Abstract
目的
评价入院时无害性急性胰腺炎评分(HAPS)对轻症急性胰腺炎(MAP)的预测效能。
方法
前瞻性纳入2016年1月~2017年8月连续就诊于四川大学华西医院的急性胰腺炎(AP)患者,计算入院时HAPS评分。根据HAPS评分将AP患者分为无害性AP组(HAPS>2)和非无害性AP组(HAPS≤2),比较两组患者的临床结局。以2012年修订的亚特兰大分级定义MAP,绘制受试者工作特征曲线(ROC),获得曲线下面积(AUC),特异性,敏感性,阳性预测值和阴性预测值。
结果
本研究共纳入703例患者,与HAPS评分预测为无害性AP组(n=182)的患者相比,非无害性AP组(n=521)患者的临床结局更差(P < 0.001)。本研究纳入MAP患者共计359例(51.1%),入院时的HAPS评分预测MAP的特异性为97.7%(95% CI: 95.4-99.0),敏感性为48.2%(95% CI: 42.9-53.3),阳性预测值为95.6%(95% CI: 91.5-98.1),阴性预测值为64.1%(95% CI: 59.8-68.2),AUC为0.749(95%CI: 0.72-0.78)。
结论
入院时HAPS评分预测MAP的效能较高。
Keywords: 无害性急性胰腺炎评分, 轻症急性胰腺炎, 预测价值
Abstract
Objective
To evaluate the efficacy of harmless acute pancreatitis score (HAPS) on admission for predicting mild acute pancreatitis (MAP).
Methods
We prospectively collected the data from consecutive AP patients admitted to West China Hospital between January, 2016 and August, 2017, and HAPS scores were calculated on admission. The clinical outcomes of the patients with harmless AP (HAPS>2) and those with non-harmless AP (HAPS≤2) were compared. MAP was defined based on the severity classification of 2012 Revised Atlanta guidelines. The area under the curve (AUC) of the receiver- operator characteristic curve (ROC), specificity, sensitivity, positive predictive value (PPV) and negative predictive value (NPV) of HAPS on admission for predicting MAP were analyzed.
Results
Of the 703 patients with AP analyzed, 182 were predicted to have harmless AP and 521 to have non- harmless AP, and the patients in the latter group had significantly worse clinical outcomes (P < 0.001). The total number of patients with MAP was 359 in the study. The specificity, the sensitivity, the PPV and NPV of HAPS on admission for predicting MAP was 97.7% (95% CI: 95.4-99.0), 48.2% (95% CI: 42.9-53.3), 95.6% (95% CI: 91.5- 98.1) and 64.1% (95% CI: 59.8- 68.2), respectively, and the AUC was 0.749 (95% CI: 0.72- 0.78).
Conclusion
HAPS score on admission can accurately predict MAP.
Keywords: harmless acute pancreatitis score, mild acute pancreatitis, prediction
急性胰腺炎(AP)是常见的消化系统急腹症之一,近年发病率呈持续增长趋势[1-2]。2012年修订的亚特兰大分级标准将AP分为轻症、中度重症和重症三大类[3]。其中,非轻症AP患者由于出现一过性或持续性器官功能衰竭(OF),和/或出现局部并发症,病程更长,病情更复杂;重症患者病情变化更快,预后更差,病死率高达30%~50%[4]。目前针对AP的特异性药物缺乏,支持治疗仍是早期的主要干预手段[5]。重症患者的OF常出现在发病后12~24 h,早期能够干预的时间窗非常狭窄,早期识别,早期积极治疗至关重要[5]。
基于此,挖掘早期预测AP严重程度方法的研究成为热点。临床评分系统是其中一大类预测方法,包括Ranson评分[6],床旁严重指数(BISAP)评分[7],Glasgow评分[8],APACHE Ⅱ评分[9],CT严重程度指数评分[10],PASS评分[11],Panc 3评分[12]等等。但上述一系列的临床评分系统或评分内容繁琐使用受限,或无法早期完成评分,或预测效能不佳[13]。能否尽早(24 h内)识别出病情较重患者且便于临床使用的评分系统?2009年Lankisch提出无害性急性胰腺炎评分(HAPS),报道其预测非重症AP的特异性高达97%[14], 为AP疾病早期预测的研究领域开辟了另一个思路。
尽管既往已有研究报道HAPS评分预测非重症AP的良好效能[15-16],但由于所采用AP严重程度分级标准不一或纳入样本量较少,其推广和应用有限,且尚无国内大样本的研究报道HAPS的预测效能评价。因此,本研究根据最新的2012年修订的亚特兰大标准[3],评价入院时的HAPS评分预测MAP的效能,以期指导早期非重症的识别。
1. 资料和方法
1.1. 研究对象
收集2016年1月~2017年8月连续就诊于四川大学华西医院的AP患者。AP的诊断根据2012年修订的亚特兰大分级指南[3]:中上腹持续性疼痛症状;血淀粉酶和/或脂肪酶升高超过正常值3倍以上;腹部彩超或CT检查符合影像学改变;以上标准满足任意两项即可确诊AP。本研究通过伦理委员会批准(批准号NO.247)。
本研究纳入标准为:确诊的AP;发病时间不超过48 h。排除标准为:AP非主要入院诊断;年龄小于18岁或大于70岁;创伤性胰腺炎、慢性胰腺炎、胰腺癌作为AP病因;合并妊娠;严重合并症,包括终末期肺病,不稳定型冠心病或充血性心力衰竭3~4级(纽约心脏病协会心衰分级),慢性肾病4~5期(KDIGO CKD指南),肝硬化(改良Child-Pugh) 2~3级;恶性肿瘤和/或免疫缺陷;(7)数据记录不完整。
1.2. 研究方法
1.2.1. 一般资料
收集患者基线资料(包括年龄、性别、体质量指数、发病时间、病因和基础疾病),入院时生命体征(体温、呼吸、心率、血压、腹膜刺激征),辅助检查(白细胞计数、红细胞压积、血肌酐)和重要临床结局(包括器官功能衰竭、局部并发症、ICU转入率、手术率、平均住院时间和病死率等)。
1.2.2. HAPS评分
根据采集数据计算入院时HAPS评分。HAPS评分标准如下:无压痛和腹肌紧张;红细胞压积正常(男性≤ 43%,女性≤ 39.6%);血肌酐正常(< 176.8 mmol/L),以上3项指标均正常定义为无害,提示MAP;1项或1项以上异常定义为非无害。
1.2.3. 病情严重程度分级
参考2012年修订的亚特兰大分级指南[3]:MAP,无器官功能衰竭和局部或系统并发症;中度重症急性胰腺炎(MSAP),出现一过性的器官功能衰竭(OF ≤ 48 h)和(或)局部或全身并发症;重症急性胰腺炎(SAP),持续性的器官功能衰竭(OF>48 h)。
1.3. 统计方法
应用SPSS 23.0和Medcalc分析软件。计量资料使用均数±标准差(正态分布)或中位数(非正态分布)表示,分类变量资料使用数字和百分比表示。连续型资料组间比较采用方差分析或秩和检验,分类变量采用χ2检验或Fisher精确检验。运用受试者工作特征(ROC)曲线计算曲线下面积(AUC)及其95%可信区间(CI)评估预测价值,并计算敏感性,特异性,阳性预测值和阴性预测值。P < 0.05为差异有统计学意义。
2. 结果
2.1. 一般基线资料
本研究共纳入AP患者703例,其中男性475例(67.6%),中位年龄45岁(37~51),中位体质量指数为25.4 kg/m2(23.0~27.7),中位发病时间为24 h(12~24)。AP病因分布:胆源性143例(20.3%),高脂血症性282例(40.1%),特发或其它病因278例(39.6%)。入院时HAPS评分各项指标中,有反跳痛的患者为395例(56.2%),肌酐大于176.8 μmol/L的患者为16例(2.3%),红细胞压积异常的患者为389例(55.3%),其中最容易出现异常的为腹肌紧张或反跳痛,其次是红细胞压积,肌酐异常者最少(表 1)。
1.
纳入患者的基线资料
Baseline characteristics of all the patients[median (IQR) or No. of cases (%)]
| Variables | Total (n=703) |
| IQR: Interquartile range; BMI: Body mass index; HAPS: Harmless acute pancreatitis score; *Male>43%, Female>39.6%. | |
| Age (year) | 45 (37-51) |
| Male | 475 (67.6) |
| BMI (kg/m2) | 25.4 (23.0-27.7) |
| Times from symptoms onset/ hours | 24(12-24) |
| Etiology | |
| Biliary | 143 (20.3) |
| Hypertriglyceridemia | 282(40.1) |
| Others | 278 (39.6) |
| HAPS | |
| Rebound tenderness | 395 (56.2) |
| Creatinine > 176.8卩mol/L | 16 (2.3) |
| Hematocrit* | 389 (55.3) |
2.2. HAPS评分预测为无害性和非无害性AP的临床结局的比较
由表 2可见,HAPS评分预测为无害性AP组患者的所有临床结局明显优于预测为非无害性AP组的患者。无害性AP与非无害性AP组相比,一过性器官功能衰竭,单个或多个持续性器官功能衰竭发生率、ICU转入率、局部并发症包括急性液体积聚和胰腺坏死发生率、手术率、病死率均显著低于非无害性AP组,差异有统计学意义(P < 0.05)。无害性组患者的平均住院日为8 d,也显著低于非无害性AP组(10 d),差异有统计学意义(P < 0.05)。
2.
HAPS评分预测为无害性AP和非无害性AP的临床结局对比
Comparisons of clinical outcomes between harmless AP and non-harmless AP groups predicted by HAPS [No. of cases (%)]
| Clinical outcomes | HarmlessAP group (n=182) | Non-Harmless AP group (n=521) | P |
| AP: Acute pancreatitis; HAPS: Harmless acute pancreatitis score; APFC: Acute pancreatic fluid collection. | |||
| Transient organ failure Single persistent organ failure |
0 | 93 (17.9) | < 0.001 |
| Respiratory | 0 | 113 (21.7) | < 0.001 |
| Circulation | 0 | 8 (1.5) | < 0.001 |
| Kidney | 0 | 17 (3.6) | < 0.001 |
| Persistent multi-organ failure | 0 | 36 (7.5) | < 0.001 |
| Intensive care unit transfer | 0 | 126 (26.4) | < 0.001 |
| Respiratory support | 0 | 100 (20.9) | < 0.001 |
| Local complication | 8(4.4) | 260 (54.4) | < 0.001 |
| APFC | 7 (3.8) | 170 (35.6) | < 0.001 |
| (ex)pancreatic necrosis | 1 (0.5) | 125 (26.2) | < 0.001 |
| Necrosectomy | 0 | 27 (5.6) | < 0.001 |
| Length of hospital stay | 8(6-11) | 10(7-16) | < 0.001 |
| Mortality | 0 | 23 (4.8) | < 0.001 |
2.3. HAPS评分预测MAP的效能
由图 1可见,本研究纳入的703例患者中,入院时HAPS评分预测为无害性AP的患者182例(25.9%),其中MAP 174例,符合率达95.6%,非MAP 8例占比4.4%;评分预测为非无害性AP有521例(74.1%),其中非MAP 336例,符合率达64.5%,MAP 185例,占比为35.8%,两者比较差异有统计学意义(P < 0.001)。
1.
根据HAPS评分预测的AP严重程度分级患者分布情况
Distribution of severity category of AP based on HAPS.
由表 3和图 2可见,在纳入的所有患者中,HAPS评分预测MAP的特异度、敏感度、阳性预测值和阴性预测值分别为97.7%、48.2%、95.6%和64.1%,AUC为0.749 (95% CI:0.72-0.78)。按照是否转院对纳入患者进行亚组分析:在310例首诊患者中,HAPS评分预测MAP的AUC为0.74,特异度、敏感度、阳性预测值和阴性预测值分别为96.0%、47.1%、96.1%和46.4%;在393例转诊患者中,HAPS评分预测MAP的AUC为0.75,特异度、敏感度、阳性预测值和阴性预测值分别为98.3%、49.7%、94.9%和75.8%。按照病因进行亚组分析:高脂血症病因的282例AP患者中,HAPS评分预测MAP的AUC为0.73,特异度、敏感度、阳性预测值和阴性预测值分别为99.3%、38.1%、98.1%和63.9%;胆源性胰腺炎患者中,HAPS评分预测MAP的AUC为0.78,特异度、敏感度、阳性预测值和阴性预测值分别为96.2%、60.9%、92.9%和75.2%。
3.
HAPS预测MAP的效能
Predictive value of HAPS for MAP[% (95% CI)]
| n | Specificity | Sensitivity | PPV | NPV | |
| HAPS: Harmless acute pancreatitis score; MAP: Mild acute pancreatitis; CI: Confidence interval; PPV: Positive predicted value; NPV: Negative predicted value; HLAP: Hyperlipidemic acute pancreatitis; BAP: Biliary acute pancreatitis. | |||||
| All patients | 703 | 97.7 (95.4-99.0) | 48.2 (42.9-53.3) | 95.6 (91.5-98.1) | 64.1 (59.8-68.2) |
| Primary patients | 310 | 96.0 (90.1-98.9) | 47.1 (40.2-54.1) | 96.1 (90.4-98.9) | 46.4 (39.4-53.4) |
| Transferred patients | 393 | 98.3 (95.8-99.5) | 49.7 (41.4-57.9) | 94.9 (87.5-98.6) | 75.8 (70.7-80.4) |
| HLAP | 282 | 99.3 (96.3-100) | 38.1 (29.8-46.8) | 98.1 (89.7-100) | 63.9 (57.3-70.1) |
| BAP | 143 | 96.2 (89.3-99.2) | 60.9 (47.9-72.9) | 92.9 (80.3-98.5) | 75.2 (65.7-83.3) |
2.
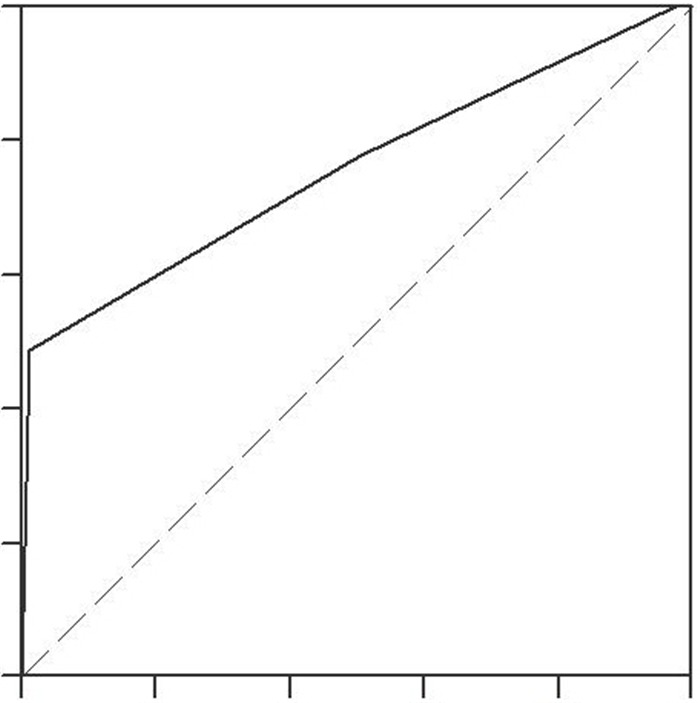
HAPS预测无害性AP的ROC曲线
ROC curve of HAPS for predicting harmless acute pancreatitis.
2.4. HAPS评分的单项参数预测MAP的效能
表 4展示了HAPS评分的3个单项参数预测MAP的效能。入院时有反跳痛的患者共有395例,其中非MAP符合率为70.6%,MAP占29.3%;入院时无反跳痛的患者共有308例,其中MAP符合率为79.5%,非MAP为20.5%,两者比较差异有统计学意义(P < 0.001)。入院时肌酐大于176.8 μmol/L的患者有16例,均为非MAP,符合率100%;入院时肌酐小于等于176.8 μmol/L的患者有687例,MAP和非MAP分别为361例(52.5%)和326例(47.4%),两者比较差异有统计学意义(P < 0.001)。入院时红细胞压积异常的患者共有389例(55.3%),其中,MAP和非MAP分别有167例(42.9%)和222例(57.1%);入院时红细胞压积正常者共有314例,其中,MAP和非MAP分别有194 (61.7%)和120例(38.2%),两者比较差异有统计学意义(P < 0.05)。
4.
比较HAPS单项指标预测MAP和非MAP的四格表
Cross table for comparison of single parameters in HAPS between MAP and Non-MAP groups
| HAPS single parameter | MAP | Non-MAP | P |
| MAP: Mild acute pancreatitis; Non-MAP: None mild acute pancreatitis. | |||
| Rebound tenderness | |||
| + | 116 | 279 | < 0.001 |
| - | 245 | 63 | |
| Creatinine > 176.8 pmol/L | |||
| + | 0 | 16 | < 0.001 |
| - | 361 | 326 | |
| Hematocrit | |||
| + | 167 | 222 | 0.002 |
| - | 194 | 120 | |
3. 讨论
AP严重程度的评估有助于治疗措施的准确选择(包括早期液体复苏和重症监护)和改善预后。目前对AP严重程度的预测主要包括临床评分系统、影像学评分和独立生化指标。自1974年第一个临床评分系统(Ranson评分)报道以来,已经有大约20个不同的评分系统报道并应用于临床[17]。其中,Ranson评分需要48 h才能完成评分,随着对AP严重程度分级更新和器官功能重要性的认识,该评分逐渐被一些新兴评分方法取代。APACHE Ⅱ是仅次于Ranson评分晚些出现,迄今为止报道预测死亡发生的准确度显著高于其他评分系统,预测重症也具有较高的敏感性和特异性[17, 18],但由于该评分包含14个评估指标,计算繁琐而限制了临床应用[9, 19]。PASS是2017年最新提出的评分,主要关于AP疾病过程的活动性程度评价,与临床结局如局部并发症发生、ICU入住、感染性坏死等相关性高;而在预测重症方面但并未表现出显著优势[20-22]。CT评分系统对AP严重程度的预测准确性与临床评分系统相似[18, 23],但由于AP通过CT反映严重程度的表现通常较临床表现滞后,在发病后7~10 d局部的表现比较完全,敏感性也偏低,因此,各指南推荐影像学检查主要用于需要明确诊断的AP,评估局部并发症和感染性坏死[2, 23]。而独立的生化指标预测效能在所有评分中表现最差,目前已经较少单独使用单个生化指标预测重症[24]。HAPS,Panc 3和BISAP评分是目前所有评分系统中操作方便并可于24 h内完成的评分系统。三者中BISAP预测重症效能最高,与APACHEⅡ相当,Panc 3与HAPS类似,准确性低于BISAP[13, 25]。但三者预测重症的均特异性较高,敏感性较低[13, 26-27],意味着许多重症患者早期预测时可能无法被识别出来。而早期识别重症患者并及时的综合救治是治疗的关键,尤其是24 h内及时的液体复苏和早期器官功能支持治疗可明显改善预后。3个评分中,HAPS预测重症的阴性预测值明显高于其他评分[13]。因此学者们开始关注是否能通过提高早期预测轻症AP的准确性来提高识别非轻症患者即中重症和重症患者的准确度,进而提高早期中重症和重症患者的救治率,HAPS的应用逐渐受到关注。
HAPS评分的参数为腹部体征、红细胞压积水平和血肌酐水平。其中,腹痛是AP患者主要的就诊原因,腹部反跳痛(或肌紧张)是重要的临床体征。红细胞压积是血液中红细胞的体积百分比,能反应血液浓缩和血液稀释水平[28],研究表明其在预测胰腺坏死和OF方面,优于其他参数[29-31]。肾脏作为SAP胰外受损的靶器官,其功能不全或衰竭被认为是SAP死亡的高危因素,血清肌酐水平是评价肾脏功能应用最广泛的参数之一[32]。HAPS评分综合以上3个参数用以早期预测MAP,具有操作简单,评估迅速,基层医师和临床经验不足的住院医师都能正确完成评分等优点而受到青睐。2009年Lankisch报道HAPS预测MAP的特异度和阳性预测值分别高达97% (89%~99%)和98% (92%~100%)[14]。德国、瑞典和印度的研究相继证实其预测MAP的价值,并证明预测为无害性AP的患者发生OF和局部并发症的风险较低[15-16]。
目前关于HAPS评分的研究国外报道仅4篇,且研究纳入病例数较少,其中采用2012年修订的亚特兰大分级标准[3]的研究仅1篇。因此,本研究是第1次针对中国西部地区AP患者,采用新的严重程度分型标准,前瞻性大样本评价入院时HAPS评分预测MAP的效能。本研究纳入患者的平均发病时间为24 h,与国外文献报道(18~24 h)相似[15-16],是国内首次具体对患者发病时间进行描述,意味着我中心的AP患者在发病早期即接受严重程度评估,也提示HAPS评分在疾病更早期阶段预测MAP的价值。
本研究中,MAP 359例,MSAP 206例和SAP 138例,与我中心报道的疾病分布特点一致[33],符合AP疾病的流行病学特征[4]。结果表明,入院时HAPS评分预测为无害性AP组的患者临床结局显著优于评分预测为非无害性AP组的患者,差异具有统计学意义(表 2),与文献报道一致。本研究中MAP占比为51.1%,HAPS预测MAP的特异度为97.7%,阳性预测值为95.6%,AUC为0.749,提示其具有较好的预测效能,与既往文献报道的结果类似[14-15]。而进一步的病因亚组分析中,比较HAPS在高脂病因和胆源性病因中的预测效能,结果显示前者的特异度(99.3%)和阳性预测值(98.1%)稍优于后者,也优于国内另一项高脂血症型AP (HLAP)的回顾性研究[34],也提示HAPS可能在预测不同病因的AP人群中具有差异性,下一步可针对不同病因的AP进行大样本的前瞻性队列研究验证。
尽管本研究结果表明HAPS在首诊与转院两组患者的预测效能相当,但根据HAPS的各项指标分布来看,在预测为无害性AP而实际为非MAP的患者中,约1/3患者在入院时表现为无反跳痛和腹肌紧张;约35%的非MAP患者入院时红细胞压积水平正常。这可能与华西医院是一家AP转诊中心有关。本研究纳入患者超过一半以上为转诊,转诊患者通常已经在当地医院接受初始治疗(包括早期的液体复苏和止痛等),而我院的首诊患者几乎由急诊入院,多数在急诊科也已接受治疗(与转诊患者情况类似)。而早期液体复苏可使部分患者的红细胞压积和肌酐降至正常,早期止痛可缓解患者腹痛,减轻腹部体征。因此,这部分非MAP患者在入院时可能表现为无反跳痛和腹肌紧张,红细胞压积和肌酐水平正常而被预测为无害性AP。提示针对已经接受过早期治疗的AP患者,对HAPS的效能解读需更谨慎。事实上,转诊AP患者在所有AP中占比很大,但既往针对这一人群的报道较少,可能HAPS在首诊未经过治疗患者中的预测价值更高,有待进一步的验证。
OSKA等[15]的研究表明,HAPS预测MAP的特异性为96%,但敏感性仅为29%。我们的研究与其结果类似。为探索敏感性低的原因,本研究进一步将HAPS评分的3个参数独立的预测效能进行比较(表 4)。结果发现,肌酐异常的16例患者均被预测为重症,这与文献报道一致;但有反跳痛或肌紧张的患者中32.1%实际是MAP,红细胞压积水平异常患者中超过一半以上(53.7%)实际是MAP。而这部分患者未被HAPS识别出来,造成了较高的漏诊率,可能会接受更集中更密集的观察监护,但是减少了非轻症患者的漏诊,增加了对中度重症和重症患者的早期监护和治疗,临床意义重大。在临床实际工作中,大多数患者为MAP,相对于敏感性偏低而言,HAPS预测的特异性和阳性预测值高意味着识别出的无害性AP患者可靠性高,进展为重症的可能性小,因此可简化这类患者一些治疗方案比如禁食时间过长、抑制胰腺分泌药物、抗生素等的使用,进一步优化患者治疗方案,节约卫生经济资源,临床意义更大。
综上所述,本研究表明入院时HAPS评分预测为无害性AP的患者的临床结局优于评分预测为非无害性AP组的患者;采用入院时HAPS评分预测MAP,具有较好的效能。
Biography
马小华,在读硕士研究生,Email: dr_maxiaohua@163.com
Funding Statement
国家重点研发计划“政府间国际科技创新合作”重点专项项目“减轻急性胰腺炎肠道功能障碍和器官功能衰竭的合作研究”(2016YFE0101800)
Contributor Information
马 小华 (Xiaohua MA), Email: dr_maxiaohua@163.com.
夏 庆 (Qing XIA), Email: xiaqing@medmail.com.cn.
References
- 1.Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. 2015;386(9988):85–96. doi: 10.1016/S0140-6736(14)60649-8. [Lankisch PG, Apte M, Banks PA. Acute pancreatitis[J]. Lancet, 2015, 386(9988): 85-96.] [DOI] [PubMed] [Google Scholar]
- 2.Hines OJ, Pandol SJ. Management of severe acute pancreatitis. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_3d6db2f8ed4f6563011cd196875370f8. BMJ. 1998;367(1):95–6. doi: 10.1136/bmj.l6227. [Hines OJ, Pandol SJ. Management of severe acute pancreatitis[J]. BMJ, 1998, 367(1): 95-6.] [DOI] [PubMed] [Google Scholar]
- 3.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11. doi: 10.1136/gutjnl-2012-302779. [Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis--2012: revision of the Atlanta classification and definitions by international consensus[J]. Gut, 2013, 62(1): 102-11.] [DOI] [PubMed] [Google Scholar]
- 4.Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis. N Engl J Med. 2016;375(20):1972–81. doi: 10.1056/NEJMra1505202. [Forsmark CE, Vege SS, Wilcox CM. Acute pancreatitis[J]. N Engl J Med, 2016, 375(20): 1972-81.] [DOI] [PubMed] [Google Scholar]
- 5.Crockett SD, Wani S, Gardner TB, et al. American gastroenterological association institute guideline on initial management of acute pancreatitis. Gastroenterology. 2018;154(4):1096–101. doi: 10.1053/j.gastro.2018.01.032. [Crockett SD, Wani S, Gardner TB, et al. American gastroenterological association institute guideline on initial management of acute pancreatitis[J]. Gastroenterology, 2018, 154(4): 1096-101.] [DOI] [PubMed] [Google Scholar]
- 6.Ranson JH, Rifkind KM, Roses DF, et al. Prognostic signs and the role of operative management in acute pancreatitis. http://cn.bing.com/academic/profile?id=aefe43f19fdce1e9dbdc360e9e7af4f6&encoded=0&v=paper_preview&mkt=zh-cn. Surg Gynecol Obstet. 1974;139(1):69–81. [Ranson JH, Rifkind KM, Roses DF, et al. Prognostic signs and the role of operative management in acute pancreatitis[J]. Surg Gynecol Obstet, 1974, 139(1): 69-81.] [PubMed] [Google Scholar]
- 7.Wu BU, Johannes RS, Sun X, et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57(12):1698–703. doi: 10.1136/gut.2008.152702. [Wu BU, Johannes RS, Sun X, et al. The early prediction of mortality in acute pancreatitis: a large population-based study[J]. Gut, 2008, 57(12): 1698-703.] [DOI] [PubMed] [Google Scholar]
- 8.Blamey SL, Imrie CW, O'neill J, et al. Prognostic factors in acute pancreatitis. Gut. 1984;25(12):1340–6. doi: 10.1136/gut.25.12.1340. [Blamey SL, Imrie CW, O'neill J, et al. Prognostic factors in acute pancreatitis[J]. Gut, 1984, 25(12): 1340-6.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larvin M, Mcmahon MJ. APACHE-Ⅱ score for assessment and monitoring of acute pancreatitis. http://d.old.wanfangdata.com.cn/NSTLQK/10.1016-S0140-6736(89)90381-4/ Lancet. 1989;2(8656):201–5. doi: 10.1016/s0140-6736(89)90381-4. [Larvin M, Mcmahon MJ. APACHE-Ⅱ score for assessment and monitoring of acute pancreatitis[J]. Lancet, 1989, 2(8656): 201-5.] [DOI] [PubMed] [Google Scholar]
- 10.Balthazar EJ, Robinson DL, Megibow AJ, et al. Acute pancreatitis: value of CT in establishing prognosis. Radiology. 1990;174(2):331–6. doi: 10.1148/radiology.174.2.2296641. [Balthazar EJ, Robinson DL, Megibow AJ, et al. Acute pancreatitis: value of CT in establishing prognosis[J]. Radiology, 1990, 174(2): 331-6.] [DOI] [PubMed] [Google Scholar]
- 11.Wu BU, Batech M, Quezada M, et al. Dynamic measurement of disease activity in acute pancreatitis: the pancreatitis activity scoring system. Am J Gastroenterol. 2017;112(7):1144–52. doi: 10.1038/ajg.2017.114. [Wu BU, Batech M, Quezada M, et al. Dynamic measurement of disease activity in acute pancreatitis: the pancreatitis activity scoring system[J]. Am J Gastroenterol, 2017, 112(7): 1144-52.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown A, James ST, Dyson T, et al. The Panc 3 score:a rapid and accurate test for predicting severity on presentation in acute pancreatitis. J Clin Gastroenterol. 2007;41(9):855–8. doi: 10.1097/01.mcg.0000248005.73075.e4. [Brown A, James ST, Dyson T, et al. The Panc 3 score:a rapid and accurate test for predicting severity on presentation in acute pancreatitis[J]. J Clin Gastroenterol, 2007, 41(9): 855-8.] [DOI] [PubMed] [Google Scholar]
- 13.Mounzer R, Langmead CJ, Wu BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis. Gastroenterology. 2012;142(7):1476–82. doi: 10.1053/j.gastro.2012.03.005. [Mounzer R, Langmead CJ, Wu BU, et al. Comparison of existing clinical scoring systems to predict persistent organ failure in patients with acute pancreatitis[J]. Gastroenterology, 2012, 142(7): 1476-82.] [DOI] [PubMed] [Google Scholar]
- 14.Lankisch PG, Weber-Dany B, Hebel K, et al. The harmless acute pancreatitis score: a clinical algorithm for rapid initial stratification of nonsevere disease. Clin Gastroenterol Hepatol. 2009;7(6):702–5. doi: 10.1016/j.cgh.2009.02.020. [Lankisch PG, Weber-Dany B, Hebel K, et al. The harmless acute pancreatitis score: a clinical algorithm for rapid initial stratification of nonsevere disease[J]. Clin Gastroenterol Hepatol, 2009, 7(6): 702-5.] [DOI] [PubMed] [Google Scholar]
- 15.Oskarsson V, Mehrabi M, Orsini N, et al. Validation of the harmless acute pancreatitis score in predicting nonsevere course of acute pancreatitis. Pancreatology. 2011;11(5):464–8. doi: 10.1159/000331502. [Oskarsson V, Mehrabi M, Orsini N, et al. Validation of the harmless acute pancreatitis score in predicting nonsevere course of acute pancreatitis[J]. Pancreatology, 2011, 11(5): 464-8.] [DOI] [PubMed] [Google Scholar]
- 16.Talukdar R, Nageshwar Reddy D. Predictors of adverse outcomes in acute pancreatitis: new horizons. Indian J Gastroenterol. 2013;32(3):143–51. doi: 10.1007/s12664-013-0306-5. [Talukdar R, Nageshwar Reddy D. Predictors of adverse outcomes in acute pancreatitis: new horizons[J]. Indian J Gastroenterol, 2013, 32 (3): 143-51.] [DOI] [PubMed] [Google Scholar]
- 17.Di MY, Liu H, Yang ZY. Prediction models of mortality in acute pancreatitis in adults: a systematic review. Ann Intern Med. 2016;165(7):482–90. doi: 10.7326/M16-0650. [Di MY, Liu H, Yang ZY. Prediction models of mortality in acute pancreatitis in adults: a systematic review[J]. Ann Intern Med, 2016, 165(7): 482-90.] [DOI] [PubMed] [Google Scholar]
- 18.Mikó A, Vigh É, Mátrai P, et al. Computed tomography severity index vs. other indices in the prediction of severity and mortality in acute pancreatitis: a predictive accuracy meta-analysis. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6718714/ Front Physiol. 2019;10(27):1002. doi: 10.3389/fphys.2019.01002. [Mikó A, Vigh É, Mátrai P, et al. Computed tomography severity index vs. other indices in the prediction of severity and mortality in acute pancreatitis: a predictive accuracy meta-analysis[J]. Front Physiol, 2019, 10(27): 1002.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rathnakar SK, Vishnu VH, Muniyappa S, et al. Accuracy and predictability of PANC-3 scoring system over APACHE Ⅱ in acute pancreatitis: a prospective study. https://www.ncbi.nlm.nih.gov/pubmed/28384928. J Clin Diagn Res. 2017;11(2):PC10–3. doi: 10.7860/JCDR/2017/23168.9375. [Rathnakar SK, Vishnu VH, Muniyappa S, et al. Accuracy and predictability of PANC-3 scoring system over APACHE Ⅱ in acute pancreatitis: a prospective study[J]. J Clin Diagn Res, 2017, 11(2): PC10-3.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buxbaum J, Quezada M, Chong B, et al. The pancreatitis activity scoring system predicts clinical outcomes in acute pancreatitis: findings from a prospective cohort study. Am J Gastroenterol. 2018;113(5):755–64. doi: 10.1038/s41395-018-0048-1. [Buxbaum J, Quezada M, Chong B, et al. The pancreatitis activity scoring system predicts clinical outcomes in acute pancreatitis: findings from a prospective cohort study[J]. Am J Gastroenterol, 2018, 113(5): 755-64.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.James B, Michael Q, Bradford C, et al. The pancreatitis activity scoring system predicts clinical outcomes in acute pancreatitis: findings from a prospective cohort study. Am J Gastroenterol. 2018;113(5):755–64. doi: 10.1038/s41395-018-0048-1. [James B, Michael Q, Bradford C, et al. The pancreatitis activity scoring system predicts clinical outcomes in acute pancreatitis: findings from a prospective cohort study[J]. Am J Gastroenterol, 2018, 113(5): 755-64.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ke L, Mao W, Li X, et al. The pancreatitis activity scoring system in predicting infection of pancreatic necrosis. Am J Gastroenterol. 2018;113(9):1393–4. doi: 10.1038/s41395-018-0112-x. [Ke L, Mao W, Li X, et al. The pancreatitis activity scoring system in predicting infection of pancreatic necrosis[J]. Am J Gastroenterol, 2018, 113(9): 1393-4.] [DOI] [PubMed] [Google Scholar]
- 23.Bollen TL, Singh VK, Maurer R, et al. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107(4):612–9. doi: 10.1038/ajg.2011.438. [Bollen TL, Singh VK, Maurer R, et al. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis[J]. Am J Gastroenterol, 2012, 107(4): 612-9.] [DOI] [PubMed] [Google Scholar]
- 24.Gomatos IP, Xu X, Ghaneh P, et al. Prognostic markers in acute pancreatitis. Expert Rev Mol Diagn. 2014;14(3):333–46. doi: 10.1586/14737159.2014.897608. [Gomatos IP, Xu X, Ghaneh P, et al. Prognostic markers in acute pancreatitis[J]. Expert Rev Mol Diagn, 2014, 14(3): 333-46.] [DOI] [PubMed] [Google Scholar]
- 25.Gray R, Cagliani J, L i, et al. Maximizing the use of scoring systems in the prediction of outcomes in acute pancreatitis. Digestion. 2019;99(2):166–71. doi: 10.1159/000490887. [Gray R, Cagliani J, Li, et al. Maximizing the use of scoring systems in the prediction of outcomes in acute pancreatitis[J]. Digestion, 2019, 99(2): 166-71.] [DOI] [PubMed] [Google Scholar]
- 26.Sumitra H, Nitesh K. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis-A prospective observational study. Int J Surg. 2018;54:76–81. doi: 10.1016/j.ijsu.2018.04.026. [Sumitra H, Nitesh K. Evaluation of the BISAP scoring system in prognostication of acute pancreatitis-A prospective observational study[J]. Int J Surg, 2018, 54: 76-81.] [DOI] [PubMed] [Google Scholar]
- 27.Yang YX, Li L. Evaluating the ability of the bedside index for severity of acute pancreatitis score to predict severe acute pancreatitis: a Meta-Analysis. http://cn.bing.com/academic/profile?id=5781279cb40feafec6e51f08556449c4&encoded=0&v=paper_preview&mkt=zh-cn. Med Princ Pract. 2016;25(2):137–42. doi: 10.1159/000441003. [Yang YX, Li L. Evaluating the ability of the bedside index for severity of acute pancreatitis score to predict severe acute pancreatitis: a Meta-Analysis[J]. Med Princ Pract, 2016, 25(2): 137-42.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walton BL, Lehmann M, Skorczewski T, et al. Elevated hematocrit enhances platelet accumulation following vascular injury. Blood. 2017;129(18):2537–46. doi: 10.1182/blood-2016-10-746479. [Walton BL, Lehmann M, Skorczewski T, et al. Elevated hematocrit enhances platelet accumulation following vascular injury[J]. Blood, 2017, 129(18): 2537-46.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koutroumpakis E, Wu B, Bakker OJ, et al. Admission hematocrit and rise in blood Urea Nitrogen at 24 h outperform other laboratory markers in predicting persistent organ failure and pancreatic necrosis in acute pancreatitis: a post Hoc analysis of three large prospective databases. Am J Gastroenterol. 2015;110(12):1707–16. doi: 10.1038/ajg.2015.370. [Koutroumpakis E, Wu B, Bakker OJ, et al. Admission hematocrit and rise in blood Urea Nitrogen at 24 h outperform other laboratory markers in predicting persistent organ failure and pancreatic necrosis in acute pancreatitis: a post Hoc analysis of three large prospective databases[J]. Am J Gastroenterol, 2015, 110(12): 1707-16.] [DOI] [PubMed] [Google Scholar]
- 30.Baillargeon JD, Orav J, Ramagopal V, et al. Hemoconcentration as an early risk factor for necrotizing pancreatitis. Am J Gastroenterol. 1998;93(11):2130–4. doi: 10.1111/j.1572-0241.1998.00608.x. [Baillargeon JD, Orav J, Ramagopal V, et al. Hemoconcentration as an early risk factor for necrotizing pancreatitis[J]. Am J Gastroenterol, 1998, 93(11): 2130-4.] [DOI] [PubMed] [Google Scholar]
- 31.Wu B, Johannes RS, Conwell DL, et al. Early hemoconcentration predicts increased mortality only among transferred patients with acute pancreatitis. Pancreatology. 2009;9(5):639–43. doi: 10.1159/000181175. [Wu B, Johannes RS, Conwell DL, et al. Early hemoconcentration predicts increased mortality only among transferred patients with acute pancreatitis[J]. Pancreatology, 2009, 9(5): 639-43.] [DOI] [PubMed] [Google Scholar]
- 32.Lipinski M, Rydzewski A, Rydzewska G. Early changes in serum creatinine level and estimated glomerular filtration rate predict pancreatic necrosis and mortality in acute pancreatitis: Creatinine and eGFR in acute pancreatitis. Pancreatology. 2013;13(3):207–11. doi: 10.1016/j.pan.2013.02.002. [Lipinski M, Rydzewski A, Rydzewska G. Early changes in serum creatinine level and estimated glomerular filtration rate predict pancreatic necrosis and mortality in acute pancreatitis: Creatinine and eGFR in acute pancreatitis[J]. Pancreatology, 2013, 13(3): 207-11.] [DOI] [PubMed] [Google Scholar]
- 33.Shi N, Liu T, Iglesia-Garcia LD, et al. Duration of organ failure impacts mortality in acute pancreatitis. http://cn.bing.com/academic/profile?id=d2035b113cf7cf7f390cc0ee19d4f5aa&encoded=0&v=paper_preview&mkt=zh-cn. Gut. 2019 doi: 10.1136/gutjnl-2019-318241. [Shi N, Liu T, Iglesia-Garcia LD, et al. Duration of organ failure impacts mortality in acute pancreatitis[J]. Gut, 2019.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.马 秀颖, 汤 茂春, 邱 雷, et al. 无害性胰腺炎评分对高脂血症性胰腺炎的评估. http://d.old.wanfangdata.com.cn/Periodical/shtddxxb201602012. 同济大学学报:医学版. 2016;2:52–6. [马秀颖, 汤茂春, 邱雷, 等.无害性胰腺炎评分对高脂血症性胰腺炎的评估[J].同济大学学报:医学版, 2016, 2: 52-6.] [Google Scholar]


