Abstract
Wound healing remained an equation with multiple variables that experts in the medical field are trying to solve. The need to find an adjuvant that can quicken the healing process is increasing with every day, as longer wound healing times raise the risk of infections. Platelet-rich plasma is a promising tool promoting faster healing in a variety of wounds (thermal wounds, burn wounds, surgeries, etc.), as a series of studies present encouraging results in patients that received platelet-rich plasma treatment. The aim of this paper is to review and comment on the useful benefits and limitations of using platelet-rich plasma as an adjuvant strategy in wound healing, emphasizing on skin related wounds.
Keywords: Wound healing, platelet-rich plasma, skin wound, skin regeneration, clinical trials.
1. Introduction
Skin tissue regeneration and a fast regeneration are important aspects in achieving a proper wound heal, in order to avoid the risk of infections, bad scarring or death (in patients with pathologies that can affect the healing process). A quicker wound healing process also presents the advantages of being more cost effective, as the patient needs less nursing time.
The human skin is a three-layered organ: the epidermis (the surface of the skin), the dermis and the hypodermis (a subcutaneous adipose layer). The skin presents nerves, sensory corpuscles, vasculature and skin appendages, such as sebaceous glands, sweat glands and hair follicles. In the process of wound healing, the epidermis is the layer that is restored through the re-epithelialization process, while the dermis and the skin appendages help the re-epithelialization process through providing nutritional and mechanical support1.
The human epidermis consists of a stratified squamous epithelium that contains keratinocytes, melanocytes and no blood vessels. After an injury is inflicted, if the injured skin region fails to re-epithelialize, this leads to infections, losing the barrier function of the organ and even death. This urges the need to a rapid wound closure by the proliferation of epithelial cells in order to restore the barrier function that is critically important for survival2.
Currently, there are multiple strategies for skin tissue regeneration, such as split-thickness skin grafts, moisture dressing or the standard wound care. However, even if all these methods present valuable advantages, they also present disadvantages. For example, skin grafts can lead to the appearance of transplant rejection3, while moisture dressing is painful - not only applying it on the wound is hurtful, but it may sometimes also affect the nerve endings4.
Notably, a plethora of studies claim and appraise the beneficial effects of platelet-rich plasma (PRP) on cellular proliferation and tissue regeneration on the ground that its molecular components, such as growth factors or cytokines, play an important role in these processes. PRP represents the compound obtained through the separation of platelets from the other cellular populations present in a blood sample. PRP shows great promise in the field of skin wound healing as the molecules and cytokines found in PRP help stimulate collagen synthesis, extracellular matrix production and processes like angiogenesis or cellular proliferation.
The aim of this review is to highlight the benefits, efficacy and limitations of PRP use in wound healing.
2. Molecular factors correlated with wound healing timeline
Wound healing naturally occurs after an injury and is an evolutionary conserved and complex process that requires an abundance of specific cells, growth factors and cytokines. These molecules form a signaling network that modulate a series of cells, including endothelial cells, fibroblasts, keratinocytes and immune cells, which are essential in wound inflammation and healing as they secrete many of the cytokines and growth factors. In these circumstances, the above mentioned cellular populations proliferate, migrate and achieve tissue repair5. The importance of wound healing stems from the fact that an untreated wound or a wound that has received improper treatment may aggravate through infections, Marjolin’s ulcer (rare and aggressive type of skin cancer that grows from burns6), other health complications or even death. These situations inconvenience especially patients that are suffering from thalassemia, anemia or diabetes, whose wound heal with difficulty compared to healthy patients, or their wounds may not close at all, depending on the size and depth of the wound.
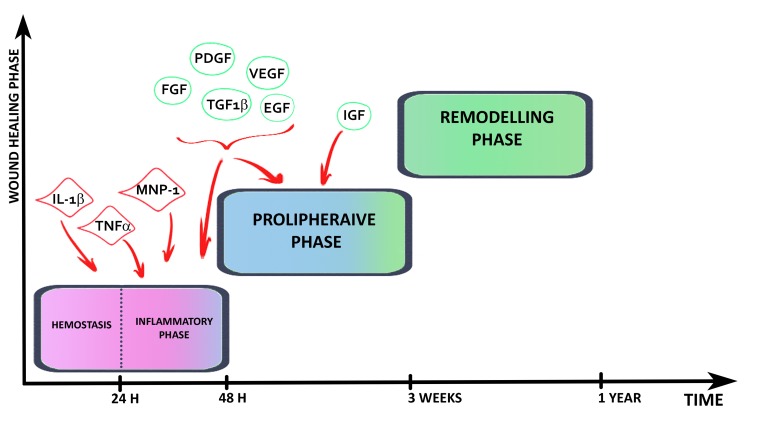
It is reported that wound healing has three main stages: the inflammatory phase, the proliferative phase and the remodeling phase. Conventionally, these stages are considered partly overlapping as a precise temporal delimitation is yet impossible to establish (see Figure 1).
Figure 1. Wound healing phases and the molecules that are required for each phase.
The cellular response in the inflammatory phase is initiated right after the lesion is inflicted and can take up to 2 days. Some studies report that this phase actually starts only one day after the injury, while the first 24 hours are considered to represent the sub-phase known as hemostasis7.
The main target during the inflammatory phase is to sanitize the injury and remove foreign bodies in order to start the proper rebuilding of the skin tissue. In such manner, the first stage is marked by the formation of a blood clot through the aggregation of platelets and thrombocytes in a fibrin network8. The blood clot acts as a barrier against microorganisms and helps organize the temporary matrix that is needed for further cellular migration events7. This process is known as the hemostasis phase, being considered by some authors as a self-standing stage9. During this phase, molecules such as IL-1β, TNF-α, MNP-1, PDGF, FGF, VEGF and TGF-β1 are recruited at the lesion site for two main reasons. On one hand, they increase blood vessel permeability which eases tissue nutrition, while on the other hand, they are involved in the formation of the granulation tissue, which is necessary for the next stage’s evolution7,10.
The second stage, called the proliferative phase, is initiated 48 hours post-injury and lasts for up to three weeks. This phase is essential for the healing process, as it is marked by the formation of a viable epithelial barrier, allowing the wound to begin proper closing8. Granulation tissue may start forming the fourth day after the injury. This tissue’s formation is characterized by collagen synthesis and fibroblastic proliferation, essential for the development of any connective tissue. Angiogenesis is essential during this period, as proper vascular irrigation brings a fresh supply of oxygen and supplements to the cells7.
For this stage, growth factors such as PDGF, VEGF, FGF, IGF, or TGF-β1 are mobilized in order to start processes like angiogenesis or reepithelialisation10.
For instance, PDGF is mitogenic for fibroblasts and helps stimulate the fibroblast proliferation and migration, VEGF promotes angiogenesis, is a powerful mitogenic for keratinocytes or endothelial cells and helps mediate the extracellular matrix synthesis and deposition and FGF enhances the endothelial and fibroblast migration and proliferation, stimulates angiogenesis and is believed to help tissue repair through skin cells growth11.
TGF-β1 helps mediate extracellular matrix formation, helps the keratinocyte migration in re-epithelialization, stimulates type I and type III collagen production, angiogenesis and enhances the proliferation of fibroblasts. Regarding wound healing, IGF is believed to stimulate cell growth and proliferation10.
The third phase, named the remodeling phase, begins at the end of the proliferative stage and lasts for up to one year or even more. This phase can be defined as an attempt to recover skin tissue’s architecture, as the granulation tissue is remodeled. During the remodeling stage a variety of cellular events occur, such as emigration processes or apoptosis affecting the majority of fibroblasts, inflammatory cells or the endothelial cells. These processes may eventually lead to scar formation, which is characterized by a decrease in the number of cells7.
3. The mechanisms of tissue regeneration
Tissue regeneration is a process that involves cellular renewal and regrowth, and it is essential in order to achieve a convenient and complete wound heal.
It is essential to mention that the length of each stage and the whole length of the healing process varies from individual to individual, and is influenced by endogenous and exogenous factors, such as diabetes, hereditary healing disorders, medication, smoking, alcoholism, nutrition, obesity and many others7,9.
The re-epithelialization of the wound accounts for 80% of the wound closure, and it depends on a series of factors such as the patient’s health conditions and genetics, as well as wound-related specifics: the location, the size, the depth or the contamination with microbes or bacteriae12.
The healing mechanism depends on the injury’s severity. If the wound is superficial and characterized by partial-thickness (only the epidermis and a small portion of the dermis are affected), it will heal mostly by re-epithelialization. However, if the wound is deep and described as having full-thickness (the epidermis and the dermis are destroyed), it will heal not only by re-epithelialization, but also through a granulation tissue formation in order to fill the gap inflicted. The main differences between a partial-thickness wound and a full-thickness wound are that the former lack the proper base which allows keratinocyte repopulation, and that, in the case remains of skin appendages, they do not regenerate until the complete dermis restoration1.
The re-epithelialization phase overlaps with the inflammatory phase, as the epithelial cells manifest a migratory activity at a few hours after the injury. In order to achieve the re-epithelialization of a partial-thickness wound, the distance needed to cover the wound is of approximately 500 µm, and, subsequently, in order to re-epithelialize a partial-thickness wound that has a 200 µm depth, a period of 8 days is needed in young human adults1.
As the re-epithelialization process is not possible without an intact dermis, the dermis structure should be further presented. The dermis consists of the papillary dermis (sub-epidermal layer) and reticular dermis, along with the dermal papillae, and the dermis consists of primarily fibroblasts and many matrix components, such as collagen, elastin or glycosaminoglycans. The different layers of the dermis have different healing capacities, with the papillary dermis that regenerates the fastest, followed by the mid-dermis and the lower reticular dermis1.
In order to restore the dermis, the void created by the wound is, as mentioned above, filled with a granulation tissue, that is deposited by fibroblasts, and it is presumed that, when the lesion is so strong that there are no fibroblasts left in the dermis, the granulation tissue is deposited by fibroblasts that belong to other regions and thus are imported. This granulation tissue consists in a population of macrophages, fibroblasts and a mix of collagen fibers, hyaluronic acid and fibronectin1.
Normally, hair follicle formation happens during embryonic development, but it has been observed that, at two or three days after the re-epithelialization of the wound, de novo hair follicles can be found in the regenerated area2.
4. Platelet-rich plasma (PRP)
PRP is a plasma enriched with platelets that contain growth factors, that are considered to help with the processes of tissue regeneration and wound healing.
One of the many applications of PRP (and possibly the best known) is in dermatocosmetology, as PRP injections help restore skin’s youth through skin and neck rejuvenation, stimulating collagen synthesis13,14.
PRP is obtained through the concentration of platelets from a normal, whole-blood sample. There are different PRP processing systems available on the market: PRP kits that use specially designed columns, kits such as GloFinn PRP, XCELL PRP System or many other, and the platelet-pheresis procedure, but the centrifugation method is the most commonly used15.
Even though an exact protocol for processing and obtaining PRP has never been established, the basic steps are always similar: blood collection using anticoagulant followed by two consecutive centrifugations. Procedural differences concern the volume of anticoagulant that is used and centrifugation time and speed. Usually, two centrifugation steps are required: the first centrifugation separates the red blood cells, while the second centrifugation concentrates the platelets - the resulting product being platelet-rich plasma.
The use of an anticoagulant is essential, as this avoids the coagulation of the collected blood. There are different methods for using an anticoagulant – one distinct method is based on using EDTA-coated tubes, while other methods are based on collecting the blood in tubes that already have a specific volume of sodium citrate, acid citrate dextrose, calcium citrate, citric acid or citrate phosphate dextrose16.
Because it is believed that the anticoagulant can affect the release of growth factors and other molecules from the platelets, an activator is added in the sample, in order to stimulate the release of soluble molecules. Some studies report using calcium chloride, thrombin, type I collagen, calcium gluconate or even a mix of calcium chloride and thrombin, while other studies report skipping this step, as it is not entirely essential17.
PRP can be generated in different forms, each one presenting various advantages and disadvantages. Liquid PRP can be easily injected at target sites, thus being the most commonly used for PRP applications. Lyophilized PRP powder allows a better grasp of the growth factors concentration18, while gel preparation of PRP exhibits antiseptic properties19.
Usually, it is recommended to use different PRP quantities and types for each application, so the PRP used is custom made for an individual to serve a specific function. The demand for tailored/custom-made PRP applications stems from the physiological differences between individuals. The age, sex, and health condition of an individual affect the platelet count, which in turn directly alters the growth factors’ concentration20.
As a result of the diverse obtaining methods for PRP and taking into account the cellular content, there are different types of PRP that present different properties– leukocyte-poor PRP (LP-PRP), leukocyte-rich PRP or leukocyte-PRP (LR-PRP), pure PRP (P-PRP) and many others21,22.
Comparisons between LP-PRP and LR-PRP have been made, in order to elucidate the effect of leukocytes in PRP`s efficacy, and it has been demonstrated that the presence of leukocytes increases significantly the acute inflammatory response23 and causes a significant amount of cellular death if injected intra-articular, affecting especially synoviocytes24.
It is widely believed that PRP’s efficacy is due to the presence of growth factors and other molecules that are released by platelets. Some of the growth factors identified in PRP are the platelet-derived growth factor (PDGF), insulin-like growth factor 1 (IGF-1), vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), epidermal growth factor (EGF) and transforming growth factor β1 (TGFβ1). Other molecules that are found in PRP are tumor necrosis factor α (TNF-α), monocyte chemotactic protein 1 (MNP-1) and interleukins (IL), such as IL-1β, IL-5, IL-6, IL-8, IL-10, IL-1 receptor antagonist (RA), vitronectin, fibronectin and fibrin25-27.
PDGF has a powerful impact on wound healing, as it is implicated in angiogenesis, activation of macrophages and mitogenesis (being a strong mitogen for muscle cells and fibroblasts) and it is directly implicated in wound healing28. There are many isoforms for this thermo-resistant molecule, named PDGF-AA, PDGF-BB, PDGF-CC, PDGF-AB, and, more recently, PDGF-DD. PDGF also stimulates cell proliferation and cell growth and helps type I collagen synthesis28,29.
IGF is a growth factor that is believed to stimulate cell growth and cellular proliferation10. There are studies that claim that, when combined with PDGF, IGF can enhance the quality of wound healing11.
The VEGF family promotes angiogenesis by stimulating the proliferation of endothelial cells and their migration and plays a key role in vasculogenesis29. Two specific isoforms of VEGF, VEGF164 and VEGF165, have a key role in vascular development, but each isoform from the rest of the VEGF family has a different role arterial development and vascular patterning30.
FGF stimulates mitogenesis in chondrocyte populations, smooth muscle cells, osteoblasts, fibroblasts and skeletal myoblasts. Thereby, this growth factor plays a key role in the process of wound healing, as it promotes cell proliferation and tissue repair29,31. It has been shown that FGF promotes type II collagen production. However, it has no effect on the type I collagen biosynthesis32. Studies mention that the FGF expression peaks when an injury is caused, suggesting its importance in wound healing, especially in cutaneous wounds33.
EGF has been proven to have a mitogenic effect and to induce cellular migration on fibroblasts and epithelial cells29. EGF affects angiogenesis and tissue regeneration by having a role in the regulation of collagen secretion and cellular proliferation10,34. EGF stimulates wound healing through epidermal regeneration and by stimulating the proliferation of dermal fibroblasts and keratinocytes11.
TGF-β1 has multiple qualities, but the most important of all its attributes, it regulates the mitogenic effect of other growth factors. Among its characteristics count: the stimulation of angiogenesis, endothelial mitogenesis, extracellular matrix, type I and type III collagen production, it enhances keratinocyte migration in reepithelialisation10,11,29.
IL-1β, IL-6 and TNF-α are usually released in immune responses and have an important role in maintaining the homeostasis35. Studies show that cytokines can mediate processes such as immunological responses or cell-to-cell communication36. IL-6 plays a key role against infections caused through injuries and can ameliorate chronic inflammation37.
It has been observed that, while IL-1β cannot be found in uninjured skin, after a lesion is induced, a significant increase in this cytokine’s expression can be found in damaged tissues within the first 30 to 90 minutes. The same increase in expression was also observed for TNF-α, and IL-6 in the wound liquid, after a surgical intervention38.
Thus, PRP has growth factors that are important in diverse processes such as angiogenesis, mitogenesis or extracellular matrix and collagen production and thus has potential in cellular proliferation, tissue regeneration and wound healing.
5. The impact of PRP on wound healing
As mentioned in the sections above, the possibility of an accelerated wound healing is crucial for a significant number of patients, which brings PRP into attention, as many researchers believe to quicken the process.
Currently, PRP is used worldwide as a skin youth rejuvenator, as it stimulates the type I collagen synthesis in the dermis, which allows the wrinkles to be partially or fully erased39. A number of studies suggest that PRP is able to stimulate the proliferation of dermal fibroblasts40,41, of keratinocytes42 and of endothelial cells43. It has also been shown that PRP may have a positive effect on epidermal regeneration, through the stimulation of cellular proliferation and migration, and also by bringing molecules involved in the process of wound healing at the lesions site44.
One experiment analyzed and compared three types of treatments for wounds in mice. The three groups were treated with PRP and human keratinocytes and fibroblasts, or with PRP and DMEM media, or with saline solution. It was observed that the group that received PRP and skin cells treatment completely healed wounds, while the other two groups did not achieve full closure of the wounds. It is mentioned that a fourth group of mice that had received only skin cells as treatment was excluded from the study, as the cells could not remain at the wound site and scattered through the encompassing tissue, proving that PRP has a positive effect on wound healing45.
Another application of PRP mixed with cells is the treatment with autologous PRP and adipose-derived stem cells (ASC). Researchers treated and analyzed the wounds of two groups, one group treated with standard wound care and the other group treated with autologous PRP and ASC. It has been observed that, while both groups had a high rate of wound closure, the group that received PRP treatment had a bigger rate of wound closure than the control group46.
The use of PRP with ASC is widely studied, as it is believed that, if cultured in keratinocyte differentiation media, ASCs differentiate in keratinocyte-like cells, which are required for proper wound healing47. Few in vivo studies with this combination are found in the literature, possibly as a result of the lack of a proper protocol. However, the existing in vivo studies show that patients that received PRP and ASC treatment presented a shorter time required for complete re-epithelialization of the wound and almost no recurrence of the chronic skin ulcer48.
An extensive analysis of studies regarding the efficacy of PRP treatment on wound healing after surgery showed that patients treated with PRP presented a significantly better vascularization through angiogenesis49, less pain50, and a 15.9 days difference between the control group and the PRP treated group in terms of healing time51.
Regarding the PRP treatment post-surgery, the use of PRP on skin flaps has proven that PRP stimulates angiogenesis through the activity of growth factors present in its composition (especially VEGF), thus further increasing flap healing52.
It has been observed, on two identical wounds induced on the same patient, that the lesion treated with PRP healed faster and left almost no scar tissue after 6 months compared to the lesion treated with thrombin. At six days after the incisions, the wound treated with thrombin developed an erythema and did not present an epithelial layer, while the wound that received PRP treatment was healthy and was covered in a thin epithelial layer25.
A PRP gel was applied to the wounds of 100 thalassemia patients (wounds such as burns or cuts), and it has been noticed that the wounds had a much shorter healing time and the lesions did not reopen while the 8 months that the patients were under surveillance16. Another study concluded that PRP treatment helps the healing time of the wounds regardless if the wound was caused by burn, cut, etc.53.
In order to assess the effect of PRP concerning the healing of burns, three groups of rats were compared, one group of rats with deep second-degree burns, one group of rats with deep second-degree burns associated with diabetes mellitus, and one group of rats with third degree burns. Each group was divided into a control subgroup and a PRP-treated subgroup. It has been noted that the groups that received PRP treatment presented an increase in the amount of collagen and the granulation tissue and the neo-epidermis was more prominent in the PRP treated rats54. A vast number of studies claim the beneficial effect that PRP holds on burn wounds through dermal regeneration and accelerated re-epithelialization55.
Studies show that PRP is able to increase the number of hair follicles and the small blood vessels around hair follicles, thus increasing hair density after two to six injections at an interval of a couple of months, with side effects such as scalp sensitivity, mild headaches, pinpoint bleeding, redness and minimal and temporary pain56. A number of articles suggest that PRP is a safe tool for treating androgenetic alopecia, which is a type of hair loss that is believed to be caused by genetics57-59.
The number of clinical trials regarding the use of PRP in wound healing is increasing year to year, which shows that the need to find a wound healing adjuvant is bigger than anytime. Clinical trials regarding the effect of PRP on wound healing, on acute deep partial thickness thermal injuries, on second and third-degree burns, or on diabetes wounds are not completed. However, there are a few studies that published promising results regarding this topic (see Table 1). It is important to mention that being an autologous compound, PRP is not toxic and doesn’t have harmful side-effects. However, in very few cases PRP extractions proved to be ineffective60.
Table 1. Examples of clinical trials employing PRP use in wound healing.
| Subject of the study | Number of patients | Results | Source |
|---|---|---|---|
| PRP in reconstructive surgery on children with retractable burn sequelae on extremities | 44 (a group received PRP treatment, a group did not receive PRP treatment) | -the wound closure time was not statistically different in the group that received PRP from the group that did not received PRP | ClinicalTrials.gov Identifier: NCT00858442 |
| Effect of PRP and Keratinocyte Suspensions on Wound Healing | 45 (a group received standard wound care, a group received PRP treatment, a group received PRP and keratinocyte treatment) | -the groups that received PRP achieved full wound closure faster and presented lower scores for pain than the group that received standard care -the group that received PRP and keratinocyte treatment showed better results than the group that received only PRP treatment | ClinicalTrials.gov Identifies: NCT00856934 |
| Use of PRP and PPP to Prevent Infection and Delayed Wound Healing | 515 patients (one group received PRP and PPP treatment, one group did not receive PRP and PPP treatment) | -the groups did not present statistically different results | ClinicalTrials.gov Identifier: NCT01639144 |
6. Conclusions
Wound healing is a crucial process for tissue homeostasis and for the well-being of the whole organism. Considering the advances made in the field of regenerative medicine, the need to find an adjuvant to aid and speed this process is more and more prominent. Numerous studies have proven that PRP modulates cellular proliferation and migration and tissue regeneration, both in in vitro and in vivo studies, it is our belief that it can be a useful tool in wound healing. Despite lack of approval by the US Food and Drug Administration (FDA), PRP therapy is gaining unproven acceptance as treatment for sports-related injuries61,62. The growth factors found in PRP supply a myriad of properties which showed that are valuable for the re-epithelialization and the regeneration of dermis processes, vital for a proper healing process, as they stimulate the same molecules that are needed in order to cure a lesion.
However, even if there are plenty of studies in scientific literature, larger studies with clinical trials and definite protocols are required, in order to clearly establish PRP’s benefic effects and the exact mechanisms involved in wound healing.
KEY POINTS
◊ PRP is a resourceful adjuvant for regenerative processes involved in wound healing
◊ It is widely believed that PRP is one of the most promising auxiliaries in wound healing, since it modulates angiogenesis and cellular proliferation
◊ Large clinical trials with specific preparation methods and rigorous treatments are required in order to clarify the effects of this tool on various wound types.
Acknowledgments
The authors are thankful to the University of Bucharest for its support.
Footnotes
Conflict of interests: The authors declare no conflicts of interest.
ASC (adipose-derived stem cell); EDTA (ethylenediaminetetraacetic acid); EGF (epidermal growth factor); FDA (Food and Drug Administration); FGF (fibroblast growth factor); IGF (insulin-like growth factor 1); IL (interleukin); LP PRP (leucocyte-poor platelet-rich plasma); LR PRP (leucocyte-rich platelet-rich plasma); MNP 1 (monocyte chemotactic protein 1); PDGF (platelet-derived growth factor); PPP (platelet-poor plasma); P PRP (pure platelet-rich plasma); PRP (plateletrich plasma); RA (interleukin 1 receptor antagonist); TGFβ1 (transforming growth factor β1); TNF-α (tumor necrosis factor α); VEGF (vascular-endothelial growth factor).
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Cellular mechanisms of skin repair in humans and other mammals. Rittié Laure. Journal of Cell Communication and Signaling. 2016;10(2):103-120. doi: 10.1007/s12079-016-0330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wound Healing and Skin Regeneration. Takeo M., Lee W., Ito M. Cold Spring Harbor Perspectives in Medicine. 2015;5(1):a023267-a023267. doi: 10.1101/cshperspect.a023267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.A Comparative Examination of the Clinical Outcome and Histological Appearance of Cryopreserved and Fresh Split-Thickness Skin Grafts. Holzer Paul W., Leonard David A., Shanmugarajah Kumaran, Moulton Krysta N., Ng Zhi Yang, Cetrulo Curtis L., Sachs David H. Journal of Burn Care & Research. 2017;38(1):e55-e61. doi: 10.1097/BCR.0000000000000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moisture Dressings: The New Standard in Wound Care. Lagana Gail, Anderson Elizabeth H. The Journal for Nurse Practitioners. 2010;6(5):366-370. [Google Scholar]
- 5.PERSPECTIVE ARTICLE: Growth factors and cytokines in wound healing. Barrientos Stephan, Stojadinovic Olivera, Golinko Michael S., Brem Harold, Tomic-Canic Marjana. Wound Repair and Regeneration. 2008;16(5):585-601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 6.Marjolin ulcers: secondary carcinomas in chronic wounds. Esther R J, Lamps L, Schwartz H S. Journal of the Southern Orthopaedic Association. 1999;8(3):181–7. [PubMed] [Google Scholar]
- 7.Wound healing - A literature review. Gonzalez Ana Cristina de Oliveira, Costa Tila Fortuna, Andrade Zilton de Araújo, Medrado Alena Ribeiro Alves Peixoto. Anais Brasileiros de Dermatologia. 2016;91(5):614-620. doi: 10.1590/abd1806-4841.20164741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cellular and molecular mechanisms of repair in acute and chronic wound healing. Martin P., Nunan R. British Journal of Dermatology. 2015;173(2):370-378. doi: 10.1111/bjd.13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Factors Affecting Wound Healing. Guo S., DiPietro L.A. Journal of Dental Research. 2010;89(3):219-229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Potential of Topical and Injectable Growth Factors and Cytokines for Skin Rejuvenation. Fabi Sabrina, Sundaram Hema. Facial Plastic Surgery. 2014;30(02):157-171. doi: 10.1055/s-0034-1372423. [DOI] [PubMed] [Google Scholar]
- 11.Is platelet-rich plasma the perfect enhancement factor? A current review. Sánchez Andrés R, Sheridan Phillip J, Kupp Leo I. The International journal of oral & maxillofacial implants. 2003;18(1):93–103. [PubMed] [Google Scholar]
- 12.Skin Wound Healing: An Update on the Current Knowledge and Concepts. Sorg Heiko, Tilkorn Daniel J., Hager Stephan, Hauser Jörg, Mirastschijski Ursula. European Surgical Research. 2016;58(1-2):81-94. doi: 10.1159/000454919. [DOI] [PubMed] [Google Scholar]
- 13.Face and neck revitalization with platelet-rich plasma (PRP): clinical outcome in a series of 23 consecutively treated patients. Redaelli Alessio, Romano Domenico, Marcianó Antonio. Journal of drugs in dermatology : JDD. 2010;9(5):466–72. [PubMed] [Google Scholar]
- 14.Evaluation of effects of platelet-rich plasma on human facial skin. Yuksel Esra Pancar, Sahin Gokhan, Aydin Fatma, Senturk Nilgun, Turanli Ahmet Yasar. Journal of Cosmetic and Laser Therapy. 2014;16(5):206-208. doi: 10.3109/14764172.2014.949274. [DOI] [PubMed] [Google Scholar]
- 15.A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting. Chahla Jorge, Cinque Mark E., Piuzzi Nicolas S., Mannava Sandeep, Geeslin Andrew G., Murray Iain R., Dornan Grant J., Muschler George F., LaPrade Robert F. The Journal of Bone and Joint Surgery. 2017;99(20):1769-1779. doi: 10.2106/JBJS.16.01374. [DOI] [PubMed] [Google Scholar]
- 16.Treatment of 100 chronic thalassemic leg wounds by plasma-rich platelets. Afradi Hojjat, Saghaei Yassaman, Kachoei Zohre A., Babaei Vahid, Teimourian Shahram. International Journal of Dermatology. 2016;56(2):171-175. doi: 10.1111/ijd.13443. [DOI] [PubMed] [Google Scholar]
- 17.Platelet-Rich Plasma: The Choice of Activation Method Affects the Release of Bioactive Molecules. Cavallo Carola, Roffi Alice, Grigolo Brunella, Mariani Erminia, Pratelli Loredana, Merli Giulia, Kon Elizaveta, Marcacci Maurilio, Filardo Giuseppe. BioMed Research International. 2016;2016:1-7. doi: 10.1155/2016/6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Platelet-Rich Plasma Powder: A New Preparation Method for the Standardization of Growth Factor Concentrations. Kieb Matthias, Sander Frank, Prinz Cornelia, Adam Stefanie, Mau-Möller Anett, Bader Rainer, Peters Kirsten, Tischer Thomas. The American Journal of Sports Medicine. 2016;45(4):954-960. doi: 10.1177/0363546516674475. [DOI] [PubMed] [Google Scholar]
- 19.Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances. Bielecki T. M., Gazdzik T. S., Arendt J., Szczepanski T., Kròl W., Wielkoszynski T. The Journal of Bone and Joint Surgery. British volume. 2007;89-B(3):417-420. doi: 10.1302/0301-620X.89B3.18491. [DOI] [PubMed] [Google Scholar]
- 20.Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. Weibrich Gernot, Kleis Wilfried K.G., Hafner Gerd, Hitzler Walter E. Journal of Cranio-Maxillofacial Surgery. 2002;30(2):97-102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 21.Effect of Leukocyte Concentration on the Efficacy of Platelet-Rich Plasma in the Treatment of Knee Osteoarthritis. Riboh Jonathan C., Saltzman Bryan M., Yanke Adam B., Fortier Lisa, Cole Brian J. The American Journal of Sports Medicine. 2015;44(3):792-800. doi: 10.1177/0363546515580787. [DOI] [PubMed] [Google Scholar]
- 22.Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Dohan Ehrenfest David M., Rasmusson Lars, Albrektsson Tomas. Trends in Biotechnology. 2009;27(3):158-167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Comparison of the Acute Inflammatory Response of Two Commercial Platelet-Rich Plasma Systems in Healthy Rabbit Tendons. Dragoo Jason L., Braun Hillary J., Durham Jennah L., Ridley Bethany A., Odegaard Justin I., Luong Richard, Arnoczky Steven P. The American Journal of Sports Medicine. 2012;40(6):1274-1281. doi: 10.1177/0363546512442334. [DOI] [PubMed] [Google Scholar]
- 24.The Effect of Platelet-Rich Plasma Formulations and Blood Products on Human Synoviocytes. Braun Hillary J., Kim Hyeon Joo, Chu Constance R., Dragoo Jason L. The American Journal of Sports Medicine. 2014;42(5):1204-1210. doi: 10.1177/0363546514525593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platelet-rich plasma: evidence to support its use. Marx Robert E. Journal of Oral and Maxillofacial Surgery. 2004;62(4):489-496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Platelet-rich plasma preparation for regenerative medicine: optimization and quantification of cytokines and growth factors. Amable Paola Romina, Carias Rosana Bizon Vieira, Teixeira Marcus Vinicius Telles, da Cruz Pacheco Italo, Corrêa do Amaral Ronaldo José Farias, Granjeiro José Mauro, Borojevic Radovan. Stem cell research & therapy. 2013;4(3):67. doi: 10.1186/scrt218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Analysis of cytokine profile and growth factors in platelet-rich plasma obtained by open systems and commercial columns. Pochini Alberto de Castro, Antonioli Eliane, Bucci Daniella Zanetti, Sardinha Luiz Roberto, Andreoli Carlos Vicente, Ferretti Mario, Ejnisman Benno, Goldberg Anna Carla, Cohen Moisés. Einstein (São Paulo) 2016;14(3):391-397. doi: 10.1590/S1679-45082016AO3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Role of platelet-derived growth factors in physiology and medicine. Andrae J., Gallini R., Betsholtz C. Genes & Development. 2008;22(10):1276-1312. doi: 10.1101/gad.1653708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Growth factor content in PRP and their applicability in medicine. Lubkowska A, Dolegowska B, Banfi G. Journal of biological regulators and homeostatic agents. 2012;26(2 Suppl 1):3S–22S. [PubMed] [Google Scholar]
- 30.The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Takahashi Hiroyuki, Shibuya Masabumi. Clinical Science. 2005;109(3):227-241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 31.Platelet-Rich Plasma: A Promising Product for Treatment of Peripheral Nerve Regeneration After Nerve Injury. Yu WenJun, Wang Jian, Yin Jun. International Journal of Neuroscience. 2011;121(4):176-180. doi: 10.3109/00207454.2010.544432. [DOI] [PubMed] [Google Scholar]
- 32.Local stimulation of articular cartilage repair by transplantation of encapsulated chondrocytes overexpressing human fibroblast growth factor 2 (FGF-2)in vivo. Kaul Gunter, Cucchiarini Magali, Arntzen David, Zurakowski David, Menger Michael D., Kohn Dieter, Trippel Stephen B, Madry Henning. The Journal of Gene Medicine. 2005;8(1):100-111. doi: 10.1002/jgm.819. [DOI] [PubMed] [Google Scholar]
- 33.Moist exposed burn ointment promotes cutaneous excisional wound healing in rats involving VEGF and bFGF. TANG QIAN-LI, HAN SHAN-SHAN, FENG JING, DI JIA-QI, QIN WEN-XI, FU JUN, JIANG QIU-YAN. Molecular Medicine Reports. 2014;9(4):1277-1282. doi: 10.3892/mmr.2014.1921. [DOI] [PubMed] [Google Scholar]
- 34.Current Evidence for Clinical Efficacy of Platelet Rich Plasma in Aesthetic Surgery: A Systematic Review. Frautschi Russell S., Hashem Ahmed M., Halasa Brianna, Cakmakoglu Cagri, Zins James E. Aesthetic Surgery Journal. 2016;37(3):353–362. doi: 10.1093/asj/sjw178. [DOI] [PubMed] [Google Scholar]
- 35.The Inflammasomes. Schroder Kate, Tschopp Jurg. Cell. 2010;140(6):821-832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Bioanalytical chemistry of cytokines – A review. Stenken Julie A., Poschenrieder Andreas J. Analytica Chimica Acta. 2015;853:95-115. doi: 10.1016/j.aca.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Interleukin-6 blockade in ocular inflammatory diseases. Mesquida M., Leszczynska A., Llorenç V., Adán A. Clinical & Experimental Immunology. 2014;176(3):301-309. doi: 10.1111/cei.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Use of platelet‐rich plasma to facilitate wound healing. Menchisheva Yuliya, Mirzakulova Ulmeken, Yui Rudolf. International Wound Journal. 2018;16(2):343-353. doi: 10.1111/iwj.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Can Platelet-rich Plasma Be Used for Skin Rejuvenation? Evaluation of Effects of Platelet-rich Plasma on Human Dermal Fibroblast. Kim Dae Hun, Je Young Jin, Kim Chang Deok, Lee Young Ho, Seo Young Joon, Lee Jeung Hoon, Lee Young. Annals of Dermatology. 2011;23(4):424. doi: 10.5021/ad.2011.23.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proliferation-Promoting Effect of Platelet-Rich Plasma on Human Adipose–Derived Stem Cells and Human Dermal Fibroblasts. Kakudo Natsuko, Minakata Tatsuya, Mitsui Toshihito, Kushida Satoshi, Notodihardjo Frederik Zefanya, Kusumoto Kenji. Plastic and Reconstructive Surgery. 2008;122(5):1352-1360. doi: 10.1097/PRS.0b013e3181882046. [DOI] [PubMed] [Google Scholar]
- 41.Platelet and growth factor concentrations in activated platelet-rich plasma: a comparison of seven commercial separation systems. Kushida Satoshi, Kakudo Natsuko, Morimoto Naoki, Hara Tomoya, Ogawa Takeshi, Mitsui Toshihito, Kusumoto Kenji. Journal of Artificial Organs. 2014;17(2):186-192. doi: 10.1007/s10047-014-0761-5. [DOI] [PubMed] [Google Scholar]
- 42.Platelet-rich plasma (PRP) and adipose-derived mesenchymal stem cells: stimulatory effects on proliferation and migration of fibroblasts and keratinocytes in vitro. Stessuk Talita, Puzzi Maria Beatriz, Chaim Elinton Adami, Alves Paulo César Martins, de Paula Erich Vinicius, Forte Andresa, Izumizawa Juliana Massae, Oliveira Carolina Caliári, Frei Fernando, Ribeiro-Paes João Tadeu. Archives of Dermatological Research. 2016;308(7):511-520. doi: 10.1007/s00403-016-1676-1. [DOI] [PubMed] [Google Scholar]
- 43.Epidermal growth factor released from platelet-rich plasma promotes endothelial cell proliferationin vitro. Bertrand-Duchesne M.-P., Grenier D., Gagnon G. Journal of Periodontal Research. 2010;45(1):87-93. doi: 10.1111/j.1600-0765.2009.01205.x. [DOI] [PubMed] [Google Scholar]
- 44.Plasma rich in growth factors promotes dermal fibroblast proliferation, migration and biosynthetic activity. Anitua E., Pino A., Orive G. Journal of Wound Care. 2016;25(11):680-687. doi: 10.12968/jowc.2016.25.11.680. [DOI] [PubMed] [Google Scholar]
- 45.Platelet-rich plasma with keratinocytes and fibroblasts enhance healing of full-thickness wounds. Law Jia Xian, Chowdhury Shiplu Roy, Saim Aminuddin Bin, Idrus Ruszymah Bt Hj. Journal of Tissue Viability. 2017;26(3):208-215. doi: 10.1016/j.jtv.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Adipose-derived Stem Cells Added to Platelet-rich Plasma for Chronic Skin Ulcer Therapy. Raposio Edoardo, Bertozzi Nicolò, Bonomini Sabrina, Bernuzzi Gino, Formentini Alessandro, Grignaffini Eugenio, Pio Grieco Michele. Wounds : a compendium of clinical research and practice. 2016;28(4):126–31. [PubMed] [Google Scholar]
- 47.Differentiation of adipose derived stem cells to keratinocyte-like cells on an advanced collagen wound matrix. Edwards N.J., Stone R., Christy R., Zhang C.K., Pollok B., Cheng X. Tissue and Cell. 2018;53:68-75. doi: 10.1016/j.tice.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Regenerative Surgery: Use of Fat Grafting Combined with Platelet-Rich Plasma for Chronic Lower-Extremity Ulcers. Cervelli V., Gentile P., Grimaldi M. Aesthetic Plastic Surgery. 2009;33(3):340-345. doi: 10.1007/s00266-008-9302-z. [DOI] [PubMed] [Google Scholar]
- 49.Evaluation of platelet-rich plasma gel potential in acceleration of wound healing duration in patients underwent pilonidal sinus surgery: A randomized controlled parallel clinical trial. Mohammadi Saeed, Nasiri Shirzad, Mohammadi Mohammad Hossein, Malek Mohammadi Ashraf, Nikbakht Mohsen, Zahed Panah Mahdi, Safar Hiva, Mostafaei Shayan, Norooznezhad Amir Hossein, Soroosh Ahmad Reza, Alimoghaddam Kamran, Ghavamzadeh Ardeshir. Transfusion and Apheresis Science. 2017;56(2):226-232. doi: 10.1016/j.transci.2016.12.032. [DOI] [PubMed] [Google Scholar]
- 50.Investigating the Effect of Autologous Platelet-Rich Plasma on Pain in Patients With Pilonidal Abscess Treated With Surgical Removal of Extensive Tissue. Mehrabi Bahar Mostafa, Ali Akbarian Mohsen, Azadmand Ali. Iranian Red Crescent Medical Journal. 2013;15(11) doi: 10.5812/ircmj.6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Effectiveness of platelet-rich plasma therapy in wound healing of pilonidal sinus surgery: A comprehensive systematic review and meta-analysis. Mostafaei Shayan, Norooznezhad Fatemeh, Mohammadi Saeed, Norooznezhad Amir Hossein. Wound Repair and Regeneration. 2017;25(6):1002-1007. doi: 10.1111/wrr.12597. [DOI] [PubMed] [Google Scholar]
- 52.The effect of platelet rich plasma on angiogenesis in ischemic flaps in VEGFR2-luc mice. Sönmez Tolga Taha, Vinogradov Alexandra, Zor Fatih, Kweider Nisreen, Lippross Sebastian, Liehn Elisa Anamaria, Naziroglu Mustafa, Hölzle Frank, Wruck Christoph, Pufe Thomas, Tohidnezhad Mersedeh. Biomaterials. 2013;34(11):2674-2682. doi: 10.1016/j.biomaterials.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 53.Platelet gel for healing cutaneous chronic wounds. Crovetti Giovanni, Martinelli Giovanna, Issi Marwan, Barone Marilde, Guizzardi Marco, Campanati Barbara, Moroni Marco, Carabelli Angelo. Transfusion and Apheresis Science. 2004;30(2):145-151. doi: 10.1016/j.transci.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Use of platelet-rich plasma in deep second- and third-degree burns. Venter Neil Grant, Marques Ruy Garcia, Santos Jeanine Salles dos, Monte-Alto-Costa Andréa. Burns. 2016;42(4):807-814. doi: 10.1016/j.burns.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 55.Platelet-rich plasma in burns. Pallua Norbert, Wolter Timm, Markowicz Marta. Burns. 2010;36(1):4-8. doi: 10.1016/j.burns.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 56.Autologous Platelet-Rich Plasma for the Treatment of Pattern Hair Loss. Singh Babu, Goldberg Lynne J. American Journal of Clinical Dermatology. 2016;17(4):359-367. doi: 10.1007/s40257-016-0196-2. [DOI] [PubMed] [Google Scholar]
- 57.Platelet-rich plasma injection is effective and safe for the treatment of alopecia. Betsi Evelyn-Evanthia, Germain Esnault, Kalbermatten Daniel F., Tremp Mathias, Emmenegger Veronique. European Journal of Plastic Surgery. 2013;36(7):407-412. [Google Scholar]
- 58.Platelet-Rich Plasma for Androgenetic Alopecia. Schiavone Giovanni, Raskovic Desanka, Greco Joseph, Abeni Damiano. Dermatologic Surgery. 2014;40(9):1010-1019. doi: 10.1097/01.DSS.0000452629.76339.2b. [DOI] [PubMed] [Google Scholar]
- 59.Management of androgenetic alopecia: a comparative clinical study between plasma rich in growth factors and topical minoxidil. Navarro M. R., Asín M., Martínez M. A., Martínez A. M., Molina C., Moscoso L., Pino A., Orive G., Anitua E. European Journal of Plastic Surgery. 2016;39(3):173-180. [Google Scholar]
- 60.Platelet-rich plasma in dermatology: Boon or a bane? Kumaran MSendhil, Arshdeep Indian Journal of Dermatology, Venereology, and Leprology. 2014;80(1):5. doi: 10.4103/0378-6323.125467. [DOI] [PubMed] [Google Scholar]
- 61.Platelet-Rich Plasma. Foster Timothy E., Puskas Brian L., Mandelbaum Bert R., Gerhardt Michael B., Rodeo Scott A. The American Journal of Sports Medicine. 2009;37(11):2259-2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 62.US Definitions, Current Use, and FDA Stance on Use of Platelet-Rich Plasma in Sports Medicine. Beitzel Knut, Allen Donald, Apostolakos John, Russell Ryan, McCarthy Mary, Gallo Gregory, Cote Mark, Mazzocca Augustus. Journal of Knee Surgery. 2014;28(01):029-034. doi: 10.1055/s-0034-1390030. [DOI] [PubMed] [Google Scholar]