Abstract
Bacterial resistance to existent antibiotherapy is a perpetual internationally-recognized problem. Year after year, there is a continuous need for novel antibacterial drugs and this research and development efforts recently resulted in few new drugs or combination of drugs proposed for the use into the clinic. This review focuses on the novel US FDA approved antibacterial agents in the last two years (2018-2019). Plazomicin, eravacycline, sarecycline, omadacycline, rifamycin (2018) and imipenem, cilastatin and relebactam combination, pretomanid, lefamulin, cefiderocol (2019) are new therapeutic options. Plazomicin aminoglycoside antibiotic targets Enterobacteriaceae infections, being mainly used for the complicated urinary tract infections. The fully synthetic fluorocycline eravacycline gained approval for the complicated intra-abdominal infections. The tetracycline-derived antibiotic sarecycline might be a useful strategy for the management of non-nodular moderate to severe acne, while the other tetracycline-derived antibiotic approved, omadacycline, may be used for the patients with acute bacterial skin and skin structure infections and community-acquired bacterial pneumonia. The already-known RNA-synthesis suppressor rifamycin is now also approved for noninvasive Escherichia Coli-caused travelers' diarrhea. Two combinatorial strategies were approved for complicated urinary tract infections, complicated intra-abdominal infections (imipenem, cilastatin and relebactam) and lung tuberculosis (pretomanid in combination with bedaquiline and linezolid). Lefamulin is a semisynthetic pleuromutilin antibiotic for community-acquired bacterial pneumonia, while cefiderocol, a cephalosporin antibiotic is the last antibacterial drug approved in 2019, for the use in complicated urinary tract infections. Despite of these new developments, there is an ongoing need and urgency to develop novel antibiotic strategies and drugs to overrun the bacterial resistance to antibiotics.
Keywords: FDA approved drugs, Plazomicin, Eravacycline, Sarecycline, Omadacycline. Rifamycin, Imipenem, Cilastatin and Relebactam, Pretomanid, Lefamulin, Cefiderocol, 2018, 2019.
1. Introduction
The run to overcome the rapid bacterial resistance started with the initial use of antibiotics. However, after decades of struggling in research and in clinical practice, this run is rather a marathon than a sprint.
Despite sustained efforts, the physicians are continuously confronting worldwide with the threat of bacterial resistance1-3. The burden on public health contributed to the creation and implementation of strategies on rational antibiotic use and on limiting the spread of resistant bacteria, the so called antibiotic stewardship4-7.
Other strategy in this direction is to optimize the existing pharmaceutical arsenal, through novel combinations and new indications8. However, the number and efficiency of these drugs is far from covering all the existing needs and to fully combat the highly adaptive bacterial microorganisms. Moreover, pan-resistant bacteria emergence has been already described9,10. Other evidences further consider the non-negligible role of environmental and agriculture-related factors11,12.
Antibiotic stewardship has proved its efficiency, but it has its own limits and challenges13-15. However, the quest for new efficient molecules have to continue, remaining a pillar of anti-multidrug resistant germs strategy16.
We briefly review here the novel antibacterial agents approved by the United States Food and Drug Administration (US FDA) during the past 2 years (2018 and 2019) with the hope to further encourage the scientific community in continuing the development of new therapeutic agents for targeting the resistance of bacteria. The recently approved antibacterial drugs and drug combinations were identified using FDA’s website (https://www. accessdata.fda.gov; www.fda.gov) and Center Watch’s site (https://www.centerwatch.com/drug-information /fda-approved-drugs/).
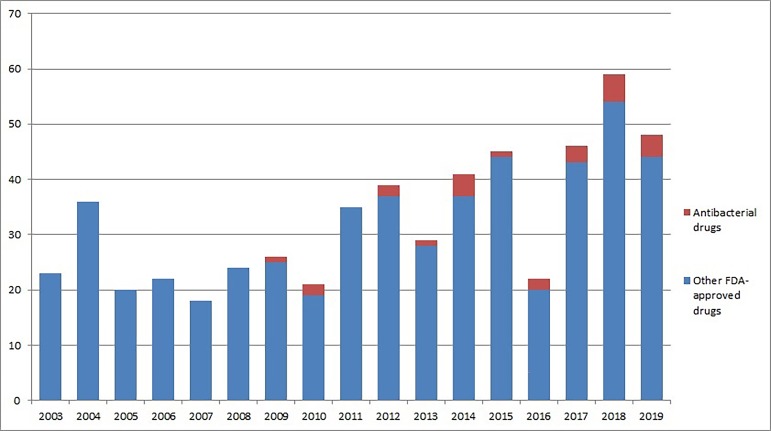
We reviewed the total number of drugs and drug combinations (blue) and number of antibacterial drugs and drug combinations (red) approved by the US FDA in the past 17 years (2003-2019) for each individual year(Figure 1, updated and modified from17). There is a clear upward trend in terms of the number of antibacterial drugs approved by year.
Figure 1. Novel FDA-approved antibacterial and non-bacterial drugs by year (last 17 years).
(updated and modified from17)
To the date of this review, 9 antibacterial drugs or combination of drugs were approved for 2018 and 2019, from a total of 107 introduced molecules. In 2018, the 5 approved antibacterial drugs or combinations of drugs represented 8.4% of the total of 59 new drugs, while in 2019 the 4 new single or combination of antibacterial drugs represented 8.33% of the 48 approved molecules. This represents a significant increase from the previous years (Figure 1), since the number of antibacterial drugs or regimens in the past two years has doubled as compared to the two previous years.
We identified 9 novel FDA approved antibacterial drugs: plazomicin aminoglycoside antibiotic, the fully synthetic fluorocycline eravacycline, tetracycline-derived antibiotics sarecycline and omadacycline, RNA synthesis suppressor rifamycin, two combinatorial strategies (imipenem (carbapenem antibiotic), cilastatin and relebactam and pretomanid (nitroimidazole) in combination with bedaquiline and linezolid), the semisynthetic pleuromutilin antibiotic lefamulin and cefiderocol, a cephalosporin antibiotic (Table 1 & Table 2). These drugs are briefly discussed by FDA-approval year in the next section.
Table 1. Main characteristics of the described antibacterial drugs FDA approved in 201818-35.
Food and Drug Administration (FDA), European Medicines Agency (EMA), complicated urinary tract infections (cUTIs), complicated intra-abdominal infections (cIAIs), community-acquired bacterial pneumonia (CABP), acute bacterial skin and skin structure infections (ABSSI), intravenous (IV), oral administration/per oral (PO), hours (h); doses and duration have to be verified in the most recent prescriptions reglementations, according to the local laws before administration.
| NAME (generic/brand/ class) | Approval status | Indication | Administration | Dose and duration |
|---|---|---|---|---|
| Plazomicin (Zemdri)/ aminoglycoside antibiotic COMPANY: Achaogen, Inc. (CA, USA) | FDA: approved in June 2018 EMA: application submitted (2018) | cUTIs; Enterobacteriaceae infections | IV infusion, every 24 hours for 4-7 days. | A. Dosage regimen (adults with CrCl>60ml/min): 15 mg/kg every 24 hours B. Dosage regimen (adults with CrCl>30 <60 ml/min): 10 mg/kg every 24h C. Dosage regimen (adults with CrCl>15 <30 ml/min): 10 mg/kg every 48h |
| Eravacycline (Xerava) / fully synthetic fluorocycline COMPANY: Tetraphase Pharmaceutical (MA, USA) | FDA: approved in August 2018 EMA: approved in September 2018 | cIAI | IV 60 min infusion, given once every 12 hours for a total of 4 to 14 days; dose is patient’s weight dependent | Adult patients (≥18 years of age) with cIAI: administer 1mg/kg, every 12h, by IV infusion (~ 60min); recommended duration of treatment is 4 to 14 days |
| Sarecycline (Seysara) / tetracycline-derived antibiotic COMPANY: Paratek Pharmaceuticals (MA, USA) and Allergan plc (USA) – acquired by Almirall SA (Spain) | FDA: approved in October 2018 EMA: not yet approved | non-nodular moderate to severe acne | PO administration with food | A. Adult <54 kg: 60 mg PO every Day 55-84 kg: 100 mg PO every Day 85-136 kg: 150 mg PO every Day B. Children ≥9 years 33-54 kg: 60 mg PO every Day 55-84 kg: 100 mg PO every Day 85-136 kg: 150 mg PO every Day If improvement after 12 weeks not observed, reassess treatment |
| Omadacycline (Nuzyra) / aminomethylcycline antibiotic, tetracycline class (inhibits 30S ribosomal subunit) COMPANY: Paratek Pharmaceuticals (MA, USA) | FDA: approved in October 2018 EMA: not yet approved | CABP, ABSSSI | Both once-daily IV and PO formulations | A. For patients with CABP, the loading dose on day 1 is 200 mg by IV infusion over 60 minutes, or 100 mg by IV infusion over 30 minutes, given twice; the maintenance dose is 100 mg by IV infusion over 30 minutes once daily, or 300 mg PO daily for a total of 7 to 14 days. B. For patients with ABSSSI, the loading dose on day 1 is 200 mg by IV infusion over 60 minutes; or 100 mg by IV infusion over 30 minutes, given twice; or, on days 1 and 2, 450 mg orally once daily. The maintenance dose is 100 mg by IV infusion over 30 minutes once daily, or 300 mg PO daily, for a total duration of 7 to 14 days |
| Rifamycin (Aemcolo) / bactericidal; inhibit bacterial DNA-dependent RNA polymerase, suppressing RNA synthesis COMPANY: Cosmo Pharmaceuticals (Ireland) | FDA: approved in November 2018 EMA: not yet approved | Travelers' diarrhea (noninvasive strains (E. coli) | PO administration | 388 mg (2 tablets) PO twice a day x 3 days |
Table 2. Main characteristics of the described antibacterial drugs FDA approved in 201936-49.
Food and Drug Administration (FDA), European Medicines Agency (EMA), complicated urinary tract infections (cUTIs), complicated intra-abdominal infections (cIAIs), community-acquired bacterial pneumonia (CABP), intravenous (IV), oral administration/per oral (PO), hours (h); doses and duration have to be verified in the most recent prescriptions reglementations, according to the local laws before administration
| NAME (generic/brand/ class) | Approval status | Indication | Administration | Dose and duration |
|---|---|---|---|---|
| Imipenem, cilastatin, relebactam (Recarbrio) COMPANY: Merck & Co (NJ, USA) | FDA: approved in July 2019 EMA: not yet approved | cUTI, cIAI | IV infusion | Injection, powder for reconstitution 500mg/500mg/250mg per vial (ie, 1.25g/vial) A. Urinary Tract Infection: 1.25 g IV every 6h x 4-14 days B. Intra-abdominal Infections 1.25 g IV every 6h x 4-14 days |
| Pretomanid / nitroimidazole, a class of novel anti-bacterial agents, in combination with bedaquiline and linezolid COMPANY: TB Alliance (NY, USA & South Africa; non-profit) | FDA: approved in August 2019 EMA: not yet approved | Drug-resistant TB (lung tuberculosis) | PO, one tablet (200ml, adult) taken once a day for 26 weeks | Pretomanid 200 mg PO/day x 26 weeks Bedaquiline 400 mg PO/day x 2 weeks, Then, 200 mg 3x/week with at least 48 h between doses for x 24 weeks (total of 26 weeks) Linezolid 1200 mg PO/day for 26 weeks |
| Lefamulin (Xenleta) / semisynthetic pleuromutilin antibiotic COMPANY: Nabriva Therapeutics (Ireland) | FDA: approved in August 2019 EMA: not yet approved | CABP | IV and PO treatment | 600 mg orally every 12 hours for 5 days or 150 mg infused IV over 60 minutes every 12 hours for 5-7 days; IV: -Mild or moderate liver dysfunction (Child-Pugh A or B): No adjustment recommended. -Severe liver dysfunction (Child-Pugh C): 150 mg IV every 24 hours Oral: -Mild liver dysfunction (Child-Pugh A): No adjustment recommended. -Moderate or severe liver dysfunction (Child-Pugh B or C): Not recommended. |
| Cefiderocol (Fetroja) / cephalosporin antibacterial COMPANY: Shionogi & Co., Ltd. (Japan) | FDA: approved in November 2019 EMA: not yet approved | cUTI | IV | 2 gram IV every 8h for 7-14 days |
2. FDA approved antibacterial drugs (2018-2019)
2.1 Plazomicin
Plazomicin sulfate (Zemdri) is a semisynthetic aminoglycoside bactericidal antibiotic drug, acting in asimilar manner to other aminoglycosides, by suppressing the 30S bacterial ribosomal subunit.Noteworthy, while other aminoglycosides can be inactivated by aminoglycoside-modifying enzymes, plazomicin is resistant to the action of these enzymes18.
Plazomicin was approved by FDA in June 2018, for targeting the infections with Gram-negative aerobic bacteria in the complicated urinary tract infections (cUTIs). Application was submitted for review by European Medicine Agency (EMA) in June 2018.
Plazomicin is administered intravenously (IV) every 24 hours for 4-7 days; it is primarily active against Gram-negative aerobic bacteria (e.g. Enterobacteriaceae family), to be used in patients over 18 years of age with cUTIs (including pyelonephritis), caused by susceptible Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis or Enterobacter cloacae18,19.
Plazomicin is supplied as single-dose vials in an amount of 500 mg/10 mL plazomicin base19. Administration recommendations can be found in Table 1 and its dosage is personalized based on the renal function and/or therapeutic monitoring of the drug, if available19. Plazomicin might be of value in patients that have resistance to their primary treatment options or who are allergic to beta-lactam antibiotics.
The most important adverse events reported with plazomicin are: nephrotoxicity (but lower incidence of nephrotoxicity than colistin), diarrhea, hypertension, headache, nausea, vomiting, hypotension20.
Plazomicin showed to be non-inferior to meropenem within the EPIC non-inferiority trial in treatment of cUTIs and even demonstrated superior microbiological eradication (81.7% versus 70.1%; 95% confidence interval (CI) 2.7-25.7)21. Plazomicin-based combinations also demonstrated decreased disease-complications and mortality when compared to colistin-based combination in the CARE trial (23.5% versus 50%; 90% CI -0.7 to 51.2)20.
2.2 Eravacycline
Eravacycline dihydrochloride (Xerava) is a fully synthetic bacteriostatic fluorocycline and a tetracycline-class antibacterial agent that binds bacterial 30S ribosomal subunit. Compared to other tetracyclines, it has two structural substitutions which makes the drug working on certain strains of Gram-positive and Gram-negative bacteria that usually have tetracycline-specific resistance mechanisms. Noteworthy, eravacycline can be used (at least in cell culture) to target Enterobacteriaceae, in the presence of certain beta-lactamases18,22.
Eravacycline was approved by FDA in August 2018 and by EMA in September 2018, being indicated in the complicated intra-abdominal infections22-24.
Eravacycline is administered IV in 60 min infusions, given once every 12 hours for a total of 4 to 14 days; dose is patient’s weight dependent (1mg/kg) and it is used in persons over 18 years of age with complicated intra-abdominal infections (cIAI) caused by susceptible microorganisms identified in the prescribing information. This is the only indication of use for eravacycline at this moment, although it may be approved for other applications in the future, similar to other tetracyclines18.
The most important adverse event reported with eravacycline in clinical trials and sometimes a cause of the treatment discontinuation is the gastrointestinal (GI) upset. Other noteworthy adverse events that can appear are infusion site reactions, nausea, and vomiting18,22.
Eravacycline was compared with ertapenem and meropenem for the treatment of cIAIs in 2 non-inferiority trials (IGNITE1 and IGNITE4), with similar clinical response rates25,26.
2.3 Sarecycline
Sarecycline hydrochloride (Seysara) is a new, narrow-spectrum tetracycline derivative. It proves antibacterial activity against skin and soft tissue pathogens, including the Cutibacterium acnes (anaerobic Gram-positive bacterium related to acne development). Similar to other tetracyclines, it possesses anti-inflammatory effects. However, it has some specific properties comparing to other tetracyclines: it seems to affect the intestinal flora less; it shows a lower rate of resistance to tetracycline-resistant Staphylococcus aureus, as well as erythromycin-resistant and clindamycin-resistant Cutibacterium acnes strains27. Sarecycline has significant effects on inflammatory lesions. However, it was also noted to show statistically significant effects on noninflammatory acneiform lesions at certain time points28.
Sarecycline was approved by FDA in October 2018, for the treatment of non-nodular moderate to severe acne. Application was also submitted for review by European Medicine Agency (EMA) in October 2018.
The drug is administrated as 1.5 mg/kg/day orally with food, in patients aged 9 and older, as a once daily antibiotic with statistically significant improvement seen as early as 3rd week. More detailed information about its administration can be found in Table 1.
In clinical trials comparing with placebo evaluating the adverse effects, nausea was reported in 3.1% of the patients treated with sarecycline versus 2.0% in patients treated with placebo; the other adverse reactions reported were found in less than 1% of female subjects treated with sarecycline: vulvovaginal mycotic infection (0.8%) and vulvovaginal candidiasis (0.6%)29.
2.4 Omadacycline
Omadacycline (Nuzyra) is an aminomethylcycline antibiotic belonging to the tetracycline class. It inhibits 30S bacterial ribosomal subunit. Compared to other tetracycline antibiotics, omadacycline has structural modifications at the C9 and C7 positions of the core tetracycline rings, enabling ribosomal protection mechanisms and stability in the efflux pump related to resistance to the tetracycline antibiotics30.
Omadacycline was approved by FDA in October 2018 for the treatment of community-acquired bacterial pneumonia (CABP) and acute bacterial skin and skin structure infections (ABSSI) and it is not yet approved by the EMA. It can be administered once-daily in both IV and PO formulations (Table 1).
Adverse events noted Omadacycline has warnings associated with tetracycline-class antibiotics, including: tooth discoloration, enamel hypoplasia and inhibition of bone growth in late pregnancy, infancy, or childhood up to 8 years of age. The most common adverse reactions (incidence ≥2%) seen in clinical trials of omadacycline are: nausea, vomiting, infusion site reactions, alanine aminotransferase increased, aspartate aminotransferase increased, gamma-glutamyl transferase increased, hypertension, headache, diarrhea, insomnia, and constipation. Omadacycline has only been studied in patients 18 years of age or older. Omadacycline’s affinity for muscarinic M2 receptors induces a transient heart rate increases and it has no effect on the QT interval31.
A meta-analysis of randomized controlled trials revealed that the clinical efficacy of omadacycline is not inferior to that of competitor drugs in the treatment of acute bacterial infections in adult patients32.
2.5 Rifamycin
Rifamycin (Aemcolo) is abactericidal minimally absorbed antibiotic that inhibits bacterial DNA-dependent RNA polymerase, by suppressing RNA synthesis. It is the first antibiotic engineered with Cosmo Pharmaceuticals’ Multi Matrix Technology, that enables colonic release of the active compound33.
It was approved by FDA in November 2018 for the treatment of the noninvasive strains of Escherichia coli causing travelers' diarrhea, in an orally dose of 388 mg (2 tablets) twice a day for 3 days but not when diarrhea is complicated by fever and/or bloody stools34.
The most important adverse reactions observed during the clinical trials are constipation (3.5%), headache (3.3%), abdominal pain (0.5%) & pyrexia (0.3%) with 1% of the patients discontinuing the treatment33,34.
In a randomized double-blind phase 3 study (ERASE), Rifamycin SV-MMX was found to be equally effective as ciprofloxacin and to not induce resistance in bacteria for the treatment of travellers' diarrhea35.
2.6 Imipenem, cilastatin and relebactam (Recarbrio)
Recarbrio is a regimen comprising of imipenem, a penem antibacterial, cilastatin, an inhibitor of the renal dehydropeptidase and relebactam, a beta-lactamase inhibitor36.
It was approved by FDA in July 2019 for the treatment of cUTIs (including pyelonephritis) and cIAI in patients 18 years of age and older who have limited or no other treatment options available. Susceptible bacteria are Gram-negative microorganisms such as Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, aerogenes and oxytoca, Pseudomonas aeruginosa, some strains of Bacteroides and other susceptible bacteria37.
It is administered as a 30 min IV infusion: 500mg/500mg/250mg per vial (1.25g/vial): 1.25 g IV every 6h x 4-14 days (for cUTI) and 1.25 g IV every 6h x 4-14 days (for cIAI)38.
Adverse events observed with this triple combination include, but are not limited to: diarrhea, nausea, headache, vomiting, increase in transaminase, phlebitis/infusion site reactions, pyrexia, hypertension36.
2.7 Pretomanid
Pretomanid is a nitroimidazole, a class of novel anti-bacterial agents. It was approved in August 2019 by the FDA to be used in combination with bedaquiline, which targets the adenosine triphosphate (ATP) synthase enzyme of the TB mycobacteria and linezolid, a synthetic antibiotic, the first of the oxazolidinone class, for the treatment of drug-resistant TB (lung tuberculosis). It is not yet approved by the EMA.
It is orally administered, one tablet (200 ml) taken once a day for 26 weeks for adults. Specifically, pretomanid 200 mg PO/day x 26 weeks, bedaquiline 400 mg PO/day x 2 weeks, then, 200 mg 3x/week with at least 48 h between doses for x 24 weeks (total of 26 weeks), and linezolid 1200 mg PO/day for 26 weeks39.
Adverse events reported with pretomanid include numbness and tingling of extremities, acne, anemia, nausea, vomiting, headache, increased transaminases, excess amylase in the blood, indigestion, decreased appetite, abdominal pain, rash, itching, sharp chest pain during breathing, increased gamma-glutamyl transferases, lower respiratory tract infection, cough, coughing up blood, back pain, visual impairment, low blood sugar (hypoglycemia), abnormal weight loss, diarrhea40.
The early efficacy reported in a recently published trial, showed that the pretomanid-containing regimens had a more significant early bactericidal activity than classical a HRZE regimen41. However, more research is needed before pretomanid can be validated as a promising therapy in tuberculosis42.
2.8 Lefamulin
Lefamulin (Xenleta) is a semisynthetic pleuromutilin antibiotic that binds to the peptidyl transferase center of the 50S bacterial ribosomal subunit, inhibiting protein synthesis within bacteria43.
It was approved by FDA in August 2019 for the treatment of CABP. Lefamulin is not yet approved by EMA.
It can be administered either as an IV infusion or PO. It is used as 600 mg orally every 12 hours for 5 days or 150 mg infused IV over 60 minutes every 12 hours for 5-7 days. For additional information please consult Table 2.
As adverse events, lefamulin can prolong the QT interval (increased risk in patients with renal failure or hepatic dysfunction), produces infusion-site reactions, diarrhea, hepatic enzyme elevations, nausea, hypokalemia, insomnia, and headache. There are multiple other drugs interactions and it should not be used in pregnant women43.
FDA approval of lefamulin was based on the results of 2 randomized, controlled double-blind, noninferiority trials called LEAP 1 and LEAP 2, which enrolled 1289 adults44,45. Results of these trials demonstrated that lefamulin was as effective as moxifloxacin in the treatment of the community-acquired bacterial pneumonia43.
2.9 Cefiderocol
Cefiderocol (Fetroja) is a siderophore cephalosporin antibacterial drug that has been developed to fight a wide range of bacterial pathogens, such as the β-lactam-resistant and carbapenem-resistant orga-nisms46.
It was approved in November 2019 for the treatment of complicated urinary tract infections. It is not yet approved by EMA.
It is administered as an IV infusion, 2 gram every 8h, for 7-14 days. Cefiderocol targets a wide range of clinically relevant gram-negative bacteria, including but not limited to the Enterobacteriaceae spp, such as Enterobacter spp, Klebsiella spp, Proteus spp, Vibrio spp, Yersinia spp, Serratia marcescens, Shigella flexneri, Salmonella spp and also nonfermenting bacterial species such as Acinetobacter and Pseudomonas46-48.
As adverse effects, cefiderocol is well tolerated, with minor reports of gastrointestinal and phlebitis. This side effect profile is similar to the profile of other cephalosporin drugs49.
Clinical trials have shown that Cefiderocol's activity against bacteria non-susceptible for meropenem and Klebsiella pneumoniae carbapenemase-producing Enterobacteriales is similar or even superior to ceftazidime-avibactam. Cefiderocol is also more potent than meropenem and ceftazidime-avibactam in targeting Pseudomonas aeruginosa (against all resistance phenotypes) and Stenotrophomonas maltophilia49.
3. Promising antimicrobials under investigation
Comparing with the last decade, we have the impression of an acceleration of antibiotic succeeded approval. However, this do not meet the urgency of WHO and United Nations calls for action50.
At the end of 2019, a total of 42 new antibiotics or new combinations are in different stages of clinical development globally, a certain number being already approved by FDA51. Iclaprim stands out by the high possibility of a soon FDA-approval. This molecule with diaminopyrimidine structure acts by inhibiting bacterial dihydrofolate reductase and it has been successfully tested in a phase 3 randomized controlled trial (REVIVE-1), showing to be non-inferior to vancomycin in treating ABSSI52. By its novel mechanism of action, lack of nephrotoxicity and capacity to suppress bacterial exotoxins, iclaprim might become an interesting player in treating resistant Gram positive germs53.
Gram-negative bacteria are protected by a double membrane envelope, which forms a highly efficient barrier to antibiotics. The external membrane contains lipopolysaccharide molecules in the outer layer and integral outer-membrane proteins (OMPs). OMPs are folded into the membrane by a protein complex called the β-barrel assembly machine (BAM), which have a central component called BamA accessible from the bacterial surface. Three recent studies report new antibiotics that seem to target BamA - therefore inhibiting the normal OMP folding that is necessary for bacterial survival - darobactin, murepavadin analogues and MRL-494.
Darobactin was efficient against multiple Gram-negative bacteria (in vitro and in infected mice): polymyxin-resistant Pseudomonas aeruginosa, β-lactam-resistant Klebsiella pneumoniae and Escherichia coli54.
Murepavadin analogues, obtained after linking the murepavadin molecule with the lipopolysaccharide binding portion of polymyxin B, displayed antibiotic activity against Klebsiella pneumoniae, Pseudomonas aeruginosa, Escherichia coli55.
MRL-494, a newly identified compound, had similar antibiotic effectiveness in vitro against both wild-type Escherichia coliand a mutant defective in outer-membrane integrity and efflux mechanisms, suggesting that this antibiotic might not need to breach the membrane to exert its activity. However, MRL-494 efficiency remains to be tested in animal models56.
Small bacterial toxins can act as antimicrobial peptides. Reducing their toxicity to human cells and retaining their antibiotic activity can open new perspectives in antibiotics development.
Recently, Nicolas et al. synthesized 4 cyclic heptapseudopeptide biomimetics, which reproduce a section of a Staphylococcus aureus toxin, PepA157. Two of the studied peptides were effective against methicillin-resistant Staphylococcus aureusin mild and severe sepsis mouse models without displaying toxicity on human erythrocytes and kidney cells, zebrafish embryos, and mice. Moreover, efficacy was also proved against Pseudomonas aeruginosa and MRSA in a mouse skin infection model. Notably, these novel compounds did not lead to resistance after serial passages for 2 weeks and 4- or 6-days’ exposure in mice.
The ability of unnatural amino acids to strengthen dynamic association with bacterial lipid bilayers and to induce membrane permeability can explain the antibiotic effect of the heptapseudopeptides57.
Delafloxacin, a new fluoroquinolone already FDA approved for the treatment of acute bacterial skin and skin structure infections, is currently the only antibiotic with in vitro activity against methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa17,58. Therefore, delafloxacin efficiency in comunity acquired pneumonia has been evaluated in recently completed Phase 3 study (NCT02679573).
Ceftobiprole medocaril, an anti-MRSA cephalosporin indicated for the treatment of complicated skin infections and community/hospital acquired pneumonia, it is still unapproved by the FDA, but currently used in some European countries and in Canada59.
The new lipoglycopeptide dalbavancin - FDA approved for acute bacterial skin and skin structure infections - has a high microbiological activity against staphylococci and enterococci. Moreover, dalbavancin has long half-life (up to 250 hours), which makes it a suitable option for a more rapid hospital discharge. However, a Phase II study which aimed to evaluate the effectiveness of dalbavancin in patients with blood stream infections or infective endocarditis was stopped early due to economic reasons (NCT03148756) and data are insufficient to support its use in this setting60,61.
4. Conclusion
There is a significant need for novel antibacterial drugs and this research and development efforts recently resulted in few new drugs or combination of drugs proposed for use into the clinic. There is a significant increase in the number of the new FDA approved drugs in the past 2 years compared to the previous years, since the number of antibacterial drugs or regimens in the past 2 years (9 antibacterial agents) is almost double the number of the ones from any 2 previous years, within the past 17 years.
The novel US FDA approved antibacterial agents in the last two years (2018-2019): plazomicin, eravacycline, sarecycline, omadacycline, rifamycin (2018) and imipenem, cilastatin and relebactam combination, pretomanid, lefamulin, cefiderocol (2019) are new players in the field of resistant bacteria treatment for specific indications. However, the number and efficiency of these new drugs is far from covering all the existing needs, to fully combat highly adaptive microorganisms. Thus, there is a real need and urgency to develop novel antibiotic strategies and drugs to overcome the bacterial resistance to antibiotics.
Through this review, we aim to further encourage the scientific community to continue the development of new therapeutic agents for targeting bacterial resistance.
Acknowledgments
We are grateful to the Fundeni Clinical Institute, Bucharest, Romania for support.
Footnotes
Conflict of interests: The authors declare no conflicts of interest.
Food and Drug Administration (FDA); European Medicines Agency (EMA); Area under the curve (AUC); New Delhi metallo-β-lactamase (NDM); Verona integron-encoded metallo-β-lactamase (VIM); Imipenemase (IMP); Complicated intra-abdominal infections (cIAIs); Complicated urinary tract infections (cUTIs); Hospital acquired bacterial pneumonia (HABP); Ventilator associated bacterial pneumonia (VABP); Acute bacterial skin and skin structure infections (ABSSI); Intravenous (IV); Central Nervous System (CNS); Hours (h); HRZE (isoniazid (H), rifampicin (R), pyrazinamide (Z) and ethambutol (E) antituberculosis combination).
DISCOVERIES is a peer-reviewed, open access, online, multidisciplinary and integrative journal, publishing high impact and innovative manuscripts from all areas related to MEDICINE, BIOLOGY and CHEMISTRY
References
- 1.Bacterial pathogens and resistance causing community acquired paediatric bloodstream infections in low- and middle-income countries: a systematic review and meta-analysis. Droz Nina, Hsia Yingfen, Ellis Sally, Dramowski Angela, Sharland Mike, Basmaci Romain. Antimicrobial resistance and infection control. 2019;8:207. doi: 10.1186/s13756-019-0673-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Plasmid evolution in carbapenemase-producing Enterobacteriaceae: a review. Kopotsa Katlego, Osei Sekyere John, Mbelle Nontombi Marylucy. Annals of the New York Academy of Sciences. 2019;1457(1):61–91. doi: 10.1111/nyas.14223. [DOI] [PubMed] [Google Scholar]
- 3.Molecular Epidemiology, Diagnostics and Mechanisms of Antibiotic Resistance in Mycobacterium tuberculosis complex in Africa: A Systematic Review of Current Reports. Osei Sekyere John, Reta Melese Abate, Maningi Nontuthuko Excellent, Fourie Petrus Bernard. J Infect. 2019 doi: 10.1016/j.jinf.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Strategies to reduce curative antibiotic therapy in intensive care units (adult and paediatric). Bretonnière Cédric, Leone Marc, Milési Christophe, Allaouchiche Bernard, Armand-Lefevre Laurence, Baldesi Olivier, Bouadma Lila, Decré Dominique, Figueiredo Samy, Gauzit Rémy, Guery Benoît, Joram Nicolas, Jung Boris, Lasocki Sigismond, Lepape Alain, Lesage Fabrice, Pajot Olivier, Philippart François, Souweine Bertrand, Tattevin Pierre, Timsit Jean-François, Vialet Renaud, Zahar Jean Ralph, Misset Benoît, Bedos Jean-Pierre. Intensive care medicine. 2015;41(7):1181–96. doi: 10.1007/s00134-015-3853-7. [DOI] [PubMed] [Google Scholar]
- 5.Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomised trial. Derde Lennie P G, Cooper Ben S, Goossens Herman, Malhotra-Kumar Surbhi, Willems Rob J L, Gniadkowski Marek, Hryniewicz Waleria, Empel Joanna, Dautzenberg Mirjam J D, Annane Djillali, Aragão Irene, Chalfine Annie, Dumpis Uga, Esteves Francisco, Giamarellou Helen, Muzlovic Igor, Nardi Giuseppe, Petrikkos George L, Tomic Viktorija, Martí Antonio Torres, Stammet Pascal, Brun-Buisson Christian, Bonten Marc J M. The Lancet. Infectious diseases. 2014;14(1):31–39. doi: 10.1016/S1473-3099(13)70295-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Catheter-related infections in chronic hemodialysis: a clinical and economic perspective. Ştefan Gabriel, Stancu Simona, Căpuşă Cristina, Ailioaie Oana Ramaiana, Mircescu Gabriel. International urology and nephrology. 2013;45(3):817–23. doi: 10.1007/s11255-012-0244-7. [DOI] [PubMed] [Google Scholar]
- 7.Antibiotic Use in the Intensive Care Unit: Optimization and De-Escalation. Campion Maureen, Scully Gail. Journal of intensive care medicine. 2018;33(12):647–655. doi: 10.1177/0885066618762747. [DOI] [PubMed] [Google Scholar]
- 8.Preventive and therapeutic strategies in critically ill patients with highly resistant bacteria. Bassetti Matteo, De Waele Jan J, Eggimann Philippe, Garnacho-Montero Josè, Kahlmeter Gunnar, Menichetti Francesco, Nicolau David P, Paiva Jose Arturo, Tumbarello Mario, Welte Tobias, Wilcox Mark, Zahar Jean Ralph, Poulakou Garyphallia. Intensive care medicine. 2015;41(5):776–95. doi: 10.1007/s00134-015-3719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prevalence of colistin resistance and mcr-1/mcr-2 genes in extended-spectrum β-lactamase/AmpC-producing Escherichia coli isolated from chickens in Canada, Senegal and Vietnam. Vounba Passoret, Rhouma Mohamed, Arsenault Julie, Bada Alambédji Rianatou, Fravalo Philippe, Fairbrother John Morris. Journal of global antimicrobial resistance. 2019;19:222–227. doi: 10.1016/j.jgar.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Liu Yi-Yun, Wang Yang, Walsh Timothy R, Yi Ling-Xian, Zhang Rong, Spencer James, Doi Yohei, Tian Guobao, Dong Baolei, Huang Xianhui, Yu Lin-Feng, Gu Danxia, Ren Hongwei, Chen Xiaojie, Lv Luchao, He Dandan, Zhou Hongwei, Liang Zisen, Liu Jian-Hua, Shen Jianzhong. The Lancet. Infectious diseases. 2016;16(2):161–8. doi: 10.1016/S1473-3099(15)00424-7. [DOI] [PubMed] [Google Scholar]
- 11.Local applications but global implications: Can pesticides drive microorganisms to develop antimicrobial resistance? Ramakrishnan Balasubramanian, Venkateswarlu Kadiyala, Sethunathan Nambrattil, Megharaj Mallavarapu. The Science of the total environment. 2019;654:177–189. doi: 10.1016/j.scitotenv.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 12.Co-selection of multi-antibiotic resistance in bacterial pathogens in metal and microplastic contaminated environments: An emerging health threat. Imran Md, Das Kirti Ranjan, Naik Milind Mohan. Chemosphere. 2019;215:846–857. doi: 10.1016/j.chemosphere.2018.10.114. [DOI] [PubMed] [Google Scholar]
- 13.Epidemiology and successful containment of a carbapenem-resistant Enterobacteriaceae outbreak in a Southern Italian Transplant Institute. Mularoni Alessandra, Martucci Gennaro, Douradinha Bruno, Campanella Ornella, Hazen Benjamin, Medaglia Alice, Arena Giuseppe, Gruttadauria Salvatore, Tuzzolino Fabio, Arcadipane Antonio, Gioè Santi, Luca Angelo, Conaldi Pier Giulio, Grossi Paolo, Gridelli Bruno. Transplant infectious disease : an official journal of the Transplantation Society. 2019;21(4):e13119. doi: 10.1111/tid.13119. [DOI] [PubMed] [Google Scholar]
- 14.Role of Education in Antimicrobial Stewardship. Gyssens Inge C. The Medical clinics of North America. 2018;102(5):855–871. doi: 10.1016/j.mcna.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 15.Antibiotic stewardship and horizontal infection control are more effective than screening, isolation and eradication. Lemmen S W, Lewalter K. Infection. 2018;46(5):581–590. doi: 10.1007/s15010-018-1137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treatment of Infections Due to MDR Gram-Negative Bacteria. Bassetti Matteo, Peghin Maddalena, Vena Antonio, Giacobbe Daniele Roberto. Frontiers in medicine. 2019;6:74. doi: 10.3389/fmed.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.New FDA approved antibacterial drugs: 2015-2017. Andrei Stefan, Valeanu Liana, Chirvasuta Radu, Stefan Mihai-Gabriel. Discoveries. 2018;6(1):e81. doi: 10.15190/d.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.New Drugs 2019, part 4. Hussar Daniel A. Nursing. 2019;49(11):34-43. [Google Scholar]
- 19.Zemdri (plazomicin) FDA Highlights of Prescribing Information. U.S. Food and Drug Administration; Accessed December 10, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210303Orig1s000lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210303Orig1s000lbl.pdf
- 20.Plazomicin: A Novel Aminoglycoside for the Treatment of Resistant Gram-Negative Bacterial Infections. Eljaaly Khalid, Alharbi Aisha, Alshehri Samah, Ortwine Jessica K, Pogue Jason M. Drugs. 2019;79(3):243–269. doi: 10.1007/s40265-019-1054-3. [DOI] [PubMed] [Google Scholar]
- 21.Once-Daily Plazomicin for Complicated Urinary Tract Infections. Wagenlehner Florian M E, Cloutier Daniel J, Komirenko Allison S, Cebrik Deborah S, Krause Kevin M, Keepers Tiffany R, Connolly Lynn E, Miller Loren G, Friedland Ian, Dwyer Jamie P. The New England journal of medicine. 2019;380(8):729–740. doi: 10.1056/NEJMoa1801467. [DOI] [PubMed] [Google Scholar]
- 22.Eravacycline: A Review in Complicated Intra-Abdominal Infections. Scott Lesley J. Drugs. 2019;79(3):315–324. doi: 10.1007/s40265-019-01067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tetraphase Pharmaceuticals Inc. Xerava (Eravacycline): US prescribing information. U.S. Food and Drug Administration; Accessed on October 21, 2018. 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211109lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/211109lbl.pdf
- 24.Xerava (Eravacycline): summary of product characteristics. European Medicines Agency; Accessed on November 1, 2018. 2018. https://www.ema.europa.eu /en/documents/product-information/xerava-epar-product-information_en.pdf https://www.ema.europa.eu /en/documents/product-information/xerava-epar-product-information_en.pdf
- 25.Assessing the Efficacy and Safety of Eravacycline vs Ertapenem in Complicated Intra-abdominal Infections in the Investigating Gram-Negative Infections Treated With Eravacycline (IGNITE 1) Trial: A Randomized Clinical Trial. Solomkin Joseph, Evans David, Slepavicius Algirdas, Lee Patrick, Marsh Andrew, Tsai Larry, Sutcliffe Joyce A, Horn Patrick. JAMA surgery. 2017;152(3):224–232. doi: 10.1001/jamasurg.2016.4237. [DOI] [PubMed] [Google Scholar]
- 26.IGNITE4: Results of a Phase 3, Randomized, Multicenter, Prospective Trial of Eravacycline vs Meropenem in the Treatment of Complicated Intraabdominal Infections. Solomkin Joseph S, Gardovskis Janis, Lawrence Kenneth, Montravers Philippe, Sway Angie, Evans David, Tsai Larry. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019;69(6):921–929. doi: 10.1093/cid/ciy1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarecycline hydrochloride for the treatment of acne vulgaris. Kaul G, Saxena D, Dasgupta A, Chopra S. Drugs of today (Barcelona, Spain : 1998) 2019;55(10):615–625. doi: 10.1358/dot.2019.55.10.3045040. [DOI] [PubMed] [Google Scholar]
- 28.Once-Daily Oral Sarecycline 1.5 mg/kg/day Is Effective for Moderate to Severe Acne Vulgaris: Results from Two Identically Designed, Phase 3, Randomized, Double-Blind Clinical Trials. Moore Angela, Green Lawrence J, Bruce Suzanne, Sadick Neil, Tschen Eduardo, Werschler Philip, Cook-Bolden Fran E, Dhawan Sunil S, Forsha Douglass, Gold Michael H, Guenthner Scott, Kempers Steven E, Kircik Leon H, Parish Jennifer L, Rendon Marta I, Rich Phoebe, Stein-Gold Linda, Tyring Stephen K, Weiss Robert A, Nasir Adnan, Schmitz Carsten, Boodhoo Terry I, Kaoukhov Alexandre, Berk David R. Journal of drugs in dermatology : JDD. 2018;17(9):987–996. [PubMed] [Google Scholar]
- 29.Sarecycline: a narrow spectrum tetracycline for the treatment of moderate-to-severe acne vulgaris. Moore Angela Yen, Charles Jean Elizze M, Moore Stephen. Future microbiology. 2019;14:1235–1242. doi: 10.2217/fmb-2019-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Omadacycline: A Review of the Clinical Pharmacokinetics and Pharmacodynamics. Rodvold Keith A, Burgos Rodrigo M, Tan Xing, Pai Manjunath P. Clinical pharmacokinetics. 2019 doi: 10.1007/s40262-019-00843-4. [DOI] [PubMed] [Google Scholar]
- 31.An Integrated Safety Summary of Omadacycline, a Novel Aminomethylcycline Antibiotic. Opal Steven, File Thomas M, van der Poll Tom, Tzanis Evan, Chitra Surya, McGovern Paul C. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019;69(Supplement_1):S40–S47. doi: 10.1093/cid/ciz398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The efficacy and safety of omadacycline in treatment of acute bacterial infection: A systemic review and meta-analysis of randomized controlled trials. Lan Shao-Huan, Chang Shen-Peng, Lai Chih-Cheng, Lu Li-Chin, Chao Chien-Ming. Medicine. 2019;98(51):e18426. doi: 10.1097/MD.0000000000018426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.FDA Approves AEMCOLO™ (Rifamycin), the First Antibiotic Approved for the Treatment of Travelers’ Diarrhea in Over a Decade. CosmoPharmaceuticals; Accessed on February 4, 2019. https://www.cosmopharma.com/news-and-media/press-releases-and-company-news/2018/ 181119 https://www.cosmopharma.com/news-and-media/press-releases-and-company-news/2018/ 181119
- 34.Rifamycin SV MMX®: A Review in the Treatment of Traveller’s Diarrhoea. Hoy Sheridan M. Clinical Drug Investigation. 2019;39(7):691-697. doi: 10.1007/s40261-019-00808-2. [DOI] [PubMed] [Google Scholar]
- 35.Rifamycin SV-MMX® for treatment of travellers’ diarrhea: equally effective as ciprofloxacin and not associated with the acquisition of multi-drug resistant bacteria. Steffen Robert, Jiang Zhi-Dong, Gracias Garcia Mónica L, Araujo Prithi, Stiess Michael, Nacak Tanju, Greinwald Roland, DuPont Herbert L. Journal of Travel Medicine. 2018;25(1) doi: 10.1093/jtm/tay116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Recarbrio (imipenem, cilastatin, and relebactam). WCG CenterWatch; Accessed on October 10, 2019. https://www.centerwatch.com/directories/1067-fda-approved-drugs/listing/4098-recarbrio-imipenem-cilastatin-and-relebactam https://www.centerwatch.com/directories/1067-fda-approved-drugs/listing/4098-recarbrio-imipenem-cilastatin-and-relebactam
- 37.FDA Approves Merck’s RECARBRIO™ (imipenem, cilastatin, and relebactam) For the Treatment of Adults with Complicated Urinary Tract and Complicated Intra-Abdominal Bacterial Infections Where Limited or No Alternative Treatment Options Are Available. Merck; Accessed on September 18, 2019. https://www.mrknewsroom.com/news-release/prescription-medicine-news/fda-approves-mercks-recarbrio-imipenem-cilastatin-and-releba https://www.mrknewsroom.com/news-release/prescription-medicine-news/fda-approves-mercks-recarbrio-imipenem-cilastatin-and-releba
- 38.Recarbrio (imipenem, cilastatin, and relebactam) FDA highlights of prescribing information [cited 2019 Nov 28]. https://www.accessdata.fda.gov/ drugsatfda_docs/label/2019/212819s000lbl.pdf. U.S. Food and Drug Administration; Accessed on November 28, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212819s000lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212819s000lbl.pdf
- 39.Pretomanid FDA highlights of prescribing information. U.S. Food and Drug Administration; Accessed on December 16, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212862Orig1s000Lbl.pdf https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/212862Orig1s000Lbl.pdf
- 40.Pretomanid. Rxlist; Accessed on November 10, 2019. https://www.rxlist.com/pretomanid-side-effects-drug-center.htm https://www.rxlist.com/pretomanid-side-effects-drug-center.htm
- 41.Bedaquiline, moxifloxacin, pretomanid, and pyrazinamide during the first 8 weeks of treatment of patients with drug-susceptible or drug-resistant pulmonary tuberculosis: a multicentre, open-label, partially randomised, phase 2b trial. Tweed Conor D, Dawson Rodney, Burger Divan A, Conradie Almari, Crook Angela M, Mendel Carl M, Conradie Francesca, Diacon Andreas H, Ntinginya Nyanda E, Everitt Daniel E, Haraka Frederick, Li Mengchun, van Niekerk Christo H, Okwera Alphonse, Rassool Mohammed S, Reither Klaus, Sebe Modulakgotla A, Staples Suzanne, Variava Ebrahim, Spigelman Melvin. The Lancet. Respiratory medicine. 2019;7(12):1048–1058. doi: 10.1016/S2213-2600(19)30366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuberculosis: experts question evidence and safety data used to approve latest drug. Krishnan Vidya. BMJ (Clinical research ed.) 2019;367:l6832. doi: 10.1136/bmj.l6832. [DOI] [PubMed] [Google Scholar]
- 43.Lefamulin (Xenleta) for community-acquired bacterial pneumonia. The Medical letter on drugs and therapeutics. 2019;61(1581):145–148. [PubMed] [Google Scholar]
- 44.Efficacy and Safety of Intravenous-to-oral Lefamulin, a Pleuromutilin Antibiotic, for the Treatment of Community-acquired Bacterial Pneumonia: The Phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) Trial. File Thomas M, Goldberg Lisa, Das Anita, Sweeney Carolyn, Saviski John, Gelone Steven P, Seltzer Elyse, Paukner Susanne, Wicha Wolfgang W, Talbot George H, Gasink Leanne B. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2019;69(11):1856–1867. doi: 10.1093/cid/ciz090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oral Lefamulin vs Moxifloxacin for Early Clinical Response Among Adults With Community-Acquired Bacterial Pneumonia: The LEAP 2 Randomized Clinical Trial. Alexander Elizabeth, Goldberg Lisa, Das Anita F, Moran Gregory J, Sandrock Christian, Gasink Leanne B, Spera Patricia, Sweeney Carolyn, Paukner Susanne, Wicha Wolfgang W, Gelone Steven P, Schranz Jennifer. JAMA. 2019 doi: 10.1001/jama.2019.15468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cefiderocol: a novel siderophore cephalosporin. Choi Justin J, McCarthy Matthew W. Expert Opinion on Investigational Drugs. 2018;27(2):193-197. doi: 10.1080/13543784.2018.1426745. [DOI] [PubMed] [Google Scholar]
- 47.Cefiderocol: Discovery, Chemistry, and In Vivo Profiles of a Novel Siderophore Cephalosporin. Sato Takafumi, Yamawaki Kenji. Clinical Infectious Diseases. 2019;69(Supplement_7):S538-S543. doi: 10.1093/cid/ciz826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.In Vitro Antibacterial Properties of Cefiderocol, a Novel Siderophore Cephalosporin, against Gram-Negative Bacteria. Ito Akinobu, Sato Takafumi, Ota Merime, Takemura Miki, Nishikawa Toru, Toba Shinsuke, Kohira Naoki, Miyagawa Satoshi, Ishibashi Naoki, Matsumoto Shuhei, Nakamura Rio, Tsuji Masakatsu, Yamano Yoshinori. Antimicrobial Agents and Chemotherapy. 2017;62(1) doi: 10.1128/AAC.01454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cefiderocol: A Siderophore Cephalosporin with Activity Against Carbapenem-Resistant and Multidrug-Resistant Gram-Negative Bacilli. Zhanel George G, Golden Alyssa R, Zelenitsky Sheryl, Wiebe Karyn, Lawrence Courtney K, Adam Heather J, Idowu Temilolu, Domalaon Ronald, Schweizer Frank, Zhanel Michael A, Lagacé-Wiens Philippe R S, Walkty Andrew J, Noreddin Ayman, Lynch Iii Joseph P, Karlowsky James A. Drugs. 2019;79(3):271–289. doi: 10.1007/s40265-019-1055-2. [DOI] [PubMed] [Google Scholar]
- 50.Interagency Coordination Group on Antimicrobial Resistance No Time to Wait: Securing the future from drug-resistant infections. World Health Organisation; Report to the Secretary-general of the United Nations.; Accessed in April 2019. https://www.who.int/antimicrobial-resistance/interagency-coordination-group/final-report/en/ https://www.who.int/antimicrobial-resistance/interagency-coordination-group/final-report/en/
- 51.Antibiotics Currently in Global Development. Pew Charitable Trusts; Accessed in September 2019.
- 52.A Phase 3, Randomized, Double-Blind, Multicenter Study to Evaluate the Safety and Efficacy of Intravenous Iclaprim Vs Vancomycin for the Treatment of Acute Bacterial Skin and Skin Structure Infections Suspected or Confirmed to be Due to Gram-Positive Pathogens: REVIVE-1. Huang David B, O'Riordan William, Overcash J Scott, Heller Barry, Amin Faisal, File Thomas M, Wilcox Mark H, Torres Antoni, Dryden Matthew, Holland Thomas L, McLeroth Patrick, Shukla Rajesh, Corey G Ralph. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;66(8):1222–1229. doi: 10.1093/cid/cix987. [DOI] [PubMed] [Google Scholar]
- 53.Iclaprim: a differentiated option for the treatment of skin and skin structure infections. Noviello Stephanie, Huang David B, Corey G Ralph. Expert review of anti-infective therapy. 2018;16(11):793–803. doi: 10.1080/14787210.2018.1536545. [DOI] [PubMed] [Google Scholar]
- 54.A new antibiotic selectively kills Gram-negative pathogens. Imai Yu, Meyer Kirsten J, Iinishi Akira, Favre-Godal Quentin, Green Robert, Manuse Sylvie, Caboni Mariaelena, Mori Miho, Niles Samantha, Ghiglieri Meghan, Honrao Chandrashekhar, Ma Xiaoyu, Guo Jason J, Makriyannis Alexandros, Linares-Otoya Luis, Böhringer Nils, Wuisan Zerlina G, Kaur Hundeep, Wu Runrun, Mateus André, Typas Athanasios, Savitski Mikhail M, Espinoza Josh L, O'Rourke Aubrie, Nelson Karen E, Hiller Sebastian, Noinaj Nicholas, Schäberle Till F, D'Onofrio Anthony, Lewis Kim. Nature. 2019;576(7787):459–464. doi: 10.1038/s41586-019-1791-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chimeric peptidomimetic antibiotics against Gram-negative bacteria. Luther Anatol, Urfer Matthias, Zahn Michael, Müller Maik, Wang Shuang-Yan, Mondal Milon, Vitale Alessandra, Hartmann Jean-Baptiste, Sharpe Timothy, Monte Fabio Lo, Kocherla Harsha, Cline Elizabeth, Pessi Gabriella, Rath Parthasarathi, Modaresi Seyed Majed, Chiquet Petra, Stiegeler Sarah, Verbree Carolin, Remus Tobias, Schmitt Michel, Kolopp Caroline, Westwood Marie-Anne, Desjonquères Nicolas, Brabet Emile, Hell Sophie, LePoupon Karen, Vermeulen Annie, Jaisson Régis, Rithié Virginie, Upert Grégory, Lederer Alexander, Zbinden Peter, Wach Achim, Moehle Kerstin, Zerbe Katja, Locher Hans H, Bernardini Francesca, Dale Glenn E, Eberl Leo, Wollscheid Bernd, Hiller Sebastian, Robinson John A, Obrecht Daniel. Nature. 2019;576(7787):452–458. doi: 10.1038/s41586-019-1665-6. [DOI] [PubMed] [Google Scholar]
- 56.A small-molecule inhibitor of BamA impervious to efflux and the outer membrane permeability barrier. Hart Elizabeth M, Mitchell Angela M, Konovalova Anna, Grabowicz Marcin, Sheng Jessica, Han Xiaoqing, Rodriguez-Rivera Frances P, Schwaid Adam G, Malinverni Juliana C, Balibar Carl J, Bodea Smaranda, Si Qian, Wang Hao, Homsher Michelle F, Painter Ronald E, Ogawa Anthony K, Sutterlin Holly, Roemer Terry, Black Todd A, Rothman Deborah M, Walker Scott S, Silhavy Thomas J. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(43):21748–21757. doi: 10.1073/pnas.1912345116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Novel antibiotics effective against gram-positive and -negative multi-resistant bacteria with limited resistance. Nicolas Irène, Bordeau Valérie, Bondon Arnaud, Baudy-Floc'h Michèle, Felden Brice. PLoS biology. 2019;17(7):e3000337. doi: 10.1371/journal.pbio.3000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.In Vitro Activity of Delafloxacin and Microbiological Response against Fluoroquinolone-Susceptible and Nonsusceptible Staphylococcus aureus Isolates from Two Phase 3 Studies of Acute Bacterial Skin and Skin Structure Infections. McCurdy S, Lawrence L, Quintas M, Woosley L, Flamm R, Tseng C, Cammarata S. Antimicrobial agents and chemotherapy. 2017;61(9) doi: 10.1128/AAC.00772-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.New and improved? A review of novel antibiotics for Gram-positive bacteria. Abbas M, Paul M, Huttner A. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2017;23(10):697–703. doi: 10.1016/j.cmi.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 60.Dalbavancin as Primary and Sequential Treatment for Gram-Positive Infective Endocarditis: 2-Year Experience at the General Hospital of Vienna. Tobudic Selma, Forstner Christina, Burgmann Heinz, Lagler Heimo, Ramharter Michael, Steininger Christoph, Vossen Matthias G, Winkler Stefan, Thalhammer Florian. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2018;67(5):795–798. doi: 10.1093/cid/ciy279. [DOI] [PubMed] [Google Scholar]
- 61.Dalbavancin for the treatment of acute bacterial skin and skin structure infections. Esposito Silvano, Noviello Silvana, Leone Sebastiano. Le infezioni in medicina. 2015;23(4):313–7. [PubMed] [Google Scholar]