Abstract
The stringent response (SR) is a highly conserved stress response in bacteria. It is composed of two factors, (i) a nucleotide alarmone, guanosine tetra- and pentaphosphate ((p)ppGpp), and (ii) an RNA polymerase-binding protein, DksA, that regulates various phenotypes, including bacterial virulence. The clinically significant opportunistic bacterial pathogen Pseudomonas aeruginosa possesses two genes, dksA1 and dksA2, that encode DksA proteins. It remains elusive, however, which of these two genes plays a more important role in SR regulation. In this work, we compared genome-wide, RNA-Seq–based transcriptome profiles of ΔdksA1, ΔdksA2, and ΔdksA1ΔdksA2 mutants to globally assess the effects of these gene deletions on transcript levels coupled with phenotypic analyses. The ΔdksA1 mutant exhibited substantial defects in a wide range of phenotypes, including quorum sensing (QS), anaerobiosis, and motility, whereas the ΔdksA2 mutant exhibited no significant phenotypic changes, suggesting that the dksA2 gene may not have an essential function in P. aeruginosa under the conditions used here. Of note, the ΔdksA1 mutants displayed substantially increased transcription of genes involved in polyamine biosynthesis, and we also detected increased polyamine levels in these mutants. Because SAM is a shared precursor for the production of both QS autoinducers and polyamines, these findings suggest that DksA1 deficiency skews the flow of SAM toward polyamine production rather than to QS signaling. Together, our results indicate that DksA1, but not DksA2, controls many important phenotypes in P. aeruginosa. We conclude that DksA1 may represent a potential target whose inhibition may help manage recalcitrant P. aeruginosa infections.
Keywords: Pseudomonas aeruginosa, quorum sensing, virulence factor, stress response, transcription regulation, DksA1, transcription factor, stringent response, gene regulation, polyamine metabolism, anaerobic respiration
Introduction
Pseudomonas aeruginosa, a Gram-negative bacterium, is an opportunistic pathogen that causes various forms of human infections (1). These include burn wound infection (2), hospital-acquired infection (3), and chronic infection in the airways of individuals with cystic fibrosis (4). Because P. aeruginosa has a wide range of habitats, this bacterium needs the ability to adapt to diverse environmental stresses and manage its survival fitness (5). To fulfill this demand, P. aeruginosa uses various transcriptional regulators to compose intricate regulatory networks that coordinate gene expression and cell signaling pathways in response to external stresses (6–8).
The stringent response (SR),2 a highly conserved regulatory system in bacteria (9–11), is mediated by the alarmone (p)ppGpp synthesized by the enzymes RelA and SpoT (12, 13). RelA synthesizes (p)ppGpp via conversion of GT(D)P and ATP to (p)ppGpp and AMP (14). SpoT is a bifunctional enzyme that can either synthesize or hydrolase (p)ppGpp, depending on the ambient conditions (14, 15). The SR regulator (p)ppGpp modulates RNA polymerase activity to control transcriptional responses in a concentration-dependent manner (15). Bacterial SR is also regulated by the transcription factor DksA (16). DksA is an RNA polymerase–binding protein that was initially characterized as a suppressor of the dnaK mutation in Escherichia coli (17). To activate the SR, DksA binds to RNA polymerase at a secondary channel to assemble a large complex with (p)ppGpp (13, 18, 19). Although the concerted actions of (p)ppGpp and DksA are required for functional SR, the deficiency of (p)ppGpp or DksA results in different transcriptome profiles and cellular phenotypes, suggesting that (p)ppGpp and DksA exert distinct roles in activating bacterial SR (18).
Currently, it is well-appreciated that SR is deeply involved in regulating bacterial virulence and metabolism in many species, including P. aeruginosa (20–26). Phenotype-level investigations have revealed that both (p)ppGpp and DksA participate in regulating quorum sensing (QS), a cell density–dependent virulence mechanism in P. aeruginosa (21, 27). The elevated intracellular production of (p)ppGpp by relA overexpression resulted in the induced synthesis of 3-oxo-dodecanoyl homoserine lactone (3-oxo-C12-HSL), a signal molecule for P. aeruginosa QS, even at low cell density (22). Consistently, the production of QS-regulated virulence determinants, such as elastase, biofilm, siderophores, and rhamnolipid, was markedly decreased in a (p)ppGpp-deficient (ppGpp0) mutant (21). However, the role of the dksA gene product has not been clearly elucidated in previous studies. The deletion of the dksA gene in P. aeruginosa resulted in reduced elastase and rhamnolipid production (28), whereas overexpression of the same dksA gene also diminished the production of virulence factors (27).
We also noted that P. aeruginosa PAO1 possesses two dksA homologs, dksA1 (PA4723) and dksA2 (PA5536), in its genome. In the aforementioned studies, dksA1 has been the gene of primary interest. The gene dksA2, found in several Pseudomonas spp., has been reported to be regulated by Zur, a zinc-responsive transcriptional regulator. Expression of P. aeruginosa dksA2 by an inducible promoter rescued the growth defect of E. coli ΔdksA and PAO1 ΔdksA1 mutants under amino acid starvation (29). In addition, pyocyanin production was re-implemented by the expression of dksA2 in a P. aeruginosa ΔdksA1 mutant. In contrast to these functional assessments of the dksA2 gene, however, transcription of the dksA2 gene was reported to be silent, when compared with that of dksA1 (29). These contradictory results warrant further studies to precisely determine the role of two DksA homologues in P. aeruginosa gene regulation.
Herein, we sought to comprehensively understand the role of dksA1 and dksA2 in P. aeruginosa biology. To achieve this goal, we constructed ΔdksA1 and ΔdksA2 single mutants and a ΔdksA1ΔdksA2 double mutant and performed transcriptome analyses with these strains. Our analysis reveals that dksA1, but not dksA2, plays important roles in regulating diverse phenotypes, such as QS, anaerobic respiration, flagellum-mediated motility, and polyamine biosynthesis. Overall, we suggest that DksA1 regulates diverse, albeit somewhat unrelated, cellular phenotypes, and therefore it could be a potential drug target for the effective control of P. aeruginosa infection.
Results
DksA1 acts as a global transcriptional regulator in P. aeruginosa
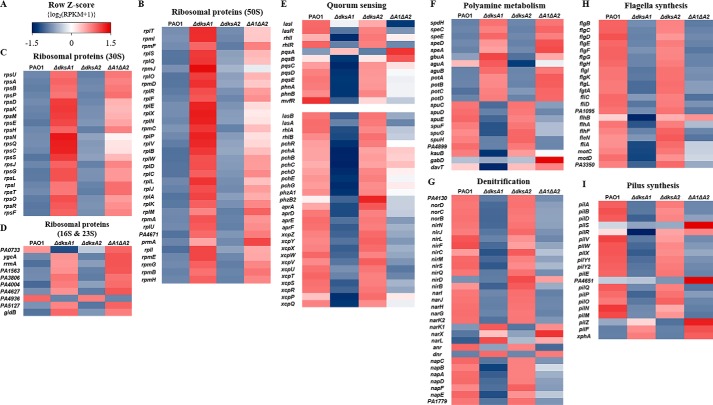
To investigate the effects of disruptions in the dksA1 and/or dksA2 genes in P. aeruginosa, transcriptomes of ΔdksA1, ΔdksA2, and ΔdksA1ΔdksA2 mutants were compared with that of the parental strain PAO1 by RNA-Seq. The row Z-score of the differential expression was calculated by processing RPKM values of targeted genes and is represented as a color-coded box in heat maps (Fig. 1A). A complete list of transcriptome results is displayed in the supporting spreadsheet.
Figure 1.
Comparative analysis of PAO1, ΔdksA1, ΔdksA2, and ΔdksA1ΔdksA2 transcriptomes. Transcript levels of genes (indicated to the left in B–I) were quantified by row Z-score (A) and represented as heat maps (B–I). The row Z-score was calculated by log2-transformed RPKM values of targeted genes. The formula to calculate row Z-scores from RPKM values was log2(RPKM + 1). The row Z-score value ranges from −1.5 to 1.5 and is represented as a color-coded box, with red and blue indicating relative up-regulation and down-regulation, respectively. B, transcript levels of genes encoding 50S ribosomal protein components. C, transcript levels of genes encoding 30S ribosomal protein components. D, transcript levels of genes encoding 16S and 23S ribosomal protein components. E, transcription of genes involved in QS and QS-regulated virulence determinants. Expression of rhlI and PQS synthesis genes was significantly decreased in ΔdksA1 and ΔdksA1ΔdksA2 mutants. Genes encoding elastase (lasB), rhamnolipid (rhlA and rhlB), pyochelin (pchR–pchG), phenazine (phzA1 and phzB1), alkaline protease (aprA–aprF), and the type 2 secretion system (xcpZ–xcpQ) were down-regulated in the presence of dksA1 gene mutation. F, transcription of genes involved in polyamine metabolism. Genes encoding proteins for polyamine biosynthesis and polyamine catabolism were differentially regulated in the absence of DksA1. G, transcription of genes encoding reductase for nitrate reductase (Nar), nitrite reductase (Nir), nitric oxide reductase (Nor), and nitrous oxide reductase (Nos) were down-regulated in dksA1 mutants. Expression of genes encoding positive regulators NarX, NarL, and DNR was increased. Transcription of genes involved in flagella synthesis and assembly (H) and type IV pili synthesis (I).
Regulation of ribosomal proteins and rRNA-related genes
We first assessed whether the deletion of dksA1 and/or dksA2 genes affects transcriptions of genes encoding ribosomal proteins and rRNA-modifying enzymes because it was reported that DksA represses transcription of genes involved in ribosome biogenesis (30). Our results demonstrate that the deletion of dksA1 clearly leads to the elevated gene transcription of major ribosomal proteins and RNA-modifying enzymes (Fig. 1, B–D). It is of note that the transcription signatures in these particular sets are indistinguishable between PAO1 and the ΔdksA2 mutant (Fig. 1, B–D). These results suggest that the product of dksA1, and not dksA2, is responsible for regulating rRNA gene transcription.
Regulation of QS
We next found that the disruption of the dksA1 gene resulted in remarkably decreased expression of QS-related genes. The gene expression to produce (i) QS signals and (ii) QS-regulated virulence determinants was invariably decreased (Fig. 1E). Transcriptions of two distinct HSL signal synthases (lasI and rhlI) and their cognate response regulators (lasR and rhlR) were noticeably reduced in the presence of the dksA1 deletion. Similarly, the expression of genes constituting the PQS synthesis operon (pqsA, pqsB, pqsC, pqsD, and pqsE) was clearly down-regulated in ΔdksA1 and ΔdksA1ΔdksA2 mutants (Fig. 1E). Consistent with these changes, the transcription of genes encoding QS-regulated virulence factors was also down-regulated. The expression of genes encoding elastase (lasB), rhamnolipid (rhlA and rhlB), phenazines (phzA1 and phzB2), pyocheline (pchR, pchA, pchB, pchC, pchD, pchE, and pchG), alkaline protease (aprA, aprD, aprE, and aprF), and the type 2 secretion system (xcp operon) became less active in the presence of the dksA1 mutation. Overall, our results suggest that DksA1 positively regulates P. aeruginosa QS, whereas DksA2 is dispensable.
Regulation of polyamine metabolism
We also noted that genes involved in the biosynthesis of polyamines using SAM as a substrate were actively transcribed in ΔdksA1 and ΔdksA1ΔdksA2 mutants (Fig. 1F). The polyamine biosynthesis pathway and genes mediating each step are shown in Fig. S3. The LuxI family proteins, LasI and RhlI, require SAM and acyl groups donated from acyl carrier proteins to produce HSL in P. aeruginosa (31). In our transcriptome analysis, expressions of speD and speE, encoding SAM decarboxylase and polyamine aminopropyltransferase, were significantly increased in ΔdksA1 and ΔdksA1ΔdksA2 mutants (Fig. 1F). Expressions of other putrescine biosynthesis genes (such as speA, aguA, aguB, and gbuA) and speC, which converts ornithine to putrescine, were also markedly up-regulated in the presence of the dksA1 mutation (Fig. 1F). In addition, spdH, encoding spermidine dehydrogenase, which converts putrescine to spermidine, was significantly up-regulated. Furthermore, the transcription of the genes in the potABCD operon encoding polyamine transport proteins was significantly increased in dksA1 mutants (Fig. 1F). In contrast, transcription of genes involved in polyamine catabolism (spuC–H) was down-regulated in ΔdksA1 and ΔdksA1ΔdksA2 mutants (Fig. 1F). Overall, our genome-wide analysis shows that when the dksA1 gene is deficient, bacterial transcription is modulated to create an environment where polyamine overproduction may occur. Once again, deficiency of the dksA2 gene induced only negligible transcriptional changes.
Regulation of anaerobic respiration
Our interest extends to the finding that dksA1 may also participate in controlling anaerobic respiration in P. aeruginosa. It has been found that P. aeruginosa can grow robustly under anaerobic conditions using alternative electron acceptors (32–34). DksA1, and not DksA2, was found to contribute to the transcription of genes involved in anaerobic respiration in P. aeruginosa. Anerobic growth, stimulated by a process called denitrification, is mediated by sequential reduction steps of nitrate (NO3−) or nitrite (NO2−) (35). The first step of denitrification is conducted by nitrate reductase (Nar) encoded by the gene cluster narK1K2GHJI. Nitrite reductase (Nir) and nitric oxide reductase (Nor), which are encoded by gene clusters nirSMCFLGHJND and norCBD, respectively, catalyze the next steps of denitrification to produce nitrous oxide. Then nitrous oxide is converted to nitrogen gas by nitrous oxide reductase encoded by the nosRZDFYL cluster (36). Our analysis clearly indicated that transcriptions of a large number of denitrification genes were remarkably suppressed in ΔdksA1 and ΔdksA1ΔdksA2 mutants (Fig. 1G). Here again, the ΔdksA2 mutant produced a transcription profile almost identical to that of PAO1 (Fig. 1G). Expression of the anr gene encoding a master anaerobic regulator, ANR, was decreased, whereas other positive regulator genes, dnr (DNR) and narXL (NarXL), exhibited increased expression in the ΔdksA1 mutant (Fig. 1G). Our results suggest that the dksA1 gene mutation probably affects P. aeruginosa anaerobic growth.
Regulation of flagella synthesis
Finally, the expression of genes engaged in flagella-mediated motility and pilus biogenesis was also noticeably attenuated in strains lacking DksA1. The transcription of genes in the flgBCDEFGHIKL cluster encoding flagellum structural components was down-regulated in the absence of DksA1 (Fig. 1H). Transcription of B-type flagellin, encoded by fliC, fliD, and PA1095, was also repressed in ΔdksA1 and ΔdksA1ΔdksA2 mutants. However, a consistent pattern of increase or decrease was not observed in the fliEFIJMNOPQR cluster involved in flagella biosynthesis and assembly and in the motAB operon encoding flagellum motor proteins among strains (see supporting Excel file). Moreover, transcription of genes encoding type IV pili, required for swarming and twitching motility, was also reduced in ΔdksA1 and ΔdksA1ΔdksA2 mutants (Fig. 1I).
DksA1 is essential for QS-mediated virulence in P. aeruginosa
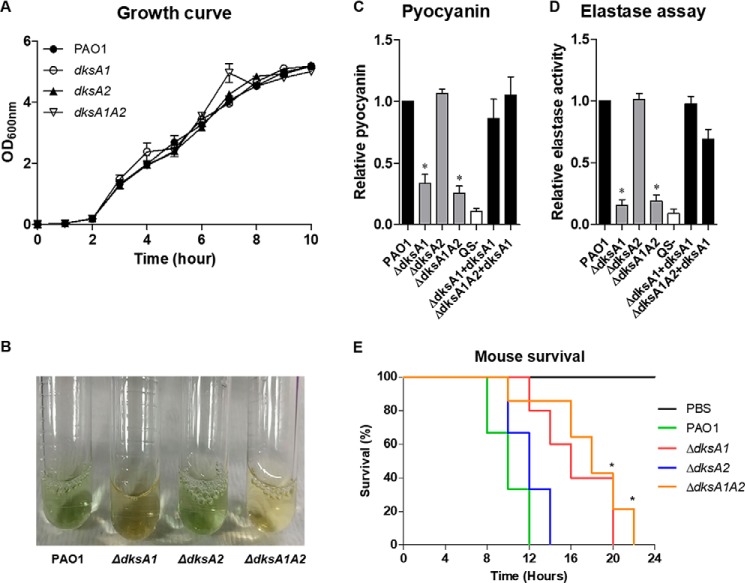
Our transcriptome analysis clearly suggests that DksA1 plays a versatile role in regulating gene transcription for various phenotypes. To examine whether the changes at the transcript level in response to the dksA1 gene mutation are reflected at the phenotype level, we conducted a series of experimental validations. First of all, we observed that all of the mutant strains exhibited an identical growth rate with their parental strain PAO1 (Fig. 2A). This finding ruled out the theory that altered transcriptome profiles in the ΔdksA1 and ΔdksA1ΔdksA2 mutants were due to slower or faster growth. During bacterial cultivation, it was noticed that strains harboring a dksA1 gene mutation failed to produce the typical green pigment, pyocyanin (Fig. 2B). When pyocyanin was quantified, the ΔdksA1 and ΔdksA1ΔdksA2 mutants produced remarkably decreased levels (Fig. 2C). The level of pyocyanin production in these two mutants was comparable with that produced in the ΔlasRΔrhlR mutant, which was reported to produce almost no pyocyanin at all. In addition, the dksA1-complemented strains showed restored pyocyanin production to the PAO1 level (Fig. 2C). Likewise, the two mutants produced markedly reduced amounts of elastase, the major virulence determinant produced by P. aeruginosa (32). When quantified, elastase produced in these two mutants was ∼20% of the level produced in PAO1. Elastase production was restored when the WT copy of the dksA1 gene was expressed (Fig. 2D). Consistent with the transcriptome profiles, elastase production in the ΔdksA2 mutant was not detected (Fig. 2D). Suppressed elastase production was further verified in a SDS-polyacrylamide gel of proteins present in concentrated bacterial culture supernatants. The elastase band was identified previously by MS and Western blotting (Fig. S1) (32, 37). As virulence factors were decreased in the absence of dksA1, the attenuated virulence of ΔdksA1 and ΔdksA1ΔdksA2 mutants was tested in vivo using 5-week-old BALB/c mice. All mice were infected with 108 cells of bacterial strains and perished within 24 h. Mice infected with PAO1 and ΔdksA2 mutants were affected at a significantly faster rate, whereas mice infected with ΔdksA1 and ΔdksA1ΔdksA2 mutants died slowly and survived for up to 22 h (Fig. 2E). This result further supported our finding that dksA1 is essential for P. aeruginosa virulence.
Figure 2.
Virulence phenotypes of ΔdksA1, ΔdksA2, and ΔdksA1ΔdksA2 mutants. A, growth curves of PAO1, ΔdksA1, ΔdksA2, and ΔdksA1ΔdksA2 strains in LB medium. Aliquots of bacterial cultures (n = 3) were withdrawn every hour to measure OD600 values. B, visual comparison of bacterial culture supernatants. Loss of pigment was clearly observed in ΔdksA1 and ΔdksA1ΔdksA2 mutants. C, pyocyanin production in each strain was quantified and normalized with that of PAO1. The values of means ± S.D. (error bars) are presented (n = 3). *, p < 0.05 versus pyocyanin production in PAO1. QS−, PAO1ΔlasRΔrhlR double mutant. Pyocyanin production was also quantified in the supernatants of dksA1-complemented strains. D, relative elastase activity of tested strains. Elastase activity was measured as described under “Experimental procedures.” *, p < 0.05 versus elastase production in PAO1. E, in vivo virulence assay. The infection dose was ∼108 cells/mouse. *, p < 0.05 versus survival rate of PAO1-infected mice. Five mice were used in each group.
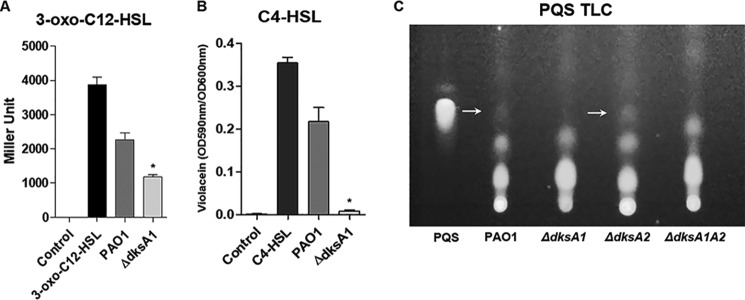
QS is operated by secretion and recognition of signaling molecules, collectively termed autoinducers (1). Because major QS-mediated phenotypes were repressed in strains devoid of DksA1, we sought to measure autoinducers to see whether the uninterrupted production of DksA1 enables the biosynthesis of QS signal molecules. Two homoserine lactone autoinducers, 3-oxo-C12-HSL and N-butyryl homoserine lactone (C4-HSL), were semiquantitatively measured using two different reporter strains, as described under “Experimental procedures.” Based on our quantification, production of 3-oxo-C12-HSL in the ΔdksA1 mutant was only half of that produced in PAO1 (Fig. 3A). When incubated with purified 3-oxo-C12-HSL (100 μg/ml), the reporter cells produced strong β-gal activity. More importantly, C4-HSL was almost completely suppressed in the ΔdksA1 mutant (Fig. 3B), suggesting that the RhlI-R QS circuit is more severely impaired when the dksA1 gene is disrupted. When cell extracts were subject to a TLC assay, loss of PQS production was also detected in ΔdksA1 and ΔdksA1ΔdksA2 mutants (Fig. 3C). As indicated by white arrows, PQS production was observed in PAO1 and ΔdksA2 mutants (Fig. 3C). In the TLC assay, purified PQS at 10 mm concentration was used as a control (Fig. 3C, far left lane). Together, our results indicate that DksA1 is required for the production of all three autoinducer molecules that mediate P. aeruginosa QS.
Figure 3.
Effects of dksA1 gene mutation on production of QS signal molecules. A, levels of 3-oxo-C12-HSL (3-oxo-C12-HSL) were measured by using an E. coli reporter strain harboring a pKDT17 plasmid. Concentrations of 3-oxo-C12-HSL are represented as β-gal activity. Purified 3-oxo-C12-HSL (100 μg/ml) was used as the positive control. *, p < 0.05 versus β-gal activity by PAO1 culture supernatant. B, levels of N-butyryl homoserine lactone (C4-HSL) was monitored by measuring the violacein production from the biosensor strain C. violaceum CV026. Violacein production was monitored by measuring absorbance at 590 nm and normalized to the OD600 values of CV026. Purified C4-HSL (100 μg/ml) was used as a positive control. *, p < 0.05 versus values by PAO1 culture supernatant. C, PQS detection by TLC. Purified PQS (10 mm, 5 μl) was used as a positive control. Samples (10 μl) were loaded on TLC plates. White arrows, spots for PQS. Error bars, S.D.
DksA is required for anaerobic respiration of P. aeruginosa
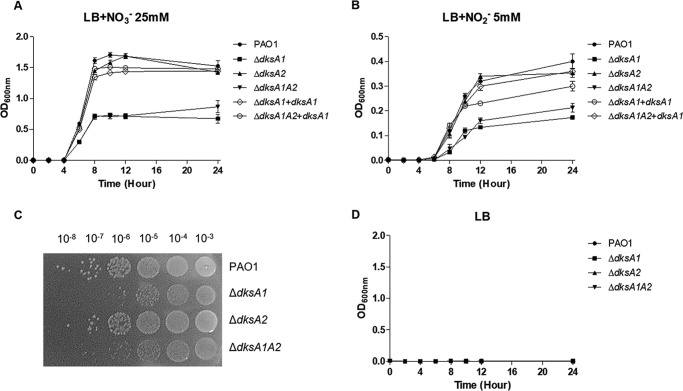
The expression of genes involved in denitrification was significantly suppressed in two mutants harboring a defective dksA1 gene, as shown in Fig. 1G. We therefore evaluated the effect of dksA1 gene disruption on P. aeruginosa anaerobic respiratory growth. When grown anaerobically with 25 mm NO3−, PAO1 and dksA1-complemented strains reached an OD600 of ∼1.5 in 24 h. In contrast, final OD600 values were only ∼0.8 in ΔdksA1 and ΔdksA1ΔdksA2 mutants under the same conditions (Fig. 4A). Again, the anaerobic growth of the ΔdksA2 mutant was indistinguishable from that of PAO1. We next tested bacterial growth using NO2− as an alternative electron acceptor. When 5 mm NO2− was used, the PAO1 and ΔdksA2 mutant grew until OD600 values reached ∼0.4 (Fig. 4B). These results further verified that P. aeruginosa anaerobic respiration is better supported by NO3− compared with NO2− (33). Under the same growth conditions, mutants possessing a defective dksA1 gene only grew up to OD600 values of ∼0.2. However, dksA1-complemented strains restored anaerobic growth similar to PAO1 (Fig. 4B). A cfu-counting assay also clearly showed growth differences between strains. More than 100-fold decreases in viable cell numbers were observed at the end of the growth experiments in ΔdksA1 and ΔdksA1ΔdksA2 mutants (Fig. 4C). Together, these results suggest that DksA1 plays a very important role in activating two major anaerobic respiration pathways in P. aeruginosa. The anaerobic growth of PAO1 and mutants was inactive in Luria–Bertani (LB) medium without alternative electron receptors (Fig. 4D).
Figure 4.
Anerobic growth of ΔdksA1, ΔdksA2, and ΔdksA1ΔdksA2 mutants. A, growth curves of PAO1, ΔdksA1, ΔdksA2 and ΔdksA1ΔdksA2 strains in LB medium supplemented with 25 mm NO3−. Aliquots of bacterial cultures (n = 3) were withdrawn every 2 h to measure OD600 values. Bacterial strains (indicated to the right) were grown anaerobically at 37 °C. B, growth curves of PAO1, ΔdksA1, ΔdksA2, and ΔdksA1ΔdksA2 strains in LB medium supplemented with 5 mm NO2−. C, defective anaerobic growth of ΔdksA1 and ΔdksA1ΔdksA2 was further verified in cfu enumeration assay. Bacterial cultures after 24 h of anaerobic growth in LB supplemented with 25 mm NO3− were serially diluted, and 10 μl of each diluent was spot-inoculated onto an LB agar plate. The plates were incubated in anaerobic conditions at 37 °C for 24 h. D, anaerobic growth was not active in plain LB medium without either alternative electron acceptor. Error bars, S.D.
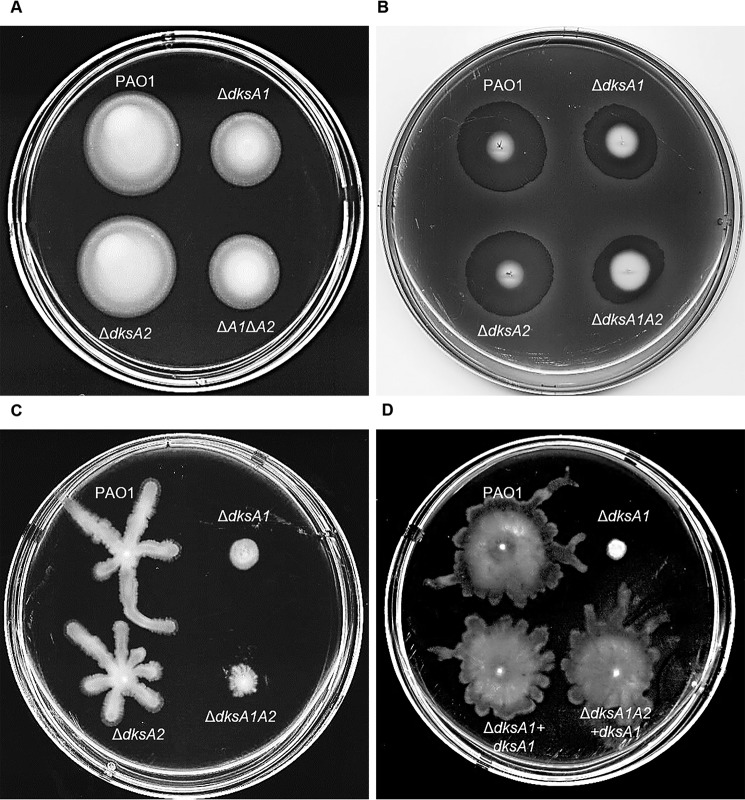
DksA1 is required for swarming motility
In P. aeruginosa, swimming motility is an ability to translocate on a sufficiently thick fluid surface, which is driven by single polar flagellum, whereas swarming motility empowers a different form of movement on a relatively thin fluid surface (38, 39). In addition, twitching motility is a type IV pili-dependent movement on a solid surface. Forming a twitch zone by twitching motility is mediated by colonial expansion inside the interstitial space of the medium (38, 40). Flagellin and pilus, which are structural components encoded by the fliC and pilA genes, are important determinants for the aforementioned motilities in P. aeruginosa (40). Because repressed gene transcription for flagella and pilus biosynthesis, including fliC and pilA, were observed in ΔdksA1 and ΔdksA1ΔdksA2 mutants, we wanted to examine motility phenotypes. As shown in Fig. 5A, swimming motility was impaired in the presence of the dksA1 gene mutation. Swimming motilities of the ΔdksA1 and ΔdksA1ΔdksA2 mutants were ∼68% of that of PAO1 or the ΔdksA2 mutant. Likewise, reduced twitching motility was detected in the mutants of the dksA1 gene (Fig. 5B). It was of particular note that among the three, the swarming motility was most significantly compromised in mutants of the dksA1 gene (Fig. 5C). The defective swarming motility was completely restored by dksA1 gene complementation (Fig. 5D). Collectively, DksA1 is critically required for the intact motility of P. aeruginosa on diverse surfaces.
Figure 5.
Effects of dksA1 gene mutation on various bacterial motilities. A, swimming motility tested on LB plates with 0.3% agar. Attenuated swimming motility is shown as the reduced size of the circular colonies of dksA1 mutants. The plate was incubated at 30 °C for 24 h without being inverted. B, twitching motility tested on an LB plate with 1% agar. Reduced size of the twitch zone is shown in ΔdksA1 and ΔdksA1ΔdksA2 mutants. The plate was incubated at 37 °C for 24 h without being inverted. The plate was stained with Coomassie Blue for better visualization of the twitch zone. C, swarming motility tested on a swarm plate. Formation of a characteristic irregular branch was not observed in ΔdksA1 or ΔdksA1ΔdksA2 mutants. The swarm plate consisted of M8 salt, 0.1% glutamate, 0.25% glucose, and 0.5% agar. Bacterial strains were inoculated on the surface of the swarm plate and incubated at 30 °C for 24 h without being inverted. D, swarming motility of the dksA1-complemented strains. The irregular branches reappeared when the dksA1 gene was complemented.
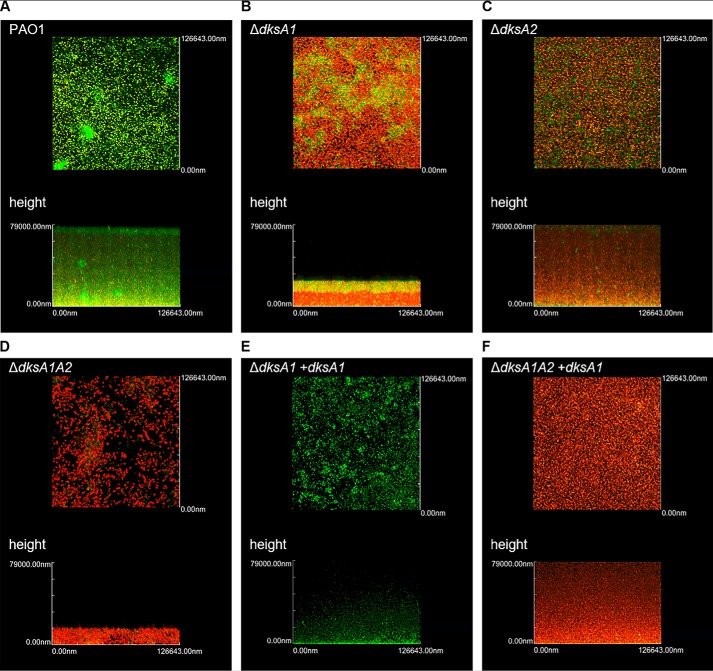
DksA1 plays a more important role in P. aeruginosa biofilm formation than DksA2
Biofilm, a mode of bacterial lifestyle with increased fitness, is a reservoir of bacterial virulence factors (41). Because the formation of biofilm depends on motility, we next evaluated the effects of the dksA1 and/or dksA2 mutations on P. aeruginosa biofilm formation. Biofilms were grown statically for 24 h on top of cover glasses and visualized via fluorescent live/dead staining using a confocal laser-scanning microscope (CLSM). The biofilm formed by PAO1 was robust with a depth of >79 μm and filled with live cells (Fig. 6A). Biofilms formed by the ΔdksA1 and ΔdksA1ΔdksA2 mutants, on the other hand, were substantially thinner and composed of mostly dead cells (Fig. 6, B and D). The biofilm architecture of the ΔdksA2 mutant was comparable with that of the PAO1 biofilm, especially in terms of the biofilm thickness and density. However, a significantly larger number of dead cells were detected in the ΔdksA2 mutant biofilm (Fig. 6C). The biofilm thickness and viability were substantially recovered when the dksA1 gene was expressed in the ΔdksA1 mutant (Fig. 6E). In addition, biofilm architecture was restored to the level of ΔdksA2 single mutant when the intact dksA1 gene was expressed in the ΔdksA1ΔdksA2 mutant (Fig. 6F). This result suggests that although disruption of DksA2 resulted in no changes in most of the phenotypes examined, biofilm viability was actually affected by the loss of DksA2. Our results also demonstrate that dksA1 gene complementation worked well in the biofilm mode of growth.
Figure 6.
CLSM images of PAO1, ΔdksA1, ΔdksA2, or ΔdksA1ΔdksA2 biofilm. One-day-old bacterial biofilms were stained with SYTO-9 (green) and PI (red) to visualize biofilm architecture and viability of constituent cells. In each panel, top and sagittal views are constructed from a stack of images taken at 84.4-nm intervals for a total of 79 μm. A, biofilm formation of WT PAO1. B, biofilm formation of the ΔdksA1 mutant with reduced thickness and with increased dead cell population (red). C, biofilm formation of the ΔdksA2 mutant with increased dead cell population. D, biofilm formation of the ΔdksA1ΔdksA2 mutant with substantially reduced thickness and increased dead cell population. E, restored thickness and viability by dksA1 gene complementation in ΔdksA1 mutant biofilm. F, biofilm formation of ΔdksA1ΔdksA2 mutant complemented with the dksA1 gene. Overall structure and viability are similar to those of the ΔdksA2 mutant biofilm. All images were captured by Olympus FV10-ASW software in two independent biofilms from four separate experiments.
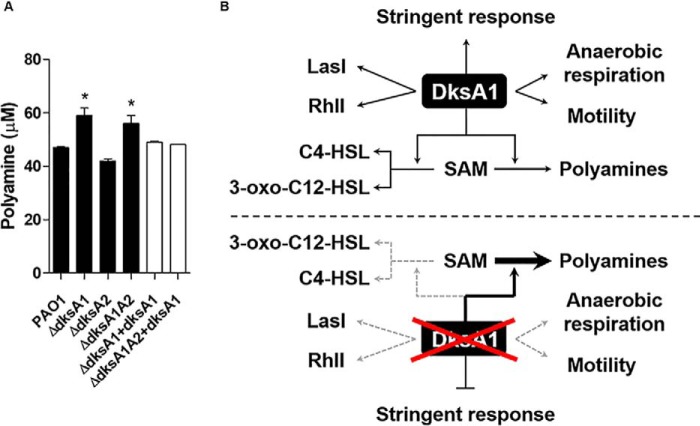
Polyamine synthesis is increased in ΔdksA1 mutants
In the transcriptome analysis, disruption of the dksA1 gene resulted in the increased expression of genes involved in polyamine metabolism (Fig. 1F). Polyamine biosynthesis is mediated by several enzymes that produce putrescine and other polyamines using amino acids and SAM as substrates (Fig. S3). Our RNA-Seq analysis also showed that the expression of genes involved in polyamine catabolism was reduced (Fig. 1F). We therefore postulated that polyamines might be accumulated in the presence of the dksA1 gene mutation. As shown in Fig. 7A, intracellular levels of total polyamines were increased by ∼30% in ΔdksA1 and ΔdksA1ΔdksA2 mutants compared with those of PAO1 and the ΔdksA2 mutant. Likewise, levels of polyamines were reduced when the dksA1 gene was complemented. These results thereby suggest that DksA1 participates in the regulation of intracellular metabolic flows, which, in turn, affects polyamine synthesis.
Figure 7.
Quantification of total polyamine production in tested strains and a model that depicts the multilayered role of DksA1. A, polyamine production in bacterial strains. Total polyamines, including putrescine, spermine, and spermidine, were measured from 100 mg of bacterial cell pellets. The values of means ± S.D. (error bars) are presented (n = 3). *, p < 0.05 versus values in PAO1. B, potential roles of DksA1 in P. aeruginosa. When DksA1 is absent (bottom half), the metabolic flow of SAM leads to elevated polyamine synthesis, inhibiting the synthesis of autoinducers. In addition to its well-characterized role in activating stringent response, DksA1 also participates in regulation of anaerobic respiration and motility.
Discussion
In this study, we showed that the disruption of DksA1 and/or DksA2 resulted in distinct transcriptional profiles in RNA synthesis, QS, virulence, motility, and anaerobic respiration in P. aeruginosa. As the gene expression profile of the ΔdksA1ΔdksA2 mutant is similar to that of the ΔdksA1 mutant, DksA1 seems to act as a major modulator of the aforementioned phenotypes. Expression of dksA1 is required to overcome various types of stress, including amino acid starvation and acidic stress (13, 42), whereas dksA2 is reported to be expressed under Zn2+ depletion (29). Moreover, phenotypes of the ΔdksA2 mutant are similar to WT PAO1 grown in nutrient-rich conditions. These observations suggest that DksA2 may be only required under specific conditions.
It has been previously suggested that DksA1 affects the expression of rhlI and translation of lasB; however, the DNA binding motif and chaperone activity of DksA were not found in E. coli (27, 43). As P. aeruginosa has a complex QS hierarchical network, reduction of C4-HSL in the supernatant negatively affects the regulation of 3-oxo-C12-HSL and the PQS system, leading to the down-regulation of virulence factor production (1). In addition, because the expression of the acylase-encoding genes, pvdQ and quiP, was increased in dksA1-disrupted mutants, reduced levels of acyl-HSL may be, in part, due to the increased acylase products.
The reduction of 3-oxo-C12-HSL and C4-HSL resulted in diminished swarming motility (44). The swarming motility in P. aeruginosa requires LasI, LasR, RhlI, and RhlR activation for rhamnolipid production, because a ΔrhlA mutant exhibited defective swarming motility. Based on this, diminished swarming motility in ΔdksA1 and ΔdksA1ΔdksA2 mutants might occur by reduced levels of 3-oxo-C12-HSL and C4-HSL. Similar to P. aeruginosa, Salmonella enterica serovar Typhimurium and Vibrio cholerae also require DksA for normal motility (25, 26).
The full expression of denitrification clusters requires two transcriptional regulators, ANR and DNR. ANR controls expression of dnr encoding DNR, and both ANR and DNR activate promoters of denitrification gene clusters (36). The anr encoding ANR is repressed by 3-oxo-C12-HSL with LasR at stationary phase (45). However, the production of C4-HSL and 3-oxo-C12-HSL was diminished, and the expression of anr was decreased in the ΔdksA1 mutant. Therefore, it is expected that the denitrification of the dksA1 mutants is regulated in a different way that depends on DksA1 rather than responding to ANR and DNR activities.
Polyamines are multifunctional metabolites associated with stress response by the modulation of gene expression, modification of membrane components, and sequestration of reactive oxygen species to protect cell components such as GC-rich DNA (46, 47). Furthermore, a positive correlation was observed between polyamine and QS in a metabolite comparison study using a ΔlasIΔrhlI mutant and PAO1, and the positive correlation is speculated to be a response to increased reactive oxygen species–induced stress resulting from QS activation (46). Moreover, polyamine synthesis requires SAM, which is an essential metabolite not only for the polyamine synthesis but also for the autoinducer synthesis (31, 48). Given this, DksA1 appears to contribute to modulate metabolic flow of the SAM between QS and polyamine synthesis (Fig. 7B). Thus, the increased gene expression and production of polyamine synthesis in the dksA1 mutants is presumed to be part of a complementary response to the dysfunction of the stress management mechanism, due to dksA1 gene disruption.
Alterations in gene expression by the disruption of dksA1 have not been fully understood in P. aeruginosa. In general, the action of DksA has been described in a concerted model whereby the interaction with (p)ppGpp and RNA polymerase (RNAP) regulates gene expression. Binding of DksA to RNAP regulates transcription in response to starvation signals by affecting the stability of an open complex (43, 49). In addition, DksA enhances the ability of RNAP to regulate gene expression in response to (p)ppGpp through direct binding to a secondary channel of RNAP in a concerted model (50). Nevertheless, recently, it has been discovered that DksA plays an independent role in the regulation of gene expression in E. coli (18, 51). The deletion of dksA in E. coli was found to globally induce changes in gene expression. Under this condition, it was proposed that the secondary channel in RNAP is occupied by a transcription factor (GreA or GreB (Gre factors)) that is structurally similar to DksA (51). Based on intracellular concentrations and RNAP affinities of DksA and Gre factors, it has been postulated that DksA preferentially occupies RNAP rather than Gre factors in E. coli under normal conditions (52). In addition, the interplay between DksA and GreA/B in a secondary channel is suggested to be critical for the regulation of inorganic polyphosphate that is required for virulence factor production (50). Furthermore, GreA has been reported to functionally compete with DksA; however, GreB is thought to have similar effects as DksA on rRNA operon (rrn) expression in E. coli (52, 53). Because P. aeruginosa harbors genes coding for the Gre factors in its genome, it will be necessary to investigate whether affected phenotypes of dksA1 mutants are caused by protein-protein interactions between RNAP and Gre factors. In addition, the characteristics of DNA sequence in the promoter region may be also responsible for the altered gene expression in the dksA1 mutants. Transcription is activated or repressed, depending on the intrinsic properties of the promoter region. Repressed target promoters, such as rrn, typically have a GC-rich discriminator region located between the −10 hexamer and +1 transcription start site, whereas activated target promoters typically contain an AT-rich sequence in this position (14).
DksA homologs, as RNAP-binding indirect transcription factors, are distributed in many bacterial species and contribute to the virulence factor production of these bacteria along with (p)ppGpp (14). Shigella flexneri, an enteric pathogen, uses DksA to acquire virulence associated genes horizontally. In addition, DksA of S. flexneri was required to spread the infection from cell to cell (54). In S. enterica, DksA is also required to control the virulence mechanism, including motility, biofilm formation, T3SS, and intestinal colonization (26). Moreover, S. enterica lacking DksA exhibits an increased LD50 in mouse infection (55). The intracellular invasive pathogen Legionella pneumophila also encodes a DksA homolog that is critical for differentiation to the transmissive form, including flagellar gene activation and cytotoxicity toward macrophage (14, 55). In addition, a ΔdksA mutant of Campylobacter jejuni, a food-poisoning pathogen, showed impaired invasion of epithelial cells and induction of interleukin-8 in vitro (14). In another enteric pathogen, V. cholerae, it was reported that the expression of cholera toxin is down-regulated when dksA is deleted through post-transcriptional regulation (56). Furthermore, P. aeruginosa lacking DksA1 showed increased susceptibility to aminoglycoside antibiotics and reduced cell viability under nutrient starvation (Fig. S2, A and B). When considering these findings, it can be suggested that DksA may be a drug target that controls bacterial infection and reduces the use of antibiotics. To screen drug candidates for anti-DksA, construction of a reporter strain is required for high-throughput positive selection. Because the DksA inhibits the expression of genes involved in ribosome synthesis, disruption of dksA induces the overexpression of ribosome synthesis (Fig. 1, B–D). Thus, applying this unique characteristic of DksA to the construction of a reporter strain would be a useful strategy for drug screening.
In summary, our research revealed that virulence and metabolism in P. aeruginosa rely on DksA1-dependent transcription, but not DksA2. QS-mediated virulence, anaerobic respiration, and motility are important phenotypes to consider when establishing anti-Pseudomonas strategies. Results provided in the current study demonstrate that all of these phenotypes are controlled by a single regulator, DksA1. We anticipate that future investigation will propose ideas to specifically and effectively target DksA1 for eradication of P. aeruginosa infection.
Experimental procedures
Ethics statement
All animal studies were performed in compliance with the guidelines provided by the Department of Animal Resources of Yonsei Biomedical Research Institute. The Committee on the Ethics of Animal Experiments at the Yonsei University College of Medicine approved this study (permit number 2018-0246).
Bacterial strains and growth conditions
All bacterial strains and plasmids used in this study are listed in Table 1. P. aeruginosa PAO1 was used as the WT strain. General experiments with PAO1 and other strains were conducted in LB medium (1% (w/v) tryptone, 0.5% (w/v) yeast extract, and 1% (w/v) sodium chloride) at 37 °C. E. coli strains in the cloning procedure were incubated in LB or in LB supplemented with 100 μg/ml of ampicillin. For P. aeruginosa, 100 μg/ml carbenicillin and 25 μg/ml irgasan were used to screen single-crossover recombinants, and LB agar supplemented with 6% sucrose instead of NaCl were used to select dksA1- and/or dksA2-deleted mutants. In the antibiotic susceptibility experiments, 3.125 μg/ml gentamycin, 12.5 μg/ml tetracycline, 400 μg/ml ampicillin, and 100 μg/ml carbenicillin were supplemented in LB.
Table 1.
Strains and plasmids used in this study
| Bacterial strains and plasmids | Genotype or description | Reference/source |
|---|---|---|
| E. coli strains | ||
| DH5αλpir | fhuA2 lac(del)U169 phoA glnV44 Φ80′ lacZ(del)M15 gyrA96 recA1 relA1 endA1 thi-1 hsdR17 λpir | |
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu Kmr λpir | |
| P. aeruginosa strains | ||
| PAO1 | WT, laboratory strain of P. aeruginosa | This study |
| PAO1ΔdksA1 | WT with dksA1 deletion | This study |
| PAO1ΔdksA2 | WT with dksA2 deletion | This study |
| PAO1ΔdksA1dksA2 | WT with dksA1 and dksA2 double deletion | This study |
| ΔlasRrhlR | WT with lasR and rhlR double deletion | |
| Other strain | ||
| C. violaceum CV026 | Biosensor for C4-HSL | |
| Plasmids | ||
| pCVD442 | Ampr suicide vector containing sacB for screening recombinant | Ref. 10 |
| pKDT17 | Ampr pTS400 plasmid containing lasR and lasB transcriptionally fused with lacZ | Ref. 61 |
| pCVD442d1 | pCVD442-containing overlapped left- and right-flanking region of dksA1 | This study |
| pCVD442d2 | pCVD442-containing overlapped left- and right-flanking region of dksA2 | This study |
| puc18-mini-Tn7t-Gm-lacZ | Ampr, Gmr, site-specific chromosomal insertion plasmid harboring promoterless lacZ | Ref. 57 |
| pTNS2 | Helper plasmid containing site-specific recombinase for chromosomal insertion | Ref. 57 |
| pD1Tn7t-comp | Complementation plasmid contacting dksA1 fragment in puc18-mini-Tn7t-Gm-lacZ | This study |
Construction of deletion mutants
To accomplish the in-frame clean deletion, two flanking regions of the targeted gene were designed to overlap. Used primer sequences are listed in Table 2. Overlapping was conducted by PCR with dksA1#1 and dksA1#4 primers. Then overlapped fragments were cloned in pCVD442 suicide vector to generate pCVD442d1. To generate the single crossover recombinant, P. aeruginosa PAO1 was conjugated with E. coli SM10 λpir harboring pCVD442d1. The conjugates were incubated on an LB agar plate containing 100 μg/ml carbenicillin and 25 μg/ml irgasan for selection of single-crossover recombinants. The selected recombinants were then transferred onto LB agar plate containing 6% sucrose to select a dksA1 gene deletion candidate. Colonies with the deletion were confirmed by PCR. To generate ΔdksA2 and ΔdksA1ΔdksA2 mutants, the same procedure was conducted in PAO1 and ΔdksA1 mutant, respectively.
Table 2.
Sequences of primers used in this study
| Primers | Sequence (5′–3′) | Description |
|---|---|---|
| dksA1#1 | ACA TGC ATG CAG GAA ATG CCT GTT GCA CGG | dksA1 left-flanking forward primer containing SphI restriction enzyme site |
| dksA1#2 | GCC GAG TTG CTT GAA TGG CCG CCT CTC ACT TT | dksA1 left-flanking reverse primer containing overlapping site to dksA1#3 |
| dksA1#3 | AGG CGG CCA TTC AAG CAA CTC GGC TCC TGA | dksA1 right-flanking forward primer containing overlapping site to dksA1#2 |
| dksA1#4 | TAA TGA GCT CCG GCG CTG GAT GAC GAA AT | dksA1 right-flanking reverse primer containing SacI restriction enzyme site |
| dksA2#1 | AAC ATG CAT GCA TCC TCA CCG AGG GTT TCG CCA | dksA2 left-flanking forward primer containing SphI restriction enzyme site |
| dksA2#2 | TTG GTT CAC GGT TGG TAA CCT CTT GAA CCT GGG AAA | dksA2 left-flanking reverse primer containing overlapping site to dksA2#3 |
| dksA2#3 | TGG TAA CCT CTT GAA CCT GGG AAA TTT AAA ACG TTA | dksA2 right-flanking forward primer containing overlapping site to dksA2#2 |
| dksA2#4 | TAA TGA GCT CTG GAG ACC GGC TTC ATC TCG AAT | dksA2 right-flanking reverse primer containing SacI restriction enzyme site |
| dksA1_compF | ATG GTA CCG TGG ATC ACA TCG TGG C | dksA1 complementation primer containing 300 bp upstream of dksA1 gene |
| dksA1_compR | ATA CTA GTA CCA GGG AAC CGA AGT GCA | dksA1 complementation primer containing 150 bp downstream of dksA1 gene |
| Mini-Tn7t_seqF | GCT TTT GAA GCT AAT TCG ATC A | Forward sequencing primer covering multiple cloning site of puc18-mini-Tn7t-Gm-lacZ |
| Mini-Tn7t_seqR | TCG GGA TCG CTA GTT AGT TA | Reverse sequencing primer covering multiple cloning site of puc18-mini-Tn7t-Gm-lacZ |
dksA1 genetic complementation
To accomplish dksA1 genetic complementation, a DNA locus that covers dksA1 ORF and its flanking sequences (300 bp upstream and 150 bp downstream) was PCR-amplified with primers listed in Table 2. The PCR product was then cloned into the puc18-mini-Tn7t-Gm-LacZ plasmid that allows chromosomal insertion into a nonfunctional region (57). The resultant plasmid was transformed into E. coli DH5α λpir and sequence-verified. The final construct, termed pD1Tn7t-comp, was then electroporated into ΔdksA1 mutants with helper plasmid pTNS2 encoding a sequence-specific recombinase enzyme. The potential clones with the intact dksA1 gene incorporated into the desired chromosomal region were selected with 50 μg/ml gentamycin. The complementation of the dksA1 gene was confirmed by DNA sequencing with primers, mini-Tn7t_seqF/R.
Elastase and pyocyanin assay
Elastase activity was measured as described previously (58). 500 μl of supernatant from overnight cultures was mixed with 1 ml of 30 mm Tris-HCl buffer containing 10 mg/ml of Elastin-Congo Red (Sigma). Then the mixture was incubated with shaking at 37 °C for 5 h. 1 ml of mixture was then transferred into a microtube and centrifuged at 13,000 rpm for 1 min. In the pyocyanin assay, 10 ml of supernatants from overnight cultures were harvested by centrifugation at 3,000 rpm for 20 min and filter-sterilized (0.2-μm pore size, Sartorius Minisart Sterile EO filters; Sartorius AG). Then 4 ml of chloroform was added to mix with 8 ml of the supernatants in a 15-ml conical tube. The supernatant mixtures were centrifuged at 3,000 rpm for 10 min to collect the blue layer fraction at the bottom of tube. 4 ml of the blue layer was transferred into a new tube, and 2 ml of 0.2 n HCl was added to produce a red-colored mixture. 1 ml of the mixture was then transferred into a microtube for centrifugation at 13,000 rpm for 1 min. Obtained resultants were measured at 520-nm absorbance (OD520).
Motility assay
To measure swimming motility, colonies of each strain grown on an LB plate were transferred onto a 0.3% agar LB plate using a sterile toothpick. The plates were then incubated at 30 °C for 24 h without being inverted. Twitching motility was measured by stabbing a sterile toothpick into 1% LB agar plates as described previously with Coomassie Blue staining (40). After incubation, the inner portion of the plate was submerged by 20 ml of Coomassie Blue for 20 min to stain the motility zone. Destaining was performed by multiple washes with destaining solution (40% methanol and 10% glacial acetic acid). Then plates were dried for 24 h at room temperature. Observation of swarming motility was performed as described previously with modification (44). In brief, 0.25% glucose and 0.1% glutamate were added into 0.5% agar-containing M8 medium, and the plates were incubated at 30 °C for 24 h.
Biofilm observation
Biofilms grown in LB for 24 h were analyzed using a CLSM as described previously (41). Biofilms were stained with 0.1% propidium iodide (Sigma) and 0.05% SYTO-9 (Life Technologies, Inc.).
Total polyamine assay
The total polyamine was measured by following the provided instructions in a Total polyamine assay kit (BioVision) with modification for applying to bacteria. Bacteria were incubated in 6 ml of LB at 37 °C for 6 h. After incubation for 6 h, 100 mg of bacterial cell pellets were then harvested to extract intracellular total polyamines. Lysis of bacterial cells was conducted by sonication at 35% amplitude for 5 s with 3-min intervals on ice.
SDS-PAGE analysis and protein identification
Proteins in supernatant were harvested by TCA (Sigma) precipitation. 1 ml of cell-free culture supernatants obtained from P. aeruginosa and mutant strains grown in LB for 16 h were mixed with 250 μl of TCA in microtubes. The mixtures were then incubated for 10 min on ice. After incubation, mixtures were centrifuged at 14,000 rpm for 5 min to harvest white pellets. Then the pellets were washed twice by ice-cold acetone. Residual acetone was removed by heat block at 95 °C for 10 min. The collected pellets were resuspended in PBS. Protein was quantified by the method of Bradford, and 10 μg of proteins were separated by 12% SDS-PAGE. The bands in the SDS-polyacrylamide gel were submitted to the Yonsei Proteome Research Center (Korea) for protein identification.
DNA manipulation
All resultant DNA were purified using a PCR/gel purification kit (Bioneer). Plasmid preparation was performed using a Plasmid mini-extraction kit (Bioneer). Restriction enzyme digestion, ligation, and agarose gel electrophoresis were performed by following standard methods. Restriction enzymes SphI and SacI (New England Biolabs, Inc.) were used to digest DNA in cloning for gene deletion. KpnI and SpeI restriction enzymes (New England Biolabs) were used in cloning for dksA1 complementation, and T4-ligase (New England Biolabs) was used to ligate DNA fragments. Transformation of E. coli was carried out by electroporation. Competent E. coli for electroporation was prepared by repeated washing with 300 mm sucrose. Electroporation settings were 2.5 kV, 25 microfarads, and 200 ohms for the 2-mm electroporation cuvette. The synthesizing of oligonucleotide primers and sequencing of DNA were performed by Macrogen (Seoul, Korea).
RNA-Seq analysis
To extract RNA, bacterial strains were grown in 25 ml of LB for 4 h at 37 °C with vigorous shaking. RNA samples were prepared by using an RNeasy minikit (Qiagen) and RNeasy Protect kit (Qiagen) following instructions provided by manufacturers. RNA samples individually prepared from three different cultures were pooled together. rRNA was removed by the Ribo-Zero rRNA removal kit (Illumina). RNA concentration was calculated by Quant-IT RiboGreen kit (Invitrogen, catalog no. R11490). To assess the integrity of the total RNA, samples were run on the TapeStation RNA screentape (Agilent Technologies, Waldbronn, Germany). Only high-quality RNA preparations, with an RNA integrity number greater than 7.0, were used for RNA library construction. Purified mRNA samples were randomly fragmented into small pieces using divalent cations under elevated temperature. The cleaved RNA fragments were copied into first-strand cDNA using SuperScript II reverse transcriptase (Invitrogen) and random primers. This was followed by second-strand cDNA synthesis using DNA polymerase I and RNase H. These cDNA fragments then went through an end repair process, the addition of a single “A” base, and then ligation of the indexing adapters. The products were then purified and enriched with PCR to create the final cDNA library. The libraries were quantified using quantitative PCR according to the qPCR Quantification Protocol Guide (KAPA Library Quantification kits for Illumina Sequencing platforms) and qualified using the TapeStation D1000 ScreenTape (Agilent Technologies). Indexed libraries were then sequenced using the HiSeq 4000 platform (Illumina). Acquisition of raw data and differential expression analysis were performed following procedures described elsewhere (59).
Autoinducer assay
PQS detection on TLC was performed as described previously with modifications (60). For PQS detection, supernatants from P. aeruginosa PAO1, ΔdksA1, ΔdksA2, and ΔdksA1ΔdksA2 grown in LB overnight were harvested by centrifugation at 3,000 rpm for 20 min. To extract PQS, 1 ml of the culture supernatants were mixed with the equivalent volume of ethyl acetate acidified by 0.02% acetic acid. The extraction was repeated two times to collect the ethyl acetate fractions. 500 μl of the collected ethyl acetate fractions were transferred into microtubes and evaporated by heat block at 60 °C in a fume hood. Residues from the evaporation were dissolved in 50 μl of solvent consisted of the acidified ethyl acetate and acetonitrile (1:1, v/v). 5 μl of the samples were loaded onto a TLC plate (TLC silica gel 60F254, Merck). In HSL measurements, C4-HSL and 3-oxo-C12-HSL were extracted from 50 ml of the culture supernatants with the equivalent volume of acidified ethyl acetate. The extraction was repeated two times to collect the ethyl acetate fraction. The collected ethyl acetate fraction was evaporated by N2 gas to dry. The residues were dissolved in 250 μl of HPLC grade ethyl acetate to obtain 20-fold concentrated extracts. Measurement of 3-oxo-C12-HSL was performed by using an E. coli strain harboring reporter plasmid pKDT17 containing a copy of lasR-lacZ transcriptional fusion (61). 100 μg/ml purified 3-oxo-C12-HSL (Sigma) was used as positive control, and the equivalent volume of extracts to the positive control was tested to β-gal activity. The Miller units from the control and samples were calculated as described previously (62). The measurement of C4-HSL was conducted through violacein productions of the Chromobacterium violaceum CV026 strain (63). The CV026 strain was incubated in 5 ml of LB supplemented with 100 μg/ml purified C4-HSL (Cayman Chemicals) as positive control. An equivalent volume of extracts to C4-HSL was used for CV026 incubation. After incubation, cell pellets in 1 ml of culture were harvested by centrifugation at 13,000 rpm for 3 min. The pellets were then resuspended using HPLC grade DMSO to dissolve violacein. Violacein was measured at 590-nm absorbance and normalized using an OD600 value of CV026.
In vivo virulence assay
A total of 25 mice aged 5 weeks were distributed into five groups, including the PBS control group. PAO1 and mutant strains were incubated in LB broth at 37 °C for 12 h. The cells were harvested by centrifugation and washed twice with PBS. Cell pellets were adjusted to 108 cells/50 μl as an initial infection dose. Mice were anesthetized with 20% (v/v) ketamine and 8% (v/v) Rompun mixed in saline. During anesthetization, 50 μl of prepared bacterial cells were inhaled directly through the nose by a pipette. The survival of the mice was monitored for 24 h with 2-h intervals. The survival of the mice was demonstrated as Kaplan–Meier curves using GraphPad Prism software.
Statistical analysis
Statistical analysis of the data in experiments was carried out by a statistics tool within the GraphPad Prism software for the paired Student's t test.
Author contributions
K. B. M. conceptualization; K. B. M. data curation; K. B. M. formal analysis; K. B. M. validation; K. B. M. investigation; K. B. M. visualization; K. B. M. methodology; K. B. M. and S. S. Y. writing-original draft; S. S. Y. supervision; S. S. Y. funding acquisition.
Supplementary Material
This work was supported by National Research Foundation (NRF) of Korea Grants 2017M3A9F3041233 and 2019R1A6A1A03032869, funded by the Korean Government. The authors declare that they have no conflicts of interest with the contents of this article.
This article contains a supporting spreadsheet and Figs. S1–S3.
- SR
- stringent response
- (p)ppGpp
- guanosine tetra- and pentaphosphate
- QS
- quorum sensing
- RPKM
- reads per kilobase per million mapped
- RNAP
- RNA polymerase
- HSL
- homoserine lactone
- PQS
- Pseudomonas quinolone signal
- OD
- optical density
- LB
- Luria–Bertani
- CLSM
- confocal laser-scanning microscope.
References
- 1. Lee J., and Zhang L. (2015) The hierarchy quorum sensing network in Pseudomonas aeruginosa. Protein Cell 6, 26–41 10.1007/s13238-014-0100-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pruitt B. A. Jr., McManus A. T., Kim S. H., and Goodwin C. W. (1998) Burn wound infections: current status. World J. Surg. 22, 135–145 10.1007/s002689900361 [DOI] [PubMed] [Google Scholar]
- 3. Montravers P., Harpan A., and Guivarch E. (2016) Current and future considerations for the treatment of hospital-acquired pneumonia. Adv. Ther. 33, 151–166 10.1007/s12325-016-0293-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chernish R. N., and Aaron S. D. (2003) Approach to resistant gram-negative bacterial pulmonary infections in patients with cystic fibrosis. Curr. Opin. Pulm. Med. 9, 509–515 10.1097/00063198-200311000-00011 [DOI] [PubMed] [Google Scholar]
- 5. Williams P., and Cámara M. (2009) Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: a tale of regulatory networks and multifunctional signal molecules. Curr. Opin. Microbiol. 12, 182–191 10.1016/j.mib.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 6. Coggan K. A., and Wolfgang M. C. (2012) Global regulatory pathways and cross-talk control Pseudomonas aeruginosa environmental lifestyle and virulence phenotype. Curr. Issues Mol. Biol. 14, 47–70 [PubMed] [Google Scholar]
- 7. Galán-Vasquez E., Luna B., and Martínez-Antonio A. (2011) The regulatory network of Pseudomonas aeruginosa. Microb. Inform. Exp. 1, 3 10.1186/2042-5783-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hwang S., Kim C. Y., Ji S. G., Go J., Kim H., Yang S., Kim H. J., Cho A., Yoon S. S., and Lee I. (2016) Network-assisted investigation of virulence and antibiotic-resistance systems in Pseudomonas aeruginosa. Sci. Rep. 6, 26223 10.1038/srep26223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boutte C. C., and Crosson S. (2013) Bacterial lifestyle shapes stringent response activation. Trends Microbiol. 21, 174–180 10.1016/j.tim.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim H. Y., Go J., Lee K. M., Oh Y. T., and Yoon S. S. (2018) Guanosine tetra- and pentaphosphate increase antibiotic tolerance by reducing reactive oxygen species production in Vibrio cholerae. J. Biol. Chem. 293, 5679–5694 10.1074/jbc.RA117.000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oh Y. T., Lee K. M., Bari W., Raskin D. M., and Yoon S. S. (2015) (p)ppGpp, a small nucleotide regulator, directs the metabolic fate of glucose in Vibrio cholerae. J. Biol. Chem. 290, 13178–13190 10.1074/jbc.M115.640466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu J., and Xie J. (2009) Magic spot: (p)ppGpp. J. Cell Physiol. 220, 297–302 10.1002/jcp.21797 [DOI] [PubMed] [Google Scholar]
- 13. Ross W., Sanchez-Vazquez P., Chen A. Y., Lee J. H., Burgos H. L., and Gourse R. L. (2016) ppGpp binding to a site at the RNAP-DksA interface accounts for its dramatic effects on transcription initiation during the stringent response. Mol. Cell 62, 811–823 10.1016/j.molcel.2016.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dalebroux Z. D., Svensson S. L., Gaynor E. C., and Swanson M. S. (2010) ppGpp conjures bacterial virulence. Microbiol. Mol. Biol. Rev. 74, 171–199 10.1128/MMBR.00046-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu X., Yu H., Zhang D., Xiong J., Qiu J., Xin R., He X., Sheng H., Cai W., Jiang L., Zhang K., and Hu X. (2016) Role of ppGpp in Pseudomonas aeruginosa acute pulmonary infection and virulence regulation. Microbiol. Res. 192, 84–95 10.1016/j.micres.2016.06.005 [DOI] [PubMed] [Google Scholar]
- 16. Vrentas C. E., Gaal T., Ross W., Ebright R. H., and Gourse R. L. (2005) Response of RNA polymerase to ppGpp: requirement for the omega subunit and relief of this requirement by DksA. Genes Dev. 19, 2378–2387 10.1101/gad.1340305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kang P. J., and Craig E. A. (1990) Identification and characterization of a new Escherichia coli gene that is a dosage-dependent suppressor of a dnaK deletion mutation. J. Bacteriol. 172, 2055–2064 10.1128/JB.172.4.2055-2064.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Magnusson L. U., Gummesson B., Joksimović P., Farewell A., and Nyström T. (2007) Identical, independent, and opposing roles of ppGpp and DksA in Escherichia coli. J. Bacteriol. 189, 5193–5202 10.1128/JB.00330-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parshin A., Shiver A. L., Lee J., Ozerova M., Schneidman-Duhovny D., Gross C. A., and Borukhov S. (2015) DksA regulates RNA polymerase in Escherichia coli through a network of interactions in the secondary channel that includes Sequence Insertion 1. Proc. Natl. Acad. Sci. U.S.A. 112, E6862–E6871 10.1073/pnas.1521365112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Khakimova M., Ahlgren H. G., Harrison J. J., English A. M., and Nguyen D. (2013) The stringent response controls catalases in Pseudomonas aeruginosa and is required for hydrogen peroxide and antibiotic tolerance. J. Bacteriol. 195, 2011–2020 10.1128/JB.02061-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schafhauser J., Lepine F., McKay G., Ahlgren H. G., Khakimova M., and Nguyen D. (2014) The stringent response modulates 4-hydroxy-2-alkylquinoline biosynthesis and quorum-sensing hierarchy in Pseudomonas aeruginosa. J. Bacteriol. 196, 1641–1650 10.1128/JB.01086-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Delden C., Comte R., and Bally A. M. (2001) Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J. Bacteriol. 183, 5376–5384 10.1128/JB.183.18.5376-5384.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boes N., Schreiber K., and Schobert M. (2008) SpoT-triggered stringent response controls usp gene expression in Pseudomonas aeruginosa. J. Bacteriol. 190, 7189–7199 10.1128/JB.00600-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pletzer D., Wolfmeier H., Bains M., and Hancock R. E. W. (2017) Synthetic peptides to target stringent response-controlled virulence in a Pseudomonas aeruginosa murine cutaneous infection model. Front. Microbiol. 8, 1867 10.3389/fmicb.2017.01867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pal R. R., Bag S., Dasgupta S., Das B., and Bhadra R. K. (2012) Functional characterization of the stringent response regulatory gene dksA of Vibrio cholerae and its role in modulation of virulence phenotypes. J. Bacteriol. 194, 5638–5648 10.1128/JB.00518-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azriel S., Goren A., Rahav G., and Gal-Mor O. (2016) The stringent response regulator DksA is required for Salmonella enterica serovar Typhimurium growth in minimal medium, motility, biofilm formation, and intestinal colonization. Infect. Immun. 84, 375–384 10.1128/IAI.01135-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jude F., Köhler T., Branny P., Perron K., Mayer M. P., Comte R., and van Delden C. (2003) Posttranscriptional control of quorum-sensing-dependent virulence genes by DksA in Pseudomonas aeruginosa. J. Bacteriol. 185, 3558–3566 10.1128/JB.185.12.3558-3566.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Branny P., Pearson J. P., Pesci E. C., Köhler T., Iglewski B. H., and Van Delden C. (2001) Inhibition of quorum sensing by a Pseudomonas aeruginosa dksA homologue. J. Bacteriol. 183, 1531–1539 10.1128/JB.183.5.1531-1539.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blaby-Haas C. E., Furman R., Rodionov D. A., Artsimovitch I., and de Crécy-Lagard V. (2011) Role of a Zn-independent DksA in Zn homeostasis and stringent response. Mol. Microbiol. 79, 700–715 10.1111/j.1365-2958.2010.07475.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perron K., Comte R., and van Delden C. (2005) DksA represses ribosomal gene transcription in Pseudomonas aeruginosa by interacting with RNA polymerase on ribosomal promoters. Mol. Microbiol. 56, 1087–1102 10.1111/j.1365-2958.2005.04597.x [DOI] [PubMed] [Google Scholar]
- 31. Ruparell A., Dubern J. F., Ortori C. A., Harrison F., Halliday N. M., Emtage A., Ashawesh M. M., Laughton C. A., Diggle S. P., Williams P., Barrett D. A., and Hardie K. R. (2016) The fitness burden imposed by synthesising quorum sensing signals. Sci. Rep. 6, 33101 10.1038/srep33101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee K. M., Yoon M. Y., Park Y., Lee J. H., and Yoon S. S. (2011) Anaerobiosis-induced loss of cytotoxicity is due to inactivation of quorum sensing in Pseudomonas aeruginosa. Infect. Immun. 79, 2792–2800 10.1128/IAI.01361-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Toyofuku M., and Yoon S. S. (2018) Nitric oxide, an old molecule with noble functions in Pseudomonas aeruginosa biology. Adv. Microb. Physiol. 72, 117–145 10.1016/bs.ampbs.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 34. Yoon M. Y., Lee K. M., Park Y., and Yoon S. S. (2011) Contribution of cell elongation to the biofilm formation of Pseudomonas aeruginosa during anaerobic respiration. PLoS ONE 6, e16105 10.1371/journal.pone.0016105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yoon S. S., Hennigan R. F., Hilliard G. M., Ochsner U. A., Parvatiyar K., Kamani M. C., Allen H. L., DeKievit T. R., Gardner P. R., Schwab U., Rowe J. J., Iglewski B. H., McDermott T. R., Mason R. P., Wozniak D. J., et al. (2002) Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell 3, 593–603 10.1016/S1534-5807(02)00295-2 [DOI] [PubMed] [Google Scholar]
- 36. Arai H. (2011) Regulation and function of versatile aerobic and anaerobic respiratory metabolism in Pseudomonas aeruginosa. Front. Microbiol. 2, 103 10.3389/fmicb.2011.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee K., Lee K. M., Go J., Ryu J. C., Ryu J. H., and Yoon S. S. (2016) The ferrichrome receptor A as a new target for Pseudomonas aeruginosa virulence attenuation. FEMS Microbiol. Lett. 363, fnw104 10.1093/femsle/fnw104 [DOI] [PubMed] [Google Scholar]
- 38. Henrichsen J. (1972) Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36, 478–503 10.1128/MMBR.36.4.478-503.1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Harshey R. M. (1994) Bees aren't the only ones: swarming in Gram-negative bacteria. Mol. Microbiol. 13, 389–394 10.1111/j.1365-2958.1994.tb00433.x [DOI] [PubMed] [Google Scholar]
- 40. Rashid M. H., and Kornberg A. (2000) Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U.S.A. 97, 4885–4890 10.1073/pnas.060030097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee K., Lee K. M., Kim D., and Yoon S. S. (2017) Molecular determinants of the thickened matrix in a dual-species Pseudomonas aeruginosa and Enterococcus faecalis biofilm. Appl. Environ. Microbiol. 83, e01182–17 10.1128/AEM.01182-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Furman R., Danhart E. M., NandyMazumdar M., Yuan C., Foster M. P., and Artsimovitch I. (2015) pH dependence of the stress regulator DksA. PLoS ONE 10, e0120746 10.1371/journal.pone.0120746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gourse R. L., Chen A. Y., Gopalkrishnan S., Sanchez-Vazquez P., Myers A., and Ross W. (2018) Transcriptional responses to ppGpp and DksA. Annu. Rev. Microbiol. 72, 163–184 10.1146/annurev-micro-090817-062444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Köhler T., Curty L. K., Barja F., van Delden C., and Pechère J. C. (2000) Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182, 5990–5996 10.1128/JB.182.21.5990-5996.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hammond J. H., Dolben E. F., Smith T. J., Bhuju S., and Hogan D. A. (2015) Links between Anr and quorum sensing in Pseudomonas aeruginosa biofilms. J. Bacteriol. 197, 2810–2820 10.1128/JB.00182-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Davenport P. W., Griffin J. L., and Welch M. (2015) Quorum sensing is accompanied by global metabolic changes in the opportunistic human pathogen Pseudomonas aeruginosa. J. Bacteriol. 197, 2072–2082 10.1128/JB.02557-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ha H. C., Sirisoma N. S., Kuppusamy P., Zweier J. L., Woster P. M., and Casero R. A. Jr. (1998) The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. U.S.A. 95, 11140–11145 10.1073/pnas.95.19.11140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chou H. T., Kwon D. H., Hegazy M., and Lu C. D. (2008) Transcriptome analysis of agmatine and putrescine catabolism in Pseudomonas aeruginosa PAO1. J. Bacteriol. 190, 1966–1975 10.1128/JB.01804-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Henard C. A., Tapscott T., Crawford M. A., Husain M., Doulias P. T., Porwollik S., Liu L., McClelland M., Ischiropoulos H., and Vázquez-Torres A. (2014) The 4-cysteine zinc-finger motif of the RNA polymerase regulator DksA serves as a thiol switch for sensing oxidative and nitrosative stress. Mol. Microbiol. 91, 790–804 10.1111/mmi.12498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gray M. J. (2019) Inorganic polyphosphate accumulation in Escherichia coli is regulated by DksA but not by (p)ppGpp. J. Bacteriol. 201, e00664–18 10.1128/JB.00664-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Aberg A., Fernández-Vazquez J., Cabrer-Panes J. D., Sánchez A., and Balsalobre C. (2009) Similar and divergent effects of ppGpp and DksA deficiencies on transcription in Escherichia coli. J. Bacteriol. 191, 3226–3236 10.1128/JB.01410-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rutherford S. T., Lemke J. J., Vrentas C. E., Gaal T., Ross W., and Gourse R. L. (2007) Effects of DksA, GreA, and GreB on transcription initiation: insights into the mechanisms of factors that bind in the secondary channel of RNA polymerase. J. Mol. Biol. 366, 1243–1257 10.1016/j.jmb.2006.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Potrykus K., Vinella D., Murphy H., Szalewska-Palasz A., D'Ari R., and Cashel M. (2006) Antagonistic regulation of Escherichia coli ribosomal RNA rrnB P1 promoter activity by GreA and DksA. J. Biol. Chem. 281, 15238–15248 10.1074/jbc.M601531200 [DOI] [PubMed] [Google Scholar]
- 54. Sharma A. K., and Payne S. M. (2006) Induction of expression of hfq by DksA is essential for Shigella flexneri virulence. Mol. Microbiol. 62, 469–479 10.1111/j.1365-2958.2006.05376.x [DOI] [PubMed] [Google Scholar]
- 55. Webb C., Moreno M., Wilmes-Riesenberg M., Curtiss R. 3rd, Foster J. W. (1999) Effects of DksA and ClpP protease on sigma S production and virulence in Salmonella typhimurium. Mol. Microbiol. 34, 112–123 10.1046/j.1365-2958.1999.01581.x [DOI] [PubMed] [Google Scholar]
- 56. Basu P., and Bhadra R. K. (2019) Post-transcriptional regulation of cholera toxin production in Vibrio cholerae by the stringent response regulator DksA. Microbiology 165, 102–112 10.1099/mic.0.000743 [DOI] [PubMed] [Google Scholar]
- 57. Choi K. H., and Schweizer H. P. (2006) mini-Tn7 insertion in bacteria with single attTn7 sites: example Pseudomonas aeruginosa. Nat. Protoc. 1, 153–161 10.1038/nprot.2006.24 [DOI] [PubMed] [Google Scholar]
- 58. Pearson J. P., Pesci E. C., and Iglewski B. H. (1997) Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 179, 5756–5767 10.1128/JB.179.18.5756-5767.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hwang W., and Yoon S. S. (2019) Virulence characteristics and an action mode of antibiotic resistance in multidrug-resistant Pseudomonas aeruginosa. Sci. Rep. 9, 487 10.1038/s41598-018-37422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Toyofuku M., Nomura N., Kuno E., Tashiro Y., Nakajima T., and Uchiyama H. (2008) Influence of the Pseudomonas quinolone signal on denitrification in Pseudomonas aeruginosa. J. Bacteriol. 190, 7947–7956 10.1128/JB.00968-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pearson J. P., Gray K. M., Passador L., Tucker K. D., Eberhard A., Iglewski B. H., and Greenberg E. P. (1994) Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc. Natl. Acad. Sci. U.S.A. 91, 197–201 10.1073/pnas.91.1.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Smale S. T. (2010) β-Galactosidase assay. Cold Spring Harb. Protoc. 2010, pdb.prot5423 10.1101/pdb.prot5423 [DOI] [PubMed] [Google Scholar]
- 63. Steindler L., and Venturi V. (2007) Detection of quorum-sensing N-acyl homoserine lactone signal molecules by bacterial biosensors. FEMS Microbiol. Lett. 266, 1–9 10.1111/j.1574-6968.2006.00501.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.