Abstract
Background
Muscle atrophy and fatty infiltration (FI) are common occurrences following rotator cuff (RC) tears. Tears of all sizes are subject to muscle degeneration. The degree of muscle degeneration following RC tears is highly correlated with repair success and functional outcomes. We have recently discovered that muscle fibro-adipogenic progenitors (FAPs) can differentiate into uncoupling protein 1 (UCP-1)–expressing beige adipocytes and induce muscle regeneration. This study evaluated the potential of local cell transplantation of beige adipose FAPs (BAT-FAPs) to treat RC muscle degeneration in a murine model of RC repair.
Methods
BAT-FAPs were isolated from muscle in UCP-1 reporter mice by flow cytometry as UCP-1+/Sca1+/PDGFR+/CD31−/CD45−/integrin α7−. C57/BL6J mice underwent supraspinatus tendon tear with suprascapular nerve transection followed by repair 2 or 6 weeks after the initial injury. At the time of repair, mice received either no additional treatment, phosphate-buffered saline injection, or BAT-FAP injection. Functional outcomes were assessed by gait analysis. Mice were humanely killed at 6 weeks after cell transplantation. Supraspinatus muscle FI, fibrosis, muscle fiber size, and vascularity were analyzed and quantified via ImageJ. Analysis of variance with post hoc Tukey test and P <.05 was used to determine statistical significance.
Results
Cell transplantation diminished fibrosis, FI, and atrophy and enhanced vascularization in both delayed repair models. Cell transplantation resulted in improved shoulder function as assessed with gait analysis in both the delayed repair models.
Conclusions
BAT-FAPs significantly reduced muscle degeneration and improved shoulder function after RC repair. BAT-FAPs hold significant promise as a therapeutic adjunct to repair for patients with advanced RC pathology.
Level of evidence
Basic Science Study; Histology; In-Vivo Animal Model
Keywords: Rotator cuff tears, rotator cuff repair, muscle atrophy, fatty infiltration, fibro-adipogenic progenitor, beige fat
Rotator cuff (RC) tears are common shoulder injuries that result in upper extremity disability and diminished quality of life. The prevalence of RC tears increases with age.2 Large and massive tears account for 40% of all RC tears and commonly result in pathologic changes within the muscle.2 In addition, small and medium tears, particularly those involving the anterior supraspinatus (SS) tendon (RC cable), are also subject to muscle degeneration.23 Pathologic muscle degeneration that follows RC tears includes muscle atrophy, fatty infiltration (FI), and fibrosis, all of which compromise RC physiology and mechanics.11 Furthermore, the degree of baseline FI and muscle atrophy are strongly related to repair success and postoperative clinical outcomes.9,17,27,30
Several animal models of RC tears and repair have been used to evaluate RC muscle degeneration and tendon to bone healing.5,7,14,20 Mathewson et al22 have reported that RC muscle properties of small mammals such as mice and rats more closely represent the human anatomy compared with larger mammals such as sheep, pigs, or cows. Furthermore, we have previously developed a murine model of chronic RC injury and repair that demonstrated that repair of a chronic injury still had moderate atrophy, FI, and abnormalities in shoulder biomechanics, similar to what is seen in human long-term cuff repairs.20,31
Previous work has identified fibro-adipogenic progenitors (FAPs) as the cellular source of fibrosis and FI following RC tears.21 On stimulation, a subset of FAPs can also differentiate into brown adipocytes (BAT) that dissipate stored energy via uncoupling protein 1 (UCP-1).10 BAT has been a recent topic of interest owing to its ability to release paracrine factors that have trophic effects on various tissues.33 Kong et al15 has described the presence of crosstalk between BAT and skeletal muscle whereby skeletal muscle growth and exercise capacity is dependent on thermogenic gene expression. We have previously shown that activation of BAT via systemic administration of a β3-adrenergic receptor agonist results in reduced muscle atrophy FI and improved shoulder function after RC repair in a mouse model of chronic injury.32 However, the disadvantage of systemic adrenergic drug administration is that it carries the risk of adverse cardiovascular and antimuscarinic side effects.26 In this study, we examine the potential of local transplantation of BAT FAPs as an alternative to systemic drug delivery to promote muscle regeneration following repair of RC tears. This would serve as a potential first step in the study of a point-of-care model of stem cell transplantation to improve muscle quality and function after RC repair.
Methods
Cell isolation/culture
Whole muscle was isolated from UCP-1 reporter mice acquired through a generous donation from Shingo Kajimura (San Francisco, CA, USA). UCP-1 reporter mice were generated by first inserting a luciferase-tdTomato cassette into the first exon of the UCP-1 gene. The following genetic construct was then inserted into the Y chromosome. Muscle was digested with 0.2% collagenase for 90 minutes followed by 0.4% Dispase (Thermo Fisher, MA, USA) for 30 minutes. FAPs were isolated as Sca1+/PDGFRα+/CD31−/CD45−/integrin α7− as previously described.28,29 FAPs were further isolated by expression of UCP-1. UCP-1(+) FAPs were then cultured in F10–20% fetal bovine serum–10 ng/mL basic fibroblast growth factor until transplantation. At the time of transplantation, cells were lifted with 0.25% trypsin–ethylenediaminetetraacetic acid and then resuspended in phosphate-buffered saline (PBS).
Rotator cuff injury and repair
Three-month-old C57/BL6J mice underwent unilateral SS tendon transection and suprascapular nerve transection, as previously described.20 The contralateral side was used as a control. For the delayed repair groups, tendon transection and suprascapular nerve transection was followed by repair 2 or 6 weeks after the initial surgery as previously described.31 For the repair surgery, mice underwent anesthesia with 3% isoflurane with oxygen. A 1-cm longitudinal skin incision was made on the right shoulder followed by division of the trapezius and deltoid to expose the RC. A 4–0 nylon suture on an FS2 reverse cutting needle was weaved through the SS tendon. As the humerus was stabilized with microdissecting forceps, the above-mentioned needle was then tunneled through the anatomic position on the greater tuberosity.
Cell injection
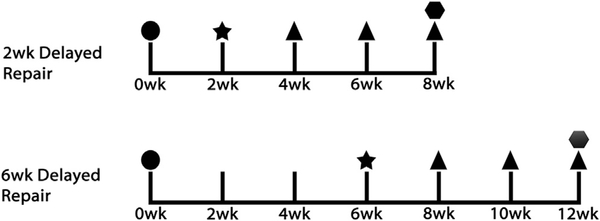
Following reattachment of the SS tendons at the time of repair, mice either received no injection, 10 μL of PBS into both proximal and distal ends of the SS, or 10 μL of 125,000 BAT-FAPs into both proximal and distal ends of the SS for a total of 250,000 cells per mouse. All injections were performed using a 28-G insulin syringe. All experiments were approved by the Institutional Animal Care and Use Committee of our institution. Experiment workflow is depicted in Figure 1.
Figure 1.
Flow diagram of experimental design (n = 12 per delayed repair group). In each repair group, 4 mice underwent repair only, 4 mice received phosphate-buffered saline injections along with repair, and 4 mice received cell injections along with repair. TT+DN, tendon transection and denervation. ●, TT+DN; ★, repair; ▲, gait analysis;  , harvest.
, harvest.
Gait analysis
DigiGait (Mouse Specifics [Framingham, MA, USA]) analysis was conducted to assess shoulder function every 2 weeks after the final surgery. All mice walked at 8 cm/s for 10 seconds on the DigiGait system. Stride length, stride duration, and stance width were the parameters chosen to assess forelimb function and calculated as previously described.12 Stride length was defined as the distance from the initial paw placement to the subsequent paw placement. Stride duration was defined as the time elapsed between initial paw placement and subsequent paw placement. Stance width was defined as the distance between the vertical axes that pass through the center of each forelimb paw.
Muscle harvesting and histology
All mice were humanely sacrificed at 6 weeks after the last surgery. Bilateral SS muscles were collected and weighed in milligrams to assess muscle atrophy. Following wet weight measurement, muscle specimens were flash-frozen by immersion in isopentane cooled with liquid nitrogen and sectioned (10 μm) with a cryostat. For immunofluorescence staining, sections were fixed with 2% paraformaldehyde for 10 minutes, washed in PBST (1XPBS–0.1% TritonX-100), blocked in 5% bovine serum albumin for 1 hour and then incubated with primary antibodies at 4°C overnight. Slides were then washed in PBS and incubated with secondary antibodies (1:200) for 1 hour at room temperature. Tissue sections were mounted with Vectashield (Vector Laboratories, Burlingame, CA, USA) with 4′,6-diamidino-2-phenylindole (DAPI). For evaluation of fibrosis and FI, staining was carried out using a Masson trichrome kit (American Mastertech [McKinney, TX, USA]) per manufacturer’s instructions. Quantification was conducted using Image J. Collagen and fat fractional area was determined as area occupied by perilipin staining or collagen fibers divided by total sample area. Vascularity was determined based on the capillary to fiber ratio, which was calculated as the total number of cells with positive CD31 staining divided by the total number of muscle fibers in the image, as previously described.
Antibodies
Primary antibodies and concentrations used for immunofluorescence in this study are as follows: rat anti-mouse CD31 (BioLegend [San Diego, CA, USA] catalog no. 102501, 1:100), goat anti-mouse perilipin (Abcam [Cambridge, MA, USA] ab61682,1:50), rabbit anti-laminin (Sigma [St. Louis, MO, USA] L9393, 1:200). Secondary antibodies used in this study are donkey anti-rabbit IgG Alexa Fluor 488 (Abcam, ab150073, 1:200), donkey anti-rat IgG Alexa Fluor 647 (Abcam, ab150155, 1:200), donkey anti-goat IgG Alexa Fluor 647 (Abcam, ab150135).
Statistical analysis
One-way analysis of variance with a post hoc Tukey test was used to analyze the difference between groups with regard to fibrosis, fatty infiltration, vascularity, and gait parameters. Significance was defined as P <.05.
Results
BAT-FAP transplantation mitigates muscle atrophy
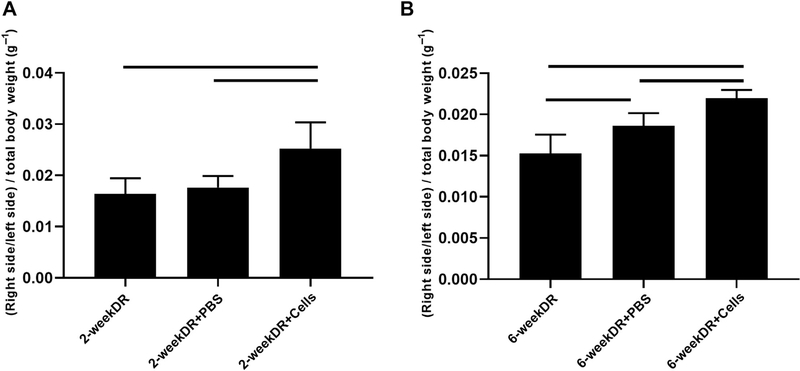
Wet muscle weights were determined by taking the ratio of the right and left SS muscle weights in milligrams and dividing that value by total body weight in grams. In the 2-week delayed repair model, mice receiving BAT-FAPs exhibited significantly less weight loss compared with those receiving PBS injection and repair alone (0.025 ± 0.03 vs. 0.018 ± 0.023 in PBS vs. 0.016 ± 0.003 with repair alone; P = .02). In the 6-week delayed repair model, mice that were treated with BAT-FAPs exhibited significantly less weight loss compared with those receiving PBS injection and repair alone (0.022 ± 0.0009 vs. 0.018 ± 0.002 in PBS vs. 0.016 ± 0.002 with repair alone; P = .001) (Fig. 2).
Figure 2.
Supraspinatus muscle wet weight change. (A) 2-week delayed repair model; (B) 6-week delayed repair model. Solid lines indicate P < .05. DR, delayed repair; PBS, phosphate-buffered saline.
BAT-FAP transplantation increases muscle fiber size
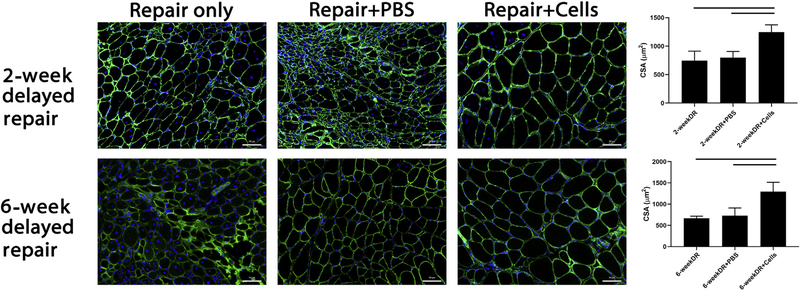
Muscle atrophy was measured microscopically via ImageJ using a cross-sectional area macro. In the 2-week delayed repair model, SS fiber cross-sectional area was significantly higher in mice receiving BAT-FAPs compared with their controls (1246 ± 128 μm2 vs. 801 ± 105 μm2 in PBS vs. 745 ± 167 μm2 with repair alone, P = .01). In the 6-week delayed repair model, SS fiber cross-sectional area was significantly higher in the mice receiving BAT-FAPs compared with those receiving PBS injection or repair alone (1294 ± 218 μm2 vs. 670 ± 45 μm2 in PBS vs. 728 ± 181 μm2 with repair alone, P = .0008) (Fig. 3).
Figure 3.
Immunofluorescence microscopy of supraspinatus (SS) fiber cross-sectional area as determined via ImageJ. Top: 2-week delayed repair model. Bottom: 6-week delayed repair model. Fiber size was significantly larger in mice receiving cell injections compared with respective controls. Solid lines indicate P < .05. PBS, phosphate-buffered saline; DAPI, 4′,6-diamidino-2-phenylindole; CSA, cross-sectional area; DR, delayed repair.
BAT-FAP transplantation reduces muscle fibrosis
The degree of fibrosis was measured as the area of collagen staining fibers divided by the total area of the image after Trichrome staining. Mice treated with BAT-FAPs exhibited significantly less fibrosis compared with those receiving PBS injection and repair alone in both 2-week delayed repair (6.5% ± 2.1% vs. 13.4% ± 1.5% in PBS vs. 17.3% ± 4% with repair alone; P = .001) and 6-week delayed repair models (7.8 ± 2.2 vs. 12.4% ± 0.8% in PBS vs. 11.9% ± 1.2% with repair alone; P = .008) (Fig. 4).
Figure 4.
Trichrome staining (×100) of the supraspinatus (SS) showed significantly more fibrosis and fatty infiltration in repair only and PBS groups compared with the group receiving cell injection. Area fraction of collagen was quantified as the area of collagen staining divided by the total area of the image. Area fraction of fat was quantified as the area occupied by fat divided by the total area of the image. Solid lines indicate P < .05. PBS, phosphate-buffered saline.
BAT-FAP transplantation reduces muscle fatty infiltration
Fatty infiltration was determined by the area of perilipin staining divided as a percentage of the total area. Mice in the cell treatment arm exhibited significantly less fatty infiltration in the SS following repair. Fat fractional area in the 2-week delayed repair model was 3.5% ± 0.6% vs. 10.7% ± 0.7% in PBS vs. 12.5% ± 1.0% with repair alone (P = .001). Fat fractional area in the 6-week delayed repair model was 8.4% ± 1.6% vs. 16.1% ± 2.4% in PBS vs. 14.8% ± 0.3% with repair alone (P = .004) (Fig. 4). Transplanted cells are seen to lie adjacent to intramuscular fat and maintain their beige phenotype 6 weeks after delivery (Fig. 5).
Figure 5.
Positional relationship of transplanted cells with intramuscular fat and blood vessels. (A) Transplanted cells lie in the region adjacent to fat and maintain their beige phenotype as seen by persistent UCP-1 expression 6 weeks post transplantation. (B) Transplanted cells cluster around blood vessels. UCP-1, uncoupling protein 1; DAPI, 4′,6-diamidino-2-phenylindole.
BAT-FAP transplantation increases SS muscle vascularity
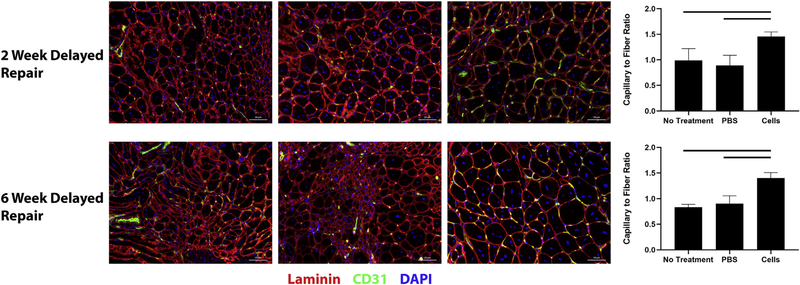
Vascularity was determined based on the capillary to fiber ratio, which was calculated as the total number of cells with positive CD31 staining divided by the total number of muscle fibers in the image. BAT-FAP transplantation resulted in significantly more vascularity in the SS in the 2-week delayed repair (1.5 ± 0.1 vs. 0.9 ± 0.2 in PBS vs. 1.0 ± 0.2 with repair alone; P = .01) and 6-week delayed repair models (1.4 ± 0.1 vs. 0.9 ± 0.2 in PBS vs. 0.8 ± 0.06 with repair alone; P = .0001) (Fig. 6). Transplanted cells also appeared to cluster around blood vessels (Fig. 5).
Figure 6.
Capillary to fiber ratio in the injured supraspinatus. Top, 2 week delayed repair model. Bottom, 6 week delayed repair model. Supraspinatus in mice receiving cell injections exhibited significantly more vascularity compared with respective controls. Solid lines indicate P < .05. PBS, phosphate-buffered saline.
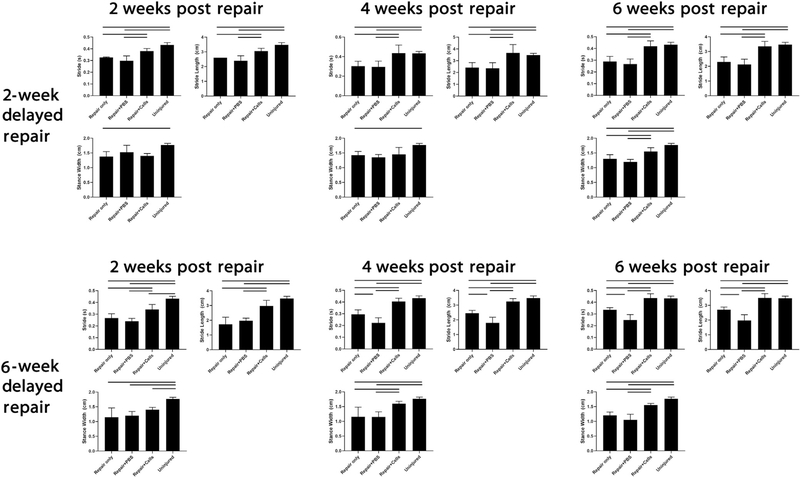
BAT-FAP transplantation improves shoulder function
In both the 2- and 6-week delayed repair models, mice receiving BAT-FAPs exhibited improved shoulder function as determined by gait analysis. In both repair models, mice receiving BAT-FAPs exhibited enhanced stride time and stride length at 2, 4, and 6 weeks post transplantation compared with mice receiving saline injections or repair alone. In the 2-week delayed repair model, stance width did not improve until 6 weeks after cell transplantation. In the 6-week delayed repair model, stance width did not improve until 4 weeks after cell transplantation (Fig. 7).
Figure 7.
Gait analysis of injured forelimb. Mice receiving cell injections exhibited significantly enhanced stride time and stride length at all time points. Solid lines indicate P < .05. PBS, phosphate-buffered saline.
Discussion
RC muscle atrophy and FI are common occurrences in patients with RC tears.11,17,23 Although tears of all sizes can result in muscle degeneration, Goutallier class 3 and 4 tears are particularly associated with larger tears, poor postrepair return to function, and high retear rates.13 The chronic nature of RC injuries has important implications for surgical repair as many patients do not present until many months or even years after the initial insult, accumulating more muscle degeneration in the interim. Our study captures this aspect of RC damage by using a delayed repair model to better reflect the protracted injury process.
There is currently no treatment for fibrosis and FI that develop after RC tears. A study by Davies et al6 demonstrated that pharmacologic inhibition of transforming growth factor–beta reduces fibrosis and FI by inducing FAP apoptosis. Wang et al32 have also shown that systemic administration of a β3-adrenergic receptor agonist results in reduced fibrosis and FI in a murine model of delayed RC repair. However, the nonselective and systemic nature of these treatments could cause unintended side effects, namely, interference with tendon to bone healing in the immediate postsurgical setting and antimuscarinic symptoms, respectively. As such, treatment that avoids the adverse side effects of systemic drug administration is important. In this study, we find that fibrosis and FI remain following isolated RC repair, which is consistent with clinical findings showing that repair does not reverse muscle degeneration.9,17,27,30 However, fibrosis and FI was significantly diminished when RC repair is augmented with intramuscular delivery of BAT-FAPs. In our delayed repair models, BAT-FAPs were delivered long after fibrosis and FI have developed, but the treatment group did decrease the amount of fibrosis and FI present. The exact mechanism by which these cells affect these changes is not entirely known. It is possible that the observed regression of fibrosis and FI is related to BAT-FAP–induced fibroblast and adipocyte death or trans-differentiation. More likely, this process was intermediated by cytokines or other factors secreted by BAT-FAPs. We have found that co-culture of BAT-FAPs with white FAPs in transwell chambers results in dedifferentiation of white fat and decreased adipogenesis of white FAPs, suggesting that secreted factors are important in mitigating these cellular changes. Future work will center on elucidating the exact mechanisms by which these cells diminish fibrosis and FI.
Rotator cuff muscle atrophy is characterized as the loss of muscle mass and myofiber size secondary to mechanical unloading and subsequent disuse. Decreased muscle mass and fiber size results in decreased postoperative strength, limited shoulder motion, and failed tendon repairs.4 Here, we show that mice exhibited significantly less muscle atrophy and greater fiber cross-sectional area when repair is augmented with BAT-FAP cell transplantation. The exact role of BAT in mitigating muscle atrophy after RC repair remains unknown. However, BAT has been shown to secrete paracrine factors known collectively as batokines that appear to have anabolic effects on skeletal muscle.33 Follistatin and IRF4, in particular, are highly expressed in BAT and promote muscle growth and functional capacity in vivo by inhibiting myostatin.15,18 It is possible that the release of batokines from BAT-FAPs may promote muscle hypertrophy and regeneration in this manner. On a macroscopic level, poor muscle quality despite successful mechanical reloading may portend further disuse and subsequent atrophy. In this study, improved muscle fiber size in the cell treatment arm translated to enhanced shoulder function.
Vascularization is important for maintaining optimal metabolic conditions and nutrient delivery to skeletal muscle during regeneration. Muscle regeneration is impaired when angiogenesis is delayed.24 We have previously found that the vascularity of the RC decreases significantly in a murine RC model.19 This phenomenon is further supported in the literature by Gigliotti et al,8 who report that vascularity decreases in human RC tears and correlate with the degree of atrophy, suggesting that improving vascularity may improve muscle growth. In the current study, we find that BAT-FAP cell transplantation significantly increases vascularity following repair. The role of BAT in angiogenesis following RC tears has yet to be determined. Vascular endothelial growth factor (VEGF) is a potent regulator of angiogenesis and is highly expressed in BAT and differentiates brown adipocytes, as described in other studies.1 VEGF has also been shown to be vital to brown adipocyte survival as blockade of VEGF signaling results in brown adipocyte apoptosis.1 It is likely that although VEGF secretion is required to maintain BAT survival and vascularization within BAT, it may also be supporting vascular growth locally within the muscle. The improvement in vascularity within the SS may contribute to improved shoulder function following repair and cell transplantation.
Various shoulder injury models have used forelimb gait analysis to evaluate shoulder function.16,25 Previous studies have shown that gait characteristics correlate well with histologic parameters of muscle degeneration following RC tears.3,32 In this study, we find that mice exhibited enhanced function in parameters that simulate flexion and abduction of the injured forelimb, suggesting that improvements in muscle quality are critical in predicting functional outcomes following RC repair. The disadvantage with using gait analysis to evaluate shoulder function, however, is that it is difficult to determine whether functional improvements are due to improvements in the mechanical properties of the RC muscles themselves. Future work will center on evaluating the contractility of isolated RC muscles after repair and cell transplantation and how it relates to overall shoulder function.
There are several limitations in this study. As with all mouse RC tear models, the SS tendon can spontaneously reattach to the humerus with scar tissue. Prior biomechanical studies demonstrate that scar tissue is significantly weaker than surgically reattached tendon and results in more functional impairment compared with repair.31 However, repair alone does not translate to full functional recovery as demonstrated in the current study, suggesting that muscle quality in the postoperative setting is just as important as tendon to bone healing in predicting functional outcomes. In addition, mouse cells were used in the current study. Stem cell antigen 1 (Sca1) is a marker specific to mouse FAPs. Markers for human FAPs are not clearly defined in the literature. Identification of human FAP surface markers are necessary to determine if results observed in this study can be obtained with human cells.
Conclusion
We demonstrate that BAT-FAPs exhibit significant regenerative potential in the setting of delayed repair of RC tears. In addition, our study highlights the importance of enhancing muscle quality on repair success and postoperative functional recovery. Localized BAT-FAP cell delivery in this study offers benefits comparable to systemic drug administration with minimal risks of adverse side effects. BAT-FAPs are directly isolated from healthy muscle and are minimally manipulated without any genetic modification prior to transplant, offering the potential for immediate point of care treatment. Future work will focus on identifying a human analog of these cells with the hopes of offering a viable therapeutic adjunct to repair for patients with advanced RC pathology.
Footnotes
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
References
- 1.Bagchi M, Kim LA, Boucher J, Walshe TE, Kahn CR, D’Amore PA.Vascular endothelial growth factor is important for brown adipose tissue development and maintenance. FASEB J 2013;27:3257–71. 10.1096/fj.12-221812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedi A, Dines J, Warren RF, Dines DM. Massive tears of the rotator cuff. J Bone Joint Surg Am 2010;92:1894–908. 10.2106/JBJS.I.01531 [DOI] [PubMed] [Google Scholar]
- 3.Carpenter JE, Thomopoulos S, Flanagan CL, DeBano CM, Soslowsky LJ. Rotator cuff defect healing: a biomechanical and histologic analysis in an animal model. J Shoulder Elbow Surg 1998;7:599–605. [DOI] [PubMed] [Google Scholar]
- 4.Chung SW, Kim SH, Tae SK, Yoon JP, Choi JA, Oh JH. Is the supraspinatus muscle atrophy truly irreversible after surgical repair of rotator cuff tears? Clin Orthop Surg 2013;5:55–65. 10.4055/cios.2013.5.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman SH, Fealy S, Ehteshami JR, MacGillivray JD, Altchek DW, Warren RF, et al. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am 2003;85:2391–402. 10.2106/00004623-200312000-00018 [DOI] [PubMed] [Google Scholar]
- 6.Davies MR, Liu X, Lee L, Laron D, Ning AY, Kim HT, et al. TGF-beta small molecule inhibitor SB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. PLoS One 2016;11:e0155486 10.1371/journal.pone.0155486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frey E, Regenfelder F, Sussmann P, Zumstein M, Gerber C, Born W, et al. Adipogenic and myogenic gene expression in rotator cuff muscle of the sheep after tendon tear. J Orthop Res 2009;27:504–9. 10.1002/jor.20695 [DOI] [PubMed] [Google Scholar]
- 8.Gigliotti D, Xu MC, Davidson MJ, Macdonald PB, Leiter JRS, Anderson JE. Fibrosis, low vascularity, and fewer slow fibers after rotator-cuff injury. Muscle Nerve 2017;55:715–26. 10.1002/mus.2538 [DOI] [PubMed] [Google Scholar]
- 9.Gladstone JN, Bishop JY, Lo IK, Flatow EL. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 2007;35: 719–28. 10.1177/0363546506297539 [DOI] [PubMed] [Google Scholar]
- 10.Gorski T, Mathes S, Krutzfeldt J. Uncoupling protein 1 expression in adipocytes derived from skeletal muscle fibro/adipogenic progenitors is under genetic and hormonal control. J Cachexia Sarcopenia Muscle 2018;9:384–99. 10.1002/jcsm.12277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures. Pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res 1994;(304):78–83. [PubMed] [Google Scholar]
- 12.Hampton TG, Stasko MR, Kale A, Amende I, Costa AC. Gait dynamics in trisomic mice: quantitative neurological traits of Down syndrome. Physiol Behav 2004;82:381–9. 10.1016/j.physbeh.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 13.Khair MM, Lehman J, Tsouris N, Gulotta LV. A systematic review of preoperative fatty infiltration and rotator cuff outcomes. HSS J 2016; 12:170–6. 10.1007/s11420-015-9465-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim HM, Galatz LM, Lim C, Havlioglu N, Thomopoulos S. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg 2012;21:847–58. 10.1016/j.jse.2011.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong X, Yao T, Zhou P, Kazak L, Tenen D, Lyubetskaya A, et al. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab 2018;28:631–43.e3. 10.1016/j.cmet.2018.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lakes EH, Allen KD. Gait analysis methods for rodent models of arthritic disorders: reviews and recommendations. Osteoarthritis Cartilage 2016;24:1837–49. 10.1016/j.joca.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lansdown DA, Lee S, Sam C, Krug R, Feeley BT, Ma CB. A prospective, quantitative evaluation of fatty infiltration before and after rotator cuff repair. Orthop J Sports Med 2017;5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee SJ, Lee YS, Zimmers TA, Soleimani A, Matzuk MM, Tsuchida K, et al. Regulation of muscle mass by follistatin and activins. Mol Endocrinol 2010;24:1998–2008. 10.1210/me.2010-0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee C, Liu M, Bertoy L, Kim HT, Feeley BT. Effect of Amibegron on blood vessel and nerve density in a mouse rotator cuff injury model. Paper presented at: Orthopaedic Research Society Annual Meeting February 2–5, 2019; Austin, TX. [Google Scholar]
- 20.Liu X, Laron D, Natsuhara K, Manzano G, Kim HT, Feeley BT. A mouse model of massive rotator cuff tears. J Bone Joint Surg Am 2012;94:e41 10.2106/JBJS.K.00620 [DOI] [PubMed] [Google Scholar]
- 21.Liu X, Ning AY, Chang NC, Kim H, Nissenson R, Wang L, et al. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J 2016;6:6–15. 10.11138/mltj/2016.6.1.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathewson MA, Kwan A, Eng CM, Lieber RL, Ward SR. Comparison of rotator cuff muscle architecture between humans and other selected vertebrate species. J Exp Biol 2014;217:261–73. 10.1242/jeb.083923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Namdari S, Donegan RP, Dahiya N, Galatz LM, Yamaguchi K, Keener JD. Characteristics of small to medium-sized rotator cuff tears with and without disruption of the anterior supraspinatus tendon. J Shoulder Elbow Surg 2014;23:20–7. 10.1016/j.jse.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 24.Ochoa O, Sun D, Reyes-Reyna SM, Waite LL, Michalek JE, McManus LM, et al. Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol 2007;293:R651–61. 10.1152/ajpregu.00069.2007 [DOI] [PubMed] [Google Scholar]
- 25.Oki S, Shirasawa H, Yoda M, Matsumura N, Tohmonda T, Yuasa K,et al. Generation and characterization of a novel shoulder contracture mouse model. J Orthop Res 2015;33:1732–8. 10.1002/jor.22943 [DOI] [PubMed] [Google Scholar]
- 26.Rosa GM, Baccino D, Valbusa A, Scala C, Barra F, Brunelli C, et al. Cardiovascular effects of antimuscarinic agents and beta3-adrenergic receptor agonist for the treatment of overactive bladder. Expert Opin Drug Safe 2018;17:487–97. 10.1080/14740338.2018.1453496 [DOI] [PubMed] [Google Scholar]
- 27.Shen PH, Lien SB, Shen HC, Lee CH, Wu SS, Lin LC. Long-term functional outcomes after repair of rotator cuff tears correlated with atrophy of the supraspinatus muscles on magnetic resonance images. J Shoulder Elbow Surg 2008;17(1 Suppl):1S–7S. 10.1016/j.jse.2007.04.014 [DOI] [PubMed] [Google Scholar]
- 28.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 2010;12:143–52. 10.1038/ncb2014 [DOI] [PubMed] [Google Scholar]
- 29.Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci 2011;124:3654–64. 10.1242/jcs.086629 [DOI] [PubMed] [Google Scholar]
- 30.Valencia AP, Lai JK, Iyer SR, Mistretta KL, Spangenburg EE, Davis DL, et al. Fatty infiltration is a prognostic marker of muscle function after rotator cuff tear. Am J Sports Med 2018;46:2161–9. 10.1177/0363546518769267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z, Liu X, Davies MR, Horne D, Kim H, Feeley BT. A mouse model of delayed rotator cuff repair results in persistent muscle atrophy and fatty infiltration. Am J Sports Med 2018;46:2981–9. 10.1177/0363546518793403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Liu X, Liu M, Lee C, Kim HT, Feeley BT. Beta-3 adrenergic receptor agonist improves rotator cuff muscle rehabilitation and shoulder function after delayed tendon repair in mice. Paper presented at: International Society of Arthroscopy, Knee Surgery, and Orthopaedic Sports Medicine Congress May 12–16, 2019; Cancun, Mexico. [Google Scholar]
- 33.Wang GX, Zhao XY, Lin JD. The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol Metab 2015;26: 231–7. 10.1016/j.tem.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]