Abstract
Post-stroke depression (PSD) is the most prevalent psychiatric complication of acute ischemic stroke. The neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR) are indicators of inflammation and are associated with stroke and depression. Therefore, the purpose of the present study was to examine the relationship between NLR/PLR and PSD. Retrospective analysis was carried out in 376 patients with first-ever acute ischemic stroke in the First Affiliated Yijishan Hospital of Wannan Medical College between March 2015 and September 2017. Patients were divided into PSD (n=104; 27.7%) and non-PSD (n=272; 72.3%) groups according to the Diagnostic and Statistical Manual of Mental Disorders-IV criteria at 6 months after stroke. Clinical data were collected retrospectively. NLR and PLR were acquired retrospectively from the routine blood tests performed at admission. A total of 120 healthy volunteers from the physical examination center in the First Affiliated Yijishan Hospital of Wannan Medical College were recruited as controls. Using logistic regression analysis, NLR (≥4.02) and PLR (≥203.74) were independently associated with PSD. NLR, odds ratio (OR) 3.926, 95% confidence intervals (CI, 2.365-7.947), P<0.001; PLR, OR 3.853, 95% CI (2.214-6.632), P=0.002. The ability of the combined index [area under the receiver operating characteristic curve, 0.701; 95% CI (0.622-0.780); P<0.001] to diagnose PSD was greater than that of either ratio alone. Higher NLRs and PLRs (≥4th quartile) were associated with PSD with a 5.79-fold (P<0.001) increase compared with lower levels of both. Higher NLRs and PLRs were found to be associated with depression 6 months after stroke, and the combined index was more meaningful than either alone in the early clinical detection of PSD.
Keywords: stroke, ischemic stroke, depression, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio
Introduction
Post-stroke depression (PSD) is one of the most prevalent psychiatric disorders following strokes, and affects nearly a third of the survivors during the first 5 years after the stroke (1). The occurrence of PSD is associated with negative clinical outcomes, including impaired cognition and delayed recovery of neurological functions. Furthermore, PSD acts as a prominent barrier to stroke rehabilitation and has been related to a reduced quality of life as well as an increasing risk of stroke recurrence and mortality (2). Thus, early diagnosis as well as efficient management of PSD must be a priority in clinical stroke rehabilitation.
Extensive investigations have suggested that inflammation plays an important role in acute ischemic stroke and depression by stimulating various pro-inflammatory markers, where the subsequent inflammation has been demonstrated to be involved in the development of PSD (3,4). Acute ischemic stroke has been demonstrated to induce the inflammatory response accompanied by a significant increase in the expression levels of inflammatory and pro-inflammatory cytokines markers, such as interleukin (IL)-1β, IL-1α, tumor necrosis factor (TNF)-α, IL-6, IL-8 and soluble TNF receptor 1 in the plasma, which are frequently found in the acute phase of stroke (5). Pro-inflammatory cytokines induce an inflammatory cascade reaction (6) and the expression of inflammatory related cytokines are strongly related with larger infarct sizes and a poorer prognosis for stroke patients (7).
Previous studies have shown that low-grade inflammation plays a critical role in the development of depression (8). Antidepressants may moderately improve depressive symptoms by reducing the levels of pro-inflammatory cytokines and increase the production of anti-inflammatory cytokines (9). Inflammatory mediators have been found to interact with key biological systems in depression (10), including altering neurotransmitter metabolism, neuroendocrine function, neural plasticity and the levels of reactive oxygen species. Similarly, a cohort study showed that the serum levels of IL-6, IL-10, TNF-α and IFN-γ were significantly higher in the PSD cohort compared to the non-PSD cohort (11). Furthermore, a recent study found that the coexistence of higher homocysteine and C-reactive protein (CRP) expression levels were independent risk factors for PSD (12).
In recent years, the neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) have emerged as well-accepted biomarkers for the assessment of overall inflammatory status. The NLR and PLR are simple and cost-effective biomarkers that can be easily derived from blood during routine examinations (13). Elevated levels of NLR and PLR have been found to be related with oxidative stress and increased cytokine production in patients with depressive disorders (14). Furthermore, the NLR and PLR are strongly related to the prognosis of infarction and thrombo-inflammatory state. The NLR has been used as an indicator to reflect the prevalence of intracranial atherosclerosis (15) and is considered to be an independent risk factor for ischemic stroke and a poorer prognosis (16). Similarly, the PLR has been used to predict poor prognoses, the rate of insufficient recanalization and the size of infarcted area following stroke (17).
In addition to the inflammation and stroke, the NLR and PLR are also considered to be related to psychiatric disorders, especially depression. The NLR has been found to be increased in patients with major depression without antidepressant therapy; however, the NLR returned to normal levels after 3 months of Selective Serotonin Reuptake Inhibitor treatment (18). Moreover, the PLR in patients with major depression has also been found to be increased (19). Recent studies have suggested that the NLR or PLR are associated with PSD 1 month following stroke occurrence (20,21), which highlighted the relationship between early PSD and the inflammation index. However, there was not clear association between these indicators and 6-month PSD. To the best of the our knowledge, the present study is the first to explore the association between the NLR/PLR and PSD at 6 months. The aim of the present study was to examine the value of the NLR and PLR as joint indicators in the diagnosis of PSD. Whether the joint index has better diagnostic value than independence index is a valuable question which may improve the diagnosis of PSD.
Materials and methods
Study population.
The present study was approved by the Ethics Committee of the First Affiliated Yijishan Hospital of Wannan Medical College. Written informed consents were signed by all participants or their relatives prior to their inclusion in the present study.
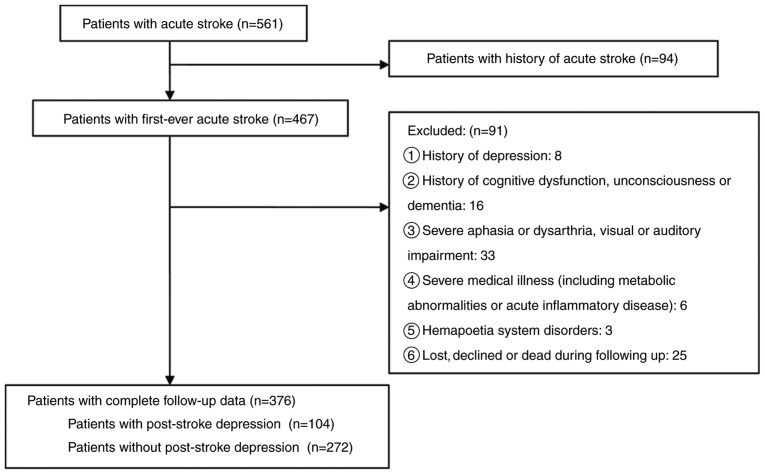
Retrospective analysis was carried out in 376 patients with first-ever acute ischemic stroke in Stroke Ward of the First Affiliated Yijishan Hospital of Wannan Medical College between March 2015 and September 2017. All of the enrolled patients met the World Health Organization Multinational Monitoring of Trends and Determinants in Cardiovascular Disease criteria defined for acute ischemic stroke (22), and the diagnoses were verified by the results from computed tomography (CT) or magnetic resonance imaging (MRI) within 24 h after admission. The exclusion criteria for patients were: i) Patients with psychosis or other psychiatric conditions, including anxiety, depression and suicidal behavior; ii) patients with central nervous system diseases including dementia, significant cognitive impairment or decreased level of consciousness; iii) patients with severe aphasia or dysarthria as well as visual or auditory impairments; iv) patients with metabolic abnormalities, tumors, significant acute inflammatory disease or other medical illness besides stroke; v) patients with hematological disorders; and vi) patients who were not followed-up or died during follow-up (Fig. 1). The diagnosis of depression was based on the structured clinical interview for the Structured Clinical Interview For Diagnostic and Statistical Manual of Mental Disorders-IV technique (23) at 6 months, followed up by telephone consultation or outpatient clinic. The severity of depressive symptoms was quantified using the Hamilton Depression Scale (HAM-D) score (24). Additionally, 120 healthy volunteers from the physical examination center in the First Affiliated Yijishan Hospital of Wannan Medical College were recruited between March 2015 and September 2017 as controls retrospectively.
Figure 1.
Study flow chart of the enrolled and excluded patients.
Clinical measurement.
Relevant clinical data were retrospectively collected from relevant medical records. Demographic data [such as age, sex, body mass index (BMI), and education level], history of conventional vascular risk factor (such as hypertension, diabetes mellitus, hypercholesterolemia and atrial fibrillation) and medical history (such smoking, alcohol consumption, previous infarctions and family history of strokes) were obtained. Stroke subtype was classified according to the Trial of Org 10172 in Acute Stroke Treatment criteria (25). Stroke severity was evaluated by trained neurologists using the National Institutes of Health Stroke Scale (NIHSS) within 24 h of admission at baseline. Cognitive function was measured using Mini Mental State Examination (MMSE) on admission (26). Functional outcomes were obtained using the modified Rankin Scale (mRS) at a 3-month follow-up. The data of CT/MRI, performed within 24 h after admission, were collected retrospectively in order to confirm diagnosis and assess the site, size and cause of the infarction. The NLR and PLR were all retrospectively obtained from the blood routine results at admission by calculating the ratios between neutrophil or platelets and lymphocytes in peripheral blood samples.
Statistical analyses.
Results are expressed as the mean ± SD or as the median (quartiles) for the continuous variables depending on whether the data were normally or not normally distributed, respectively. Categorical variables are presented as percentages. Proportions were compared using the χ2 test, and Student's t-test and two-way ANOVA were employed for the normally distributed variables, while the Mann-Whitney U test was employed for the non-normally distributed variables. Spearman's rank correlation was used for bivariate correlations. The association between the NLR or PLR and the NIHSS score and HAM-D score were also assessed using linear regression models with multivariate adjustments for possible confounders; the NLR and PLR were dichotomized using a median split. The influence of the NLR/PLR separately or jointly on PSD was examined using binary logistic regression analyses, resulting in odds ratios (ORs) and 95% CI. Multiple-adjusted logistic regression models were also used, allowing for the adjustment for potential confounding factors. A receiver operating characteristic curve analysis was used to identify the cutoff points for the NLR and PLR levels at admission with the greatest sensitivity and specificity to predict PSD at the 6-month follow-up. All statistical analyses were performed using SPSS for Windows (version 19.0; IBM Corp.). P<0.05 was considered to indicate a statistically significant difference.
Results
Clinical characteristics of study samples.
Among all follow-up patients, 224 (59.57%) patients were male, and the average age was 61.37±10.34 years. In total, 104 (27.66%) patients were diagnosed with PSD at the end of 6 months follow-up. The basic characteristics of the 376 patients with and without PSD are presented in Table I.
Table I.
Baseline clinical characteristics in patients with and without post-stroke depression at 6 months.
| Variant | Non-PSD (n=272) | PSD (n=104) | Normal control (n=120) | P-valuea | P-valueb |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, years Mean ± SD | 60.64±10.02 | 61.74±10.52 | 60.21±10.37 | 0.348 | 0.275 |
| Female, % | 101 (37.13) | 51 (49.04) | 52 (43.33) | 0.035 | 0.393 |
| Educational, years | 5 (0-7) | 3 (0-6) | 0.032 | ||
| BMI, kg/m2 | 22.24 (20.67-24.58) | 26.73 (22.87-30.12) | 0.016 | ||
| Widowhood, % | 28 (10.29) | 36 (34.62) | <0.001 | ||
| Vascular risk factors, % | |||||
| Hypertension | 186 (68.38) | 72 (69.23) | 0.874 | ||
| Hyperlipidemia | 85 (31.25) | 33 (31.73) | 0.928 | ||
| Diabetes mellitus | 116 (42.62) | 48 (46.15) | 0.540 | ||
| Coronary heart disease | 79 (29.04) | 34 (32.69) | 0.490 | ||
| Atrial fibrillation | 81 (29.78) | 35 (33.65) | 0.467 | ||
| Active smokers | 101 (37.13) | 43 (41.35) | 0.452 | ||
| Alcohol consumption | 91 (33.46) | 36 (34.62) | 0.832 | ||
| Type of stroke etiology, % | 0.390 | ||||
| Atherothrombotic | 156 (57.35) | 63 (60.57) | |||
| Lacunar | 73 (26.84) | 15 (14.42) | |||
| Cardioembolic | 6 (2.21) | 7 (6.73) | |||
| Others | 37 (13.60) | 19 (18.27) | |||
| Lesion location, % | 0.459 | ||||
| Frontal | 55 (20.22) | 17 (16.35) | |||
| Parietal | 31 (11.40) | 13 (12.50) | |||
| Temporal | 22 (8.09) | 11 (10.58) | |||
| Occipital | 26 (9.56) | 9 (8.65) | |||
| Basal ganglia | 84 (30.88) | 29 (27.88) | |||
| Posterior fossa | 51 (18.75) | 20 (19.23) | |||
| Others | 3 (1.10) | 5 (4.81) | |||
| Hospital stay, days | 15 (7-23) | 16 (8-27) | 0.261 | ||
| Baseline NIHSS score | 8 (6-11) | 9 (7-12) | <0.001 | ||
| Baseline MMSE score | 28 (25-30) | 26 (22-28) | 0.024 | ||
| mRS at 3 months | 2 (1-2) | 2 (1-4) | 0.072 | ||
| NLR | 2.83 (2.31-3.79) | 3.81 (2.52-4.67) | 1.96 (1.54-2.57) | <0.001 | <0.001 |
| PLR | 112.04 (89.25-143.73) | 159.74 (124.87-246.05) | 112.17 (87.36,142.3) | <0.001 | <0.001 |
Data are presented as the mean ± SD, or median (inter-quartile range).
aPSD vs. non-PSD and
bPSD vs. normal controls. BMI, body mass index; IQR, interquartile range; MMSE, mini mental state examination; mRS, modified Rankin scale; NIHSS, National Institutes of Health stroke scale; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PSD, post-stroke depression.
Relationship between the NLR and PLR, and the stroke characteristics.
The median value [interquartile range (IQR)] of NLR or PLR for all patients with stroke was significantly higher than that of the normal subjects, the NLR in patients and in healthy subjects was 2.87 (2.39-4.02) and 1.96 (1.54-2.57), respectively (P<0.001). The PLR in patients and in healthy subjects was 125.31 (103.21-203.74) and 112.17 (87.36,142.3), respectively (P<0.001). Moreover, the NLR and PLR were observed to increase with the extent of damage caused by the stroke, as defined by the NIHSS score. There was a positive correlation observed between the NLR and the NIHSS score (r=0.289, P<0.001), and a similar correlation between the PLR and the NIHSS score (r=0.237, P<0.001). Similarly, Raised HAM-D scores at the 6-month follow up were also found to correspond with increased NLRs and PLRs (r=0.206, P=0.003; r=0.201, P=0.001, respectively). Additionally, when patient MRI data was available (n=261), a positive correlation was also observed between the infarct volume of patients as well as the NLRs (r=0.166, P=0.032). No correlation was observed between the PLR and the infarct volumes (P=0.071). There was a positive correlation between the lesion volumes and HAM-D scores (r=0.251, P<0.001).
NLRs, PLRs and PSD.
Patients with PSD showed significantly higher NLR levels at admission than the ones without PSD [3.81 (2.52-4.67) vs. 2.83 (2.31-3.79), respectively, P<0.001]. Similarly, PLR levels in patients with PSD were higher than those in patients without PSD [159.74 (124.87-246.05) vs. 112.04 (89.25-143.73), respectively, P<0.001]. Patients with PSD were predominantly female compared to the non-PSD group. The PSD group also had a lower average education level, higher BMI, a higher proportion of patients living alone, higher stroke severity and worse cognition. However, there was no association between lesion location, etiological subtype, functional outcomes according to mRS as well as vascular risk factors and PSD.
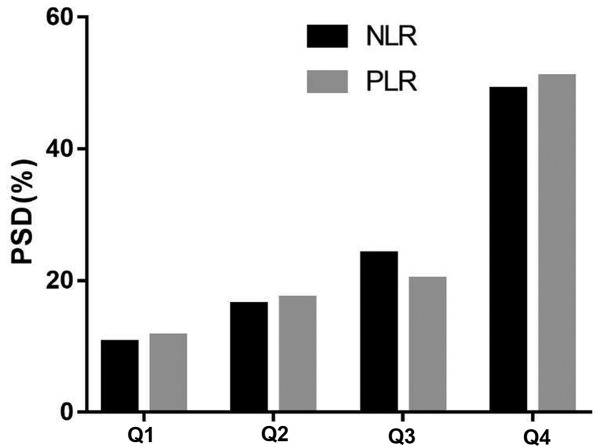
The quartiles of NLR and PLR values in the PSD group were observed to have a significant difference from those in the non-PSD group (P<0.0001) (Table II). The PSD distribution across the NLR quartiles ranged between 10.58-49.04% between the first and fourth quartile, respectively (Fig. 2). The corresponding distribution for PLR was 11.54-50.96% between the first and fourth quartile, respectively (Fig. 2).
Table II.
The NLR and PLR quartiles of patients.
| PLR and NLR % | PSD (n=104) | Non-PSD (n=272) | P-value |
|---|---|---|---|
| NLR, % | <0.001 | ||
| Quartile 1 | 11 (10.58) | 81 (29.78) | 0.001 |
| Quartile 2 | 17 (16.35) | 73 (26.84) | 0.033 |
| Quartile 3 | 25 (24.04) | 71 (26.10) | 0.681 |
| Quartile 4 | 51 (49.04) | 47 (17.28) | <0.001 |
| PLR, % | <0.001 | ||
| Quartile 1 | 12 (11.54) | 78 (28.68) | 0.005 |
| Quartile 2 | 18 (17.31) | 76 (27.94) | 0.033 |
| Quartile 3 | 21 (20.19) | 70 (25.74) | 0.262 |
| Quartile 4 | 53 (50.96) | 48 (17.65) | <0.001 |
NLR, neutrophil-to-lymphocyte ratio; PLR platelet-to-lymphocyte ratio; PSD, post-stroke depression.
Figure 2.
The incidence of PSD according to the baseline NLR or PLR quartiles. NLR, neutrophil to lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; PSD, post-stroke depression, Q, quartile.
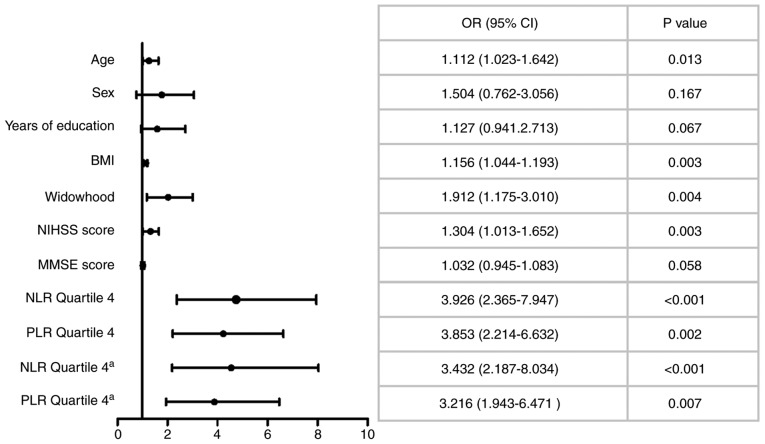
In the multivariate logistic regression analysis, analyzing quartile 1 to quartile 3 of the NLR data [upper quartile, <4.02; median, 2.87 (2.39-4.02)] and the PLR data [upper quartile, <203.74; median, 121.31 (103.21-203.74)], using all stroke patients as a reference respectively, after adjusting for other multiple confounding factors, the fourth quartile of the NLR data [OR 3.926, 95% CI (2.365-7.947), P<0.001] and the PLR [OR 3.853, 95% CI (2.214-6.632), P=0.002] were significantly associated with PSD (Fig. 3). Moreover, age, BMI, widowhood and NIHSS score were associated with PSD.
Figure 3.
Multivariable logistic regression analyses depicting the associations of admission NLRPLR and other baseline characteristics with 6-month PSD. BMI, body mass index; CI, confidence interval; MMSE, mini mental state examination; NIHSS, National Institutes of Health Stroke Scale; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet to lymphocyte ratio. aMultivariate regression model included infarct volume (n=261).
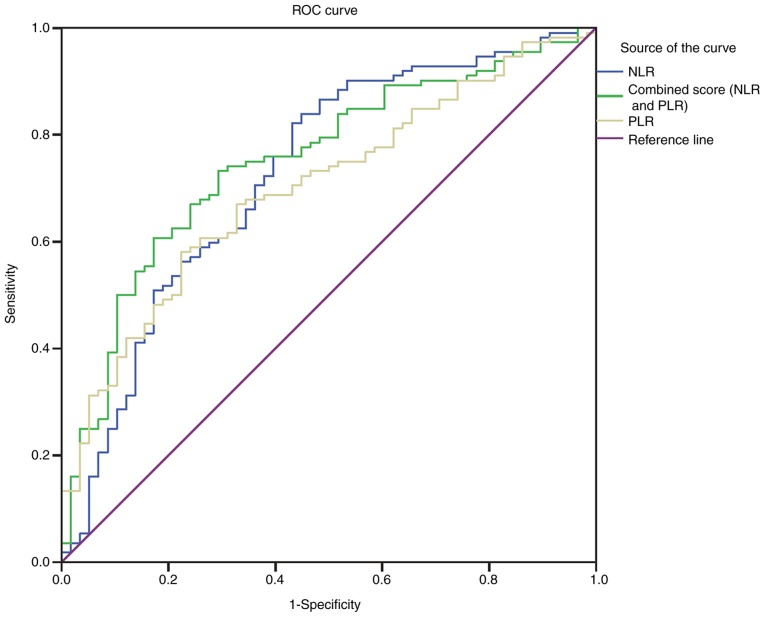
With an area under the curve (AUC) of 0.726 (95% CI, 0.643-0.809), the NLR showed a greater discriminatory ability to predict PSD than the PLR [AUC 0.701, 95% CI (0.622-0.780); P<0.001] (Fig. 4). Furthermore, the PLR improved the ability of NLR to diagnose PSD [AUC of the combined model 0.751, 95% CI (0.675-0.827); P<0.001] (Fig. 4).
Figure 4.
ROC analysis of NLRPLR in the 6-month PSD. ROC curve demonstrating the sensitivity as a function of specificity for predicting the post-stroke depression within 6 months, based on the levels of the NLR and PLR. NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ROC, receiving operator curve.
The lower level of both the NLR and the PLR (both <quartile 4) were used as a reference, and the group with higher levels of both NLR and PLR (≥quartile 4; NLR ≥4.02 and PLR ≥203.74) was used to predict PSD with an OR of 5.79 (95% CI, 3.03-9.45; P<0.001) compared with the group with lower levels of both factors (<quartile 4) after adjustment for multiple confounding factors (age, BMI, widowhood, NIHSS score, MMSE score, vascular risk factors and the type of stroke etiology). This showed that higher levels of both NLR and PLR (≥quartile 4; NLR ≥4.02 and PLR ≥203.74) exhibited superior predictive value in evaluating the risk of PSD compared with lower levels of both factors.
Discussion
Although previous studies have analyzed the significance of the NLR or the PLR in predicting 1 month PSD, the current study paper included these two common inflammatory indicators and analyzed their respective and combined abilities to predict PSD 6 months after stroke. It was found that high NLRs and PLRs were strongly associated with the risk of PSD at 6 months after the adjustment by variables, and it was also found that the ability of the combined index to diagnose PSD was greater than that of either of the inflammatory indicators alone. Higher NLRs and PLRs, ≥quartile 4, was associated with PSD at a 5.79-fold (P<0.001) increase compared with the lower levels of both ratios. The combined index was much more meaningful than the independent indexes in the early clinical detection of PSD. This present study combined the two common indicators and found that the combined forecasting ability was stronger than the single forecasting ability. It is believed that the NLR and PLR may be readily available prognostic markers for PSD.
A number of studies have reported that NLR is associated with the poor prognosis for ischemic diseases (27). Previous studies have demonstrated that increased neutrophils, a high number of which have been found atherosclerotic plaques at various stages of atherosclerosis, may damage endothelial cells, and they. Chronic inflammation represented by NLR was also reported to be associated with a number of vascular risk factors, including hypertension, diabetes and hyperlipidemia (28,29). During infarction progression, neutrophils appear to be the first leukocytes to reach the ischemic brain, and in turn activate the inflammatory process (30). Furthermore, once they have reached the site of injury, the highest levels of pro-inflammatory cytokines and biomarkers of inflammation coincide with the largest recruitment and activation of neutrophils (31). The release of these inflammatory cytokines further amplifies the immune cascade, leading to cellular dysfunction and the production of reactive oxygen species. Inflammation changes the function of the intracranial neuroendocrine, and at the same time reduces the synthesis and secretion of monoamine neurotransmitters, leading to the development of PSD (32). Meanwhile, Lymphocytes are an immune cell related to regulation and protection. The decrease of lymphocyte numbers, reflects the pathological stress state of the body, thus a low count indicates a poor prognosis (33).
Increasing evidence has shown that inflammation is involved in the etiology of depression. There are a number of cytokines which are believed to have a large impact on the development and progression of depression, including inflammatory and pro-inflammatory cytokines as well as cytokines produced by the hypothalamic-pituitary-adrenal (HPA) axis (34). The excess production of these cytokines in patients with depression, may affect neurotransmission through the overactivation of the HPA axis (35). It has also been shown that major depressive disorder is affected by excessive oxidative stress. Both the NLR and PLR have been shown to demonstrate the degree of inflammation, and are also relatively inexpensive and easy to measure in the clinic (36-39). As such, raised NLRs and PLRs have also been shown to be associated with increased cytokine production and oxidative stress and have been used to measure the systemic inflammatory response. Furthermore, an increased NLR and dysfunctional platelets have also been linked to patients with psychiatric disorders including depression (18,40-42).
Platelets can act as an inflammatory indicator, and higher platelet activation serves as a predictor of inflammation. Activated platelets play an important role in psychiatric disorders, including depression (43,44), and high levels are considered to be a risk factor for an increased incidence of cardiovascular and cerebrovascular diseases (45). The activation of platelets, mediated by a number of inflammatory factors, including cytokines, serotonin, glutamate, dopamine and P-selectin, serves an important role in psychiatric disorders (46). In particular, serotonin has been previously reported to be involved in the activation of plasma platelets and accelerate aggregation (47). Meanwhile, activated platelets participate in the regulation of the permeability of endothelial cells and recruitment of mononuclear cell through the release of pro-inflammatory factors. Depression is a result of platelet activation, which is potentiated by elevated serotonin and epinephrine levels (48). There are high levels of serotonin and glutamate in platelet dense granules, and serotonin receptors (5HT2A) and serotonin transporter (SERT) on the surface of platelets (49). Inflammation induces platelet activation which is accompanied by the release of serotonin and glutamate. Pro-inflammatory factors which derive from activated platelets, take part in regulating and maintaining the inflammatory reaction, and are thought to play an important role in depression (50).
The activation of platelets is not only considered to be a reciprocal causation of inflammation, but also closely related to infarction and mental disorders, especially depression (51). Previous studies have shown that there is a significant correlation between inflammatory responses and the development of atherosclerosis, platelet aggregation, plaque rupture and intravascular thrombosis (52). Nemeroff and Musselmanl (53) demonstrated that platelet dysnfunction may increase the risk of a patient developing depression. Activated platelets may induce the formation of a thrombus in patients suffering from depression, and increased serotonin levels may further alter the functions of platelets in these patients (54). It has previously been shown that an increased PLR is associated with severe major depression and that it may lead to psychotic symptoms (51). Raised PLRs have also been associated with acute ishemic stroke patients (55). Both of these results are consistent with the findings of the current study, further highlighting that PSD patients have raised PLR levels when compared to non-PSD patients. Therefore, as a stable inflammatory indicator, PLR, which reflects the inflammatory state of stroke and depression, can also be considered as an available prognostic marker for PSD.
There are some limitations with the present study. Firstly, this was a single-center retrospective study. Due to the limitations of the retrospective study, it was not possible to collect psychosocial factors associated with PSD. Secondly, some patients with severe aphasia or severe disease were unable to complete the follow-up were examinations and were thus excluded from the study, which may have introduced bias to the results. Thirdly, the NLR and PLR values were collected only once within 24 h on admission; however, there may be a dynamic change during the development of PSD. As such, it is necessary to pay attention to the correlation between the NLR and PLR changes at various time points after stroke and the prediction of PSD in prospective study.
Although the study had some limitations, the present study demonstrated the important relationship between the NLRPLR on admission and PSD 6 months after stroke in south China in a Han population, which may be involved in the pathophysiological mechanisms behind PSD depression. Further multicenter prospective studies are needed to confirm the relationship between NLRPLR and PSD, and to predict whether inhibiting the inflammatory response could prevent the occurrence of PSD.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
WD was involved in all aspects of the study, including study design, data analysis and the revision of the article. JHu analyzed the data and prepared the manuscript. WZ collected and analyzed general patient data. ZZ and JHan interpreted the images and evaluated the clinical data of stroke patients. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the ethics committee of the First Affiliated Yijishan Hospital of Wannan Medical College. All subjects gave written informed consent in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Towfighi A, Ovbiagele B, El Husseini N, Hackett ML, Jorge RE, Kissela BM, Mitchell PH, Skolarus LE, Whooley MA, Williams LS. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; and Council on Quality of Care and Outcomes Research: Poststroke depression: A scientific statement for healthcare professionals from the American heart association/American stroke association. Stroke. 2017;48(e30-e43) doi: 10.1161/STR.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 2.Guajardo VD, Terroni L, Sobreiro Mde F, Zerbini MI, Tinone G, Scaff M, Iosifescu DV, de Lucia MC, Fráguas R. The influence of depressive symptoms on quality of life after stroke: A prospective study. J Stroke Cerebrovasc Dis. 2015;24:201–209. doi: 10.1016/j.jstrokecerebrovasdis.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Ferrari F, Villa RF. The neurobiology of depression: An integrated overview from biological theories to clinical evidence. Mol Neurobiol. 2017;54:4847–4865. doi: 10.1007/s12035-016-0032-y. [DOI] [PubMed] [Google Scholar]
- 4.Becker KJ. Inflammation and the silent sequelae of stroke. Neurotherapeutics. 2016;13:801–810. doi: 10.1007/s13311-016-0451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeng L, Wang Y, Liu J, Wang L, Weng S, Chen K, Domino EF, Yang GY. Pro-inflammatory cytokine network in peripheral inflammation response to cerebral ischemia. Neurosci Lett. 2013;548:4–9. doi: 10.1016/j.neulet.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Waisman A, Hauptmann J, Regen T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015;129:625–637. doi: 10.1007/s00401-015-1402-7. [DOI] [PubMed] [Google Scholar]
- 7.Bodhankar S, Chen Y, Vandenbark AA, Murphy SJ, Offner H. Treatment of experimental stroke with IL-10-producing B-cells reduces infarct size and peripheral and CNS inflammation in wild-type B-cell-sufficient mice. Metab Brain Dis. 2014;29:59–73. doi: 10.1007/s11011-013-9474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel A. Review: The role of inflammation in depression. Psychiatr Danub. 2013;25 (Suppl 2)(S216-S223) [PubMed] [Google Scholar]
- 9.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maes M. Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:664–675. doi: 10.1016/j.pnpbp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Su JA, Chou SY, Tsai CS, Hung TH. Cytokine changes in the pathophysiology of poststroke depression. Gen Hosp Psychiatry. 2012;34:35–39. doi: 10.1016/j.genhosppsych.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 12.Yin J, Zhong C, Zhu Z, Bu X, Xu T, Guo L, Wang X, Zhang J, Cui Y, Li D, et al. Elevated circulating homocysteine and high-sensitivity C-reactive protein jointly predicts post-stroke depression among Chinese patients with acute ischemic stroke. Clin Chim Acta. 2018;479:132–137. doi: 10.1016/j.cca.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Arbel Y, Finkelstein A, Halkin A, Birati EY, Revivo M, Zuzut M, Shevach A, Berliner S, Herz I, Keren G, Banai S. Neutrophil/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients undergoing angiography. Atherosclerosis. 2012;225:456–460. doi: 10.1016/j.atherosclerosis.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Kasama T, Miwa Y, Isozaki T, Odai T, Adachi M, Kunkel SL. Neutrophil-derived cytokines: Potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:273–279. doi: 10.2174/1568010054022114. [DOI] [PubMed] [Google Scholar]
- 15.Nam KW, Kwon HM, Jeong HY, Park JH, Kim SH, Jeong SM. High neutrophil to lymphocyte ratios predict intracranial atherosclerosis in a healthy population. Atherosclerosis. 2018;269:117–121. doi: 10.1016/j.atherosclerosis.2017.12.035. [DOI] [PubMed] [Google Scholar]
- 16.Goyal N, Tsivgoulis G, Chang JJ, Malhotra K, Pandhi A, Ishfaq MF, Alsbrook D, Arthur AS, Elijovich L, Alexandrov AV. Admission neutrophil-to-lymphocyte ratio as a prognostic biomarker of outcomes in large vessel occlusion strokes. Stroke. 2018;49:1985–1987. doi: 10.1161/STROKEAHA.118.021477. [DOI] [PubMed] [Google Scholar]
- 17.Tekesin A, Tunç A. Inflammatory markers are beneficial in the early stages of cerebral venous thrombosis. Arq Neuropsiquiatr. 2019;77:101–105. doi: 10.1590/0004-282X20190001. [DOI] [PubMed] [Google Scholar]
- 18.Demircan F, Gözel N, Kılınç F, Ulu R, Atmaca M. The impact of red blood cell distribution width and neutrophil/lymphocyte ratio on the diagnosis of major depressive disorder. Neurol Ther. 2016;5:27–33. doi: 10.1007/s40120-015-0039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai L, Xu L, Wei L, Chen W. Relationship of mean platelet volume To MDD: A retrospective study. Shanghai Arch Psychiatry. 2017;29:21–29. doi: 10.11919/j.issn.1002-0829.216082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang G, Chen H, Wang Q, Hong X, Hu P, Xiao M, Shu M, He J. High platelet-to-lymphocyte ratio are associated with post-stroke depression. J Affect Disord. 2019;246:105–111. doi: 10.1016/j.jad.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Luan X, Zhao K, Qiu H, Liu Y, Tu X, Tang W, He J. The association between neutrophil-to-lymphocyte ratio and post-stroke depression. Clin Chim Acta. 2018;486:298–302. doi: 10.1016/j.cca.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Huang H, Zheng T, Wang S, Wei L, Wang Q, Sun Z. Serum 25-hydroxyvitamin D predicts early recurrent stroke in ischemic stroke patients. Nutr Metab Cardiovasc Dis. 2016;26:908–914. doi: 10.1016/j.numecd.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 23.First MB, Gibbon M. The structured clinical interview for DSM-IV Axis I disorders (SCID-I) and the structured clinical interview for DSM-IV Axis II disorders (SCID-II) In: Comprehensive Handbook of Psychological Assessment, Vol. 2. Personality assessment. Hilsenroth MJ and Segal DL (eds.). John Wiley & Sons Inc., Hoboken NJ, 134-143, 2004. [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brott T, Adams HP Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V. Measurements of acute cerebral infarction: A clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Qun S, Tang Y, Sun J, Liu Z, Wu J, Zhang J, Guo J, Xu Z, Zhang D, Chen Z, et al. Neutrophil-to-lymphocyte ratio predicts 3-month outcome of acute ischemic stroke. Neurotox Res. 2017;31:444–452. doi: 10.1007/s12640-017-9707-z. [DOI] [PubMed] [Google Scholar]
- 28.Buyukkaya E, Karakas MF, Karakas E, Akcay AB, Tanboga IH, Kurt M, Sen N. Correlation of neutrophil to lymphocyte ratio with the presence and severity of metabolic syndrome. Clin Appl Thromb Hemost. 2014;20:159–163. doi: 10.1177/1076029612459675. [DOI] [PubMed] [Google Scholar]
- 29.Balta S, Celik T, Mikhailidis DP, Ozturk C, Demirkol S, Aparci M, Iyisoy A. The relation between atherosclerosis and the neutrophil-lymphocyte ratio. Clin Appl Thromb Hemost. 2016;22:405–411. doi: 10.1177/1076029615569568. [DOI] [PubMed] [Google Scholar]
- 30.Zhang RL, Chopp M, Chen H, Garcia JH. Temporal profile of ischemic tissue damage, neutrophil response, and vascular plugging following permanent and transient (2H) middle cerebral artery occlusion in the rat. J Neurol Sci. 1994;125:3–10. doi: 10.1016/0022-510x(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 31.Ciree A, Michel L, Camilleri-Broet S, Jean Louis F, Oster M, Flageul B, Senet P, Fossiez F, Fridman WH, Bachelez H, Tartour E. Expression and activity of IL-17 in cutaneous T-cell lymphomas (mycosis fungoides and Sezary syndrome) Int J Cancer. 2004;112:113–120. doi: 10.1002/ijc.20373. [DOI] [PubMed] [Google Scholar]
- 32.Celikbilek A, Ismailogullari S, Zararsiz G. Neutrophil to lymphocyte ratio predicts poor prognosis in ischemic cerebrovascular disease. J Clin Lab Anal. 2014;28:27–31. doi: 10.1002/jcla.21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gibson PH, Cuthbertson BH, Croal BL, Rae D, El-Shafei H, Gibson G, Jeffrey RR, Buchan KG, Hillis GS. Usefulness of neutrophil/lymphocyte ratio as predictor of new-onset atrial fibrillation after coronary artery bypass grafting. Am J Cardiol. 2010;105:186–191. doi: 10.1016/j.amjcard.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Furtado M, Katzman MA. Examining the role of neuroinflammation in major depression. Psychiatry Res. 2015;229:27–36. doi: 10.1016/j.psychres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Li W, Ling S, Yang Y, Hu Z, Davies H, Fang M. Systematic hypothesis for post-stroke depression caused inflammation and neurotransmission and resultant on possible treatments. Neuro Endocrinol Lett. 2014;35:104–109. [PubMed] [Google Scholar]
- 36.Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: An update. Expert Rev Cardiovasc Ther. 2016;14:573–577. doi: 10.1586/14779072.2016.1154788. [DOI] [PubMed] [Google Scholar]
- 37.Li H, Zhou Y, Ma Y, Han S, Zhou L. The prognostic value of the platelet-to-lymphocyte ratio in acute coronary syndrome: A systematic review and meta-analysis. Kardiol Pol. 2017;75:666–673. doi: 10.5603/KP.a2017.0068. [DOI] [PubMed] [Google Scholar]
- 38.Li DY, Hao XY, Ma TM, Dai HX, Song YS. The prognostic value of platelet-to-lymphocyte ratio in urological cancers: A meta-analysis. Sci Rep. 2017;7(15387) doi: 10.1038/s41598-017-15673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chandrashekara S, Mukhtar Ahmad M, Renuka P, Anupama KR, Renuka K. Characterization of neutrophil-to-lymphocyte ratio as a measure of inflammation in rheumatoid arthritis. Int J Rheum Dis. 2017;20:1457–1467. doi: 10.1111/1756-185X.13157. [DOI] [PubMed] [Google Scholar]
- 40.Demir S, Atli A, Bulut M, İbiloğlu AO, Güneş M, Kaya MC, Demirpençe Ö, Sır A. Neutrophil-lymphocyte ratio in patients with major depressive disorder undergoing no pharmacological therapy. Neuropsychiatr Dis Treat. 2015;11:2253–2258. doi: 10.2147/NDT.S89470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ivković M, Pantović-Stefanović M, Dunjić-Kostić B, Jurišić V, Lačković M, Totić-Poznanović S, Jovanović AA, Damjanović A. Neutrophil-to-lymphocyte ratio predicting suicide risk in euthymic patients with bipolar disorder: Moderatory effect of family history. Compr Psychiatry. 2016;66:87–95. doi: 10.1016/j.comppsych.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Mayda H, Ahsen A, Bağcioğlu E, Öztürk A, Bahçeci B, Soyuçok E, Başpinar E, Ulu MS. Effect of increased neutrophil-to-lymphocyte ratio (NLR) and decreased mean platelet volume (MPV) values on inflammation in acute mania. Noro Psikiyatr Ars. 2016;53:317–320. doi: 10.5152/npa.2016.10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morel-Kopp MC, McLean L, Chen Q, Tofler GH, Tennant C, Maddison V, Ward CM. The association of depression with platelet activation: Evidence for a treatment effect. J Thromb Haemost. 2009;7:573–581. doi: 10.1111/j.1538-7836.2009.03278.x. [DOI] [PubMed] [Google Scholar]
- 44.Ormonde do Carmo MB, Mendes-Ribeiro AC, Matsuura C, Pinto VL, Mury WV, Pinto NO, Moss MB, Ferraz MR, Brunini TM. Major depression induces oxidative stress and platelet hyperaggregability. J Psychiatric Res. 2015;61:19–24. doi: 10.1016/j.jpsychires.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Gokdemir MT, Karakilcik AZ, Gokdemir GS. Prognostic importance of paraoxonase, arylesterase and mean platelet volume efficiency in acute ischaemic stroke. J Pak Med Assoc. 2017;67:1679–1683. [PubMed] [Google Scholar]
- 46.Dietrich-Muszalska A, Wachowicz B. Platelet haemostatic function in psychiatric disorders: Effects of antidepressants and antipsychotic drugs. World J Biol Psychiatry. 2017;18:564–574. doi: 10.3109/15622975.2016.1155748. [DOI] [PubMed] [Google Scholar]
- 47.Zafar MU, Paz-Yepes M, Shimbo D, Vilahur G, Burg MM, Chaplin W, Fuster V, Davidson KW, Badimon JJ. Anxiety is a better predictor of platelet reactivity in coronary artery disease patients than depression. Eur Heart J. 2010;31:1573–1582. doi: 10.1093/eurheartj/ehp602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazza MG, Lucchi S, Tringali AGM, Rossetti A, Botti ER, Clerici M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: A meta-analysis. Prog Neuropsychopharmacol Boil Psychiatry. 2018;84:229–236. doi: 10.1016/j.pnpbp.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 49.McCorvy JD, Wacker D, Wang S, Agegnehu B, Liu J, Lansu K, Tribo AR, Olsen RHJ, Che T, Jin J, Roth BL. Structural determinants of 5-HT2B receptor activation and biased agonism. Nat Struct Mol Biol. 2018;25:787–796. doi: 10.1038/s41594-018-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu CS, Adibfar A, Herrmann N, Gallagher D, Lanctot KL. Evidence for inflammation-associated depression. Curr Top Behav Neurosci. 2017;31:3–30. doi: 10.1007/7854_2016_2. [DOI] [PubMed] [Google Scholar]
- 51.Kayhan F, Gündüz Ş, Ersoy SA, Kandeğer A, Annagür BB. Relationships of neutrophil-lymphocyte and platelet-lymphocyte ratios with the severity of major depression. Psychiatry Res. 2017;247:332–335. doi: 10.1016/j.psychres.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 52.Esenwa CC, Elkind MS. Inflammatory risk factors, biomarkers and associated therapy in ischaemic stroke. Nat Rev Neurol. 2016;12:594–604. doi: 10.1038/nrneurol.2016.125. [DOI] [PubMed] [Google Scholar]
- 53.Nemeroff CB, Musselman DL. Are platelets the link between depression and ischemic heart disease? Am Heart J. 2000;140 (4 Suppl)(S57-S62) doi: 10.1067/mhj.2000.109978. [DOI] [PubMed] [Google Scholar]
- 54.Musselman DL, Tomer A, Manatunga AK, Knight BT, Porter MR, Kasey S, Marzec U, Harker LA, Nemeroff CB. Exaggerated platelet reactivity in major depression. Am J Psychiatry. 1996;153:1313–1317. doi: 10.1176/ajp.153.10.1313. [DOI] [PubMed] [Google Scholar]
- 55.Altintas O, Altintas MO, Tasal A, Kucukdagli OT, Asil T. The relationship of platelet-to-lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone endovascular therapy. Neurol Res. 2016;38:759–765. doi: 10.1080/01616412.2016.1215030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.