Abstract
Objective
We examined the benefits of a community‐based program combining physical exercise, cognitive training, and education on dementia and lifestyle habits.
Methods
This crossover open‐label trial included 141 community‐dwelling elderly people with suspected mild cognitive decline (MCD). Subjects were assigned to a 6‐month intervention‐first/6‐month observation‐second (INT‐OBS) group or an OBS‐INT group. The 6‐month intervention consisted of 2 h of physical exercise, cognitive training, and classroom study or rest once weekly. Primary outcome was change in Touch Panel‐type Dementia Assessment Scale (TDAS) score.
Results
TDAS score improved significantly during the intervention period compared with the observation period for all subjects (P < 0.05). Some physical functions also improved significantly during the intervention period compared with the observation period in the OBS‐INT group (P < 0.05).
Interpretation
This community‐based program improved both cognitive and physical function in elderly people with suspected MCD.
Introduction
Numerous studies have examined physical and cognitive training programs for prevention of cognitive decline. According to recent systematic reviews, there is insufficient evidence for the cognitive benefits of single‐component physical activity.1 Alternatively, cognitive training can improve intellectual function, but these gains are generally restricted to the training domain.2 The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) study reported that multidomain intervention including diet, exercise, cognitive training, and vascular risk monitoring improved multidomain neuropsychological test performance.3 Based on these findings, the following interventions are considered vital for preventing cognitive decline; exercise combining aerobic and strength training, cognitive training engaging multiple domains, and education to improve lifestyle habits. In addition, retrospective studies have reported a significant association between higher frequency of past stimulating leisure activities and reduced dementia risk.4, 5 Conversely, a follow‐up study of people aged 35–55 years for an average of 24 years found a negative association between physical activity and dementia onset.6 Nonetheless, physical inactivity in people over the age of 65 years is considered a strong risk factor for dementia onset.7 Therefore, it is essential that intervention be sustained through habit formation.
Many previous studies have focused on either physical activity or cognitive training to prevent cognitive decline. Recently, dementia prevention classes have been instituted by local governments throughout Japan, but the contents are determined by each local government, suggesting that these programs will vary in efficacy.8 About 15 years ago, Kotoura town and Houki town in Tottori Prefecture, Japan, initiated dementia prevention classes centered on exercise, cognitive training, and interpersonal communication. Based on this approach, we have developed a unique cognitive decline prevention program that combines physical exercise, cognitive training, and education on dementia and lifestyle habits. An inexpensive, easily administered, and standardized program not requiring highly trained personnel is required for broad application at the community level. Therefore, this study aims to verify the effectiveness of this program for suspected cognitive decline by a method that local government and general medical staff perform participant selection, intervention, and primary evaluation.
Subjects and Methods
Subjects
About 300 community‐dwelling elderly people in Houki town (Tottori Prefecture, Japan), who had not received Certification of Needed Long‐Term Care, were over 65 years old, and scored 10–13 points on a dementia screening test administered via touch‐pad computer (Japanese version produced by Nihon Kohden Corporation, Tokyo, Japan, and sold commercially as model MSP‐1000) between 1 April 2016 and 31 August 2017 were considered as candidates. The maximum possible score on the MSP‐1000 is 15 points. When the cutoff value is 12 points or less, the sensitivity and specificity for distinguishing Alzheimer’s disease patients from healthy controls are 96% and 86%, respectively.9 This study included subjects with MSP‐1000 scores of 10–13 in order to include mild cognitive decline (MCD) but exclude severe cognitive decline. Subjects who were unable to perform physical exercises because of serious heart disease or were unable to rise from a sitting position were also excluded. The period for obtaining consent was from 1 July 2017 to 31 August 2017, and consent was obtained from 147 subjects. Magnetic resonance imaging (MRI) was also conducted with additional consent for subjects without contraindications such as medical implants. However, consent was withdrawn by six subjects before baseline assessment, so the final cohort included 141 elderly subjects with suspected MCD.
The study was approved by the ethics committee of Tottori University (approval number: 1705B025) and conformed to the principles of the Declaration of Helsinki. The study was registered at the UMIN Clinical Trials Registry (UMIN000027792). Subjects provided informed consent for participation after a full explanation of the study protocol. If the subject has already been diagnosed with dementia, a guardian’s consent was obtained in writing.
Procedures
The examination and intervention schedule of this crossover open‐label trial is shown in Figure 1. We performed the following examinations at baseline; cognitive function test, physical function tests, blood tests, carotid ultrasonography, lifestyle questionnaire, and MRI (for those providing additional consent). Subsequently, the subjects were assigned to receive the intervention for the first 6 months and then observation for 6 months (INT‐OBS group) or the observation and intervention in reverse order (OBS‐INT group) by stratified randomization considering age and sex and using a computer generated random number. In addition, the number of subjects per classroom providing the intervention program was set at 11–15. Five classrooms were held for both groups. The INT‐OBS group participated in a 2‐h program once a week for 6 months after the baseline test, and the OBS‐INT group spent 6 months without any intervention. After that, we repeated the same test battery except for MRI. After the second test battery, the INT‐OBS group was observed for 6 months, whereas the OBS‐INT group participated in the 2‐hour program once weekly for 6 months. Finally, we performed the test battery again, including MRI for those providing additional consent.
Figure 1.
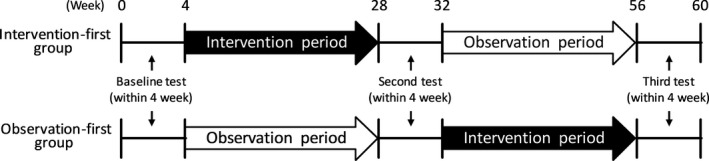
Schedule of this study.
Intervention program
The program consists of 50 min of physical exercise, 20 min of break time or education on dementia and lifestyle habits, and 50 min of cognitive training. The education component was delivered once every 4 weeks (six times in total), and days without education were used for a rest period. Each classroom was operated by an instruction team composed of one occupational therapist, one public health nurse or nurse, and one adviser with knowledge of dementia prevention.
The physical exercise program included the following activities centered on aerobic and strength training; preparation exercise (10 min) consisting of deep breathing, scapula exercise, trunk rotation, pelvic exercise, and lower limb stretch, followed by aerobic and strength exercises (35 min) consisting of one‐leg standing, stepping, cognitive tasks performed while stepping, chair squats, side steps, walking, and a final cooling down period (5 min) consisting of the preparation exercise. The intensity range of the aerobic and strength training exercises were 3–4 metabolic equivalents (METs).
The cognitive training performed in this study focused on eight domains, including visual–spatial perception, attention function, recent memory, working memory, mathematical ability, ability to think, executive function, and judgment function (Table S1), and it was designed to stimulate various cognitive domains. Weekly cognitive training was conducted according to the schedule shown in Table S2; an introductory component (10 min), followed by an individual activity (15 min), a group activity (20 min), and a summary of the class (5 min). Each 8‐week training session was considered to be one set, and three sets of the same content were implemented. Instead of conducting fixed activities, the contents and difficulty levels were adjusted as appropriate for each classroom at the discretion of the instructor.
Lectures on dementia and lifestyle habits were recorded on DVD and played during the session. Six themes were presented in the following order by experts in each field; “What is dementia?,” “Relationship between dementia and lifestyle‐related diseases,” “Relationship between dementia prevention and lifestyle habits,” “Relationship between dementia prevention and social exchange,” “Early consultation and response,” and finally “To create a town where dementia can be prevented.”
Assessments
Cognitive function tests, physical function tests, blood tests, carotid ultrasonography, and questionnaire surveys were conducted on the same day during the 4 week test period, whereas MRI was performed on a different day. The examiner was not involved in classroom operation and was blinded to group assignment. The cognitive function tests were evaluated using a computer. The evaluation methods of other tests were unified among the examiners.
Cognitive function test
To assess cognitive function, we used the Touch Panel‐type Dementia Assessment Scale (TDAS) (Nihon Kohden Corporation, Tokyo, Japan),10 a modified version of the Alzheimer’s Disease Assessment Scale‐Cognitive Subscale (ADAS‐Cog)11 in which subjects enter answers directly into a touch panel‐type computer following instructions. The TDAS clarifies the level of cognitive dysfunction using nine examination items, which included “word recognition,” “following simple commands,” “visual–spatial perception,” “accuracy of the order of a process,” “naming fingers,” “orientation,” “money calculation,” “object recognition,” and “clock time recognition.” Scores range from 0 (all correct answers) to 101 (all incorrect answers).
Physical function tests
We tested the following parameters of physical condition and musculoskeletal function: grip strength, sit‐to‐stand test, open‐eye one‐leg standing time, and anteflexion on sitting. Grip strength was measured twice for the dominant or stronger hand, and the better value was recorded. The sit‐to‐stand result was recorded as the number of times standing and sitting within 30 sec with both hands in front of the chest. We measured open‐eye one‐leg standing time twice (60 sec maximum) and recorded the better value. We measured the extent of long seat body anteflexion twice and recorded the better value. On the day of the examination, some subjects in poor physical condition could not complete all items.
Blood tests
Blood samples were collected, and total cholesterol, HDL cholesterol, LDL cholesterol, blood glucose, HbA1c, total protein, and albumin were measured by Tottori Health Service Association (Tottori, Japan).
Ultrasonography
Carotid plaques in intra‐ and extracranial arteries were evaluated using an ultrasonic diagnostic system (UF‐760AG; Fukuda Denshi Co., Ltd., Tokyo, Japan). Carotid plaques were measured in B‐mode using a 7.5 MHz transducer. Plaques were defined by intima‐media thickness (IMT) greater than 1.1 mm. The IMT measurements were performed from the near and far walls along four segments of the bilateral carotid arteries: the common carotid artery (CCA) proximal to the starting point, CCA proximal to the carotid bifurcation, the carotid bifurcation, and internal carotid artery.12 The plaque score (PS) was calculated by summing maximum plaque thicknesses for the eight segments.
Lifestyle questionnaire
A self‐administered questionnaire about daily life was completed by each participant. The survey included items on physical activity, intellectual activity, socialization, sleep quality, sleep time, napping frequency, meals, self‐rated health, residence status, drinking habit, and smoking.
MRI analysis
MRI was performed at baseline and with the third test battery by a mobile MRI car (Freeill Co. Ltd., Gunma, Japan) equipped with a 1.5 T system (ECHELON RX; Hitachi, Ltd., Tokyo, Japan). Three‐dimensional volumetric acquisition of T1‐weighted gradient echo sequences in the sagittal plane were acquired (thin slices) and analyzed using a voxel‐based specific regional analysis system for Alzheimer’s disease (VSRAD advance®, Eisai Co, Ltd, Tokyo, Japan).13, 14 VSRAD advance allows for measurement of atrophy in the target volume of interest (VOI), in this case, the medial temporal structures including the entire entorhinal cortex, hippocampus, and amygdala. VSRAD advance automatically calculates z‐scores for the target VOI. The higher the z‐score was considered the greater the atrophy of the medial temporal region.
Outcomes
The primary outcome of this study was the change in cognitive performance measured using TDAS, whereas the secondary outcomes were the effects on physical function measured using physical function tests, including lifestyle‐related disease measured using blood tests and ultrasonography, lifestyle habits evaluated using a questionnaire, and the degree of medial temporal region atrophy measured using MRI.
Statistics
The sample size was originally defined as about 300 subjects (150 per arm), which was about the entire number of people with MSP‐1000 scores of 10–13 points during screening. However, only 147 subjects provided consent for this study. We were unable to infer the effect size in the sample size calculation due to the lack of previous randomized controlled trial evaluating cognitive decline prevention programs using TDAS. Therefore, we did not perform a power analysis in this study.
To verify the efficacy of the program that combined physical exercise, cognitive training, and education on dementia and lifestyle habits, the analysis excluded subjects who participated in less than two‐thirds of the classes and those who could not perform all evaluations at the baseline, and at the second and third tests (Per Protocol Set). Although such subjects were excluded from the analysis, participation in the program was not canceled unless there was an intention to withdraw consent. The SPSS statistical software package (version 25, IBM Japan, Tokyo, Japan) was used for all statistical analyses. The Shapiro–Wilk test was used to assess the normal distribution of data and Levene’s test to assess equality of variance. Differences in baseline demographics and background characteristics between groups were assessed using Student’s t‐test, Welch’s t‐test, Mann–Whitney U test, or chi‐squared test as indicated. Changes in test results among baseline, second, and third test batteries were evaluated by one‐way repeated measures analysis of variance or the Friedman test. The Bonferroni correction was used for post hoc pairwise comparisons. Changes in the results of VSRAD between the baseline and the third test were evaluated using the Wilcoxon signed‐rank test. For questionnaire results, the ordinal scale was evaluated using the chi‐square test. In addition, for each test item, the difference between the test results before and after the intervention period or before and after the observation period was calculated, and a Student’s t‐test, Welch’s t‐test, or Mann–Whitney U test was performed to assess respective changes. All statistical tests were two‐sided, and an α‐level of 0.05 was considered statistically significant.
Results
Subjects and baseline characteristics
The subject flow during the study is summarized in Figure 2. Of the 141 subjects who performed the baseline test, five were excluded because they were unable to perform all the tests, so 70 subjects remained in the INT‐OBS group and 66 in the OBS‐INT group. Of these, 63 in the INT‐OBS group and 65 in the OBS‐INT group remained for the second test battery, whereas 60 in the INT‐OBS group and 54 in the OBS‐INT group remained for the third test battery. The INT‐OBS and OBS‐INT groups were similar at the baseline (Table 1). The mean age of the subjects was 77.3 (standard deviation [SD], 6.3) years and 47 (34.6%) subjects were men.
Figure 2.
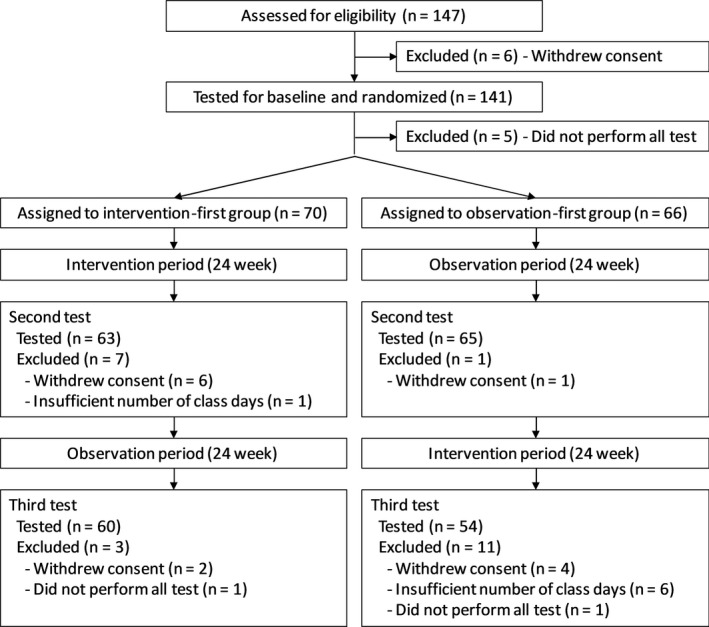
Flow chart of the subjects of this study.
Table 1.
Baseline characteristics of subjects.
| Characteristics | All subjects | INT‐OBS group | OBS‐INT group |
|---|---|---|---|
| (n = 136) | (n = 70) | (n = 66) | |
| Age, mean ± SD | 77.3 ± 6.3 | 77.6 ± 6.8 | 77.0 ± 5.6 |
| Sex (male), n (%) | 47 (34.6%) | 24 (34.3%) | 23 (34.8%) |
| Medical history, n (%) | |||
| Dementia | 6 (4.4%) | 3 (4.3%) | 3 (4.5%) |
| Hypertension | 72 (52.9%) | 35 (50.0%) | 37 (56.1%) |
| Dyslipidemia | 17 (12.5%) | 10 (14.3%) | 7 (10.6%) |
| Diabetes mellitus | 30 (22.1%) | 15 (21.4%) | 15 (22.7%) |
| Cognitive function, mean ± SD | |||
| TDAS score | 7.0 ± 5.7 | 7.2 ± 5.9 | 6.8 ± 5.5 |
| Physical function, mean ± SD | |||
| Grip strength (kg) | 24.8 ± 6.6 | 23.8 ± 6.0 | 26.0 ± 6.9 |
| Sit‐to‐stand test (n/30 sec) | 16.6 ± 5.3 | 16.6 ± 5.4 | 16.7 ± 5.3 |
| One‐leg standing (sec) | 23.4 ± 23.2 | 23.8 ± 24.3 | 23.1 ± 22.0 |
| Anteflexion on sitting (cm) | 31.7 ± 8.2 | 30.9 ± 8.6 | 32.5 ± 7.7 |
| Ultrasonography, mean ± SD | |||
| Plaque scores (mm) | 2.7 ± 2.5 | 2.5 ± 2.5 | 2.9 ± 2.4 |
| Blood tests, mean ± SD | |||
| Total cholesterol (mg/dL) | 193.0 ± 32.4 | 195.2 ± 34.8 | 190.7 ± 29.6 |
| HDL cholesterol (mg/dL) | 58.9 ± 13.0 | 59.1 ± 13.3 | 58.8 ± 12.6 |
| LDL cholesterol (mg/dL) | 108.4 ± 26.3 | 111.2 ± 26.1 | 105.4 ± 26.2 |
| Glucose (mg/dL) | 122.8 ± 45.3 | 124.1 ± 51.8 | 121.4 ± 37.0 |
| HbA1c (%) | 5.8 ± 0.7 | 5.8 ± 0.8 | 5.7 ± 0.5 |
| Total protein (g/dL) | 7.1 ± 0.4 | 7.2 ± 0.4 | 7.1 ± 0.4 |
| Albumin (g/dL) | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.3 |
| MRI, mean ± SD | |||
| Number, n | 105 | 52 | 53 |
| VARAD (z‐score) | 1.4 ± 0.8 | 1.5 ± 0.8 | 1.4 ± 0.8 |
INT, intervention; OBS, observation; TDAS, Touch Panel‐type Dementia Assessment Scale; VSRAD, voxel‐based specific regional analysis system for Alzheimer’s disease.
Cognitive function
Table 2 shows the results of cognitive evaluation using the TDAS. The total TDAS score did not change significantly in the INT‐OBS group but significantly improved at the third test compared with baseline in the OBS‐INT group (P < 0.05). Figure 3A shows the changes before and after the intervention or observation period. In the combined INT‐OBS and OBS‐INT group, the TDAS score significantly improved during the intervention period compared with the observation period (mean ± SD, –1.05 ± 3.92 vs. 0.74 ± 4.78 points; 95% CI, –2.90 to –0.67; P < 0.05). Furthermore, Table S3 shows the results of comparing changes in the TDAS without combining the INT‐OBS and OBS‐INT group.
Table 2.
Results of cognitive test, physical examination, ultrasonography test, and MRI test.
| INT‐OBS group§ | OBS‐INT group¶ | INT‐OBS vs. OBS‐INT group (P value) | ||||
|---|---|---|---|---|---|---|
| Mean ± SD | P value | Mean ± SD | P value | INT period | OBS period | |
| TDAS | ||||||
| Baseline test | 7.2 ± 5.9 | 0.434 | 6.8 ± 5.5 | 0.034† | 0.431 | 0.187 |
| Second test | 6.8 ± 5.9 | 7.3 ± 7.1 | ||||
| Third test | 7.7 ± 7.0 | 4.9 ± 4.3 | ||||
| Grip strength (kg) | ||||||
| Baseline test | 23.8 ± 6.0 | <0.001†‡ | 26.0 ± 6.9 | <0.001†‡ | <0.001 | <0.001 |
| Second test | 24.0 ± 6.5 | 25.2 ± 7.0 | ||||
| Third test | 26.0 ± 6.3 | 26.5 ± 7.7 | ||||
| Sit‐to‐stand test (n/30s) | ||||||
| Baseline test | 16.6 ± 5.4 | <0.001†‡ | 16.7 ± 5.3 | <0.001*, †, ‡ | 0.011 | 0.012 |
| Second test | 17.8 ± 6.0 | 17.2 ± 5.0 | ||||
| Third test | 19.8 ± 6.5 | 20.1 ± 5.4 | ||||
| One‐leg standing time (sec) | ||||||
| Baseline test | 23.8 ± 24.3 | <0.001*, † | 23.1 ± 22.0 | <0.001*, † | 0.197 | 0.064 |
| Second test | 31.9 ± 22.9 | 32.4 ± 23.7 | ||||
| Third test | 36.6 ± 21.7 | 39.5 ± 21.3 | ||||
| Anteflexion on sitting (cm) | ||||||
| Baseline test | 30.9 ± 8.6 | <0.001† | 32.5 ± 7.7 | <0.001†‡ | 0.342 | 0.003 |
| Second test | 32.2 ± 6.7 | 31.9 ± 7.9 | ||||
| Third test | 34.3 ± 7.4 | 35.0 ± 7.7 | ||||
| Plaque scores (mm) | ||||||
| Baseline test | 2.5 ± 2.5 | 2.9 ± 2.4 | ||||
| Second test | 2.9 ± 2.6 | 0.231 | 3.4 ± 2.7 | 0.189 | 0.049 | 0.051 |
| Third test | 2.6 ± 2.3 | 2.8 ± 2.2 | ||||
| VSRAD (z‐score) | ||||||
| Baseline test | 1.46 ± 0.79 | 0.361 | 1.42 ± 0.83 | 0.871 | – | |
| Second test | – | – | ||||
| Third test | 1.35 ± 0.55 | 1.40 ± 0.83 | ||||
INT, intervention; OBS, observation; TDAS, Touch Panel‐type Dementia Assessment Scale; VSRAD, voxel‐based specific regional analysis system for Alzheimer’s disease.
Significant difference between Baseline and Second test by post hoc test (P < 0.05).
Significant difference between Baseline and Third test by post hoc test (P < 0.05).
Significant difference between Second and Third test by post hoc test (P < 0.05).
Sample sizes for tests other than VSRAD analysis are n = 70 (Baseline test), n = 63 (Second test), and n = 60 (Third test). For VSRAD analysis, sample sizes are n = 52 (Baseline test) and n = 41 (Third test).
Sample sizes for test other than VSRAD analysis are n = 66 (Baseline test), n = 65 (Second test), and n = 54 (Third test). For VSRAD analysis, sample sizes are n = 53 (Baseline test) and n = 43 (Third test).
Figure 3.
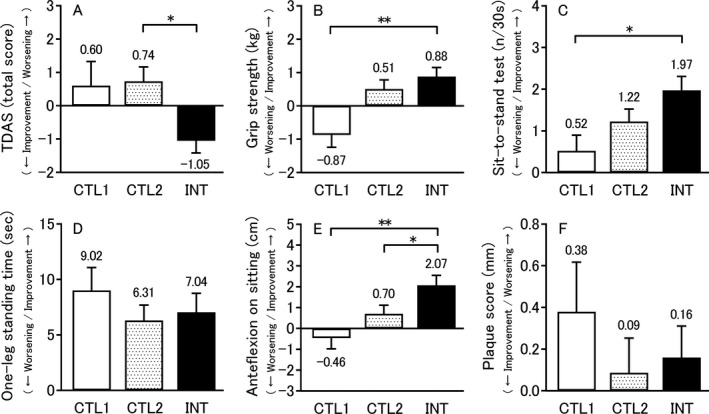
Mean changes from pre‐intervention to post‐intervention or pre‐observation to post‐observation in the TDAS score (A), physical examination performances (B–E), and plaque status by carotid ultrasonography (F). All data are expressed as mean ± standard error, and the number on the error bar is the mean value. Sample sizes are n = 65 (CTL1), n = 125 (CTL2), and n = 117 (INT). *P < 0.05, **P < 0.01. TDAS, Touch Panel‐type Dementia Assessment Scale; CTL1, mean change from pre‐observation to post‐observation in observation‐first group only (Control 1); CTL2, mean change from pre‐observation to post‐observation in all subjects (Control 2); INT, mean change from pre‐intervention to post‐intervention in all subjects (Intervention).
Physical function
Table 2 also summarizes results of the physical function tests. The physical function changed significantly both the INT‐OBS and OBS‐INT group for all items (grip strength, sit‐to‐stand test, one‐leg standing, and anteflexion on sitting, all P < 0.001). Comparison of the test results, the changes before and after the intervention period between the INT‐OBS and OBS‐INT groups showed a significant improvement in grip strength and sit‐to‐stand test performance in the OBS‐INT group (P < 0.001 and P = 0.011, respectively). Alternatively, there were significant decreases in grip strength, sit‐to‐stand test performance, and anteflexion on sitting in the OBS‐INT group across the changes before and after the observation period (P < 0.001, P = 0.012, P = 0.003, respectively). Figure 3B–E illustrates the changes before and after the intervention or observation period. In the combined INT‐OBS and OBS‐INT group, a significant improvement in anteflexion on sitting was observed following the intervention period (mean ± SD, 2.07 ± 5.17 vs. 0.70 ± 4.60 cm; 95% CI, 0.12–2.61; P < 0.05). On the other hand, when only the results for the OBS‐INT group before and after the observation period were used as the control results, significant improvements in grip strength (mean ± SD, 0.88 ± 2.92 vs. –0.87 ± 2.96 kg; 95% CI, 0.85–2.65; P < 0.01), sit‐to‐stand test performance (mean ± SD, 1.97 ± 3.59 vs. 0.52 ± 3.01 times/30 sec; 95% CI, 0.41–2.49; P < 0.05), and anteflexion on sitting (mean ± SD, 2.07 ± 5.17 vs. –0.46 ± 4.11 cm; 95% CI, 1.05–4.01; P < 0.01) were observed after the intervention. Furthermore, Table S3 shows the results of comparing changes in the physical function tests without combining the INT‐OBS and OBS‐INT group.
Other tests
Table 2 shows the results of carotid ultrasonography. There was no change in PS across the three tests in either group, but there was a significant improvement in the OBS‐INT group over the intervention period (P = 0.049). Figure 3F presents the changes before and after the intervention or observation. There was no significant difference in PS before and after the intervention period versus before and after the observation period. Table 2 shows the results of VSRAD. The degree of medial temporal atrophy did not change significantly in either group.
Tables S3 and S4 and Figure S1 summarize blood test results. The lifestyle questionnaire results are shown in Tables S5 and S6.
Costs
Potential expenses incurred may include daily allowances for the three classroom leaders, venue costs, computer costs, projector costs, and consumables used for cognitive training. However, there was no venue fee as we used classrooms under the jurisdiction of the local government (Houki Town). We also used computers and a projector owned by the local government (Houki Town). Therefore, the actual amount spent in this study was less than 20,000 yen per class for session leaders and consumables. There were few train stations near the venues in Houki town, so transportation was often inconvenient. However, we set up multiple venues, and we arranged for each participant to attend a venue as close to home as possible. In addition, the schedule was set according to that of the local demand bus. As a result, only 7 subjects were excluded from the analysis due to poor attendance (less than two‐thirds classroom participation). The average number of sessions attended was 21.4/person, for an average participation rate of 89.1%. While the transportation costs were paid by the subject, the cost is only 100 yen (about one American dollar) per trip.
Discussion
By comparing changes in TDAS scores and physical function tests between the observation and intervening periods, this crossover trial demonstrated that our program can improve cognitive function (CTL2 vs. INT in Fig. 3) and some physical functions in elderly people with MCD (CTL1 vs. INT in Figs. 3B, C, and E). Occupational therapists, public health nurses, and nurses were assigned as instructors in each classroom for program implementation, but cognitive function was evaluated by computer and physical function without using special equipment. Therefore, this program can be introduced even in local communities without the participation of experts.
It has been reported that exercise improves memory function in mice, including AD model mice, by triggering hippocampal neurogenesis and increasing brain‐derived neurotrophic factor expression.15 In addition, physical activity reduces the risk of cardiovascular disease,16 and prefrail or frail older adults can reduce frailty by performing interventions combining resistance exercise, nutritional education, and psychosocial programs.17 This is also expected to improve cognitive function, advance physical function, and promote health through physical activity. However, it is also important to select subjects who will benefit the most from these activities. A study of patients with mild‐to‐moderate dementia found that moderate‐ to high‐intensity aerobic and strength exercise training improved physical fitness but not cognitive function.18 Although the current study did not use strict diagnostic criteria for inclusion, these benefits were likely obtained by selecting subjects with extremely MCD using a dementia screening test (MSP‐1000). Since the MSP‐1000 is a computerized test, it is possible to conduct uniform screening anywhere. Further, the intervention program was designed to engage different cognitive domains every week for overall cognitive improvement. A previous study concluded that cognitive training improves cognitive performance in the trained domain.2 In addition, TDAS is a test evaluating the overall cognitive function. We accurately evaluated the effects of a cognitive training setting that stimulated various cognitive domains. This computer‐based test does not require specialists; therefore, it is useful as a test that can be performed by other local governments. It was also designed as a group activity because social isolation is a risk factor for dementia.7, 19 Indeed, the classroom structure led to the formation of a community, and the increased interpersonal communication may have contributed synergistically to the improvement in cognitive function.
A study conducted in non‐demented older adults reported that hippocampal volume, as measured by MRI, increased in subjects who participated in aerobic exercise training.20 In the current study, this result was not observed, possibly because of the interval between the completion of the intervention and the MRI scan (MRI scans were not performed during the second test). Further, a study examining the effects of multicomponent exercise in mild cognitive impairment (MCI) reported whole brain cortices atrophy rather just medial temporal lobe atrophy, especially in amnesic MCI.21 These effects warrant further examination.
Low cost is a prerequisite for widespread implementation by local municipalities. The greatest expense incurred by this program was labor, which is not easily reduced as a difference in effectiveness between classrooms (with different instructors) has been documented in a dementia prevention program,8 so we believe that professional instructors are necessary to ensure reliability.
A practical problem encountered in the implementation of this program was transportation, as cognitive impairment usually precludes driving.22 In Japan, people who have been diagnosed with dementia need to return their driver’s license. Nonetheless, the participation rate in the classroom was 89.1%, which may have been facilitated by conducting the program in multiple locations (i.e., closer to individual participants) and coordinating the schedule with local demand bus operational hours.
This study has several limitations. First, although there was a significant difference in cognitive function among subjects (Fig. 3A), we conducted only three test batteries, and there was no washout period, so test results before and after the observation period in the INT‐OBS group may not be a pure comparison control. However, no significant difference was observed in cognitive function change during the observation period between INT‐OBS and OBS‐INT groups (Table 2). Therefore, there was no carryover effect, and cognitive function declined to the same extent as when no intervention was received after intervention in the INT‐OBS group. Therefore, all subjects may be used as comparison controls for evaluation of cognitive function. On the other hand, there was a significant difference in physical function across the observation period between INT‐OBS and OBS‐INT groups except for balance (Table 2), so a carryover effect is possible in the INT‐OBS group. Based on these considerations, it was considered appropriate to use results obtained from the OBS‐INT group before and after the observation period as the control for comparison of the intervention effects (Fig. 3B–E). Second, the study was conducted in only one locale. In interventional trials, it has been reported that single‐center trials show larger treatment effects than multicenter trials.23 However, 10 separate classes were held. In addition, the evaluation was performed using computers and based on unified standards, which may serve to reduce methodological variability across locations. Third, subjects were not definitively diagnosed with MCI or dementia. Therefore, it cannot be stated whether this program can improve cognitive function in dementia or MCI. Further examination is necessary to verify whether continued implementation of this program can prevent or delay the onset of MCI or dementia. Fourth, only 150/300 candidates participated in the study, which may have introduced a selection bias because these subjects may have been be the most motivated. Whether the same effect can be obtained for the nonparticipation MCD population in this study remains to be determined. For future studies, it is important to encourage as many potential participants as possible to participate in the classroom.
We have developed a unique cognitive decline prevention program combining physical exercise, cognitive training, and education on dementia and lifestyle habits that can be applied in a community setting for elderly residents with suspected cognitive decline. This program appears to improve cognitive and physical function at low cost. Therefore, the program may be easily instituted in other areas.
Author Contributions
M.K. and K.U. designed the study. T.M. and S.A. developed the physical exercise program. S.T., Y.T., C.M., and M.H. developed the cognitive training program. K.W‐I., A.T., Y.K., and M.I. developed the education program. M.K. and T.K. implemented the tests. M.K. analyzed the data. M.K. wrote the first draft of the manuscript. All authors contributed to writing the final draft manuscript and approved publication.
Conflict of Interest
M.K., T.K., K.W‐I., S.T., A.T., Y.T., S.A., Y.K., C.M., M.H., and M.I. have no conflicts of interest to declare. K.U. owns a patent on the Touch Panel‐type Dementia Assessment Scale and receives royalties from Nihon Kohden Corporation.
Supporting information
Figure S1. Mean changes from pre‐intervention to post‐intervention or pre‐observation to post‐observation for blood test parameters.
Table S1. Definitions of each cognitive function assessed by the program.
Table S2. Schedule of cognitive training.
Table S3. Mean changes in the cognitive test, physical examination, ultrasonography test, and blood tests.
Table S4. Blood test results.
Table S5. Results of lifestyle questionnaire in intervention‐first group.
Table S6. Results of lifestyle questionnaire in observation‐first group.
Acknowledgment
We thank the staff of the Senior Citizens Welfare Division, Mutual Welfare Support Bureau, Department of Health and Welfare, Tottori Prefecture, and Health measures section, Houki Town who were involved in the management of the council for study execution. We also thank the people who participated in this study, and the staff involved in classroom management (including occupational therapists by Tottori Association of Occupational Therapists), testing, financial support, or in creating a DVD on education for dementia and lifestyle habits. This study was conducted with grants from the Nippon Foundation and Tottori Prefecture.
Funding Information
This study was conducted with grants from the Nippon Foundation and Tottori Prefecture.
Funding Statement
This work was funded by Nippon Foundation grant ; Tottori Prefecture grant .
References
- 1. Brasure M, Desai P, Davila H, et al. Physical activity interventions in preventing cognitive decline and Alzheimer‐type dementia: a systematic review. Ann Intern Med 2018;168:30–38. [DOI] [PubMed] [Google Scholar]
- 2. Butler M, McCreedy E, Nelson VA, et al. Does cognitive training prevent cognitive decline?: a systematic review. Ann Intern Med 2018;168:63–68. [DOI] [PubMed] [Google Scholar]
- 3. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;385:2255–2263. [DOI] [PubMed] [Google Scholar]
- 4. Verghese J, Lipton RB, Katz MJ, et al. Leisure activities and the risk of dementia in the elderly. N Engl J Med 2003;348:2508–2516. [DOI] [PubMed] [Google Scholar]
- 5. Akbaraly TN, Portet F, Fustinoni S, et al. Leisure activities and the risk of dementia in the elderly: results from the Three‐City Study. Neurology 2009;73:854–861. [DOI] [PubMed] [Google Scholar]
- 6. Sabia S, Dugravot A, Dartigues JF, et al. Physical activity, cognitive decline, and risk of dementia: 28 year follow‐up of Whitehall II cohort study. BMJ 2017;357:j2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 8. Ito Y, Urakami K. Evaluation of dementia‐prevention classes for community‐dwelling older adults with mild cognitive impairment. Psychogeriatrics 2012;12:3–10. [DOI] [PubMed] [Google Scholar]
- 9. Inoue M, Jinbo D, Nakamura Y, et al. Development and evaluation of a computerized test battery for Alzheimer's disease screening in community‐based settings. Am J Alzheimers Dis Other Dementiasr 2009;24:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Inoue M, Jimbo D, Taniguchi M, et al. Touch Panel‐type Dementia Assessment Scale: a new computer‐based rating scale for Alzheimer's disease. Psychogeriatrics 2011;11:28–33. [DOI] [PubMed] [Google Scholar]
- 11. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–1364. [DOI] [PubMed] [Google Scholar]
- 12. Handa N, Matsumoto M, Maeda H, et al. Ultrasonic evaluation of early carotid atherosclerosis. Stroke 1990;21:1567–1572. [DOI] [PubMed] [Google Scholar]
- 13. Hirata Y, Matsuda H, Nemoto K, et al. Voxel‐based morphometry to discriminate early Alzheimer's disease from controls. Neurosci Lett 2005;382:269–274. [DOI] [PubMed] [Google Scholar]
- 14. Matsuda H, Mizumura S, Nemoto K, et al. Automatic voxel‐based morphometry of structural MRI by SPM8 plus diffeomorphic anatomic registration through exponentiated lie algebra improves the diagnosis of probable Alzheimer disease. Am J Neuroradiol 2012;33:1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Choi SH, Bylykbashi E, Chatila ZK, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer's mouse model. Science 2018;361:eaan8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christmas C, Andersen RA. Exercise and older patients: guidelines for the clinician. J Am Geriatr Soc 2000;48:318–324. [DOI] [PubMed] [Google Scholar]
- 17. Seino S, Nishi M, Murayama H, et al. Effects of a multifactorial intervention comprising resistance exercise, nutritional and psychosocial programs on frailty and functional health in community‐dwelling older adults: a randomized, controlled, cross‐over trial. Geriatr Gerontol Int 2017;17:2034–2045. [DOI] [PubMed] [Google Scholar]
- 18. Lamb SE, Sheehan B, Atherton N, et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ 2018;361:k1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saito T, Murata C, Saito M, et al. Influence of social relationship domains and their combinations on incident dementia: a prospective cohort study. J Epidemiol Community Health 2018;72:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011;108:3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Suzuki T, Shimada H, Makizako H, et al. A randomized controlled trial of multicomponent exercise in older adults with mild cognitive impairment. PLoS ONE ONE 2013;8:e61483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hird MA, Egeto P, Fischer CE, et al. A systematic review and meta‐analysis of on‐road simulator and cognitive driving assessment in Alzheimer's disease and mild cognitive impairment. J Alzheimers Dis 2016;53:713–729. [DOI] [PubMed] [Google Scholar]
- 23. Dechartres A, Boutron I, Trinquart L, et al. Single‐center trials show larger treatment effects than multicenter trials: evidence from a meta‐epidemiologic study. Ann Intern Med 2011;155:39–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mean changes from pre‐intervention to post‐intervention or pre‐observation to post‐observation for blood test parameters.
Table S1. Definitions of each cognitive function assessed by the program.
Table S2. Schedule of cognitive training.
Table S3. Mean changes in the cognitive test, physical examination, ultrasonography test, and blood tests.
Table S4. Blood test results.
Table S5. Results of lifestyle questionnaire in intervention‐first group.
Table S6. Results of lifestyle questionnaire in observation‐first group.


