neuroimaging Initiative, Fagan AM., Miller BL., Boxer AL., Seeley WW., Butovsky O., Barres BA., Paul SM., Holtzman DM., 2017. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 539, 523–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Kanekiyo T, Yang L, Linthicum D, Shinohara M, Fu Y, Price L, Frisch-Daiello JL, Han X, Fryer JD, Bu G, 2016. APOE2 eases cognitive decline during aging: clinical and preclinical evaluations. Ann. Neurol 79, 758–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Sayer LM, Monnier VM, Perry G 1996. Oxidative posttranslational modifications in Alzheimer disease. A possible pathogenic role in the formation of senile plaques and neurofibrillary tangles. Mol. Chem. Neuropathol 28, 41–48. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Weisgraber KH, Huang DY, Dong LM, Salvesen GS, Pericak-Vance M, Schmechel D, Saunders AM, Goldgaber D, Roses AD, 1993. Binding of human apolipoprotein E to synthetic amyloid beta peptide: isoform-specific effects and implications for late-onset Alzheimer disease. Proc. Nat. Acad. Sci. USA 90, 8098–8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam R, Roediger F, Jordan B, Mattson MP, Keller JN, Waeg G, Butterfield DA, 1997. The lipid peroxidation product, 4-hydroxy-2-trans-nonenal, alters the conformation of cortial synaptosomal membrane proteins. J. Neurochem 69, 1161–1169. [DOI] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Butterfield DA, 2013. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic. Biol. Med 62, 157–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchantchou F, Graves M, Rogers E, Ortiz D, Shea TB, 2005. N-acetyl cysteine alleviates oxidative damage to central nervous system of ApoE-deficient mice following folate and vitamin E-deficiency. J. Alzheimers Dis. 7, 135–138. [DOI] [PubMed] [Google Scholar]
- Tudorache IF, Trusca VG, Gafencu AV, 2017. Apolipoprotein E – a multifunctional protein with implications in various pathologies as a result of its structural features. Comput. Struct. Biotechnol. J 15, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdile G, Fuller SJ, Martins RN 2015. The role of type 2 diabetes in neurodegeneration. Neurobiol. Dis 84, 22–38. [DOI] [PubMed] [Google Scholar]
- Winkler K, Scharnagl H., Tisljar U., Hoschützky H., Friedrich I., Hoffmann MM., Hüttinger M., Wieland H., März W., 1999. Competition of Abeta amyloid peptide and apolipoprotein E for receptor-mediated endocytosis. J. Lipid Res 40, 447–455. [PubMed] [Google Scholar]
- Yamazaki Y, Zhao N, Caulfield TR, Liu C-C, Bu G 2019. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nature Rev. Neurol 15, 501–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Petanceska SS, Montine TJ, Holtzman DM, Schmidt SD, Parker CA, Callahan MJ, Lipinski WJ, Bisgaier CL, Turner BA, Nixon RA, Martins RN, Ouimet C, Smith JD., Davies P., Laska E., Ehrlich ME., Walker LC., Mathews PM., Gandy S., 2004. Aging, gender and APOE isotype modulate metabolism of Alzheimer's Abeta peptides and F-isoprostanes in the absence of detectable amyloid deposits. J. Neurochem 90, 1011–1018. [DOI] [PubMed] [Google Scholar]