Abstract
Increasing bacterial resistance to antibiotics is a worldwide ongoing issue. Urgent need for new antibacterial agents has resulted in significant research efforts, with new molecules proposed for use in clinical practice. However, as highlighted by many groups this process does not have an optimal rhythm and efficacy, to fully combat highly adaptive germs, particularly in the intensive care units. This review focuses on the last three years of novel FDA approved antibacterial agents (2015-2017): ceftazidime/avibactam, obiltoxaximab, bezlotoxu-mab, delafloxacin, meropenem/vaborbactam, ozenoxacin. Ceftazidime/avibactam and meropenem/ vaborbactam are new players in the field of resistant bacteria treatment. Ceftazidime/avibactam is validated in selected patients with complicated urinary or intra-abdominal infections, hospital and ventilator-associated pneumonia. Meropenem/ vaborbactam gained approval for the cases of complicated urinary tract infections. Other potential indications are under investigation, widened and validated by future studies. Obiltoxaximab is a monoclonal antibody that can be used in the prevention and treatment of inhalational anthrax. Bezlotoxumab monoclonal antibody is an useful and specific tool for the management of recurrent Clostridium difficile infection. Delafloxacin is approved for patients with acute skin or skin structure infections. Despite recent progress, it is imperative to continue the development of new antibiotic drugs and new strategies to counteract resistance to antibiotics.
Keywords: FDA approved drugs, ceftazidime, avibactam, obiltoxaximab, bezlotoxumab, delafloxacin, vaborbactam, vabomere, ozenoxacin, malacidin, teixobactin, 2015, 2016, 2017
1. Introduction
Antibiotics discovery and clinical use is undoubtedly one of the pillars of modern medicine. Modern medicine saw a continuous competition between new antibacterial drug research and the ability of bacteria to develop resistance1. New classes of antibiotics were created, old drugs regained interest and paradigm changes were proposed2-4. However, this process seems to have diminished its pace and, after less than a century since the first clinical use of an antibiotic, bacterial resistance to antibiotics is a major concern of current medical practice and research5. The great influenza pandemics offers an unfortunate insight of a post-antibiotic era.
In the intensive care unit, dealing with resistant bacteria is a daily struggle. A careful antibiotic stewardship combined with public health prevention measures are vigorously promoted, in order to lower the incidence of resistant bacteria2,6-9. Despite sustained efforts, some bacteria highly susceptible to develop resistance, such as Pseudomonas aeruginosa or Acinetobacter baumannii, end up being treated by not so new drugs like colimycin3,10,11. From this perspective, the intensive care physician is looking regularly to the research field, expecting an ideal antibiotic: specific, effective, well tolerated and with no long term induced resistance.
In this present review, we briefly address the new antibacterial agents approved during the recent years by FDA, as a hope to reinforce the current therapeutic armamentarium. New antibacterial agents were identified using FDA (www.accessdata.fda.gov, www.fda.gov) and Center Watch sites (https://www.centerwatch.com/drug-information/fda-approved-drugs/).
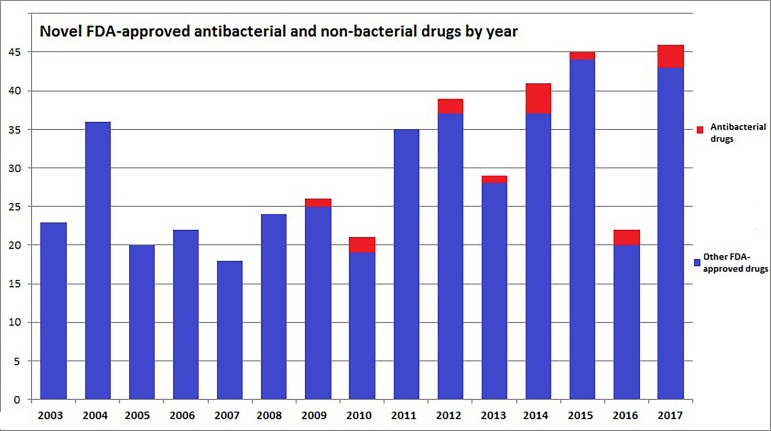
The number of FDA-approved antibacterial drugs and the total novel molecules for each year during the past 15 years is summarized in Figure 1. A tendency of increasing the number of approved molecules by year can be noticed. Furthermore, visibly more antibacterial molecules have been approved in recent years compared with previous years.
Figure 1. Novel FDA-approved antibacterial and non-bacterial drugs by year (last 15 years).
To the date of this review, no antibacterial drug has been approved for 2018, from a total of 6 newly introduced molecules. In 2017, the 3 approved antibacterial drugs represented 6.5% of the total of 46 new drugs. 2016 brought 2 new drugs, respectively 9% of the 22 approved molecules. In 2015, only one antibacterial agent was approved by FDA (2.2% from a total of 45).
We identified 6 novel FDA approved antibacterial drugs: the 3rd generation cephalosporin and β-lactamase inhibitor combination ceftazidime/avibactam in 2015; two monoclonal antibodies, obiltoxaximab and bezlotoxumab in 2016; a new fluoroquinolone, delafloxacin, a combination of meropenem with the β-lactamase inhibitor vaborbactam, vabomere, and a non-fluorinated quinolone, ozenoxacin, all in 2017 (Table 1). These drugs are briefly discussed further by FDA-approval year.
Table 1. Main characteristics of the described antibacterial drugs15,16,23,30,31,34,43,47,53.
Food and Drug Administration (FDA), European Medicines Agency (EMA), complicated intra-abdominal infections (cIAIs), complicated urinary tract infections (cUTIs), hospital acquired bacterial pneumonia (HABP), ventilator associated bacterial pneumonia (VABP), acute bacterial skin and skin structure infections (ABSSI), intravenous (iv), hours (h), therapy (ther.).
| NAME (generic/brand/ class) | Approval status | Indication | Administration | Dose and duration |
|---|---|---|---|---|
| Ceftazidime/avibactam / avycaz (USA), zavicefta (Europe) / combination of ceftazidime, 3rd generation cephalosporin, with avibactam, a β-lactamase inhibitor COMPANY: Allergan Inc (USA), Pfizer (Europe) | FDA: 1. since 2015 in combination with metronidazole (cIAIs cUTIs 2. since 01/02/2018 for HABP and VABP EMA: 1. since 2016 for cIAIs, cUTIs, HABP, VABP 2. infections due to aerobic Gram-negative organisms (adults - limited options) | CIAIs, cUTIs, HABP, VABP | Ceftazidime 2g and avibactam 0.5g. IV infusion over 2h | 1. For cIAIs and cUTIs, ceftazidime 2g / avibactam 0.5g every 8 hours, for 5-14 days. 2. For HABP and VABP, ceftazidime 2g / avibactam 0.5g /8h for 7-14 days. 3. Adaptation of doses in case of renal function impairment. |
| Obiltoxaximab/ Anthim/ Monoclonal antibody COMPANY : Elusys Therapeutics | FDA: approved in March 2016 EMA: not approved | Inhalational anthrax | Diluted in 0.9% Sodium Chloride,IV infusion over 1 hour and 30 minutes | Adult patients 16 mg/kg. In paediatric patients, weight adaptation needed, greater than 40 kg - 16 mg/kg, 15 to 40 kg - 24 mg/kg, less than or equal to 15 kg - 32 mg/kg |
| Bezlotoxumab/ Zinplava/ Monoclonal antibody COMPANY: Merck Sharp & Dohme Limited | FDA: Approved in October 2016 EMA: Approved in January 2017 | Prevention of CDI recurrence, in >18 years old patients with antibiotic ther. | Diluted solution iv infusion over 60 min using a low-protein binding 0.2-5 µm in-line or add-on filter. | Recommended dosage is 10 mg/kg. Not evaluated in patients below 18 years of age |
| Delafloxacin/ Baxdela/ fluoroquinolones COMPANY: Melinta Therapeutics | FDA: approved in June 2017 for ABSSI EMA: application in March 2018 | ABSSI | IV infusion or oral use | 300 mg/12h for 5-14 days iv infusion over 1h 450 mg/12h orally for 5 to 14 days. Renal adaptation needed. |
| Meropenem-Vaborbactam/ Vabomere/ combination of meropenem, and vaborbactam, a β-lactamase inhibitor COMPANY: Melinta Therapeutics | FDA: approved in August 2017 for adults with cUTI, including pyelonephritis EMA: marketing authorization application submitted in July 2017 | cUTI, including pyelonephritis | Single-dose vials containing 2 g (1 g meropenem and 1 g vaborbactam) as a sterile, dry powder (iv) | 4g administered over 3 hours by intravenous infusion every 8 hours for up to 14 days. Renal adaptation needed. |
| Ozenoxacin/ xepi/ non-fluorinated quinolone COMPANY: Medimetrics Pharm. | FDA: approved in Dec. 2017 for the treatment of impetigo Staph. Aureus, Strept. pyogenes (> 2 months old) | Impetigo | Pale-yellow 1% cream, Topical use only | Topically applications to the affected area twice a day for 5 days |
2. FDA approved antibacterial drugs (2015-2017)
2.1 Ceftazidime/avibactam
Ceftazidime/avibactam is a combination of ceftazidime, a third generation cephalosporin, with avibactam, a β-lactamase inhibitor12. Ceftazidime inhibits peptidoglycan synthesis by inhibiting penicillin-binding proteins, resulting in cell wall instability and cell death13. Avibactam is a synthetic non-β-lactam β-lactamase inhibitor that inhibits the activities of Ambler class A and C β-lactamases and of some Ambler class D enzymes, but not the B1 metallo-β-lactamases, such as the New Delhi metallo-β-lactamase (NDM), Verona integron-encoded metallo-β-lactamase (VIM) and Imipenmase (IMP)14.
Cmax and area under the curve (AUC) of ceftazidime proportionally increase with the dose; avibactam demonstrates linear pharmacokinetics across the dosage range15. Ceftazidime, as well as avibactam, are excreted via kidneys as unchanged drugs15. Less than 10% of ceftazidime and 5.7% to 8.2% of avibactam are protein bound15. Volumes of distribution of ceftazidime and avibactam are 17 L and 22.2 L respectively15.
In patients with impaired renal function, the serum half-life of ceftazidime is prolonged and a dosage adjustment is recommended16. Moreover, in patients with complicated intra-abdominal infections and creatinine clearance of 30-50 ml/min, ceftazidime/avibactam has been found to have lower efficacy17.
Adverse reactions that have been described are hypersensitivity reactions, including anaphylaxis, Clostridium difficile-associated diarrhea and Central Nervous System reactions, such as seizures, especially in patients with renal impairment15.
Ceftazidime/avibactam is indicated for the treatment of complicated intra-abdominal infections and complicated urinary tract infections caused by the following susceptible Gram-negative microorganisms: Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterobacter cloacae, Klebsiella oxytoca, Citrobacter freundii complex and Pseudomonas aeruginosa, in patients 18 years or older, based on clinical trials which proved its non-inferiority when compared to carbapenems17-19. In complicated intra-abdominal infections, combination therapy of ceftazidime/avibactam and metronidazole is recommended15-17.
Recent approval for hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia based on the Reprove study, a phase 3, multicenter, double-blind, randomized trial which included 870 patients, make ceftazidime/avibactam a viable therapeutic option for hospital-acquired pneumonia20.
Ceftazidime/avibactam might be valuable for the Intensive Care Unit (ICU) patient when is also used as carbapebem-sparing antibiotic6. However, a retrospective case series argued the quick emergence of resistance in treated patients21,22.
2.2 Obiltoxaximab
Obiltoxaximabis a monoclonal antibody directed against the protective antigen (PA) of Bacillus anthracis, thus preventing it from binding to cellular receptors23. It is a chimeric agent, consisting of enhanced 14B7VH and VL genes connected to human ν1 and K constants, which was derived from the murine monoclonal antibody 14B7, with mutations resulting in a 50-fold increase in affinity and corresponding neutralizing capability24.
Obiltoxaximab is the second monoclonal antibody approved for the treatment of inhalational anthrax, the other being raxibacumab25. It can be administered as pre-exposure prophylaxis, in which case it is the only therapeutic option, and in confirmed cases in combination with the appropriate antimicrobials24.
As clinical trials with intentional exposure of humans to anthrax are unethical, its efficacy was examined in multiple studies conducted in two animal models of inhalational anthrax, including New Zealand White rabbits (two studies) and cynomolgus macaques (4 studies) at disease onset following lethal challenge with aerosolized Bacillus anthracis spores26. In these studies, obiltoxaximab monotherapy neutralized PA and increased survival across the range of disease severity, indicating clinical benefit of toxin neutralization in both early and late stages of inhalational anthrax26,27.
The human dose was selected and justified by comparing observed drug exposures in animals to observed exposures in healthy and infected humans28. In humans at a dose of 16 mg/kg IV obiltoxaximab AUC was >2 times that in animals, while maximum serum concentrations were comparable28.
Obiltoxaximab has a black box warning due to severe hypersensitivity reactions that have been reported during infusion, including anaphylaxis; a premedication with diphenhydramine is recommended23. Although not yet approved, recent studies on humans using obiltoxaximab via intramuscular route showed good efficacy with no hypersensitivity reactions29.
2.3 Bezlotoxumab
Bezlotoxumab is a fully human monoclonal IgG1 antibody directed against Clostridium difficile toxin B30. Bezlotoxumab exerts its action by impeding the binding of toxin B to colonic cells and consequently preventing development of C. difficile infection31. By neutralizing the toxin B, bezlotoxumab attenuates pro-inflammatory responses in vitro and reduces damage to epithelial tissue of colonic explants32,33. It has no direct antimicrobial activity against C. difficile, it has low immunogenicity and is generally well tolerated34. Recent data suggest that it is also cost-effective when administered together with standard-of-care antibiotics35.
FDA approval in October 2016 was obtained based on two double-blind, randomized, placebo-controlled, phase 3 clinical trials, Modify I and II, which involved 2655 adults treated for primary or recurrent C. difficile infection. In these trials, bezlotoxumab was associated with a lower rate of recurrent infection and had a safety profile similar to that of placebo36. Based on the population studied in the trials, it has been proposed that the risk factors justifying treatment with bezlotoxumab are: age over 65 years, history of previous C. difficile infection, immunosuppression and presence of virulent strain or severe C. difficile infection37.
Another monoclonal antibody, actoxumab, directed against C. difficile toxin A, is available38. Modify trials did not show any benefit in adding actoxumab to bezlotoxumab. On the contrary, the rates of sustained cure were lower compared to bezlotoxumab alone36.
2.4 Delafloxacin
Delafloxacin is a new fluoroquinolone with a potential role in the treatment of acute bacterial skin and skin structure infections, in adults. It was shown to be active against Gram‐positive pathogens (Staph. aureus, including methicillin-resistant, methicillin-susceptible isolates, Staph. haemolyticus, Staph. lugdunensis, Strep. agalactiae Strep. anginosus group and Enterococcus faecalis) and some Gram-negative bacteria (Escherichia coli, Enterobacter cloacae, Klebsiella pneumoniae, Pseudomonas aeruginosa)39.
Its mechanism of action consists of inhibition of the activity of bacterial DNA topoisomerase IV and DNA gyrase (topoisomerase II), thus interfering with bacterial DNA replication by preventing the relaxation of positive supercoils introduced as part of the elongation process40. Due to its chemical structure, delafloxacin is weakly acid, unlike other fluoroquinolones, thus displaying a preserved antibacterial action through a reduced minimum inhibitory concentration in environments with low pH40. It is highly protein bound (84%), primarily to albumin, has a large distribution volume (48 L) and has a half-life of 3.7-8.5 hours, with a peak serum concentration of 7.45 mg/L after a 1 hour infusion40. Its clearance is ensured in approximately equal proportions by renal and non-renal pathways40.
Delafloxacin received FDA approval after demonstrating its non-inferiority to the combination of vancomycin and aztreonam in two phase 3 studies, in adult patients with acute bacterial skin and skin structure infections (PROCEED Study Group)41-43. Its efficacy in community-acquired bacterial pneumonia is currently under investigation in a phase III clinical trial (NCT02679573)44.
The drug is contraindicated in patients with known hypersensitivity to delafloxacin or any of the fluoroquinolone class of antibacterial drugs. Its common side effects include nausea, diarrhea, headache, transaminase elevations and vomiting43. Of note, FDA issued a black box warning related to the risk of tendinitis, tendon rupture, peripheral neuropathy, CNS effects, exacerbation of myasthenia gravis, hypersensitivity reactions and Clostridium Difficile-associated diarrhoea40,43.
Unlike other fluoroquinolones, it does not seem to elevate the risk of QTc interval prolongation on the EKG, or phototoxicity. Its use appears to be safe in patients with renal disease or hepatic impairment40,43,45,46.
2.5 Vabomere
Vabomere is the first combination of a carbapenem and a β-lactamase inhibitor, consisting of meropenem, a broad spectrum carbapenem antibacterial, and vaborbactam, a β-lactamase inhibitor approved by the FDA in August 2017 for the treatment of complicated urinary infections (including pyelonephritis) in adult patients47.
Meropenem-susceptible microorganisms include Gram-negative bacteria like Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae species complex or Pseudomonas aeruginosa48. After binding to penicillin-binding proteins, meropenem inhibits the final step of peptidoglycan synthesis in bacterial cell walls and thus the biosynthesis of cell walls, leading to bacterial lysis49. Vaborbactam, a β-lactamase inhibitor, has no antibacterial efficacy on its own, but it blocks carbapenemases produced by Klebsiella pneumoniae and other β-lactamases, which could cause degradation of meropenem, thus leading to in vitro activity against nearly all (99%) of Klebsiella pneumoniae carbapenemase producing Enterobacteriaceae49.
Meropenem is 2% protein bound, has a distribution volume of 20.2 L and a half-life of 2.3 hours49. Vaborbactam is 33% protein bound, has a distribution volume of 18.6 L and a half-life of 2.2 hours49. While vaborbactam is not well metabolized and is excreted in the urine over a 2 day period, approximately 30% of the meropenem dose is metabolized by hydrolysis of the β-lactam ring to an inactive form, which is excreted in the urine, and between 40-60% of the meropenem dose is excreted unchanged within 2 days49.
Meropenem-vaborbactam received FDA approval after demonstrating its superiority over piperacilline-tazobactam for the treatment of complicated urinary tract infections, including acute pyelonephritis, in a phase 3, multicenter, randomized, double-blind, double-dummy study including 550 patients (TANGO I trial)50. Due to its activity against multi-drug resistant bacteria, meropenem-vaborbactam shows promising implications in the treatment of ventilator-associated pneumonia (TANGO II trial)51,52. A phase III, multicenter, prospective, randomized, double-blinded TANGO III trial comparing vabomere and piperacilline/tazobactam in hospital-acquired or ventilator-associated pneumonia is launced and estimated to be completed in 2010 (NCT03006679). The drug is contraindicated in patients with hypersensitivity to any of the two components or to other drugs in the same class, and the most frequently encountered adverse reactions include headache, phlebitis or infusion site reactions, and diarrhea47. Rare but severe side effects include hypersensitivity reactions, seizures (especially in patients treated with valproic acid), Clostridium difficile-associated diarrhea, thrombocytopenia, neuromotor impairment, development of drug resistant bacteria and overgrowth of nonsusceptible organisms49. Precautions should be taken and doses should be adapted for the patients with renal function impairment53.
2.6 Ozenoxacin
Ozenoxacin, a non-fluorinated quinolone, received FDA approval in December 2017 for the topical treatment of impetigo caused by Staphylococcus aureus or Streptococcus pyogenes in adult and pediatric patients older than 2 months54.
The drug is bactericidal against susceptible microorganisms through inhibition of bacterial DNA replication enzymes, DNA gyrase A and topoisomerase IV54. After topical application, the majority of ozenoxacin plasma samples were below the limit of quantification, suggesting no systemic absorption. Thus, the distribution, metabolism and excretion of ozenoxacin have not been investigated in humans55.
FDA approval was granted after a phase 3 randomized, double-blind, multicenter study proved the efficacy and safety of ozenoxacin in the treatment of impetigo56. Adverse reactions, such as rosacea or seborrheic dermatitis, were rarely reported, but prolonged use may result in overgrowth of nonsusceptible bacteria and fungi54.
3. Antimicrobials under investigation
Other drugs or combinations are in different stages of clinical research. World Health organization extensively reviewed, from a global health perspective, the antibacterial agents in clinical development in 2017, with focus on innovativeness and expected activity on priority pathogens57. Despite the total number of 33 new antibiotic entities and 9 new biological agents targeting global priority pathogens, the 7 drugs against Mycobacterium tuberculosis and the 9 agents against C. difficile, the review noticed an insufficient “clinical pipeline” and “a lack of potential treatment options for priority resistant bacteria, especially for multidrug- and extensively drug-resistant Gram-negative pathogens”, including resistant to anti-tuberculosis treatment57.
A recent article by Bassetti M. et al. reviews the potential drugs in treating ventilator-associated pneumonia51. Beside the already FDA-approved tedizolid (2014), ceftolozane/tazobactam (2014), ceftazidime/avibactam (2015) and meropenem/vaborbactam (2017), there are studies concerning cefiderocol, imipenem/relebactam, ceftaroline/avibactam, aztreonam/avibactam, plazomicin, eravacyclin, murapavadin51. From this list, cefiderocol stands out, since its FDA and EMA approvals are expected in 201858.
Cefiderocol, also known as S-649266, is a siderophore cephalosporine showing potency against Gram-negative bacteria58. A particular capacity to chelate iron makes it able to penetrate the outer bacterial membrane through bacterial iron-transporting systems59. A large study using clinical collections from North America and Europe evaluated the bacterial spectrum of cefiderocol and found a good activity against Gram-negative bacteria, even for resistant species, like meropenem-non susceptible Enterobacteriaceae (MIC <4 mcg/ml for 97% of isolates)60. This effect was also noted for Pseudomonas aeruginosa and, interestingly, for Acinetobacter baumanni, making this new molecule particularly valuable in the case of difficult to treat resistant infections60. 3 Phase III clinical trials are evaluating the potential role and safety of cefiderocol in treating carbapanem-resistant enterobacteria infections, ventilator associated pneumonia or urinary tract infections, and their results are awaited with interest (NCT02714595, NCT03032380, NCT02321800).
Recently, the future perspectives in antimicrobial research have become a little more optimistic. Description of a specific isolation chip by Nichols et al. that allows the identification of new antibiotic sources in soil microorganisms has opened the gates for new discoveries61. Antimicrobial discovery had been significantly slowed down by the difficulties in culturing environmental microorganisms that were concerning 99% of the species62. These methodological improvements lead to the discovery of new classes of antibiotics. One very promising anti-Gram-positive bacteria recently described is teixobactin, an 11-residue, macrocyclic depsipeptide, first identified by Ling et al. in 2015, which possesses a very strong inhibitory action on peptidoglycan synthesis63. New species of β-proteobacteria temporarily named Eleftheria terrae have been used during the process63. This molecule proved to be extremely potent in vitro against Staphylococcus aureus, including methicillin-resistant variants (minimal inhibitory concentration (MIC) 0.25 mcg/ml), Mycobacterium tuberculosis (MIC 0.125 mcg/ml), Vancomycin-resistant Enterococcous faecium (MIC 0.5 mcg/ml), Clostridium Difficile (MIC 5 ng/ml) and Bacillus anthracis (MIC20 ng/ml), but not on Gram-negative bacteria63. During this study, no resistance was observed and teixobactin shown good efficacy and tolerance in methicillin-resistant Staphylococcus aureus (MRSA) induced-sepsis mice models63.
This strong bactericidal effect can be explained by its ability of blocking the cell wall synthesis through synergistic inhibition of peptidoglycan and teichoic acid formation, by binding the precursor lipid II and lipid III, causing cell wall injury and the destruction of bacterial cell64. No study in humans has yet been performed and the road to clinical practice might be long, but there are hopes that this new class could be the long awaited solution for the burden of MRSA and VRE (Vancomycin-resistant Enterococcus) infections, as well as for the resistant strains of Mycobacterium tuberculosis65.
Another newly discovered antibiotic class using a culture-independent approach are the malacidins, Hover et al. publishing their results in 201866. The malacidins have a lipopeptidic structure, with protidic core, which includes 4 non-proteinogeic aminoacids66. The 10 members of this class are differentiated by a methylene group at the end of the lipidic branch66. Malacidin A revealed a calcium-dependent bactericidal in vitro and in vivo effect on Gram-positive bacteria like Staphylococcus aureus, including vancomycin-resistant variants. No resistance was detected66. As in the case of teixobactin, the potential clinical benefit is significant and the evolution of the studies on malacidins is followed with a great interest.
4. Conclusion
The last 3 years brought new antimicrobial drugs available for clinical use. Some agents like obiltoxaximab, bezlotoxumab antibodies and ozenoxacin target narrow areas of interest. Future studies and clinical practice will define the place of delafloxacin among the other acute skin infection treatments, and likewise concerning the role of the new combinations - ceftazidime/avibactam and meropenem/vaborbactam - in the practical management resistant bacterial infections in the Intensive Care Unit. The recent discovery of new antibiotic classes and the augmentation of the source pool for further research have brought a glimmer of optimism. But the road to actual clinical benefit might be long and the past experience has taught us that resistance can develop even for very promising molecules. A shared and vigorous research effort is continuously needed in order to improve the therapeutic options for the increasingly resistant and highly adaptable germs.
Acknowledgments
We are grateful to the Centre Hospitalier Lyon Sud, Pierre Benite, France for support.
Footnotes
Conflict of interests: The authors declare no conflicts of interest.
Food and Drug Administration (FDA); European Medicines Agency (EMA); Area under the curve (AUC); New Delhi metallo-β-lactamase (NDM); Verona integron-encoded metallo-β-lactamase (VIM); Imipenmase (IMP); Complicated intra-abdominal infections (cIAIs); Complicated urinary tract infections (cUTIs); Hospital acquired bacterial pneumonia (HABP); Ventilator associated bacterial pneumonia (VABP); Acute bacterial skin and skin structure infections (ABSSI); Intravenous (IV); Central Nervous System (CNS); Hours (h);
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Antibacterial resistance worldwide: causes, challenges and responses. Levy Stuart B, Marshall Bonnie. Nature Medicine. 2004;10(12s):S122-S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 2.Strategies to reduce curative antibiotic therapy in intensive care units (adult and paediatric) Bretonnière Cédric, Leone Marc, Milési Christophe, Allaouchiche Bernard, Armand-Lefevre Laurence, Baldesi Olivier, Bouadma Lila, Decré Dominique, Figueiredo Samy, Gauzit Rémy, Guery Benoît, Joram Nicolas, Jung Boris, Lasocki Sigismond, Lepape Alain, Lesage Fabrice, Pajot Olivier, Philippart François, Souweine Bertrand, Tattevin Pierre, Timsit Jean-François, Vialet Renaud, Zahar Jean Ralph, Misset Benoît, Bedos Jean-Pierre. Intensive Care Medicine. 2015;41(7):1181-1196. doi: 10.1007/s00134-015-3853-7. [DOI] [PubMed] [Google Scholar]
- 3.In vivo efficacy of glycopeptide-colistin combination therapies in a Galleria mellonella model of Acinetobacter baumannii infection. Hornsey M, Wareham D W. Antimicrobial agents and chemotherapy. 2011;55(7):3534–7. doi: 10.1128/AAC.00230-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A resurgence of β-lactamase inhibitor combinations effective against multidrug-resistant Gram-negative pathogens. Bush Karen. International journal of antimicrobial agents. 2015;46(5):483–93. doi: 10.1016/j.ijantimicag.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 5.General principles of antibiotic resistance in bacteria. Martinez Jose L. Drug discovery today. Technologies. 2014;11:33–9. doi: 10.1016/j.ddtec.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Bassetti Matteo, De Waele Jan J, Eggimann Philippe, Garnacho-Montero Josè, Kahlmeter Gunnar, Menichetti Francesco, Nicolau David P, Paiva Jose Arturo, Tumbarello Mario, Welte Tobias, Wilcox Mark, Zahar Jean Ralph, Poulakou Garyphallia. Intensive care medicine. 2015;41(5):776–95. doi: 10.1007/s00134-015-3719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Impact of a program combining pre-authorization requirement and post-prescription review of carbapenems: an interrupted time-series analysis. Delory T, De Pontfarcy A, Emirian A, About F, Berdougo B, Brun-Buisson C, Lesprit P. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2013;32(12):1599–604. doi: 10.1007/s10096-013-1918-5. [DOI] [PubMed] [Google Scholar]
- 8.Prevention of Ventilator-Associated Pneumonia: The Multimodal Approach of the Spanish ICU "Pneumonia Zero" Program. Álvarez-Lerma Francisco, Palomar-Martínez Mercedes, Sánchez-García Miguel, Martínez-Alonso Montserrat, Álvarez-Rodríguez Joaquín, Lorente Leonardo, Arias-Rivera Susana, García Rosa, Gordo Federico, Añón José M, Jam-Gatell Rosa, Vázquez-Calatayud Mónica, Agra Yolanda. Critical care medicine. 2018;46(2):181–188. doi: 10.1097/CCM.0000000000002736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Derde Lennie P G, Cooper Ben S, Goossens Herman, Malhotra-Kumar Surbhi, Willems Rob J L, Gniadkowski Marek, Hryniewicz Waleria, Empel Joanna, Dautzenberg Mirjam J D, Annane Djillali, Aragão Irene, Chalfine Annie, Dumpis Uga, Esteves Francisco, Giamarellou Helen, Muzlovic Igor, Nardi Giuseppe, Petrikkos George L, Tomic Viktorija, Martí Antonio Torres, Stammet Pascal, Brun-Buisson Christian, Bonten Marc J M. The Lancet. Infectious diseases. 2014;14(1):31–39. doi: 10.1016/S1473-3099(13)70295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Population Pharmacokinetics of Colistin Methanesulfonate and Colistin in Critically Ill Patients with Acute Renal Failure Requiring Intermittent Hemodialysis. Jacobs M, Grégoire N, Mégarbane B, Gobin P, Balayn D, Marchand S, Mimoz O, Couet W. Antimicrobial agents and chemotherapy. 2016;60(3):1788–93. doi: 10.1128/AAC.01868-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mechanisms of antimicrobial resistance in Gram-negative bacilli. Ruppé Étienne, Woerther Paul-Louis, Barbier François. Annals of intensive care. 2015;5(1):61. doi: 10.1186/s13613-015-0061-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceftazidime-avibactam: a novel cephalosporin/β-lactamase inhibitor combination. Zhanel George G, Lawson Christopher D, Adam Heather, Schweizer Frank, Zelenitsky Sheryl, Lagacé-Wiens Philippe R S, Denisuik Andrew, Rubinstein Ethan, Gin Alfred S, Hoban Daryl J, Lynch Joseph P, Karlowsky James A. Drugs. 2013;73(2):159–77. doi: 10.1007/s40265-013-0013-7. [DOI] [PubMed] [Google Scholar]
- 13.Ceftazidime-avibactam: an evidence-based review of its pharmacology and potential use in the treatment of Gram-negative bacterial infections. Lagacé-Wiens Philippe, Walkty Andrew, Karlowsky James A. Core evidence. 2014;9:13–25. doi: 10.2147/CE.S40698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.In vitro antibacterial activity of the ceftazidime-avibactam (NXL104) combination against Pseudomonas aeruginosa clinical isolates. Levasseur Premavathy, Girard Anne-Marie, Claudon Monique, Goossens Herman, Black Michael T, Coleman Kenneth, Miossec Christine. Antimicrobial agents and chemotherapy. 2012;56(3):1606–8. doi: 10.1128/AAC.06064-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avycaz FDA Highlights of prescribing information. Food and Drug Administration. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/206494s003lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/206494s003lbl.pdf
- 16.Zavicefta EMA summary of product characteristics. Food and Drug Administration. 2018. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/ 004027/WC500210234.pdf http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/ 004027/WC500210234.pdf
- 17.Efficacy and Safety of Ceftazidime-Avibactam Plus Metronidazole Versus Meropenem in the Treatment of Complicated Intra-abdominal Infection: Results From a Randomized, Controlled, Double-Blind, Phase 3 Program. Mazuski John E, Gasink Leanne B, Armstrong Jon, Broadhurst Helen, Stone Greg G, Rank Douglas, Llorens Lily, Newell Paul, Pachl Jan. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;62(11):1380–1389. doi: 10.1093/cid/ciw133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ceftazidime-avibactam or best available therapy in patients with ceftazidime-resistant Enterobacteriaceae and Pseudomonas aeruginosa complicated urinary tract infections or complicated intra-abdominal infections (REPRISE): a randomised, pathogen-directed, phase 3 study. Carmeli Yehuda, Armstrong Jon, Laud Peter J, Newell Paul, Stone Greg, Wardman Angela, Gasink Leanne B. The Lancet. Infectious diseases. 2016;16(6):661–673. doi: 10.1016/S1473-3099(16)30004-4. [DOI] [PubMed] [Google Scholar]
- 19.Efficacy and safety of ceftazidime-avibactam versus imipenem-cilastatin in the treatment of complicated urinary tract infections, including acute pyelonephritis, in hospitalized adults: results of a prospective, investigator-blinded, randomized study. Vazquez José A, González Patzán Luis Demetrio, Stricklin David, Duttaroy Dipesh D, Kreidly Zouheir, Lipka Joy, Sable Carole. Current medical research and opinion. 2012;28(12):1921–31. doi: 10.1185/03007995.2012.748653. [DOI] [PubMed] [Google Scholar]
- 20.Ceftazidime-avibactam versus meropenem in nosocomial pneumonia, including ventilator-associated pneumonia (REPROVE): a randomised, double-blind, phase 3 non-inferiority trial. Torres Antoni, Zhong Nanshan, Pachl Jan, Timsit Jean-François, Kollef Marin, Chen Zhangjing, Song Jie, Taylor Dianna, Laud Peter J, Stone Gregory G, Chow Joseph W. The Lancet. Infectious diseases. 2018;18(3):285–295. doi: 10.1016/S1473-3099(17)30747-8. [DOI] [PubMed] [Google Scholar]
- 21.Clinical Outcomes, Drug Toxicity, and Emergence of Ceftazidime-Avibactam Resistance Among Patients Treated for Carbapenem-Resistant Enterobacteriaceae Infections. Shields Ryan K, Potoski Brian A, Haidar Ghady, Hao Binghua, Doi Yohei, Chen Liang, Press Ellen G, Kreiswirth Barry N, Clancy Cornelius J, Nguyen M Hong. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;63(12):1615–1618. doi: 10.1093/cid/ciw636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Editorial Commentary: Ceftazidime-Avibactam and Carbapenem-Resistant Enterobacteriaceae: “We're Gonna Need a Bigger Boat”. Spellberg Brad, Bonomo Robert A. Clinical Infectious Diseases. 2016;63(12):1619-1621. doi: 10.1093/cid/ciw639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ANTHIM (obiltoxaximab). FDA Highlights of prescribing information. Food and Drug Administration. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125509lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125509lbl.pdf
- 24.Obiltoxaximab: Adding to the Treatment Arsenal for Bacillus anthracis Infection. Hou Audrey W, Morrill Amanda M. The Annals of pharmacotherapy. 2017;51(10):908–913. doi: 10.1177/1060028017713029. [DOI] [PubMed] [Google Scholar]
- 25.Approval of Raxibacumab for the Treatment of Inhalation Anthrax Under the US Food and Drug Administration “Animal Rule”. Tsai Chia-Wei, Morris Stephen. Frontiers in Microbiology. 2015;6 doi: 10.3389/fmicb.2015.01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Efficacy Projection of Obiltoxaximab for Treatment of Inhalational Anthrax across a Range of Disease Severity. Yamamoto Brent J, Shadiack Annette M, Carpenter Sarah, Sanford Daniel, Henning Lisa N, O'Connor Edward, Gonzales Nestor, Mondick John, French Jonathan, Stark Gregory V, Fisher Alan C, Casey Leslie S, Serbina Natalya V. Antimicrobial agents and chemotherapy. 2016;60(10):5787–95. doi: 10.1128/AAC.00972-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obiltoxaximab Prevents Disseminated Bacillus anthracis Infection and Improves Survival during Pre- and Postexposure Prophylaxis in Animal Models of Inhalational Anthrax. Yamamoto Brent J, Shadiack Annette M, Carpenter Sarah, Sanford Daniel, Henning Lisa N, Gonzales Nestor, O'Connor Edward, Casey Leslie S, Serbina Natalya V. Antimicrobial agents and chemotherapy. 2016;60(10):5796–805. doi: 10.1128/AAC.01102-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Animal-to-Human Dose Translation of Obiltoxaximab for Treatment of Inhalational Anthrax Under the US FDA Animal Rule. Nagy C F, Mondick J, Serbina N, Casey L S, Carpenter S E, French J, Guttendorf R. Clinical and translational science. 2017;10(1):12–19. doi: 10.1111/cts.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Safety, Pharmacokinetics, and Immunogenicity of Obiltoxaximab After Intramuscular Administration to Healthy Humans. Nagy Christa F, Leach Timothy S, King Alex, Guttendorf Robert. Clinical pharmacology in drug development. 2017 doi: 10.1002/cpdd.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Center for Drug Evaluation and Research. Application Number: 761046Orig1s000. Summary Review. Food and Drug Administration. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/ 2016/761046Orig1s000Approv.pdf https://www.accessdata.fda.gov/drugsatfda_docs/nda/ 2016/761046Orig1s000Approv.pdf
- 31.Center for Drug Evaluation and Research. Application Number: 761046Orig1s000. Microbiology/ Virology Review. Food and Drug Administration. 2016. https://www.accessdata. fda.gov/ drugsatfda_docs/nda/2016/761046Orig1s000 https://www.accessdata. fda.gov/ drugsatfda_docs/nda/2016/761046Orig1s000
- 32.Mechanism of action and epitopes of Clostridium difficile toxin B-neutralizing antibody bezlotoxumab revealed by X-ray crystallography. Orth Peter, Xiao Li, Hernandez Lorraine D, Reichert Paul, Sheth Payal R, Beaumont Maribel, Yang Xiaoyu, Murgolo Nicholas, Ermakov Grigori, DiNunzio Edward, Racine Fred, Karczewski Jerzy, Secore Susan, Ingram Richard N, Mayhood Todd, Strickland Corey, Therien Alex G. The Journal of biological chemistry. 2014;289(26):18008–21. doi: 10.1074/jbc.M114.560748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bezlotoxumab: A Review in Preventing Clostridium difficile Infection Recurrence. Deeks Emma D. Drugs. 2017;77(15):1657–1663. doi: 10.1007/s40265-017-0809-y. [DOI] [PubMed] [Google Scholar]
- 34.Center for Drug Evaluation and Research. Application Number: 761046Orig1s000. Pharmacology Review. Food and Drug Administration. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761046Orig1s000PharmR.pdf https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761046Orig1s000PharmR.pdf
- 35.Cost-effectiveness of Bezlotoxumab Compared With Placebo for the Prevention of Recurrent Clostridium difficile Infection. Prabhu Vimalanand S, Dubberke Erik R, Dorr Mary Beth, Elbasha Elamin, Cossrow Nicole, Jiang Yiling, Marcella Stephen. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;66(3):355–362. doi: 10.1093/cid/cix809. [DOI] [PubMed] [Google Scholar]
- 36.Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. Wilcox Mark H., Gerding Dale N., Poxton Ian R., Kelly Ciaran, Nathan Richard, Birch Thomas, Cornely Oliver A., Rahav Galia, Bouza Emilio, Lee Christine, Jenkin Grant, Jensen Werner, Kim You-Sun, Yoshida Junichi, Gabryelski Lori, Pedley Alison, Eves Karen, Tipping Robert, Guris Dalya, Kartsonis Nicholas, Dorr Mary-Beth. New England Journal of Medicine. 2017;376(4):305-317. doi: 10.1056/NEJMoa1602615. [DOI] [PubMed] [Google Scholar]
- 37.Preventing recurrence of Clostridium difficile infection: bezlotoxumab. Evidence update from NICE. National Institute for Health and Care Excellence. 2017. https://www.nice.org.uk/advice/es13/chapter/Evidence-review https://www.nice.org.uk/advice/es13/chapter/Evidence-review
- 38.Epitopes and Mechanism of Action of the Clostridium difficile Toxin A-Neutralizing Antibody Actoxumab. Hernandez Lorraine D., Kroh Heather K., Hsieh Edward, Yang Xiaoyu, Beaumont Maribel, Sheth Payal R., DiNunzio Edward, Rutherford Stacey A., Ohi Melanie D., Ermakov Grigori, Xiao Li, Secore Susan, Karczewski Jerzy, Racine Fred, Mayhood Todd, Fischer Paul, Sher Xinwei, Gupta Pulkit, Lacy D. Borden, Therien Alex G. Journal of Molecular Biology. 2017;429(7):1030-1044. doi: 10.1016/j.jmb.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clinical and pharmacokinetic drug evaluation of delafloxacin for the treatment of acute bacterial skin and skin structure infections. Bassetti Matteo, Pecori Davide, Cojutti Piergiorgio, Righi Elda, Pea Federico. Expert Opinion on Drug Metabolism & Toxicology. 2017;13(11):1193-1200. doi: 10.1080/17425255.2017.1386654. [DOI] [PubMed] [Google Scholar]
- 40.What Is Old Is New Again: Delafloxacin, a Modern Fluoroquinolone. Cho Jonathan C., Crotty Matthew P., White Bryan P., Worley Marylee V. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2017;38(1):108-121. doi: 10.1002/phar.2050. [DOI] [PubMed] [Google Scholar]
- 41.Efficacy and safety of delafloxacin compared with vancomycin plus aztreonam for acute bacterial skin and skin structure infections: a Phase 3, double-blind, randomized study. Pullman J, Gardovskis J, Farley B, Sun E, Quintas M, Lawrence L, Ling R, Cammarata S. Journal of Antimicrobial Chemotherapy. 2017;72(12):3471-3480. doi: 10.1093/jac/dkx329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.A comparison of the efficacy and safety of intravenous followed by oral delafloxacin with vancomycin plus aztreonam for the treatment of acute bacterial skin and skin structure infections: a phase 3, multinational, double-blind, randomized study. O'Riordan William, McManus Alison, Teras Juri, Poromanski Ivan, Cruz-Saldariagga Maria, Quintas Megan, Lawrence Laura, Liang ShuJui, Cammarata Sue. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018 doi: 10.1093/cid/ciy165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baxdela (delafloxacin). FDA Highlights of prescribing information. Food and Drug Administration. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208610s000.208611s000lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208610s000.208611s000lbl.pdf
- 44.A phase 3, multicenter, randomized, double‐blind, comparator‐controlled study to evaluate the safety and efficacy of intravenous to oral delafloxacin in adult subjects with community‐acquired bacterial pneumonia. ClinicalTrials.gov. 2018. https://clinicaltrials.gov/ct2/show/NCT02 679573 https://clinicaltrials.gov/ct2/show/NCT02 679573
- 45.Pharmacokinetics of Intravenous Delafloxacin in Patients With End-Stage Renal Disease. Hoover Randall, Alcorn Harry, Lawrence Laura, Paulson Susan K, Quintas Megan, Cammarata Sue K. Journal of clinical pharmacology. 2018;58(7):913–919. doi: 10.1002/jcph.1099. [DOI] [PubMed] [Google Scholar]
- 46.Clinical Pharmacology of Delafloxacin in Patients With Hepatic Impairment. Hoover Randall, Marbury Thomas C, Preston Richard A, Quintas Megan, Lawrence Laura E, Paulson Susan K, Luke David R, Cammarata Sue K. Journal of clinical pharmacology. 2017;57(3):328–335. doi: 10.1002/jcph.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vabomere FDA Highlights of prescribing information. Food and Drug Administration. 2017. https://www.accessdata.fda.gov /drugsatfda_docs/label/2017/209776lbl.pdf https://www.accessdata.fda.gov /drugsatfda_docs/label/2017/209776lbl.pdf
- 48.Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations. Zhanel George G, Lawrence Courtney K, Adam Heather, Schweizer Frank, Zelenitsky Sheryl, Zhanel Michael, Lagacé-Wiens Philippe R S, Walkty Andrew, Denisuik Andrew, Golden Alyssa, Gin Alfred S, Hoban Daryl J, Lynch Joseph P, Karlowsky James A. Drugs. 2018;78(1):65–98. doi: 10.1007/s40265-017-0851-9. [DOI] [PubMed] [Google Scholar]
- 49.Meropenem/Vaborbactam, the First Carbapenem/β-Lactamase Inhibitor Combination. Cho Jonathan C, Zmarlicka Monika T, Shaeer Kristy M, Pardo Joe. The Annals of pharmacotherapy. 2018;52(8):769–779. doi: 10.1177/1060028018763288. [DOI] [PubMed] [Google Scholar]
- 50.Effect of Meropenem-Vaborbactam vs Piperacillin-Tazobactam on Clinical Cure or Improvement and Microbial Eradication in Complicated Urinary Tract Infection: The TANGO I Randomized Clinical Trial. Kaye Keith S, Bhowmick Tanaya, Metallidis Symeon, Bleasdale Susan C, Sagan Olexiy S, Stus Viktor, Vazquez Jose, Zaitsev Valerii, Bidair Mohamed, Chorvat Erik, Dragoescu Petru Octavian, Fedosiuk Elena, Horcajada Juan P, Murta Claudia, Sarychev Yaroslav, Stoev Ventsislav, Morgan Elizabeth, Fusaro Karen, Griffith David, Lomovskaya Olga, Alexander Elizabeth L, Loutit Jeffery, Dudley Michael N, Giamarellos-Bourboulis Evangelos J. JAMA. 2018;319(8):788–799. doi: 10.1001/jama.2018.0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.New antibiotics for ventilator-associated pneumonia. Bassetti Matteo, Vena Antionio, Castaldo Nadia, Righi Elda, Peghin Maddalena. Current opinion in infectious diseases. 2018;31(2):177–186. doi: 10.1097/QCO.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 52.Meropenem-Vaborbactam vs. Best Available Therapy for Carbapenem-Resistant Enterobacteriaceae Infections in TANGO II: Outcomes in Immunocompromised Patients. Paterson David L, Kwak Eun J, Bhowmick Tanaya, Alexander Elizabeth, Loutit Jeffrey S, Zhang Shu, Dudley Michael N, Walsh Thomas J. Open Forum Infectious Diseases. 2017;4(suppl_1):S537-S537. [Google Scholar]
- 53.Single-Dose Pharmacokinetics and Safety of Meropenem-Vaborbactam in Subjects with Chronic Renal Impairment. Rubino Christopher M., Bhavnani Sujata M., Loutit Jeffery S., Lohse Brooke, Dudley Michael N., Griffith David C. Antimicrobial Agents and Chemotherapy. 2018;62(3) doi: 10.1128/AAC.02103-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ozenoxacin. FDA Highlights of prescribing information. Food and Drug Administration. 2017. https://www.accessdata.fda.gov/ drugsatfda_docs/label/2017/208945lbl.pdf https://www.accessdata.fda.gov/ drugsatfda_docs/label/2017/208945lbl.pdf
- 55.Systemic bioavailability and safety of twice-daily topical ozenoxacin 1% cream in adults and children with impetigo. Gropper Savion, Cepero Ana Luisa, Santos Benjamin, Kruger Dawie. Future Microbiology. 2014;9(8s):S33-S40. doi: 10.2217/fmb.14.85. [DOI] [PubMed] [Google Scholar]
- 56.Ozenoxacin 1% cream in the treatment of impetigo: a multicenter, randomized, placebo- and retapamulin-controlled clinical trial. Gropper Savion, Albareda Nuria, Chelius Klaus, Kruger Dawie, Mitha Ismail, Vahed Yacoob, Gani Mashra, García-Alonso Fernando. Future microbiology. 2014;9(9):1013–23. doi: 10.2217/fmb.14.78. [DOI] [PubMed] [Google Scholar]
- 57.Antibacterial agents in clinical development. World Health Organization. 2017. http://www.who.int/medicines/news/2017/IAU_AntibacterialAgentsClinicalDevelopment_webfinal_2017_09_19.pdf http://www.who.int/medicines/news/2017/IAU_AntibacterialAgentsClinicalDevelopment_webfinal_2017_09_19.pdf
- 58.Cefiderocol: a novel siderophore cephalosporin. Choi Justin J, McCarthy Matthew W. Expert opinion on investigational drugs. 2018;27(2):193–197. doi: 10.1080/13543784.2018.1426745. [DOI] [PubMed] [Google Scholar]
- 59.Catechol Siderophore Transport by Vibrio cholerae. Wyckoff Elizabeth E, Allred Benjamin E, Raymond Kenneth N, Payne Shelley M. Journal of bacteriology. 2015;197(17):2840–9. doi: 10.1128/JB.00417-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.In Vitro Activity of the Siderophore Cephalosporin, Cefiderocol, against a Recent Collection of Clinically Relevant Gram-Negative Bacilli from North America and Europe, Including Carbapenem-Nonsusceptible Isolates (SIDERO-WT-2014 Study). Hackel Meredith A, Tsuji Masakatsu, Yamano Yoshinori, Echols Roger, Karlowsky James A, Sahm Daniel F. Antimicrobial agents and chemotherapy. 2017;61(9) doi: 10.1128/AAC.00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Use of Ichip for High-Throughput In Situ Cultivation of "Uncultivable" Microbial Species. Nichols D., Cahoon N., Trakhtenberg E. M., Pham L., Mehta A., Belanger A., Kanigan T., Lewis K., Epstein S. S. Applied and Environmental Microbiology. 2010;76(8):2445-2450. doi: 10.1128/AEM.01754-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Platforms for antibiotic discovery. Lewis Kim. Nature reviews. Drug discovery. 2013;12(5):371–87. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 63.A new antibiotic kills pathogens without detectable resistance. Ling Losee L, Schneider Tanja, Peoples Aaron J, Spoering Amy L, Engels Ina, Conlon Brian P, Mueller Anna, Schäberle Till F, Hughes Dallas E, Epstein Slava, Jones Michael, Lazarides Linos, Steadman Victoria A, Cohen Douglas R, Felix Cintia R, Fetterman K Ashley, Millett William P, Nitti Anthony G, Zullo Ashley M, Chen Chao, Lewis Kim. Nature. 2015;517(7535):455–9. doi: 10.1038/nature14098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dual Targeting of Cell Wall Precursors by Teixobactin Leads to Cell Lysis. Homma Tomoyuki, Nuxoll Austin, Gandt Autumn Brown, Ebner Patrick, Engels Ina, Schneider Tanja, Götz Friedrich, Lewis Kim, Conlon Brian P. Antimicrobial agents and chemotherapy. 2016;60(11):6510–6517. doi: 10.1128/AAC.01050-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.A new antibiotic and the evolution of resistance. Arias Cesar A, Murray Barbara E. The New England journal of medicine. 2015;372(12):1168–70. doi: 10.1056/NEJMcibr1500292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Culture-independent discovery of the malacidins as calcium-dependent antibiotics with activity against multidrug-resistant Gram-positive pathogens. Hover Bradley M, Kim Seong-Hwan, Katz Micah, Charlop-Powers Zachary, Owen Jeremy G, Ternei Melinda A, Maniko Jeffrey, Estrela Andreia B, Molina Henrik, Park Steven, Perlin David S, Brady Sean F. Nature microbiology. 2018;3(4):415–422. doi: 10.1038/s41564-018-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]