Abstract
Purpose
Osteopontin (OPN) is a neuroprotective factor in the retina that improves photoreceptor survival. The aim of the present study was to investigate whether human RPE cells express and respond to OPN.
Methods
Hypoxia and chemical hypoxia were induced by cell culture in 0.25% O2 and the addition of CoCl2, respectively. Hyperosmolarity was produced by the addition of 100 mM NaCl or 200 mM sucrose. Gene expression was quantified with real-time reverse transcription (RT)–PCR, and protein secretion was investigated with enzyme-linked immunosorbent assay (ELISA). Nuclear factor of activated T cell 5 (NFAT5) was depleted with siRNA.
Results
The acutely isolated RPE cells and the cultured RPE cells expressed OPN. OPN gene expression was induced by hypoxia and hyperosmotic media, as well as by exogenous bFGF. High extracellular NaCl and hypoxia induced secretion of OPN. Hyperosmotic expression of the OPN gene was mediated by the p38 MAPK and ERK1/2 signal transduction pathways, and the transcriptional activities of CREB and NFAT5. The hypoxic expression of the OPN gene was mediated by the PI3K signal transduction pathway and caspase-mediated, necrosis-related pathways. Phospholipases A2 were involved in mediating hyperosmotic and hypoxic OPN gene expression. Autocrine or paracrine P2Y2 receptor signaling induced by extracellular ATP contributed to hyperosmotic expression of the OPN gene whereas activation of A1 receptors by extracellularly formed adenosine contributed to thypoxic OPN gene expression. Autocrine or paracrine VEGF signaling exerted an inhibitory effect on expression of the OPN gene. Exogenous OPN induced expression and secretion of bFGF, but not of VEGF.
Conclusions
The data indicated that RPE cells produce and respond to OPN; OPN expression is, in part, induced by the cellular danger signal ATP. RPE-derived neuroprotective factors such as bFGF may contribute to the prosurvival effect of OPN on photoreceptor cells.
Introduction
Retinal diseases such as age-related macular degeneration, retinitis pigmentosa, and glaucoma are characterized by degeneration of photoreceptors or inner retinal neurons or both. Various neurotrophic factors, growth factors, and cytokines have been shown to promote the survival of photoreceptors and neurons in the retina. Among other factors, brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor (GDNF), and basic fibroblast growth factor (bFGF) rescue photoreceptors and retinal neurons from degeneration [1]. The survival of photoreceptors and neurons induced by growth and neurotrophic factors is mediated by a direct autocrine or paracrine effect, for example, of bFGF produced in photoreceptor segments [2,3], and by an indirect mode involving retinal glial cells which release prosurvival factors, in particular bFGF, upon stimulation with neurotrophins [1,4-6]. Researchers showed, for example, that photoreceptor cell-derived GDNF stimulates the production of various factors such as bFGF, brain-derived neurotrophic factor, GDNF, and osteopontin (OPN) in Müller glial cells which promote photoreceptor survival [6‒8].
OPN, also known as secreted phosphoprotein 1 (SPP1) and early T lymphocyte activation 1 (Eta-1), is a phosphorylated glycoprotein [9]. OPN exists as an immobilized component of the extracellular matrix and as a soluble, multifunctional cytokine that plays important roles in promoting inflammation, tissue remodeling, fibrosis, and angiogenesis [10‒18]. In the neuroretina, OPN is localized to retinal ganglion cells, activated microglia, and Müller glia [8,19‒23]. OPN is upregulated under various pathological conditions, such as ischemia, glaucoma, and retinal light damage [10,24], and protects retinal ganglion cells and photoreceptors from death [8,25]. In addition to interactions with extracellular matrix components, secreted OPN is a ligand of CD44 receptor variants and cell surface integrins [9,13,16,26,27]. Retinal injury and degeneration stimulate the expression of CD44 in reactive glial cells [28‒31].
RPE cells play crucial roles in the maintenance of photoreceptor integrity and function. A major function of the RPE is phagocytosis and digestion of membrane discs that are shed from the tips of photoreceptor outer segments [32]. Because the discs contain high amounts of peroxidized lipids and protein adducts, this function protects the photoreceptors from photooxidative damage. Dysfunction and degeneration of RPE cells are crucially involved in pathogenesis of age-related macular degeneration (AMD) [33]. Age-related dysregulation of protein and lipid recycling and degradation pathways in RPE cells [34,35] results in lipofuscin accumulation within the RPE and drusen deposition beneath the RPE. Accumulated lipoproteins constitute a hydrophobic barrier that adversely affects the transport of oxygen and nutrients from the choriocapillaris to photoreceptors [36]. In addition, normal aging and AMD are associated with a decrease in choroidal blood flow [37,38]. Inadequate choroidal perfusion and lipoprotein accumulation lead to hypoxia of the outer retina that stimulates the growth of choroidal vessels resulting in the development of neovascular AMD [36]. Photoreceptor degeneration is a key pathological event in end-stage AMD [33].
It was shown that the survival of photoreceptors is supported by Müller cell-derived OPN [7,8]. With the exception of one study that showed expression of OPN in the ARPE-19 cell line in response to stimulation with glyoxal [39], there is no knowledge regarding the production of OPN in RPE cells. The aim of the present study was to investigate whether OPN is expressed and secreted by human RPE cells, and to determine which intracellular signal transduction molecules and cell surface receptors mediate the expression of OPN in cells. In addition, it was investigated whether exogenous OPN modulates the production of angiogenic vascular endothelial growth factor (VEGF) and bFGF in RPE cells. We tested two conditions that are implicated in pathogenesis of AMD in situ: hypoxia and extracellular hyperosmolarity. The involvement of the latter condition is suggested by the fact that systemic hypertension is a risk factor of AMD [40,41]. The main condition that causes acute hypertension is the increase in extracellular osmolarity due to a high extracellular salt (NaCl) level [42,43]. Because the use of antihypertensive medication is not associated with the risk of AMD [44,45], it was suggested that conditions that cause hypertension rather than hypertension per se may aggravate AMD [46].
Methods
Materials
Cell culture components and solutions were purchased from Gibco BRL (Paisley, UK). Recombinant human VEGF-A165, bFGF, heparin-binding epidermal growth factor-like growth factor (HB-EGF), hepatocyte growth factor (HGF), IL-1 receptor antagonist, OPN, platelet-derived growth factor (PDGF)-BB, transforming growth factor β1 (TGF-β1), and tumor necrosis factor-α (TNFα) were purchased from R&D Systems (Abingdon, UK). Recombinant human interleukin-1β (IL-1β) and placental growth factor (PlGF)-2 were from Reliatech (Braunschweig, Germany). 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX), Gö6976, H-89, the inhibitor of hypoxia-inducible transcription factor-1 (HIF-1), LY294002, necrostatin-1, inactive necrostatin-1, PD98059, PP2, SP600125, and SU1498 were obtained from Calbiochem (Bad Soden, Germany). Ac-DEVD-CHO, Ac-IETD-CHO, Ac-YVAD-CMK, AG1478, Stattic, and z-VAD were from Enzo Life Science (Lausen, Switzerland). 666–15, A-438079, adenosine, adenosine 5′-triphosphate (ATP), AG490, AR-C 118925XX, ARL-67156, caffeic acid phenethyl ester (CAPE), 8-(3-chlorostyryl) caffeine (CSC), 2-chloro-N-cyclopentyl-2’-methyladenosine (2'-MeCCPA), MRS2179, MRS2365, MRS2768, SB203580, and SR11302 were from Tocris (Ellisville, MO). Uridine 5′-triphosphate (UTP) and dithiothreitol were from Carl Roth (Karlsruhe, Germany), and PD173074 was kindly provided by Pfizer (Karlsruhe, Germany). Human-specific small interfering RNA (siRNA) against nuclear factor of activated T cell 5 (NFAT5) and nontargeted scrambled siRNA were obtained from Santa Cruz Biotechnology (Heidelberg, Germany). 4’,6-Diamidino-2-phenylindole (DAPI) was from Invitrogen (Paisley, UK). AG1296, 2-aminoethoxydiphenyl borate, apyrase, 4-bromophenacyl bromide, N-nitrobenzylthioinosine (NBTI), 1,10-phenanthroline, SB431542, and all other agents used were from Sigma-Aldrich (Taufkirchen, Germany), unless stated otherwise. The following antibodies were used for immunocytochemistry: rabbit anti-human OPN (1:100; HPA027541; Sigma-Aldrich), fluorescein isothiocyanate-coupled monoclonal anti-pan cytokeratin (1:50; Sigma-Aldrich), and Alexa Fluor 568-conjugated goat anti-rabbit (1:400; A11036; Invitrogen).
Cell culture
The study followed the tenets of the Declaration of Helsinki for research involving human subjects and the ARVO statement for the use of human subjects. The use of human material was approved by the Ethics Committee of the University of Leipzig (#745, 07/25/2011). Post-mortem eyes from human cornea donors without reported eye disease were obtained within 48 h of death with written informed consent from the donors’ relatives for the use of retinal tissue in basic science. The RPE cells were prepared and cultured as described [47]. Cell lines derived from different donors were used in passages 3 to 5. Near-confluent cultures were growth arrested in medium without serum for 16 h, and subsequently, serum-free media with and without test substances were added. Hyperosmotic media were made up by addition of NaCl (100 mM) or sucrose (200 mM). The hypoosmotic medium (60% osmolarity) was made up by adding distilled water. The cells were preincubated with pharmacological inhibitors for 30 min.
RNA extraction and cDNA synthesis
Total RNA was extracted with the InviTrap Spin Universal RNA Mini Kit (Stratec Molecular, Berlin, Germany). The quality of the RNA was analyzed with agarose gel electrophoresis. The A260/A280 ratio of the optical density was measured using the NanoDrop 1000 device (peQLab, Erlangen, Germany), and was between 1.95 and 2.03 for all RNA samples, indicating sufficient quality. After treatment with DNase I (Roche, Mannheim, Germany), cDNA was synthesized from 1 µg total RNA using the RevertAid H Minus First Strand cDNA Synthesis kit (Fermentas, St. Leon-Roth, Germany).
RT–PCR
Reverse transcription (RT)–PCR was performed with the Taq PCR Master Mix kit (Qiagen, Hilden, Germany) and the primer pairs described in Table 1. One microliter of the first-strand mixture and 0.25 µM of each gene-specific sense and anti-sense primers were used for amplification in a final volume of 20 µl. Amplification was performed for 40 cycles with the PTC-200 Thermal Cycler (MJ Research, Watertown, MA). Each cycle consisted of 30 s at 94 °C, 60 s at 58 °C, and 1 min at 72 °C.
Table 1. Primer pairs used in PCR experiments.
| Gene / Accession number | Gene ID | OMIM number | Primer sequences (5′-3′) | Product (bp) |
|---|---|---|---|---|
|
ACTB |
60 |
102630 |
F: ATGGCCACGGCTGCTTCCAGC |
237 |
|
NM_001101 |
|
|
R: CATGGTGGTGCCGCCAGACAG |
|
|
OPN |
6696 |
166490 |
F: GCCGAGGTGATAGTGTGGTT |
178 |
|
NM_001251830.1 |
|
|
R: CAATCAGAAGGCGCGTTCAG |
|
|
CD44 |
960 |
107269 |
F: CCGCTATGTCCAGAAAGGA |
195 |
|
NM_001202557.1 |
|
|
R: CTGTCTGTGCTGTCGGTGAT |
|
|
NFAT5 |
10725 |
604708 |
F: TCACCATCATCTTCCCACCT |
174 |
|
NM_006599.3 |
|
|
R: CTGCAATAGTGCATCGCTGT |
|
|
VEGFA188, 164, 120 |
7422 |
192240 |
F: CCTGGTGGACATCTTCCAGGAGTA |
479; 407; |
|
NM_003376.5 |
|
|
R: CTCACCGCCTCGGCTTGTCACA |
275 |
|
NM_001287044.1 |
|
|
|
|
|
NM_001025370.2 |
|
|
|
|
|
bFGF |
2247 |
134920 |
F: AGAGCGACCCTCACATCAAG |
234 |
| NM_001361665.1 | R: ACTGCCCAGTTCGTTTCAGT |
s, sense. as, anti-sense.
Real-time RT–PCR
Real-time RT–PCR was performed with the Single-Color Real-Time PCR Detection System (Bio-Rad, Munich, Germany) using the primer pairs described in Table 1. The PCR solution contained 1 µl cDNA, a specific primer set (0.2 µM each), and 7.5 µl of a 2X mastermix (iQ SYBR Green Supermix; Bio-Rad) in a final volume of 15 µl. The following conditions were used: initial denaturation and enzyme activation (one cycle at 95 °C for 3 min); denaturation, amplification, and quantification, 45 cycles at 95 °C for 30 s, 58 °C for 20 s, and 72 °C for 45 s; melting curve, 55 °C with the temperature gradually (0.5 °C) increased up to 95 °C. The amplified samples were analyzed with standard agarose gel electrophoresis. mRNA expression was normalized to the level of β-actin (ACTB, Gene ID: 60; OMIM: 102630) mRNA. The changes in mRNA expression were calculated according to the 2-ΔΔCT method [48].
ELISA
The cells were stimulated with iso- and hyperosmotic media (+ 100 mM NaCl, 200 mM sucrose), with CoCl2 (150 µM)-containing, serum-free medium, or with OPN (10 ng/ml) in serum-free medium. The supernatants were collected after 6 and 24 h, and the levels of OPN, VEGF-A165, and bFGF in the cultured media (100 µl) were determined with enzyme-linked immunosorbent assay (ELISA; R&D Systems).
siRNA transfection
Cells were seeded at 7 × 104 cells per well in 12-well culture plates and were allowed to grow to a confluency of 60–80%. Thereafter, the cells were transfected with NFAT5 siRNA (5 nM) and nontargeted siRNA (5 nM), respectively, using HiPerfect reagent (Qiagen) in F-10 medium containing 10% fetal bovine serum according to the manufacturer’s instructions. After 48 h, the medium was removed, and fresh medium without serum was added for 5 h. Thereafter, serum-free medium containing high (+ 100 mM) NaCl and CoCl2 (150 µM), respectively, was added for 24 h.
Immunocytochemistry
The cultures were fixed with 4% paraformaldehyde for 15 min on ice. After several washing steps in prechilled PBS (1X; 155 mM NaCl, 1.54 mM KH2PO4, 2.71 mM Na2HPO4-7H2O pH 7.2; Invitrogen), the cultures were incubated in PBS containing 0.3% Triton X-100 for 15 min at room temperature (RT). Blocking of nonspecific antibody binding was performed with PBS containing 10% normal goat serum and 0.3% Triton X-100 for 2 h at RT. Primary antibodies diluted in blocking solution were incubated at 4 °C overnight. After several washing steps in PBS plus 0.3% Triton X-100, the secondary antibody was applied for 1 h at RT. After additional washing steps, DAPI (1:10,000) was added for 15 min at RT. The coverslips were mounted with Fluorescence Mounting Medium (DakoCytomation, Glostrup, Denmark). Images were taken with an epifluorescence microscope (Olympus BX40, Olympus, Essex, UK), a charge-coupled device (CCD) camera (Olympus XM10), and cellSens software (Olympus).
Statistical analysis
For each test, at least three independent experiments using cells from different donors were performed. Data are expressed as means ± standard error of the mean (SEM). Statistical analysis was performed with Prism (GraphPad Software, San Diego, CA). Statistical significance was determined with one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test and the Mann–Whitney U test. A p value of less than 0.05 was considered statistically significant.
Results
OPN gene expression in RPE cells
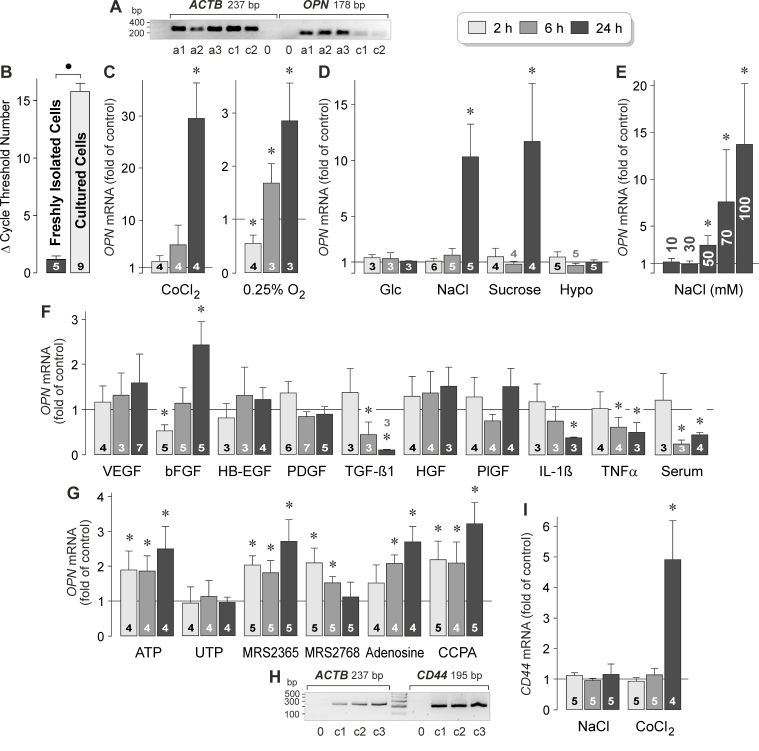
RT–PCR was performed to compare the levels of OPN (Gene ID: 6696; OMIM: 166490) mRNA in RPE cells that had been acutely isolated from the eyes of post-mortem donors without reported eye disease and in cultured human RPE cell lines derived from different donors. As shown in Figure 1A, the acutely isolated cells and the cultured cells contained OPN gene transcripts. The level of OPN mRNA was strongly reduced in the cultured cells compared to the acutely isolated cells, as indicated by the statistically significantly (p<0.05) increased cycle number necessary for the detection of the transcript (Figure 1B).
Figure 1.
Regulation of OPN gene expression and OPN secretion in RPE cells. A: Presence of OPN gene transcripts in RPE cells. To confirm the correct lengths of the PCR products, agarose gel electrophoresis was performed using products obtained from acutely isolated RPE cells (a) and cultured RPE cell lines (c) derived from different post-mortem donors. Negative controls (0) were performed by adding double-distilled water instead of cDNA as the template. The β-actin (ACTB) mRNA level was used to normalize the OPN mRNA level in real-time reverse transcription (RT)–PCR. B: Expression level of the OPN gene in acutely isolated RPE cells and cultured RPE cell lines, as revealed with real-time RT–PCR. Each bar represents the cycle number necessary for the detection of the transcript. C‒F: The OPN mRNA level in cultured RPE cell lines was determined with real-time RT–PCR after stimulation of the cells for 2, 6, and 24 h (as indicated by the panels of the bars), and is expressed as the fold of unstimulated control. C: Effects of chemical hypoxia and cell culture in a 0.25% O2 atmosphere on OPN gene expression. Chemical hypoxia was induced with the addition of CoCl2 (150 µM) to the culture medium. D: Effects of hyperglycemia induced by the addition of high (25 mM) glucose, extracellular hyperosmolarity induced by the addition of high (+ 100 mM) NaCl and sucrose (200 mM), respectively, and extracellular hypoosmolarity (60% osmolarity) on OPN gene expression. E: Dose-dependence of the effect of high extracellular NaCl on the OPN mRNA level. Ten millimoles to 100 mM NaCl were added to the culture medium, as indicated in the bars. Data were obtained in six independent experiments using cell lines from different donors. F: Effects of inflammatory and growth factors on the expression of the OPN gene. The following factors were tested: VEGF, bFGF, HB-EGF, PDGF-BB, TGF-β1, HGF, PlGF, IL-1β, and TNFα (each at 10 ng/ml). In addition, fetal calf serum (10%) was tested. G: Effects of purinergic receptor agonists on the expression of the OPN gene. The following agents were tested: ATP (50 µM), UTP (50 µM), the selective P2Y1 agonist MRS2365 (200 nM), the selective P2Y2 agonist MRS2768 (100 µM), adenosine (50 µM), and the selective A1 agonist 2’-MeCCPA (100 nM). H: Presence of CD44 gene transcripts in RPE cells. Agarose gel electrophoresis was performed using products obtained from cultured RPE cell lines derived from different donors (1‒3). Negative controls (0) were performed by adding double-distilled water instead of cDNA as the template. I: Effects of a hyperosmotic medium (+ 100 mM NaCl) and chemical hypoxia induced with the addition of CoCl2 (150 µM) on the expression of the CD44 gene. The numbers of independent experiments using cell lines from different donors are indicated in or above the bars. Statistically significant difference between acutely isolated and cultured cells: ●p<0.05. Statistically significant difference versus unstimulated control: *p<0.05.
Regulation of OPN gene expression
To investigate which pathogenic conditions induce the expression of the OPN gene in RPE cells, we stimulated cultured cell lines from different donors with the hypoxia mimetic CoCl2 [49], high (25 mM) glucose, and hyper- and hypoosmotic media, and performed real-time RT–PCR. As shown in Figure 1C, CoCl2-induced chemical hypoxia induced a statistically significant (p<0.05) increase in OPN gene expression after 24 h of stimulation. Cell culture in a 0.25% O2 atmosphere induced biphasic regulation of OPN gene expression with down- and upregulation after 2 and 6‒24 h of stimulation, respectively (Figure 1C). Treatment of the cells with CoCl2 caused a higher increase in the OPN mRNA level than cell culture in 0.25% O2 (Figure 1C). The data suggest that CoCl2-induced expression of the OPN gene is mediated by several mechanisms that may include hypoxia, alteration of the metal ion homeostasis, and oxidative stress. Expression of the OPN gene remained unaltered in the presence of high glucose (Figure 1D). Extracellular hyperosmolarity was induced with the addition of 100 mM NaCl or 200 mM sucrose (which caused equal increases in the osmolarity). As shown in Figure 1D, hyperosmotic media induced statistically significant (p<0.05) increases in OPN gene expression after 24 h of stimulation. The amplitudes and time-dependencies of the effects of high NaCl and sucrose were similar (Figure 1D). This result suggests that the NaCl-induced expression of the OPN gene was caused by the elevation of the extracellular osmolarity, but not by the alterations of the transmembrane sodium and chloride gradients. The effect of high NaCl on the OPN mRNA level was dose-dependent; statistically significant (p<0.05) increases were found when more than 30 mM NaCl was added to the culture medium (Figure 1E). The hypoosmotic medium did not alter the cellular level of OPN mRNA (Figure 1D). The data suggest that the OPN gene in RPE cells is transcriptionally activated by hypoxia and extracellular hyperosmolarity.
To investigate whether the expression of the OPN gene in RPE cells is regulated by inflammatory and growth factors, we stimulated the cells with various cytokines. As shown in Figure 1F, exogenous VEGF, HB-EGF, PDGF, HGF, and PlGF did not alter the level of OPN transcripts in RPE cells. bFGF induced biphasic regulation of the expression of the OPN gene, with down- and upregulation after 2 and 24 h of stimulation, respectively (Figure 1F). The cellular level of the OPN gene transcripts was time-dependently decreased during stimulation with TGF-β1, IL-1β, TNFα, and fetal calf serum (Figure 1F).
Extracellular ATP is an endogenous danger signal which is released in large quantities by stressed cells [50]. In many cell systems, such as retinal glial cells [50], autocrine or paracrine purinergic signaling is mediated by osmo- or mechanosensitive release of ATP, and subsequent activation of purinergic receptors is involved in the early cellular response to osmotic stress. Extracellular ATP acts at the purinergic metabotropic (P2Y) and ionotropic (P2X) receptors. RPE cells are known to express multiple purinergic receptor subtypes, including P2Y, P2X, and adenosine receptors [51,52]. The expression level of the OPN gene in the RPE cells was increased by exogenous ATP, but not UTP (Figure 1G). The selective P2Y1 receptor agonist MRS2365 also induced an increase in the OPN mRNA level in RPE cells while the selective P2Y2 receptor agonist MRS2768 induced a transient increase (Figure 1G). In addition, exogenous adenosine and the selective A1 receptor agonist 2’-MeCCPA stimulated the expression of the OPN gene in RPE cells (Figure 1G).
OPN is a ligand of CD44 receptor variants [26,27]. RPE cells contained CD44 (Gene ID: 960; OMIM: 107269) gene transcripts (Figure 1H). The expression level of the CD44 gene was unaltered under hyperosmotic conditions and upregulated after 24 h of stimulation with the hypoxia mimetic CoCl2 (Figure 1I).
Regulation of OPN secretion
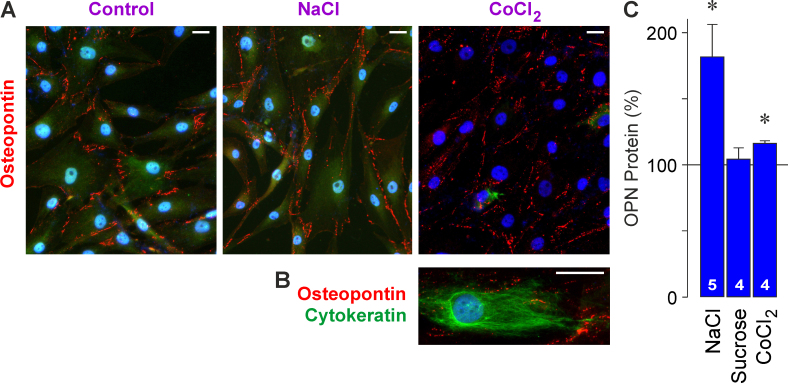
Cultured RPE cells were labeled with an antibody against OPN (Figure 2A). The punctate OPN immunoreactivities were arranged in lines along the borders of the cell somata and processes (Figure 2A). The distribution of the OPN immunoreactivity was not altered in response to high extracellular NaCl and the hypoxia mimetic CoCl2 compared to control (Figure 2A). There was no colocalization of OPN and cytokeratin immunoreactivities (Figure 2B), suggesting that OPN was mainly localized near the plasma membrane.
Figure 2.
OPN protein in cultured RPE cells. A: Cell cultures were immunolabeled with an antibody against OPN (red). The cell nuclei were stained with 4’,6-diamidino-2-phenylindole (DAPI; blue). The cells were cultured for 24 h under unstimulated control conditions and in the presence of high (+ 100 mM) NaCl and the hypoxia mimetic CoCl2 (150 µM), respectively. B: Double-immunolabeling of OPN (red) and cytokeratin (green). C: OPN secretion from RPE cells. The OPN protein level was determined with enzyme-linked immunosorbent assay (ELISA) in the media of cells cultured for 24 h in the presence of high (+ 100 mM) NaCl, sucrose (200 mM), and CoCl2 (150 µM). The data are expressed as percent of unstimulated control (100%). The numbers of independent experiments using cell lines from different donors are indicated in the bars. Statistically significant difference versus unstimulated control: *p<0.05. Scale bars in A and B, 20 µm.
High extracellular NaCl, but not sucrose-induced extracellular hyperosmolarity, stimulated the secretion of OPN from RPE cells (Figure 2C). The data suggest that alterations of the transmembrane sodium and chloride gradients, but not the increase in extracellular osmolarity, induce the secretion of OPN. Chemical hypoxia caused a moderate increase in the OPN protein level in the cultured media (Figure 2C).
Intracellular signaling involved in osmotic and hypoxic expression of the OPN gene
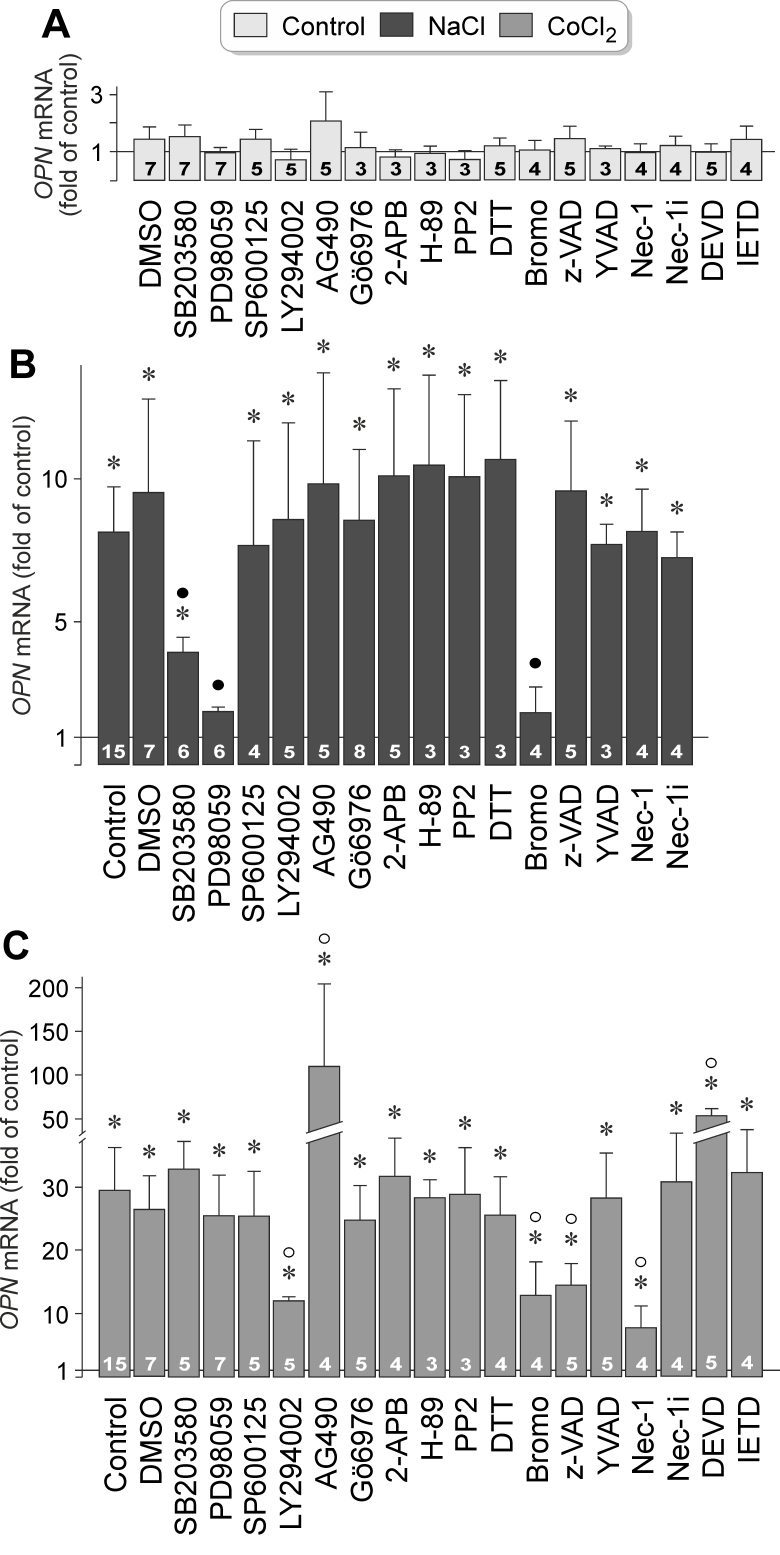
Extracellular hyperosmolarity and hypoxia induced delayed upregulation of OPN gene expression in RPE cells (Figure 1C,D). To investigate which signal transduction pathways are involved in mediating OPN gene expression, we tested different inhibitory agents in cells that were stimulated for 24 h with high (+ 100 mM) NaCl and hypoxia mimetic CoCl2, respectively. The agents tested did not alter statistically significantly (p>0.05) OPN gene expression under unstimulated control conditions (Figure 3A). As shown in Figure 3B, hyperosmotic OPN gene expression was statistically significantly (p<0.05) decreased by the inhibitor of p38 mitogen-activated protein kinase (p38 MAPK) activation, SB203580, the inhibitor of extracellular signal-regulated kinases 1 and 2 (ERK1/2) activation, PD98059, and the inhibitor of phospholipases A2 (PLA2), 4-bromophenacyl bromide. Many other inhibitors of various signaling molecules, including inhibitors of phosphatidylinositol-3 kinase (PI3K)-related kinases (LY294002) and c-Jun NH2-terminal kinase (JNK; SP600125), did not alter hyperoosmotic expression of the OPN gene (Figure 3B).
Figure 3.
Intracellular signaling involved in osmotic and hypoxic expression of the OPN gene in RPE cells. The level of OPN mRNA was determined with real-time RT–PCR in cells cultured for 24 h in iso- (control; A) and hyperosmotic (+ 100 mM NaCl) media (B), and in the presence of CoCl2 (150 µM; C), respectively. The following agents were tested: the inhibitor of p38 MAPK activation, SB203580 (10 µM), the inhibitor of ERK1/2 activation, PD98059 (20 µM), the JNK inhibitor SP600125 (10 µM), the inhibitor of PI3K-related kinases, LY294002 (5 µM), the JAK2 Inhibitor AG490 (10 µM), the inhibitor of protein kinase Cα/β, Gö6976 (1 µM), the inhibitor of store-operated calcium entry channels, inositol trisphosphate receptors, and transient receptor potential channels, 2-aminoethoxydiphenyl borate (2-APB; 100 μM), the protein kinase A inhibitor H-89 (1 µM), the inhibitor of Src tyrosine kinases, PP2 (100 nM), the reducing agent dithiothreitol (DTT; 3 mM), the PLA2 inhibitor 4-bromophenacyl bromide (Bromo; 300 µM), the pan-caspase inhibitor z-VAD (30 µM), the caspase-1 inhibitor Ac-YVAD-CMK (YVAD; 500 nM), the inhibitor of programmed necrosis, necrostatin-1 (Nec-1; 30 µM), inactive necrostatin-1 (Nec-1i; 30 µM) which was tested as negative control, the caspase-3 inhibitor Ac-DEVD-CHO (DEVD; 100 µM), and the caspase-8 inhibitor Ac-IETD-CHO (IETD; 100 µM). Vehicle control was made with dimethyl sulfoxide (DMSO; 1:1000). The numbers of independent experiments using cell lines from different donors are indicated in the bars. Statistically significant difference versus unstimulated control: *p<0.05. Statistically significant difference versus NaCl control: ●p<0.05. Statistically significant difference versus CoCl2 control: ○p<0.05.
Hypoxic activation of the OPN gene was statistically significantly (p<0.05) decreased by the inhibitor of PI3K-related kinases, LY294002, and the inhibitor of PLA2, 4-bromophenacyl bromide (Figure 3C). The inhibitor of Janus kinase 2 (JAK2), AG490, induced a statistically significant (p<0.05) increase in OPN gene expression under hypoxic conditions (Figure 3C). We also found that hypoxic expression of the OPN gene was statistically significantly (p<0.05) suppressed by a broad-spectrum caspase inhibitor, z-VAD, and an inhibitor of programmed necrosis, necrostatin-1 [53] (Figure 3C). Although the caspase-3-specific inhibitor Ac-DEVD-CMK increased the hypoxic activation of the OPN gene, caspase-1- and caspase-8-specific inhibitors, and the inactive derivative of necrostatin-1, had no effects (Figure 3C). The data indicate that hyperosmotic and hypoxic expression of the OPN gene in RPE cells is mediated by activation of different signal transduction pathways. Hyperosmotic expression of the OPN gene is mediated by activation of p38 MAPK and ERK1/2 signal transduction pathways, but hypoxic expression of the OPN gene is mediated by the PI3K signal transduction pathway and caspase-mediated, necrosis-related pathways. Activation of PLA2 is involved in mediating OPN gene expression under both conditions.
Extracellular signaling involved in osmotic and hypoxic expression of the OPN gene
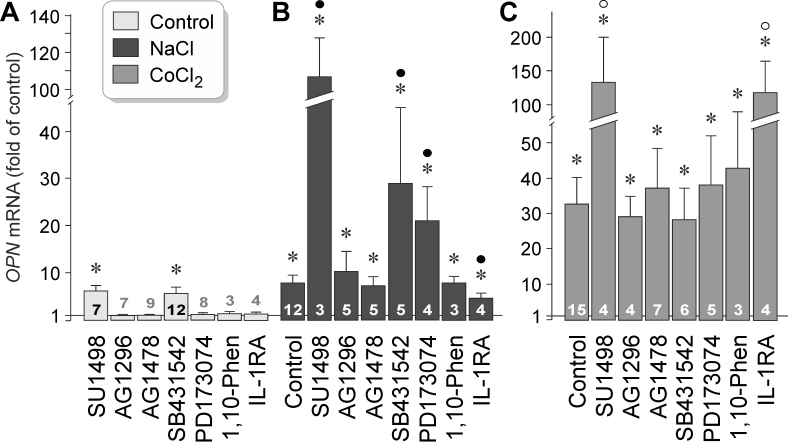
It was shown that extracellular hyperosmolarity induces release of various growth factors, such as VEGF, bFGF, and TGF-β1 from RPE cells [54,55]. To investigate whether autocrine or paracrine growth factor signaling is required for hyperosmotic and hypoxic expression of the OPN gene, we tested inhibitors of the VEGF receptor-2 (SU1498), the PDGF receptor tyrosine kinase (AG1296), the epidermal growth factor (EGF) receptor tyrosine kinase (AG1478), of TGF-β1 superfamily activin receptor-like kinase receptors (SB431542), and the FGF receptor kinase (PD173074), as well as an IL-1 receptor antagonist. As shown in Figure 4A, inhibitors of VEGF receptor-2 (SU1498) and TGF-β1 superfamily activin receptor-like kinase receptors (SB431542) induced statistically significant (p<0.05) increases in OPN gene expression under unstimulated control conditions. The data suggest that constitutive secretion of VEGF and TGF-β1 inhibits OPN gene expression in RPE cells. Hyperosmotic expression of the OPN gene was statistically significantly (p<0.05) decreased by the IL-1 receptor antagonist, and increased in the presence of inhibitors of VEGF receptor-2, TGF-β1 superfamily activin receptor-like kinase receptors, and the FGF receptor kinase (Figure 4B). Inhibitors of PDGF and EGF receptor tyrosine kinases (AG1296, AG1478) had no effects on hyperosmotic expression of the OPN gene (Figure 4B). In addition, the broad-spectrum metalloproteinase inhibitor 1,10-phenanthroline had no effect, suggesting that metalloproteinase-mediated release of growth factors from the extracellular matrix plays no role in hyperosmotic expression of the OPN gene. Under hypoxic conditions, the antagonist of VEGF receptor-2, SU1498, and the IL-1 receptor antagonist induced strong increases in OPN gene expression while the other inhibitory agents tested had no effects (Figure 4C). The data suggest that autocrine or paracrine VEGF signaling exerts an inhibitory effect on OPN gene expression under control, hyperosmotic, and hypoxic conditions. Autocrine or paracrine TGF-β1 signaling inhibits expression of the OPN gene under control and hyperosmotic conditions, while autocrine or paracrine IL-1 signaling stimulates hyperosmotic expression and inhibits hypoxic expression of the OPN gene.
Figure 4.
Receptor-mediated signaling involved in osmotic and hypoxic expression of the OPN gene in RPE cells. The level of OPN mRNA was determined with real-time reverse transcription (RT)–PCR in cells cultured for 24 h in iso- (control; A) and hyperosmotic (+ 100 mM NaCl) media (B), and in the presence of CoCl2 (150 µM; C), respectively. The following agents were tested: the inhibitor of the VEGF receptor-2, SU1498 (10 µM), the inhibitor of the PDGF receptor tyrosine kinase, AG1296 (10 µM), the inhibitor of the EGF receptor tyrosine kinase, AG1478 (600 nM), the inhibitor of TGF-β1 superfamily activin receptor-like kinase receptors, SB431542 (10 µM), the FGF receptor kinase inhibitor, PD173074 (500 nM), the broad-spectrum metalloproteinase inhibitor 1,10-phenanthroline (1,10-Phen; 10 µM), and a human recombinant IL-1 receptor antagonist (IL-1RA; 1 µg/ml). The numbers of independent experiments using cell lines from different donors are indicated in or above the bars. Statistically significant difference versus unstimulated control: *p<0.05. Statistically significant difference versus NaCl control: ●p<0.05. Statistically significant difference versus CoCl2 control: ○p<0.05.
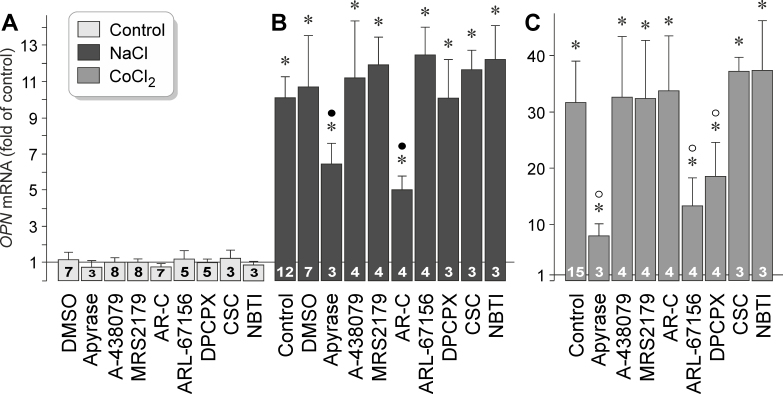
We found that extracellular ATP and adenosine stimulated OPN gene expression in RPE cells under control conditions (Figure 1G). To investigate whether purinergic receptor signaling is involved in mediating hyperosmotic and hypoxic expression of the OPN gene, we tested pharmacological receptor antagonists. The agents tested did not alter OPN gene expression under unstimulated control conditions (Figure 5A). We found that hyperosmotic and hypoxic expression of the OPN gene was statistically significantly (p<0.05) decreased in the presence of the ATP/ADP phosphohydrolase apyrase (Figure 5B,C). Because apyrase removes extracellular ATP and ADP by enzymatic hydrolyzation, the data suggest that the release of endogenous ATP is required for the full induction of OPN gene expression in RPE cells. Hyperosmotic expression of the OPN gene was also decreased by the selective inhibitor of P2Y2 receptors, AR-C 118925XX (Figure 5B). The following inhibitory agents had no effects on hyperosmotic OPN gene expression: the P2X7 receptor antagonist A-438079, the P2Y1 receptor antagonist MRS2179, the ecto-ATPase inhibitor ARL-67156, the adenosine A1 receptor antagonist DPCPX, the adenosine A2A receptor antagonist CSC, and the antagonist of nucleoside transporters, NBTI (Figure 5B). The data suggest that extracellular ATP and autocrine or paracrine P2Y2 receptor signaling is required for the full induction of OPN gene expression under hyperosmotic conditions.
Figure 5.
Purinergic receptor signaling involved in mediating osmotic and hypoxic expression of the OPN gene in RPE cells. The level of OPN mRNA was determined with real-time reverse transcription (RT)–PCR in cells cultured for 24 h in iso- (control; A) and hyperosmotic (+ 100 mM NaCl) media (B), and in the presence of CoCl2 (150 µM; C), respectively. The following agents were tested: the ATP/ADP phosphohydrolase apyrase (10 U/ml), the P2X7 receptor antagonist A-438079 (50 nM), the P2Y1 receptor antagonist MRS2179 (30 µM), the P2Y2 receptor antagonist AR-C 118925XX (AR-C; 10 µM), the ecto-ATPase inhibitor ARL-67156 (50 µM), the adenosine A1 receptor antagonist DPCPX (50 nM), the adenosine A2A receptor antagonist CSC (200 nM), and the antagonist of nucleoside transporters, NBTI (10 µM). The numbers of independent experiments using cell lines from different donors are indicated in or above the bars. Statistically significant difference versus unstimulated control: *p<0.05. Statistically significant difference versus NaCl control: ●p<0.05. Statistically significant difference versus CoCl2 control: ○p<0.05.
Hypoxic expression of the OPN gene was statistically significantly (p<0.05) decreased by ATP/ADP phosphohydrolase apyrase, the ecto-ATPase inhibitor ARL-67156 (which blocks the conversion of ATP to ADP/AMP), and the antagonist of adenosine A1 receptors, DPCPX (Figure 5C). All other inhibitory agents tested had no effects. The data suggest that the release of endogenous ATP, extracellular degradation of ATP, and autocrine or paracrine activation of A1 receptors by adenosine are involved in mediating the hypoxic expression of the OPN gene in RPE cells.
Transcription factor activities involved in hyperosmotic expression of the OPN gene
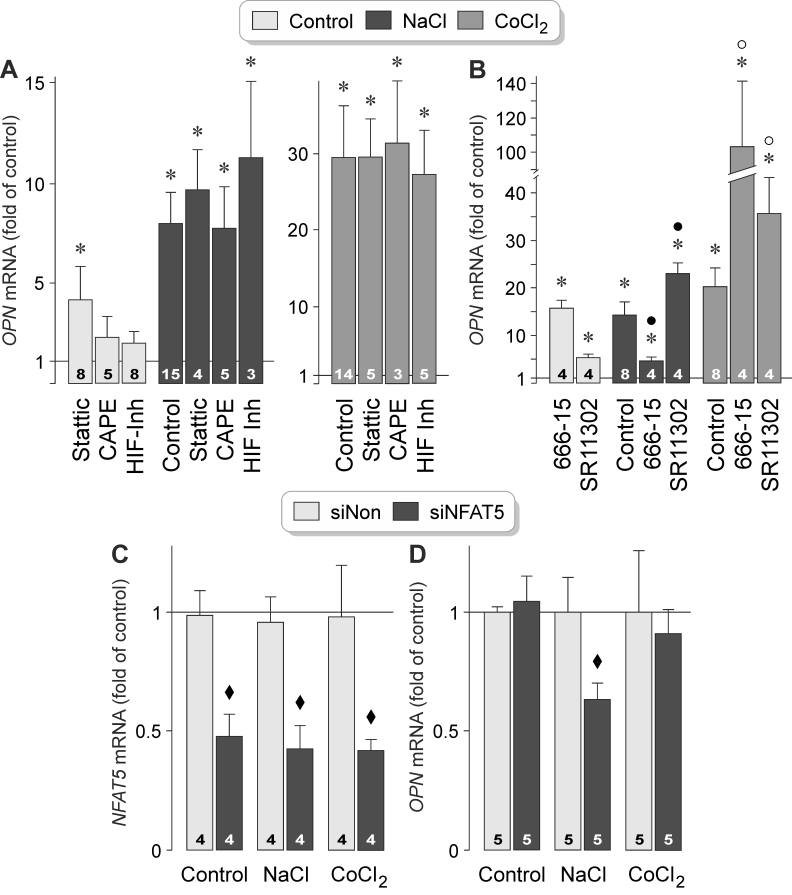
Hyperosmotic stress was shown to induce expression and activation of various transcription factors in RPE cells, including HIF-1α, nuclear factor-κB (NF-κB), and NFAT5 [54]. To investigate which transcription factors mediate hyperosmotic and hypoxic expression of the OPN gene, we tested inhibitory agents in cells stimulated with high NaCl or the hypoxia mimetic CoCl2. As shown in Figure 6A, hyperosmotic and hypoxic expression of the OPN gene remained unaltered in the presence of the inhibitor of signal transducer and activator of transcription 3 (STAT3), Stattic [56], the NF-κB inhibitor CAPE [57], and a HIF-1 inhibitor [58]. Hyperosmotic expression of the OPN gene was statistically significantly (p<0.05) decreased by the CREB inhibitor 666–15, and increased by the AP-1 inhibitor SR11302 (Figure 6B). Both inhibitors increased statistically significantly (p<0.05) hypoxic expression of the OPN gene (Figure 6B). The data suggest that hyperosmotic expression of the OPN gene in RPE cells is (in part) mediated by the transcriptional activity of CREB while the transcriptional activity of AP-1 inhibits hyperosmotic OPN gene expression. Hypoxic expression of the OPN gene is inhibited by the transcriptional activities of CREB and AP-1. Stattic, 666–15, SR11302 induced statistically significant (p<0.05) increases in OPN gene expression under unstimulated control conditions (Figure 6A,B), suggesting that the transcriptional activities of STAT3, AP-1, and CREB inhibit constitutive expression of the OPN gene in RPE cells.
Figure 6.
The transcriptional activities of CREB and NFAT5 contribute to induction of osmotic, but not hypoxic, expression of the OPN gene in RPE cells. The level of OPN mRNA was determined with real-time reverse transcription (RT)–PCR in cells cultured for 24 h in iso- (control) and hyperosmotic (+ 100 mM NaCl) media, and in the presence of CoCl2 (150 µM), respectively. A: The following agents were tested: the STAT3 inhibitor Stattic (1 µM), the NF-κB inhibitor CAPE (5 µM), and a HIF-1 inhibitor (HIF Inh; 5 µM). B: Effects of the CREB inhibitor 666–15 (250 nM) and the AP-1 inhibitor SR11302 (5 µM) on the expression of the OPN gene. C, D: Effects of NFAT5 siRNA (siNFAT5; 5 nM) and nontargeted scrambled siRNA (siNon; 5 nM) on the levels of the NFAT5 mRNA (C) and OPN mRNA (D). The numbers of independent experiments using cell lines from different donors are indicated in the bars. Statistically significant difference versus unstimulated control: *p<0.05. Statistically significant difference versus NaCl control: ●p<0.05. Statistically significant difference versus CoCl2 control: ○p<0.05. Statistically significant difference versus nontargeted siRNA: ♦p<0.05.
To investigate whether the activity of the transcription factor NFAT5, which is critically involved in cellular adaptation to hyperosmotic stress in various cell systems [59], contributes to expression of the OPN gene, we knocked down NFAT5 with siRNA. Transfection with NFAT5 siRNA reduced the level of NFAT5 (Gene ID10725; OMIM 604708) gene transcripts by 50‒60% in cells cultured in control and hyperosmotic media, and in the presence of CoCl2, respectively (Figure 6C). We found no differences in the level of OPN gene transcripts between cells transfected with NFAT5 siRNA and nontargeted scrambled siRNA cultured under unstimulated control and hypoxic conditions (Figure 6D). Under hyperosmotic conditions, cells transfected with NFAT5 siRNA displayed a statistically significantly (p<0.05) lower level of OPN mRNA compared to cells transfected with nontargeted siRNA (Figure 6D). The data suggest that hyperosmotic, but not the hypoxic, expression of the OPN gene is mediated by the transcriptional activities of CREB and NFAT5.
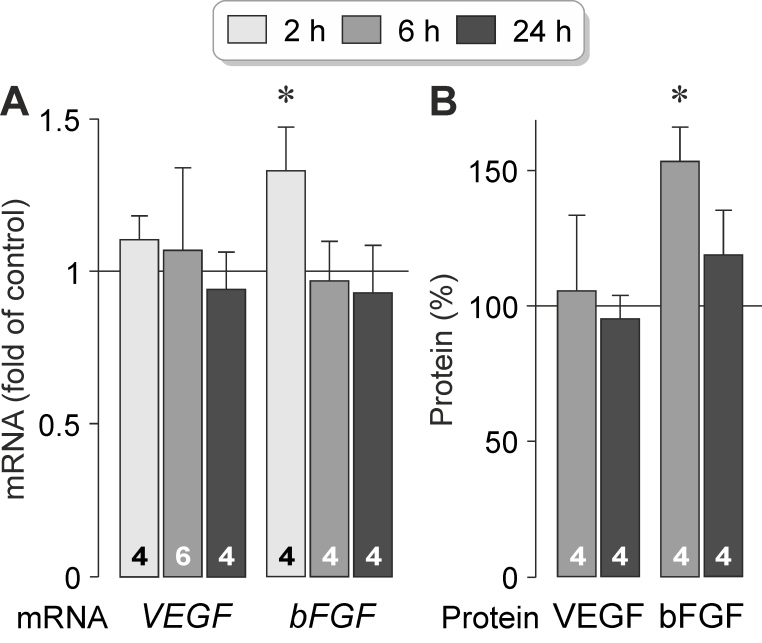
OPN stimulates the expression and secretion of bFGF
To investigate whether OPN influences the production of VEGF and bFGF in RPE cells, we stimulated cultured cells with exogenous OPN. As shown in Figure 7A, administration of OPN increased statistically significantly (p<0.05) expression of the bFGF (Gene ID: 2247; OMIM: 134920) gene after 2 h of stimulation and had no effect on expression of the VEGF (Gene ID: 7422; OMIM: 192240) gene. Exogenous OPN also stimulated statistically significantly (p<0.05) secretion of the bFGF protein from RPE cells after 6 h of stimulation and had no effect on secretion of VEGF (Figure 7B). The data indicate that exogenous OPN stimulates production and secretion of bFGF, but not VEGF, in RPE cells.
Figure 7.
Exogenous OPN stimulates expression and secretion of bFGF in RPE cells. A: Effects of exogenous OPN (10 ng/ml) on expression of the VEGF and bFGF genes. B: Effects of exogenous OPN (10 ng/ml) on the secretion of the VEGF-A165 and bFGF proteins. The data were obtained in cells stimulated for 2, 6, and 24 h, as indicated in the panels of the bars. The mRNA levels were determined with real-time reverse transcription (RT)–PCR and are expressed as folds of unstimulated control. Protein levels in the cultured media were determined with enzyme-linked immunosorbent assay (ELISA) and are expressed as percent of unstimulated control (100%). The numbers of independent experiments using cell lines from different donors are indicated in the bars. Statistically significant difference versus unstimulated control: *p<0.05.
Discussion
OPN is a neuroprotective factor in the retina which supports the survival of retinal ganglion cells and photoreceptors [8,25]. It was shown that OPN is expressed by ganglion cells, microglia, and Müller glia in the retina [8,19‒23]. However, there is little knowledge regarding production of OPN in the RPE that normally mediates the support of photoreceptor integrity and function [32]. The aim of the present study was to investigate whether RPE cells express and secrete OPN. We found that acutely isolated and cultured RPE cells contain OPN mRNA (Figure 1A), and that cultured RPE cells display OPN immunoreactivity (Figure 2A). The level of OPN mRNA was strongly decreased in the cultured cells compared to the acutely isolated cells (Figure 1B). The reason for downregulation of OPN gene expression in cultured cells compared to acutely isolated cells is unclear. Because hypoxia stimulates OPN gene expression in cultured cells (Figure 1C), it could be that acutely isolated cells had post-mortem hypoxia. It could also be that the cellular environment in situ, for example, the extracellular matrix, may stimulate expression of the OPN gene in RPE cells.
It was shown that expression of OPN in the neuroretina is upregulated under various pathological conditions, such as ischemia, glaucoma, and retinal light damage [10,24]. We found that expression of the OPN gene in RPE cells increased under hypoxic conditions (Figure 1C). Expression of the OPN gene also increased under hyperosmotic conditions (Figure 1D). Extracellular hyperosmolarity, a condition that causes hypertension in vivo [42,43], was suggested to be implicated in the development of AMD [46]. We found that secretion of OPN from RPE cells is strongly induced by high extracellular NaCl but not by extracellular hyperosmolarity induced with the addition of sucrose (Figure 2C). The data indicated that high extracellular osmolarity induces expression of the OPN gene; secretion of OPN is induced by high extracellular NaCl. Hypoxia induces moderate secretion of OPN from RPE cells (Figure 2C). In addition, expression of the OPN gene is stimulated by (long-term) administration of bFGF (Figure 1F).
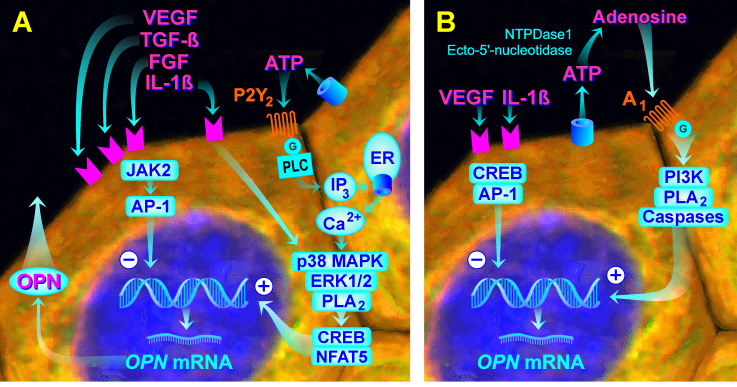
Figure 8A,B show a schematic summary of signal transduction pathways found in the present study to be implicated in regulation of OPN gene expression in cultured RPE cells under hyperosmotic and hypoxic conditions. Expression of the OPN gene is mediated by different intracellular and receptor-mediated signal transduction pathways under both conditions. It was shown that high extracellular NaCl induces phosphorylation of various key intracellular signal transduction molecules, including p38 MAPK and ERK1/2 in RPE cells [54]. Hyperosmotic expression of the OPN gene is mediated by the p38 MAPK and ERK1/2 signal transduction pathways (Figure 3B), and the transcriptional activities of CREB and NFAT5 (Figure 6B,D). Hypoxic expression of the OPN gene is mediated by the PI3K signal transduction pathway and caspase-mediated, necrosis-related pathways (Figure 3C). The activity of PLA2 is involved in mediating OPN gene expression under both conditions (Figure 3B,C).
Figure 8.
Schematic summary of signal transduction pathways that are implicated in regulation of expression of the OPN gene in cultured RPE cells under hyperosmotic and hypoxic conditions. A: High NaCl-induced extracellular hyperosmolarity induces release of VEGF, TGF-β1, FGF, IL-1β, and ATP from the cells. The first three factors inhibit, and IL-1β and ATP stimulate, OPN gene expression. The effect of ATP is mediated in part by autocrine or paracrine activation of the P2Y2 receptors. Hyperosmotic OPN gene expression is mediated by the activities of p38 MAPK, ERK1/2, and PLA2, and the transcriptional activities of CREB and NFAT5. Inhibitory effects on hyperosmotic expression of the OPN gene are mediated (in part) by JAK2 and AP-1. High NaCl, but not extracellular hyperosmolarity, also stimulates the secretion of the OPN protein from RPE cells. B: Hypoxia induces release of VEGF, IL-1β, and ATP from the cells. VEGF and IL-1β inhibit OPN gene expression. ATP is extracellularly degraded to adenosine which activates A1 receptors resulting in stimulation of OPN gene expression. Hypoxic expression of the OPN gene is mediated (in part) by the activities of PI3K, PLA2, and caspases. Inhibitory effects on hypoxic expression of the OPN gene are (in part) mediated by CREB and AP-1.
Autocrine or paracrine P2Y2 receptor signaling induced by extracellular ATP is required for full hyperosmotic expression of the OPN gene (Figure 5B). However, hypoxic expression of the OPN gene is at least in part induced by autocrine or paracrine activation of adenosine A1 receptors (Figure 5C). Extracellular adenosine can be provided by the release from cells via equilibrative nucleoside transporters or by extracellular formation through enzymatic dephosphorylation of ATP [50]. The inhibitory effects of the ATP/ADP phosphohydrolase apyrase and the ecto-ATPase inhibitor ARL-67156, and the absence of an effect of the antagonist of nucleoside transporters, NBTI (Figure 5C), suggest that adenosine, which activates A1 receptors, is provided by extracellular degradation of ATP and not by transporter-mediated release from the cells. The data indicated that hypoxia and extracellular hyperosmolarity trigger the release of the endogenous danger signal molecule ATP from RPE cells which induces receptor-mediated upregulation of OPN gene expression. The result that the ATP/ADP phosphohydrolase apyrase also decreased hyperosmotic expression of the OPN gene (Figure 5B) suggest that (in addition to ATP which activates P2Y2 receptors) degradation products of ATP, which activate purinergic receptor subtypes not investigated in this study, may be involved in mediating the full expression of the OPN gene. The involvement of different receptor subtypes in mediating the OPN gene under hyperosmotic and CoCl2-stimulated conditions may be explained by the partly different intracellular signal transduction pathways activated under these conditions (Figure 3B,C). Further research is required to determine the contributions of different purinergic receptor subtypes in mediating OPN gene expression in RPE cells under various conditions.
We found that hypoxic expression of the OPN gene in RPE cells is (in part) mediated by activation of caspase-dependent, necrosis-related pathways (Figure 3C). Although a pan-caspase inhibitor decreased hypoxic expression of the OPN gene, agents that specifically inhibited various caspase types did not suppress expression (Figure 3C). The type of caspase involved in mediating hypoxic expression of the OPN gene remains to be determined in future investigations. It was shown that CoCl2-induced chemical hypoxia is not associated with an alteration in the RPE cell viability [60]. This suggests that the necrosis-related pathways activated by hypoxia mediate intracellular signal transduction, but do not induce necrosis. We also found that inhibition of IL-1 receptors resulted in differing regulation of OPN gene expression under hyperosmotic and hypoxic conditions (Figure 4B,C). This suggests that both conditions induce the release of IL-1β from the cells. However, because a caspase-1-specific inhibitor had no effects (Figure 3B,C), production of IL-1β is not involved in mediating OPN gene expression.
Exogenous OPN stimulated the gene expression and secretion of bFGF, but not of VEGF, in RPE cells (Figure 7A,B). bFGF is a major neuroprotective factor in the retina [61] that protects photoreceptors from death via increased phosphorylation of CREB and subsequent upregulation of its prosurvival transcriptional targets [62]. Because (long-term) administration of bFGF stimulates the expression of OPN (Figure 1F), there is positive feedback regulation between bFGF and OPN in RPE cells that may contribute to the inhibition of photoreceptor degeneration under pathological conditions.
Inhibitors of various growth factor receptors increased the expression of the OPN gene in RPE cells under different conditions (Figure 4A‒C). In particular, the inhibitor of VEGF receptor-2, SU1498, induced a strong increase in OPN gene expression under hyperosmotic and hypoxic conditions (Figure 4B,C). The data suggest that autocrine or paracrine VEGF signaling exerts an inhibitory effect on OPN gene expression in RPE cells. VEGF is the most relevant angiogenic factor induced by retinal hypoxia, for example, in neovascular AMD [63]. Photoreceptor degeneration is a key pathological event in end-stage AMD [33]. The data suggest that in ischemic-hypoxic retinal disorders, VEGF may contribute to photoreceptor degeneration by downregulating neuroprotective factors in RPE cells. Anti-VEGF agents are clinically used in the treatment of neovascular AMD [64]. It is conceivable but remains to be proven that the clinical effects of anti-VEGF agents include induction of the neuroprotective factor OPN in RPE cells.
We found statistically significant effects of high NaCl on the expression of the OPN gene when more than 30 mM NaCl were added to the culture medium (Figure 1E). It is generally accepted that the highest pathological blood osmolarity in human subjects is around 360 mOsm/kg which can be achieved with the addition of 40 mM NaCl to the culture medium [65,66]. However, less well appreciated is that the local extracellular NaCl concentration in the interstitium may be considerably higher (up to 250 mM) than the plasma concentration of NaCl (about 140 mM) [67,68]. Therefore, the present results may have relevance for in vivo conditions.
RPE cells may secrete OPN under pathological conditions (Figure 2C), and OPN induces the expression and secretion of bFGF (Figure 7A,B). The data suggested that RPE cell-derived OPN may contribute to the prosurvival effect of OPN on photoreceptors previously described to be mediated by Müller cells [8]. However, it is unclear whether administration of exogenous OPN may represent a viable approach to protect photoreceptors from degeneration, for example, in AMD, because OPN is also a proinflammatory and angiogenic signaling molecule [10,12,14,16‒18]. Therefore, separation of the cell protective effect from the proinflammatory and angiogenic effects of OPN, for example, by the development of small OPN-derived peptides, might be helpful. Various subdomains of OPN were described that are involved in mediating different functions or bind to different integrins [69‒71]. However, further research is required to reveal which subdomains of OPN are required for its prosurvival effect on photoreceptors.
Acknowledgments
The authors thank Ute Weinbrecht for excellent technical assistance.
References
- 1.Bringmann A, Iandiev I, Pannicke T, Wurm A, Hollborn M, Wiedemann P, Osborne NN, Reichenbach A. Cellular signaling and factors involved in Müller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009;28:423–51. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Wen R, Cheng T, Li Y, Cao W, Steinberg RH. α2-Adrenergic agonists induce basic fibroblast growth factor expression in photoreceptors in vivo and ameliorate light damage. J Neurosci. 1996;16:5986–92. doi: 10.1523/JNEUROSCI.16-19-05986.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsushima M, Yamada H, Yamamoto C, Miyashiro M, Ogata N, Uyama M. Expression of basic fibroblast growth factor and fibroblast growth factor receptor 1 in the experimental retinal vein occlusion model. Nippon Ganka Gakkai Zasshi. 1997;101:564–70. [PubMed] [Google Scholar]
- 4.Wen R, Song Y, Cheng T, Matthes MT, Yasumura D, LaVail MM, Steinberg RH. Injury-induced upregulation of bFGF and CNTF mRNAs in the rat retina. J Neurosci. 1995;15:7377–85. doi: 10.1523/JNEUROSCI.15-11-07377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Müller cells and other cells of the inner retina, but not photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:927–36. [PubMed] [Google Scholar]
- 6.Harada C, Harada T, Quah HMA, Maekawa F, Yoshida K, Ohno S, Wada K, Parada LF, Tanaka K. Potential role of glial cell line-derived neurotrophic factor receptors in Müller glial cells during light-induced retinal degeneration. Neuroscience. 2003;122:229–35. doi: 10.1016/s0306-4522(03)00599-2. [DOI] [PubMed] [Google Scholar]
- 7.Hauck SM, Kinkl N, Deeg CA, Swiatek-de Lange M, Schöffmann S, Ueffing M. GDNF family ligands trigger indirect neuroprotective signaling in retinal glial cells. Mol Cell Biol. 2006;26:2746–57. doi: 10.1128/MCB.26.7.2746-2757.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Río P, Irmler M, Arango-González B, Favor J, Bobe C, Bartsch U, Vecino E, Beckers J, Hauck SM, Ueffing M. GDNF-induced osteopontin from Müller glial cells promotes photoreceptor survival in the Pde6brd1 mouse model of retinal degeneration. Glia. 2011;59:821–32. doi: 10.1002/glia.21155. [DOI] [PubMed] [Google Scholar]
- 9.Giachelli CM, Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615–22. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- 10.Takagi H, Suzuma K, Otani A, Oh H, Koyama S, Ohashi H, Watanabe D, Ojima T, Suganami E, Honda Y. Role of vitronectin receptor-type integrins and osteopontin in ischemia-induced retinal neovascularization. Jpn J Ophthalmol. 2002;46:270–8. doi: 10.1016/s0021-5155(02)00482-3. [DOI] [PubMed] [Google Scholar]
- 11.Pardo A, Gibson K, Cisneros J, Richards TJ, Yang Y, Becerril C, Yousem S, Herrera I, Ruiz V, Selman M, Kaminski N. Up-regulation and profibrotic role of osteopontin in human idiopathic pulmonary fibrosis. PLoS Med 2005; 2:0891–0903. [DOI] [PMC free article] [PubMed]
- 12.Naldini A, Leali D, Pucci A, Morena E, Carraro F, Nico B, Ribatti D, Presta M. Cutting edge: IL-1β mediates the proangiogenic activity of osteopontin-activated human monocytes. J Immunol. 2006;177:4267–70. doi: 10.4049/jimmunol.177.7.4267. [DOI] [PubMed] [Google Scholar]
- 13.Cui R, Takahashi F, Ohashi R, Gu T, Yoshioka M, Nishio K, Ohe Y, Tominaga S, Takagi Y, Sasaki S, Fukuchi Y, Takahashi K. Abrogation of the interaction between osteopontin and αvβ3 integrin reduces tumor growth of human lung cancer cells in mice. Lung Cancer. 2007;57:302–10. doi: 10.1016/j.lungcan.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 14.Du XL, Jiang T, Sheng XG, Gao R, Li QS. Inhibition of osteopontin suppresses in vitro and in vivo angiogenesis in endometrial cancer. Gynecol Oncol. 2009;115:371–6. doi: 10.1016/j.ygyno.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 15.Kohan M, Breuer R, Berkman N. Osteopontin induces airway remodeling and lung fibroblast activation in a murine model of asthma. Am J Respir Cell Mol Biol. 2009;41:290–6. doi: 10.1165/rcmb.2008-0307OC. [DOI] [PubMed] [Google Scholar]
- 16.Dai J, Peng L, Fan K, Wang H, Wei R, Ji G, Cai J, Lu B, Li B, Zhang D, Kang Y, Tan M, Qian W, Guo Y. Osteopontin induces angiogenesis through activation of PI3K/AKT and ERK1/2 in endothelial cells. Oncogene. 2009;28:3412–22. doi: 10.1038/onc.2009.189. [DOI] [PubMed] [Google Scholar]
- 17.Sabo-Attwood T, Ramos-Nino ME, Eugenia-Ariza M, Macpherson MB, Butnor KJ, Vacek PC, McGee SP, Clark JC, Steele C, Mossman BT. Osteopontin modulates inflammation, mucin production, and gene expression signatures after inhalation of asbestos in a murine model of fibrosis. Am J Pathol. 2011;178:1975–85. doi: 10.1016/j.ajpath.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Yan W, Lu X, Qian C, Zhang J, Li P, Shi L, Zhao P, Fu Z, Pu P, Kang C, Jiang T, Liu N, You Y. Overexpression of osteopontin induces angiogenesis of endothelial progenitor cells via the αvβ3/PI3K/AKT/eNOS/NO signaling pathway in glioma cells. Eur J Cell Biol. 2011;90:642–8. doi: 10.1016/j.ejcb.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Ju WK, Kim KY, Cha JH, Kim IB, Lee MY, Oh SJ, Chung JW, Chun MH. Ganglion cells of the rat retina show osteopontin-like immunoreactivity. Brain Res. 2000;852:217–20. doi: 10.1016/s0006-8993(99)02140-x. [DOI] [PubMed] [Google Scholar]
- 20.Hikita ST, Vistica BP, Jones HR, Keswani JR, Watson MM, Ericson VR, Ayoub GS, Gery I, Clegg DO. Osteopontin is proinflammatory in experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2006;47:4435–43. doi: 10.1167/iovs.06-0064. [DOI] [PubMed] [Google Scholar]
- 21.Chidlow G, Wood JP, Manavis J, Osborne NN, Casson RJ. Expression of osteopontin in the rat retina: effects of excitotoxic and ischemic injuries. Invest Ophthalmol Vis Sci. 2008;49:762–71. doi: 10.1167/iovs.07-0726. [DOI] [PubMed] [Google Scholar]
- 22.Deeg CA, Eberhardt C, Hofmaier F, Amann B, Hauck SM. Osteopontin and fibronectin levels are decreased in vitreous of autoimmune uveitis and retinal expression of both proteins indicates ECM re-modeling. PLoS One. 2011;6:e27674. doi: 10.1371/journal.pone.0027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wahl V, Vogler S, Grosche A, Pannicke T, Ueffing M, Wiedemann P, Reichenbach A, Hauck SM, Bringmann A. Osteopontin inhibits osmotic swelling of retinal glial (Müller) cells by inducing release of VEGF. Neuroscience. 2013;246:59–72. doi: 10.1016/j.neuroscience.2013.04.045. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Wu W, Dentchev T, Zeng Y, Wang J, Tsui I, Tobias JW, Bennett J, Baldwin D, Dunaief JL. Light damage induced changes in mouse retinal gene expression. Exp Eye Res. 2004;79:239–47. doi: 10.1016/j.exer.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Birke MT, Neumann C, Birke K, Kremers J, Scholz M. Changes of osteopontin in the aqueous humor of the DBA2/J glaucoma model correlated with optic nerve and RGC degenerations. Invest Ophthalmol Vis Sci. 2010;51:5759–67. doi: 10.1167/iovs.10-5558. [DOI] [PubMed] [Google Scholar]
- 26.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1). Science. 1996;271:509–12. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 27.Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–61. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaitin MH, Ankrum MT, Wortham HS. Distribution of CD44 in the retina during development and the rds degeneration. Brain Res Dev Brain Res. 1996;94:92–8. doi: 10.1016/0165-3806(96)00046-6. [DOI] [PubMed] [Google Scholar]
- 29.Kuhrt H, Härtig W, Grimm D, Faude F, Kasper M, Reichenbach A. Changes in CD44 and ApoE immunoreactivities due to retinal pathology of man and rat. J Hirnforsch. 1997;38:223–9. [PubMed] [Google Scholar]
- 30.Chaitin MH, Brun-Zinkernagel AM. Immunolocalization of CD44 in the dystrophic rat retina. Exp Eye Res. 1998;67:283–92. doi: 10.1006/exer.1998.0510. [DOI] [PubMed] [Google Scholar]
- 31.Krishnamoorthy R, Agarwal N, Chaitin MH. Upregulation of CD44 expression in the retina during the rds degeneration. Brain Res Mol Brain Res. 2000;77:125–30. doi: 10.1016/s0169-328x(00)00035-8. [DOI] [PubMed] [Google Scholar]
- 32.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–81. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 33.Roth F, Bindewald A, Holz FG. Keypathophysiologic pathways in age-related macular disease. Graefes Arch Clin Exp Ophthalmol. 2004;242:710–6. doi: 10.1007/s00417-004-0976-x. [DOI] [PubMed] [Google Scholar]
- 34.Kaarniranta K, Sinha D, Blasiak J, Kauppinen A, Vereb Z, Salminen A, Boulton ME, Petrovski G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9:973–84. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrington DA, Sinha D, Kaarniranta K. Defects in retinal pigment epithelial cell proteolysis and the pathology associated with age-related macular degeneration. Prog Retin Eye Res. 2016;51:69–89. doi: 10.1016/j.preteyeres.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlingemann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004;242:91–101. doi: 10.1007/s00417-003-0828-0. [DOI] [PubMed] [Google Scholar]
- 37.Dallinger S, Findl O, Strenn K, Eichler HG, Wolzt M, Schmetterer L. Age dependence of choroidal blood flow. J Am Geriatr Soc. 1998;46:484–7. doi: 10.1111/j.1532-5415.1998.tb02471.x. [DOI] [PubMed] [Google Scholar]
- 38.Ehrlich R, Kheradiya NS, Winston DM, Moore DB, Wirostko B, Harris A. Age-related ocular vascular changes. Graefes Arch Clin Exp Ophthalmol. 2009;247:583–91. doi: 10.1007/s00417-008-1018-x. [DOI] [PubMed] [Google Scholar]
- 39.Roehlecke C, Valtink M, Frenzel A, Goetze D, Knels L, Morawietz H, Funk RH. Stress responses of human retinal pigment epithelial cells to glyoxal. Graefes Arch Clin Exp Ophthalmol. 2016;254:2361–72. doi: 10.1007/s00417-016-3463-2. [DOI] [PubMed] [Google Scholar]
- 40.Sperduto RD, Hiller R. Systemic hypertension and age-related maculopathy in the Framingham Study. Arch Ophthalmol. 1986;104:216–9. doi: 10.1001/archopht.1986.01050140070022. [DOI] [PubMed] [Google Scholar]
- 41.Klein R, Klein BE, Tomany SC, Cruickshanks KJ. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 2003;110:1273–80. doi: 10.1016/S0161-6420(03)00599-2. [DOI] [PubMed] [Google Scholar]
- 42.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–56. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 43.He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA. Plasma sodium: ignored and underestimated. Hypertension. 2005;45:98–102. doi: 10.1161/01.HYP.0000149431.79450.a2. [DOI] [PubMed] [Google Scholar]
- 44.Hyman L, Schachat AP, He Q, Leske MC. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch Ophthalmol. 2000;118:351–8. doi: 10.1001/archopht.118.3.351. [DOI] [PubMed] [Google Scholar]
- 45.Van Leeuwen R, Tomany SC, Wang JJ, Klein R, Mitchell P, Hofman A, Klein BE, Vingerling JR, Cumming RG, de Jong PT. Is medication use associated with the incidence of early age-related maculopathy? Pooled findings from 3 continents. Ophthalmology. 2004;111:1169–75. doi: 10.1016/j.ophtha.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 46.Bringmann A, Hollborn M, Kohen L, Wiedemann P. Intake of dietary salt and drinking water: Implications for the development of age-related macular degeneration. Mol Vis. 2016;22:1437–54. [PMC free article] [PubMed] [Google Scholar]
- 47.Chen R, Hollborn M, Grosche A, Reichenbach A, Wiedemann P, Bringmann A, Kohen L. Effects of the vegetable polyphenols epigallocatechin-3-gallate, luteolin, apigenin, myricetin, quercetin, and cyanidin in retinal pigment epithelial cells. Mol Vis. 2014;20:242–58. [PMC free article] [PubMed] [Google Scholar]
- 48.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 49.An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature. 1998;392:405–8. doi: 10.1038/32925. [DOI] [PubMed] [Google Scholar]
- 50.Reichenbach A, Bringmann A. Purinergic signaling in retinal degeneration and regeneration. Neuropharmacology. 2016;104:194–211. doi: 10.1016/j.neuropharm.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Housley GD, Bringmann A, Reichenbach A. Purinergic signaling in special senses. Trends Neurosci. 2009;32:128–41. doi: 10.1016/j.tins.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Prager P, Hollborn M, Steffen A, Wiedemann P, Kohen L, Bringmann A. P2Y1 receptor signaling contributes to high salt-induced priming of the NLRP3 inflammasome in retinal pigment epithelial cells. PLoS One. 2016;11:e0165653. doi: 10.1371/journal.pone.0165653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 54.Hollborn M, Vogler S, Reichenbach A, Wiedemann P, Bringmann A, Kohen L. Regulation of the hyperosmotic induction of aquaporin 5 and VEGF in retinal pigment epithelial cells: Involvement of NFAT5. Mol Vis. 2015;21:360–77. [PMC free article] [PubMed] [Google Scholar]
- 55.Veltmann M, Hollborn M, Reichenbach A, Wiedemann P, Kohen L, Bringmann A. Osmotic induction of angiogenic growth factor expression in human retinal pigment epithelial cells. PLoS One. 2016;11:e0147312. doi: 10.1371/journal.pone.0147312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schust J, Sperl B, Hollis A, Mayer TU, Berg T. Stattic: A small-molecule inhibitor of STAT3 activation and dimerization. Chem Biol. 2006;13:1235–42. doi: 10.1016/j.chembiol.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-κB. Proc Natl Acad Sci USA. 1996;93:9090–5. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee K, Lee JH, Boovanahalli SK, Jin Y, Lee M, Jin X, Kim JH, Hong YS, Lee JJ. (Aryloxyacetylamino)benzoic acid analogues: A new class of hypoxia-inducible factor-1 inhibitors. J Med Chem. 2007;50:1675–84. doi: 10.1021/jm0610292. [DOI] [PubMed] [Google Scholar]
- 59.Cheung CY, Ko BC. NFAT5 in cellular adaptation to hypertonic stress ‒ regulations and functional significance. J Mol Signal. 2013;8:5. doi: 10.1186/1750-2187-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Doktor F, Prager P, Wiedemann P, Kohen L, Bringmann A, Hollborn M. Hypoxic expression of NLRP3 and VEGF in cultured retinal pigment epithelial cells: Contribution of P2Y2 receptor signaling. Purinergic Signal. 2018;14:471–84. doi: 10.1007/s11302-018-9631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faktorovich EG, Steinberg RH, Yasumura D, Matthes MT, LaVail MM. Photoreceptor degeneration in inherited retinal dystrophy delayed by basic fibroblast growth factor. Nature. 1990;347:83–6. doi: 10.1038/347083a0. [DOI] [PubMed] [Google Scholar]
- 62.O’Driscoll C, O’Connor J, O’Brien CJ, Cotter TG. Basic fibroblast growth factor-induced protection from light damage in the mouse retina in vivo. J Neurochem. 2008;105:524–36. doi: 10.1111/j.1471-4159.2007.05189.x. [DOI] [PubMed] [Google Scholar]
- 63.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 64.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379:1728–38. doi: 10.1016/S0140-6736(12)60282-7. [DOI] [PubMed] [Google Scholar]
- 65.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–22. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496:513–7. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci USA. 2004;101:10673–8. doi: 10.1073/pnas.0403139101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Müller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15:545–52. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 69.Lai CF, Seshadri V, Huang K, Shao JS, Cai J, Vattikuti R, Schumacher A, Loewy AP, Denhardt DT, Rittling SR, Towler DA. An osteopontin-NADPH oxidase signaling cascade promotes pro-matrix metalloproteinase 9 activation in aortic mesenchymal cells. Circ Res. 2006;98:1479–89. doi: 10.1161/01.RES.0000227550.00426.60. [DOI] [PubMed] [Google Scholar]
- 70.Kahles F, Findeisen HM, Bruemmer D. Osteopontin: A novel regulator at the cross roads of inflammation, obesity and diabetes. Mol Metab. 2014;3:384–93. doi: 10.1016/j.molmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li M, Wang L, Putnis CV. Energetic basis for inhibition of calcium phosphate biomineralization by osteopontin. J Phys Chem B. 2017;121:5968–76. doi: 10.1021/acs.jpcb.7b04163. [DOI] [PubMed] [Google Scholar]