Abstract
Background.
Cognitive impairment is a core feature of psychotic disorders, but the profile of impairment across adulthood, particularly in African-American populations, remains unclear.
Methods.
Using cross-sectional data from a case–control study of African-American adults with affective (n = 59) and nonaffective (n = 68) psychotic disorders, we examined cognitive functioning between early and middle adulthood (ages 20–60) on measures of general cognitive ability, language, abstract reasoning, processing speed, executive function, verbal memory, and working memory.
Results.
Both affective and nonaffective psychosis patients showed substantial and widespread cognitive impairments. However, comparison of cognitive functioning between controls and psychosis groups throughout early (ages 20–40) and middle (ages 40–60) adulthood also revealed age-associated group differences. During early adulthood, the nonaffective psychosis group showed increasing impairments with age on measures of general cognitive ability and executive function, while the affective psychosis group showed increasing impairment on a measure of language ability. Impairments on other cognitive measures remained mostly stable, although decreasing impairments on measures of processing speed, memory and working memory were also observed.
Conclusions.
These findings suggest similarities, but also differences in the profile of cognitive dysfunction in adults with affective and nonaffective psychotic disorders. Both affective and nonaffective patients showed substantial and relatively stable impairments across adulthood. The nonaffective group also showed increasing impairments with age in general and executive functions, and the affective group showed an increasing impairment in verbal functions, possibly suggesting different underlying etiopathogenic mechanisms.
Keywords: Life-span, neuropsychology, psychosis, schizophrenia, trajectory
Introduction
Cognitive impairment is a core feature of psychotic disorders, manifesting before onset of symptoms (Jones et al., 1994; Davidson et al., 1999), persisting during illness remission (Addington and Addington, 1993; Brissos et al., 2011), and predicting multiple functional outcomes (Green et al., 2000; Fett et al., 2011). Studies of cognitive function in schizophrenia patients have almost unequivocally reported medium to large impairments across most cognitive domains (Fioravanti et al., 2005; Reichenberg and Harvey, 2007), but findings from studies of cognitive functioning in other psychosis patients have been more mixed, with evidence of similarities (Schatzberg et al., 2000; Fleming et al., 2004; Bora et al., 2009; Reichenberg et al., 2009; Zanelli et al., 2010) and differences (Mojtabai et al., 2000; Glahn et al., 2006; MacCabe et al., 2013; Mollon et al., 2018) between diagnoses. Thus, the profile of cognitive impairment in affective and nonaffective psychosis diagnoses remains unclear.
Questions also remain regarding the course of cognitive impairment in psychotic disorders. There is evidence for cognitive decline between premorbid and post-onset stages of psychotic disorders (Seidman et al., 2006; Meier et al., 2013; Gur et al., 2014; Mollon et al., 2018). However, both cross-sectional (Hyde et al., 1994; Chen et al., 1996; Mockler et al., 1997; Fucetola et al., 2000) and longitudinal (Szöke et al., 2008; Bozikas and Andreou, 2011; Samamé et al., 2014) studies have generally reported stabilization of impairments after illness onset, with some exceptions (Bilder et al., 1992; Davidson et al., 1995; Harvey et al., 1999; Friedman et al., 2001). However, previous studies examining the course of cognitive impairment after illness onset have several limitations. First, the length of follow-up in longitudinal studies has been short, with few exceeding 2 years and even fewer exceeding 5 years. Second, many of these studies, particularly those with cross-sectional data, did not include control groups, making it difficult to establish whether age-associated cognitive changes reflect pathological processes or normal aging. Third, age has been categorized into broad periods, rather than investigated as a continuous factor, making it difficult to fully disentangle the effect of age on cognitive functioning. Fourth, the results regarding specific cognitive measures are mixed, with evidence for decline relative to controls on measures of executive function (Heilbronner et al., 2016), and visual and verbal memory (Gur et al., 1998; Albus et al., 2002; Burdick et al., 2006), but also stable processing speed impairments (Bonner-Jackson et al., 2010). Finally, studies on the course of cognitive impairment across adulthood have mostly focused separately on nonaffective psychotic disorders, such as schizophrenia, and affective psychoses, such as bipolar disorder. Therefore, knowledge regarding the similarities and differences between affective and nonaffective psychotic disorders in terms of the profile of cognitive impairment across adulthood remains scarce. Finally, the roles of potential confounders, such as antipsychotic medications, cannabis use, and psychiatric comorbidity have not been comprehensively examined. Thus, the course of cognitive impairments across adulthood in psychotic disorders, particularly as pertaining to specific cognitive measures and diagnoses, remains uncertain.
In this study, we examined cognitive functioning in a population-based, case–control, cross-sectional study of African-American adults with affective and nonaffective psychotic disorders. African-Americans are underrepresented in psychiatry research and there is a need for more data on cognitive functioning in adults with psychotic disorders from this population. The aims of the study were to (1) establish the profile of cognitive impairment in affective and nonaffective psychosis patients across early and middle adulthood (ages 20–60), and (2) examine the effect of important potential confounders, including antipsychotic medication, cannabis use, psychiatric comorbidity, substance dependence, symptom severity, duration of psychosis, education, and functioning.
Methods
Subjects
All subjects were African-Americans from the Hartford area. Cognitive data were available for 134 patients with psychosis and 254 controls. The sample was determined from a data freeze on 17 January 2018, prior to any analyses presented herein, and reflects a subset of the planned sample for the study. Patients had various psychotic diagnoses, which are described below. Neither patients nor controls were excluded for having non-psychotic psychiatric disorders. Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV diagnoses were made using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID) (First et al., 2002) and a consensus process (Glahn et al., 2007). Subjects with a history of major non-psychiatric medical disorders (history of strokes, HIV/AIDS, history of traumatic brain injury, epilepsy, hepatitis, chronic myelogenous leukemia, cancer, history of seizures, history of coma or unconsciousness, and severe tremors) or with an intelligence quotient of below 70 were excluded. All subjects provided informed consent. The review boards at Hartford Hospital and Yale University approved the study. Sample characteristics are provided in Table 1.
Table 1.
Sample characteristics
| Controls (n = 231) | Affective psychosis (n = 59) | Nonaffective psychosis (n = 68) | ||||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age (s.d.) | 39.2 | (12.9) | 41.9 | (11.4) | 41.4 | (12.0) |
| Male (%) | 114 | (49.4) | 28 | (47.5) | 37 | (54.4) |
| Education (%) | ||||||
| High school | 109 | (47.2) | 36 | (61.0) | 42 | (61.8) |
| College | 113 | (48.9) | 23 | (39.0) | 25 | (36.8) |
| Graduate school | 9 | (3.9) | 0 | - | 1 | (1.5) |
| Maternal education (%) | ||||||
| High school | 121 | (61.1) | 35 | (76.1) | 33 | (66.0) |
| College | 63 | (31.8) | 9 | (19.6) | 13 | (26.0) |
| Graduate school | 14 | (7.1) | 2 | (4.3) | 4 | (8.0) |
| Months employed in past year (s.d.) | 6.4 | (5.1) | 3.0 | (4.4) | 1.8 | 3.1 |
| Severity of psychotic symptoms (s.d.) | 0.7 | 1.4 | 20.1 | 8.6 | 17.9 | 8.4 |
| Duration of psychosis in years (s.d.) | 0 | - | 17.0 | (10.6) | 16.9 | (12.1) |
| Psychiatric diagnoses | ||||||
| Number of diagnoses (s.d.) | 1.0 | (1.2) | 3.3 | (1.1) | 2.7 | (1.3) |
| Anxiety disorders (%) | 13 | (5.6) | 12 | (20.3) | 6 | (8.8) |
| Depression (%) | 17 | (7.4) | 7 | (11.9) | 9 | (13.2) |
| PTSD (%) | 19 | (8.2) | 20 | (33.9) | 14 | (20.6) |
| Cannabis dependence (%) | 29 | (12.6) | 11 | (18.6) | 12 | (17.6) |
| Alcohol dependence (%) | 16 | (6.9) | 4 | (6.8) | 9 | (13.2) |
| Any substance dependence (%) | 43 | (18.6) | 13 | (22.0) | 20 | (29.4) |
| Antipsychotics (%) | ||||||
| Typical | 0 | - | 4 | (6.8) | 10 | (14.7) |
| Atypical | 2 | (0.9) | 32 | (54.2) | 34 | (50.0) |
| Current cannabis use (%) | ||||||
| Social | 42 | (18.3) | 9 | (15.8) | 6 | (9.4) |
| Abuse or dependence | 34 | (14.8) | 12 | (21.1) | 15 | (23.4) |
| General functioning | ||||||
| GAF score (s.d.) | 79.8 | (9.1) | 58.2 | (9.6) | 57.8 | 8.7 |
| Social functioning (%) | ||||||
| Excellent | 95 | (41.3) | 8 | (13.6) | 16 | (23.9) |
| Good | 102 | (44.4) | 24 | (40.7) | 27 | (40.3) |
| Fair | 29 | (12.6) | 16 | (27.1) | 17 | (25.4) |
| Poor | 4 | (1.7) | 11 | (18.6) | 7 | (10.4) |
Cognitive functioning
Subjects completed a cognitive test battery (‘Charlie’, https://github.com/sammosummo/Charlie). This cognitive battery has been described elsewhere (Mathias et al., 2017, 2018) and a summary of the measures is included in online Supplementary Table S1. These measures have been used extensively in prior research, so that their psychometric properties and cognitive demands are well understood. In addition to the measures directly indexed by the cognitive battery, we derived a general composite score (g) as the first component of principal component analyses using all cognitive tests. Correlations between all test scores can be seen in online Supplementary Fig. S1.
Statistical analyses
The statistical programing language R (R Development Core Team, 2008) was used for all analyses. Due to the more limited number of patients younger than 20 years and older than 60 years, we restricted analyses to individuals aged 20–60 years of age. Patients were categorized into two groups: (1) affective psychosis (n = 59), comprising diagnoses of schizoaffective disorder (n = 40), psychotic bipolar disorder (n = 12), and psychotic major depression (n = 7), and (2) nonaffective psychosis (n = 68), comprising diagnoses of schizophrenia (n = 56), psychosis not otherwise specified (n = 10), and brief psychotic disorder (n = 2). These patient groups were compared to controls in all subsequent analyses.
In order to examine group differences in cognitive functioning, as well as the effect of age on these differences, cross-sectional cognitive data from ages 20 to 60 were used to generate cognitive charts for each group and cognitive test. All test scores were trans-formed to z scores, with higher scores indicating better performance. Individual z scores adjusted for sex were subsequently used to calculate percentiles for each age stratum and 5-year sliding windows were applied to increase accuracy of percentile estimation (Buuren and Fredriks, 2001; Vorstman et al., 2015). Online Supplementary Fig. S2 shows g charts for control, affective psychosis and nonaffective psychosis groups, which were generated by connecting percentiles using local regression smoothing.
Group differences in cognitive charts (i.e. 50th percentiles) were tested using regression analysis. Group and age effects, as well as group-by-age interactions were included in all models. Natural cubic splines (Smith, 1979; Lin and Zhang, 1999; Benedetti and Abrahamowicz, 2004) were used to model the non-linear effect of age on cognitive charts as illustrated in online Supplementary Fig. S3, which shows cognitive charts for control, affective psychosis, and nonaffective psychosis groups with local regression smoothing (Buuren and Fredriks, 2001). Alternative models were evaluated with the Akaike information criterion (AIC) (Akaike, 1992) and Bayesian information criterion (BIC) (Schwarz, 1978) fit indices, which are information theory based statistics useful for comparing the relative fit of several models. Models with lower AIC and BIC values are thought to be relatively better fitting. Online Supplementary Table S2 shows AIC and BIC indices of these alternative models, indicating that a model with age as a natural cubic spline with three knots (i.e. one internal knot at median age 40 years and two boundary knots at ages 20 and 60) was a better fit to the data than a model with age as a linear function. Moreover, a model with age as a natural cubic spline with three knots was also a better fit than a model with age as a natural cubic spline with four knots [i.e. two internal knots at the 33rd (age = 34) and 66th (age = 46) percentiles, and two boundary knots at ages 20 and 60]. Finally, a model with group and age effects, as well as a group-by-age interaction, was a better fit than a model with only group and age effects. Thus, all models included group and age effects, as well as group-by-age interactions, and age as a natural cubic spline with three knots. Modeling age as a natural cubic spline with three knots (i.e. one internal knot at median age 40 years and two boundary knots at ages 20 and 60) enabled examination of group-by-age interactions on cognitive charts in early adulthood (i.e. between ages 20 and 40), as well as middle adulthood (i.e. between ages 40 and 60).
In order to examine the effect of potential confounders, individual test scores were subsequently adjusted for (1) antipsychotic medication, (2) cannabis use, (3) psychiatric comorbidity (i.e. number of non-psychotic psychiatric diagnoses), (4) current substance dependence, (5) lifetime substance dependence (i.e. including remitted substance dependence), (6) severity of psychotic symptoms as measured by the Lifetime Dimensions of Psychosis Scale (LDPS) (Levinson et al., 2002), (7) duration of psychosis, (8) education, (9) maternal education, (10) employment in the past year, (11) general functioning as measured by the Global Assessment of Functioning (GAF) scale (Hall, 1995) and (12) social functioning. Individual test scores were also adjusted for sex. These adjusted scores were then used to generate cognitive charts as described above. To control for multiple testing, the false discovery rate (FDR) was set at 5% (Benjamini and Yekutieli, 2001).
Results
Affective and nonaffective psychosis patients show widespread cognitive impairments
Figure 1 depicts effect sizes (ESs) of overall cognitive impairment for the affective and nonaffective psychosis groups, and Table 2 shows group effects on each cognitive chart for the affective and nonaffective psychosis groups. Since cognitive test scores were z transformed, β coefficients correspond to standardized ESs, with values of 0.2, 0.5 and 0.8 indicating small, medium and large ESs, respectively (Cohen, 1992). The nonaffective psychosis group showed statistically significant impairments on all cognitive measures, with a large g impairment (β = −0.82, p < 0.001), medium impairments on the Wechsler Test of Adult Reading (WTAR) (β = −0.67, p < 0.001), semantic fluency (β = −0.45, p <0.001), Trail-Making A and B (β = −0.60, p < 0.001; β = −0.61, p < 0.001), digit-symbol coding (β = −0.63, p < 0.001), California Verbal Learning Test (CVLT) recall and recognition (β = −0.50, p < 0.001 and β = −0.43, p < 0.001), digit span forward and backward (β = −0.57, p < 0.001; β = −0.46, p < 0.001), and letter-number sequencing (β = −0.65, p < 0.001), and small impairments on vocabulary (β = −0.27, p < 0.001) and matrix reasoning (β = −0.29, p < 0.001). All impairments remained statistically significant after FDR correction.
Fig. 1.
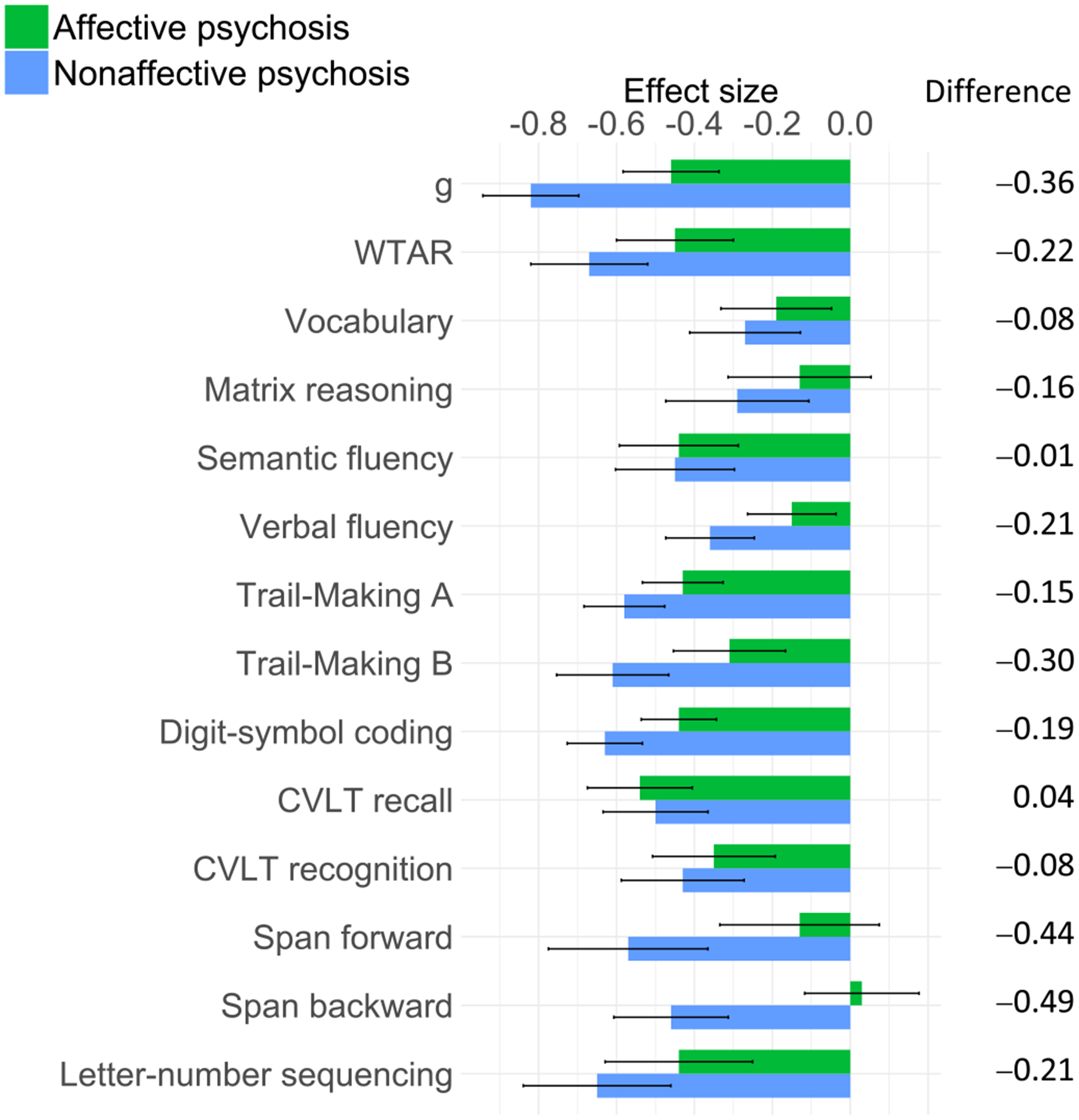
ES of overall impairment on each cognitive measure for affective and nonaffective psychosis groups.
Table 2.
Group and group-by-age interaction effects of regression analyses adjusting for sex
| Group effect | Group-by-age interaction Early adulthood | Group-by-age interaction Middle adulthood | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive measure | β | p | pFDR | β | p | pFDR | ΔES | β | p | pFDR | ΔES |
| Affective psychosis | |||||||||||
| g | −0.46 | <0.001 | <0.001 | 0.45 | 0.204 | 0.317 | 0.17 | 0.42 | 0.034 | 0.078 | 0.31 |
| WTAR | −0.45 | <0.001 | <0.001 | 0.19 | 0.642 | 0.817 | −0.18 | 1.36 | <0.001 | <0.001 | 1.29 |
| Vocabulary | −0.19 | 0.011 | 0.013 | −0.92 | 0.026 | 0.093 | −0.63 | 0.51 | 0.024 | 0.068 | 0.70 |
| Matrix reasoning | −0.13 | 0.169 | 0.198 | 1.39 | 0.013 | 0.060 | 0.68 | 0.54 | 0.076 | 0.134 | 0.22 |
| Semantic fluency | −0.44 | <0.001 | <0.001 | 0.64 | 0.144 | 0.288 | 0.11 | 1.17 | <0.001 | <0.001 | 1.01 |
| Verbal fluency | −0.15 | 0.010 | 0.013 | −0.13 | 0.705 | 0.822 | −0.04 | −0.18 | 0.343 | 0.437 | −0.15 |
| Trail-Making A | −0.43 | <0.001 | <0.001 | −0.33 | 0.318 | 0.445 | −0.15 | −0.17 | 0.330 | 0.437 | −0.10 |
| Trail-Making B | −0.31 | <0.001 | <0.001 | 0.08 | 0.859 | 0.925 | 0.03 | 0.04 | 0.850 | 0.850 | 0.03 |
| Digit-symbol coding | −0.44 | <0.001 | <0.001 | 0.76 | 0.008 | 0.057 | 0.32 | 0.54 | 0.001 | 0.003 | 0.36 |
| CVLT recall | −0.54 | <0.001 | <0.001 | 1.34 | <0.001 | 0.001 | 0.60 | 0.77 | <0.001 | <0.001 | 0.45 |
| CVLT recognition | −0.35 | <0.001 | <0.001 | 0.86 | 0.082 | 0.192 | 0.42 | 0.31 | 0.252 | 0.391 | 0.11 |
| Span forward | −0.13 | 0.200 | 0.216 | 1.03 | 0.079 | 0.192 | 0.62 | −0.18 | 0.579 | 0.660 | −0.40 |
| Span backward | 0.03 | 0.670 | 0.670 | 0.00 | 0.999 | 0.999 | −0.11 | 0.50 | 0.049 | 0.098 | 0.49 |
| Letter-number sequencing | −0.44 | <0.001 | <0.001 | 0.79 | 0.174 | 0.305 | 0.42 | 0.16 | 0.613 | 0.660 | −0.02 |
| Nonaffective psychosis | |||||||||||
| g | −0.82 | <0.001 | <0.001 | −0.84 | 0.019 | 0.095 | −0.60 | 0.60 | 0.002 | 0.007 | 0.78 |
| WTAR | −0.67 | <0.001 | <0.001 | −0.49 | 0.230 | 0.358 | −0.45 | 0.82 | <0.001 | 0.002 | 0.91 |
| Vocabulary | −0.27 | <0.001 | <0.001 | 0.46 | 0.259 | 0.362 | 0.09 | 0.81 | <0.001 | 0.002 | 0.69 |
| Matrix reasoning | −0.29 | 0.003 | 0.003 | 0.00 | 0.999 | 0.999 | 0.03 | −0.13 | 0.661 | 0.771 | −0.13 |
| Semantic fluency | −0.45 | <0.001 | <0.001 | −0.04 | 0.932 | 0.999 | −0.15 | 0.61 | 0.011 | 0.026 | 0.61 |
| Verbal fluency | −0.36 | <0.001 | <0.001 | −0.67 | 0.061 | 0.172 | −0.38 | 0.01 | 0.939 | 0.939 | 0.16 |
| Trail-Making A | −0.58 | <0.001 | <0.001 | −0.42 | 0.195 | 0.358 | −0.19 | −0.24 | 0.185 | 0.323 | −0.14 |
| Trail-Making B | −0.61 | <0.001 | <0.001 | −1.54 | <0.001 | 0.006 | −0.89 | 0.10 | 0.657 | 0.771 | 0.44 |
| Digit-symbol coding | −0.63 | <0.001 | <0.001 | 0.63 | 0.027 | 0.095 | 0.30 | 0.22 | 0.156 | 0.313 | 0.08 |
| CVLT recall | −0.50 | <0.001 | <0.001 | 0.01 | 0.979 | 0.999 | 0.31 | 1.28 | <0.001 | <0.001 | 1.26 |
| CVLT recognition | −0.43 | <0.001 | <0.001 | 0.62 | 0.208 | 0.358 | −0.27 | 0.25 | 0.350 | 0.544 | 0.11 |
| Span forward | −0.57 | <0.001 | <0.001 | 1.36 | 0.021 | 0.095 | 0.52 | 1.17 | <0.001 | 0.002 | 0.85 |
| Span backward | −0.46 | <0.001 | <0.001 | 0.16 | 0.728 | 0.927 | 0.06 | 0.15 | 0.564 | 0.771 | 0.11 |
| Letter-number sequencing | −0.65 | <0.001 | <0.001 | −0.94 | 0.107 | 0.250 | −0.53 | −0.03 | 0.919 | 0.939 | 0.18 |
Bolded estimates signify statistical significance (P < 0.05).
WTAR, Wechsler Test of Adult Reading; FDRCorrected for multiple testing (FDR = 0.05); ΔES, effect size of change (in S.D.).
The affective psychosis group showed statistically significant impairments on all cognitive measures, except matrix reasoning, and span forward and backward, with medium impairments on g (β = −0.46, p < 0.001), WTAR (β = −0.45, p < 0.001), semantic fluency (β = −0.44, p < 0.001), Trail-Making A (β = −0.43, p <0.001), digit-symbol coding (β = −0.44, p < 0.001), CVLT recall (β = −0.54, p < 0.001) and letter-number sequencing (β = −0.44, p < 0.001), and small impairments on vocabulary (β = −0.19, p = .013), verbal fluency (β = −0.15, p = .013), Trail-Making B (β = −0.31, p < 0.001), and CVLT recognition (β = −0.35, p <0.001). All impairments remained statistically significant after FDR correction. While the nonaffective psychosis group showed larger impairments than the affective psychosis group on all measures except CVLT recall, these differences were small, with only differences on span forward and backward reaching a magnitude of medium ES (Fig. 1).
Cognitive charts in early adulthood
Figure 2 shows cognitive charts for control, affective psychosis and nonaffective psychosis groups. Age-associated decline was apparent in all groups and in all cognitive measures. However, age-associated group differences were also observed. Table 2 shows group-by-age interactions and ESs on each cognitive chart during early (i.e. between ages 20 and 40) and late (i.e. between ages 40 and 60) adulthood for affective and nonaffective psychosis groups. During early adulthood, the nonaffective psychosis group showed increasing impairments on Trail-Making B (β = −1.54, p = 0.006), and g (β = −0.84, p = 0.019), although the latter did not reach statistical significance after FDR correction. As illustrated in Fig. 2, the nonaffective psychosis group showed no impairment on Trail-Making B at age 20 (0.02 S.D. above controls), but a large impairment had emerged by age 40 (0.87 S.D. below controls), indicating an increase in deficit of 0.89 S.D. The g impairment increased from 0.49 S.D. at age 20 to 1.09 at age 40, indicating an increase in deficit of 0.60 S.D.
Fig. 2.
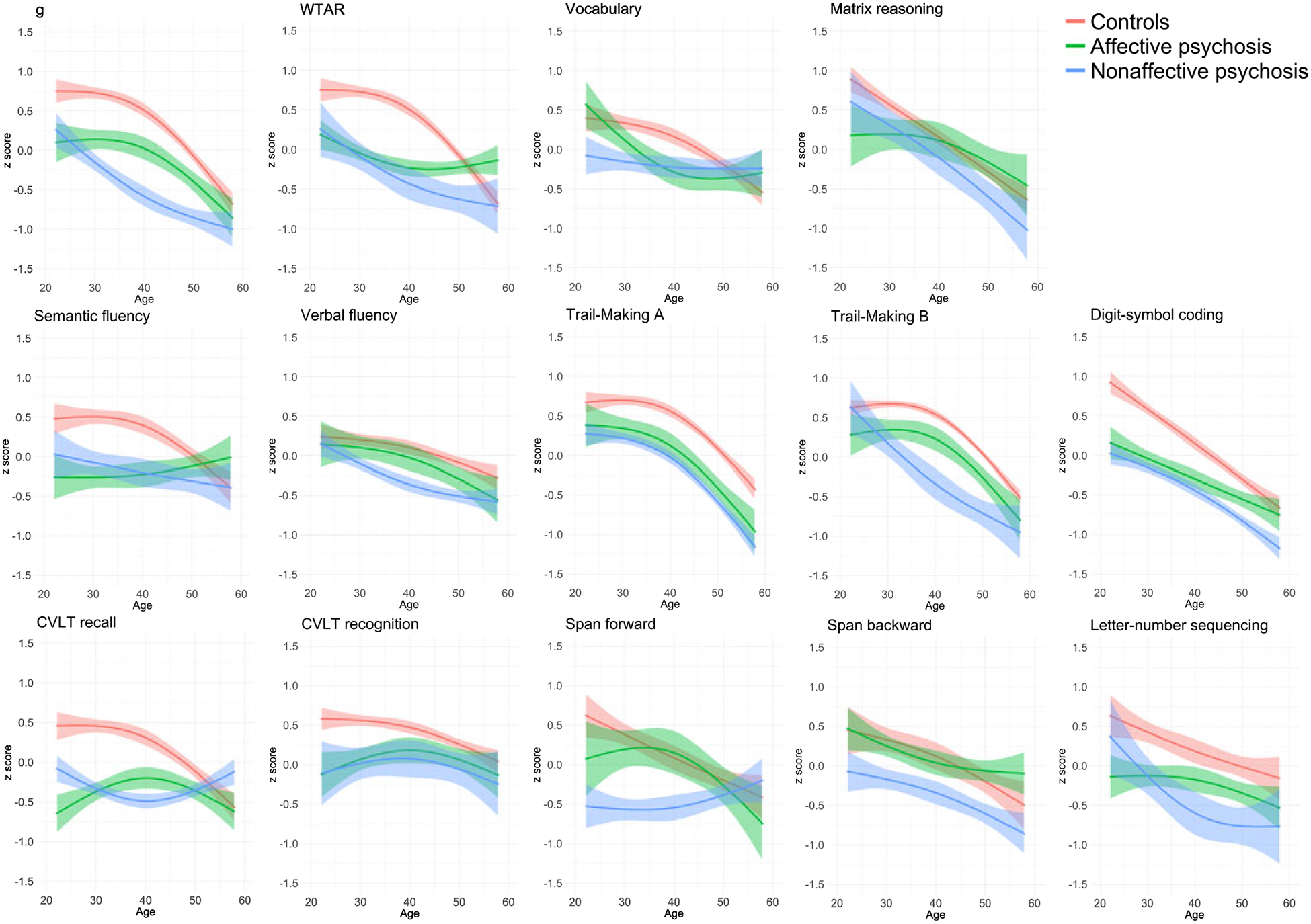
Cognitive charts for control, affective psychosis and nonaffective psychosis groups.
Similarly, the affective psychosis group showed an increasing impairment during early adulthood on vocabulary (β = −0.92, p = 0.026), although this did not reach statistical significance after FDR correction. As seen in Fig. 2, the affective psychosis group did not show a vocabulary impairment at age 20 (0.18 S.D. above controls), but by age 40 a medium impairment (0.45 S.D. below controls) had emerged, indicating an increase in deficit of 0.63 S.D. On the other hand, the affective psychosis group showed a decreasing impairment during early adulthood on CVLT recall (β = 1.34, p = 0.001), so that the impairment decreased from 1.11 S.D. to 0.51 S.D. between ages 20 and 40, indicating a decrease in deficit of 0.60 S.D.
Cognitive charts in middle adulthood
During middle adulthood (i.e. between ages 40 and 60), both the affective and nonaffective psychosis groups showed decreasing impairments on WTAR (affective: β = 1.36, p < 0.001; nonaffective: β = 0.82, p = 0.002), semantic fluency (affective: β = 1.17, p < 0.001; nonaffective: β = 0.61, p = 0.026) and CVLT recall (affective: β = 0.77, p < 0.001; nonaffective: β = 1.28, p < 0.001) (Table 2). Additionally, the affective psychosis group showed a decreasing impairment on the digit-symbol coding (β = 0.54, p = 0.003), and the nonaffective psychosis group on g (β = 0.60, p = 0.007), vocabulary (β = 0.81, p = 0.002), and span forward (β = 1.17, p = 0.002). In all cases, cognitive impairments present at age 40 were negligible or no longer present at age 60 (Fig. 2).
Group-by-age interactions in early and middle adulthood on verbal fluency, Trail-Making A, CVLT recognition, and letter-number sequencing were non-significant for both groups, suggesting stable deficits on these measures throughout adulthood. The affective psychosis group also showed a stable g impairment throughout adulthood, and the nonaffective group showed stable matrix reasoning and span backward deficits. Table 3 summarizes cognitive impairments across early and middle adulthood for both psychosis groups.
Table 3.
Summary of cognitive impairments throughout early and middle adulthood
| Affective psychosis | Nonaffective psychosis | |||
|---|---|---|---|---|
| Early adulthood | Middle adulthood | Early adulthood | Middle adulthood | |
| g | – | – | ↓ | ↑ |
| WTAR | – | ↑ | – | ↑ |
| Vocabulary | ↓ | ↑ | – | ↑ |
| Matrix reasoning | None | – | – | |
| Semantic fluency | – | ↑ | – | ↑ |
| Verbal fluency | – | – | – | – |
| Trail-Making A | – | – | – | – |
| Trail-Making B | – | – | ↓ | – |
| Digit-symbol coding | ↑ | ↑ | ↑ | – |
| CVLT recall | ↑ | ↑ | – | ↑ |
| CVLT recognition | – | – | – | – |
| Span forward | None | ↑ | ↑ | |
| Span backward | None | – | – | |
| Letter-number sequencing | – | – | – | – |
–, Stable impairment; ↑, relatively smaller age-associated decline (decreasing impairment); ↓, relatively greater age-associated decline (increasing impairment).
Confounders do not account for cognitive impairments or deviations in cognitive charts
Online Supplementary Tables S3–S14 show group and group-by-age interaction effects after adjustment for potential confounders. Results were similar when adjusting for antipsychotic medication, cannabis use, psychiatric comorbidity, and both current and lifetime substance dependence (online Supplementary Tables S3–S7). The nonaffective psychosis group showed small to large cognitive impairments and an increasing impairment on Trail-Making B during early adulthood. The affective psychosis group showed small to medium impairments, and both groups showed decreasing impairments on several measures during middle adulthood.
When adjusting for severity of psychotic symptoms (online Supplementary Table S7), the nonaffective psychosis group showed small cognitive impairments and an increasing impairment on Trail-Making B during early adulthood, but impairments in the affective psychosis group were attenuated. When adjusting for duration of psychosis (online Supplementary Table S8), the increasing impairment on Trail-Making B during early adulthood in the nonaffective psychosis group no longer reached statistical significance and the affective psychosis group showed only a small impairment on CVLT recall. However, duration of psychosis and age are correlated (online Supplementary Fig. S4), making it difficult to disentangle their effects.
Both groups showed small to medium impairments when adjusting for education (online Supplementary Table S9) and decreasing impairments on several measures during middle adulthood. Interestingly, the increasing impairment on Trail-Making B during early adulthood in the nonaffective psychosis group no longer reached statistical significance, but the affective psychosis group showed a significantly increasing impairment on vocabulary and verbal fluency during this period. However, the causal nature of the relationship between education and cognition is complex, making these results challenging to interpret. Adjusting for maternal education, on the other hand, attenuated the increasing impairment on vocabulary in the affective group, but the nonaffective group showed increasing impairments on Trail-Making B and letter number sequencing (online Supplementary Table S11).
When adjusting for employment, both groups showed small cognitive impairments across adulthood and decreasing impairments on several measures during middle adulthood (online Supplementary Table S12). The affective psychosis group showed an increasing impairment on vocabulary during early adulthood, and the nonaffective group showed increasing impairments on g and Trail-Making B. Adjusting for general functioning attenuated impairments on most cognitive measures across adulthood in both the affective and nonaffective groups (online Supplementary Table S13), but the nonaffective group still showed an increasing impairment on Trail-Making B in early adulthood. On the other hand, impairments across adulthood remained small to large in both groups when adjusting for social functioning (online Supplementary Table S14), but the increasing impairment on Trail-Making B during early adulthood in the nonaffective group no longer reached FDR-corrected significance.
Discussion
Using cross-sectional data from a population-based, case–control, adult sample of African-American psychosis patients, we found substantial and widespread cognitive impairments, in line with an expansive literature on cognitive dysfunction in psychotic disorders (Fioravanti et al., 2005; Glahn et al., 2007; Reichenberg and Harvey, 2007; Bora et al., 2009). Both affective and nonaffective psychosis patients showed impairments on measures of general cognitive ability, language, abstract reasoning, processing speed, executive function, verbal memory and working memory, but the nonaffective group showed somewhat larger impairments on almost all cognitive measures. Examining cognitive charts throughout adulthood also revealed age-associated group differences. Specifically, the nonaffective psychosis group showed increasing impairments on g and Trail-Making B during early adulthood (between ages 20 and 40). The affective psychosis group showed an increasing impairment on vocabulary during this period. Impairments on other cognitive measures remained mostly stable, although decreasing impairments on measures of processing speed, memory and working memory were also observed, mostly in middle adulthood (between ages 40 and 60). These findings add to knowledge about the profile of cognitive dysfunction across adulthood in psychotic disorders in several ways.
First, we found that the nonaffective psychosis group showed larger impairments than the affective psychosis group across all cognitive measures except for verbal memory recall. These differences were generally small, however, with only differences on span forward and backward reaching a magnitude of medium ES. Previous studies have also reported small differences in the magnitude of cognitive impairment between psychotic disorders (Bora et al., 2009; Zanelli et al., 2010). Our findings lend support to the notion that differences in cognitive impairment between affective and nonaffective psychotic disorders are more quantitative than qualitative (Reichenberg et al., 2009) since both affective and non-affective psychosis groups showed a similar profile of impairment, with decrements across most cognitive measures. Interestingly, the affective psychosis group did not show impairments on the digit span forward and backward, measures of working memory. Differential working memory impairments in affective and nonaffective psychotic disorders have been reported previously, albeit on measures of spatial rather than verbal working memory (Gooding and Tallent, 2002; Pirkola et al., 2005; Glahn et al., 2006). Nevertheless, working memory impairments may represent a somewhat specific vulnerability to nonaffective psychosis.
Second, examining cognitive charts across early and middle adulthood in affective and nonaffective psychosis patients revealed differences between groups, as well as cognitive measures. Specifically, the nonaffective psychosis group showed increasing impairments on g and Trail-Making B during early adulthood. The affective psychosis group showed an increasing impairment on vocabulary during early adulthood. While evidence suggests that psychosis patients exhibit cognitive decline between premorbid and post-onset periods of illness (Seidman et al., 2006; Meier et al., 2013; Mollon et al., 2018), studies have mostly reported stable cognitive impairments throughout adulthood (Hyde et al., 1994; Chen et al., 1996; Mockler et al., 1997; Fucetola et al., 2000; Szöke et al., 2008; Bozikas and Andreou, 2011; Samamé et al., 2014). Our findings suggest that, for certain cognitive measures, impairments may continue to increase during early adulthood. Increasing impairments on g and Trail-Making B in nonaffective psychosis patients are in line with evidence that impairments in these domains constitute some of the largest in schizophrenia (Reichenberg and Harvey, 2007). Moreover, previous studies have also reported increasing impairment in some measures of executive function during early adulthood (Fucetola et al., 2000; Heilbronner et al., 2016). The increasing impairment in vocabulary seen in the affective psychosis group, on the other hand, suggests that this and other language measures may be a poor indicator of premorbid cognitive ability. Nevertheless, impairments on the majority of cognitive measures remained static throughout adulthood with stable impairments on measures of processing speed, verbal memory and working memory throughout adulthood, again, suggesting a similar profile of impairment across psychotic disorders. Interestingly, we found that both patient groups showed decreasing impairments on measures of general cognitive ability, language, processing speed, memory and working memory, particularly during middle adulthood. For the most part, these effects reflect acceleration of age-associated differences in controls v. stabilization in patients, which is in line with evidence of age-associated cognitive decline in the general population well before late adulthood (Salthouse, 2009; Singh-Manoux et al., 2012; Hartshorne and Germine, 2015; Siman-Tov et al., 2016). However, whether this stabilization in patients continues to later adulthood warrants further investigation since there is evidence for further cognitive decline in older adults with psychotic disorders (Harvey et al., 1999; Friedman et al., 2001; Rajji and Mulsant, 2008).
Third, we examined the effect of a number of potential con-founders and found that, for the most part, these factors could not account for the differences across groups and cognitive measures. Overall impairments remained significant across most cognitive measures in the nonaffective psychosis group when adjusting for antipsychotic medication, cannabis use, psychiatric comorbidity, duration of psychosis, education, maternal education, employment, and social functioning. Overall impairments in the affective psychosis group showed greater attenuation by these factors, but remained significant across most cognitive measures when adjusting for antipsychotic medication, cannabis use and education. Overall impairments in both psychosis groups showed greatest attenuation when adjusting for symptom severity and general functioning, although the increasing impairment on Trail-Making B in the nonaffective group and increasing impairment on vocabulary in the affective group remained significant. Duration of psychosis was the only factor to attenuate increasing impairments in both psychosis groups, likely due to the fact that duration of psychosis and age are correlated. Nevertheless, our findings are in line with evidence that cognitive dysfunction, particularly in non-affective psychotic disorders, cannot be explained by antipsychotic medications (Mohamed et al., 1999; Hill et al., 2004), cannabis use (Løberg and Hugdahl, 2009; Yucel et al., 2012), and symptom severity (Aleman et al., 1999; O’Leary et al., 2000). Moreover, while there is substantial evidence for psychiatric comorbidity and substance dependence in psychotic disorders (Mueser et al., 1992; Cassano et al., 1998; Buckley et al., 2008), few studies have examined their effects on cognitive dysfunction (Pencer and Addington, 2003; McGurk et al., 2009; D’Souza and Markou, 2012). While our findings suggest that cognitive impairment, particularly in non-affective psychotic dis-order, cannot be broadly explained by psychiatric comorbidity, and both current and lifetime substance dependence, more research on the cognitive correlates of specific comorbidities, such as depression and alcohol dependence, is warranted. Similarly, while we were able to examine the effect of current antipsychotic medications on cognitive impairment, future studies on the effect of lifetime medications would be informative, particularly since a substantial proportion of psychotic patients use different and even multiple antipsychotics throughout the course of the illness (Stahl and Grady, 2004; Gallego et al., 2012).
This study has some limitations. First, the cognitive charts were calculated using cross-sectional data, and replication with longitudinal data is needed. However, there are obvious challenges and pitfalls to collecting longitudinal data, especially spanning several decades, and length of follow-up in longitudinal studies has so far been limited to 10 years (Bozikas and Andreou, 2011). Moreover, we adjusted for several potential confounders, including education and antipsychotic medication. Nevertheless, future examination of the influence of other potential cohort factors, such as health and environment, is needed. Second, our sample comprises African-American adults, and replication in samples of other ethnicities is required. However, African-Americans, like other minority groups, are underrepresented in psychiatry research, and were specifically chosen as the study population for this reason. Third, while our study advances knowledge regarding the cognitive profile of affective and nonaffective psychoses across adulthood, we were not able to subdivide these groups further to examine the profile of cognitive impairment in specific disorders, such as psychotic major depression. Fourth, due to the limited number of participants aged younger than 20 and older than 60, we were not able to examine age-associated differences in cognitive functioning beyond this age range. In the general population, cognitive changes are more likely to occur after the age of 65 (Czaja et al., 2006; Deary et al., 2009) and indeed there is evidence for further cognitive decline in older adults with psychotic disorders (Harvey et al., 1999; Friedman et al., 2001; Rajji and Mulsant, 2008). Finally, cognitive impairments in this study were somewhat smaller than those reported in the literature, possibly due to the fact that neither patients nor controls were excluded for having non-psychotic psychiatric disorders. Nevertheless, since psychiatric comorbidity is prevalent in psychotic disorders, our findings add valuable insight into cognitive dysfunction in psychosis.
In conclusion, we found substantial and widespread cognitive impairments across measures of general cognitive ability, language, abstract reasoning, processing speed, executive function, verbal memory and working memory in adults with both affective and nonaffective psychotic disorders. Moreover, during early adulthood, the nonaffective psychosis group showed increasing impairments on measures of general cognitive ability and executive function, while the affective psychosis group showed an increasing impairment in language ability. On most cognitive measures, however, impairments remained relatively stable throughout adulthood. Results remained largely unchanged when adjusting for antipsychotic medication, cannabis use, psychiatric comorbidity, substance dependence, symptom severity, duration of psychosis and education. Our findings extend previous knowledge regarding the profile of cognitive dysfunction across adulthood in affective and nonaffective psychotic disorders, suggesting both similarities and differences across groups and measures, as well as static and dynamic impairments. Different underlying etiopathogenic mechanisms may underlie the divergent cognitive impairments seen in affective and nonaffective psychosis.
Supplementary Material
Acknowledgements.
The authors would like to thank all study participants.
Financial support. This work was supported by the National Institute of Mental Health (NIMH) (Grant no. R01MH106324-01).
Footnotes
Supplementary material. The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718003938.
References
- Addington J and Addington D (1993) Premorbid functioning, cognitive functioning, symptoms and outcome in schizophrenia. Journal of Psychiatry and Neuroscience 18, 18. [PMC free article] [PubMed] [Google Scholar]
- Akaike H. (1992). Information theory and an extension of the maximum likelihood principle In Breakthroughs in statistics. Springer, New York, NY, pp. 610–624. [Google Scholar]
- Albus M, Hubmann W, Scherer J, Dreikorn B, Hecht S, Sobizack N and Mohr F (2002) A prospective 2-year follow-up study of neurocognitive functioning in patients with first-episode schizophrenia. European Archives of Psychiatry and Clinical Neuroscience 252, 262–267. [DOI] [PubMed] [Google Scholar]
- Aleman A, Hijman R, de Haan EH and Kahn RS (1999) Memory impairment in schizophrenia: a meta-analysis. American Journal of Psychiatry 156, 1358–1366. [DOI] [PubMed] [Google Scholar]
- Benedetti A and Abrahamowicz M (2004) Using generalized additive models to reduce residual confounding. Statistics in Medicine 23, 3781–3801. [DOI] [PubMed] [Google Scholar]
- Benjamini Y and Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Annals of Statistics 29, 1165–1188. [Google Scholar]
- Bilder RM, Lipschutz-Broch L, Reiter G, Geisler SH, Mayerhoff DI and Lieberman JA (1992) Intellectual deficits in first-episode schizophrenia: evidence for progressive deterioration. Schizophrenia Bulletin 18, 437. [DOI] [PubMed] [Google Scholar]
- Bonner-Jackson A, Grossman LS, Harrow M and Rosen C (2010) Neurocognition in schizophrenia: a 20-year multi-follow-up of the course of processing speed and stored knowledge. Comprehensive Psychiatry 51, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Yucel M and Pantelis C (2009) Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: meta-analytic study. British Journal of Psychiatry 195, 475–482. [DOI] [PubMed] [Google Scholar]
- Bozikas VP and Andreou C (2011) Longitudinal studies of cognition in first episode psychosis: a systematic review of the literature. Australian and New Zealand Journal of Psychiatry 45, 93–108. [DOI] [PubMed] [Google Scholar]
- Brissos S, Dias VV, Balanza-Martinez V, Carita AI and Figueira ML (2011) Symptomatic remission in schizophrenia patients: relationship with social functioning, quality of life, and neurocognitive performance. Schizophrenia Research 129, 133–136. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Miller BJ, Lehrer DS and Castle DJ (2008) Psychiatric comorbidities and schizophrenia. Schizophrenia Bulletin 35, 383–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdick KE, Goldberg JF, Harrow M, Faull RN and Malhotra AK (2006) Neurocognition as a stable endophenotype in bipolar disorder and schizophrenia. The Journal of Nervous and Mental Disease 194, 255–260. [DOI] [PubMed] [Google Scholar]
- Buuren SV and Fredriks M (2001) Worm plot: a simple diagnostic device for modelling growth reference curves. Statistics in Medicine 20, 1259–1277. [DOI] [PubMed] [Google Scholar]
- Cassano GB, Pini S, Saettoni M, Rucci P and Dell’Osso L (1998) Occurrence and clinical correlates of psychiatric comorbidity in patients with psychotic disorders. The Journal of Clinical Psychiatry 59, 60–68. [DOI] [PubMed] [Google Scholar]
- Chen EY, Lam L, Chen R, Nguyen D and Chan C (1996) Prefrontal neuropsychological impairment and illness duration in schizophrenia: a study of 204 patients in Hong Kong. Acta Psychiatrica Scandinavica 93, 144–150. [DOI] [PubMed] [Google Scholar]
- Cohen J (1992) A power primer. Psychological Bulletin 112, 155. [DOI] [PubMed] [Google Scholar]
- Czaja SJ, Charness N, Fisk AD, Hertzog C, Nair SN, Rogers WA and Sharit J (2006) Factors predicting the use of technology: findings from the Center for Research and Education on Aging and Technology Enhancement (CREATE). Psychology and Aging 21, 333–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza MS and Markou A (2012) Schizophrenia and tobacco smoking comorbidity: nAChR agonists in the treatment of schizophrenia-associated cognitive deficits. Neuropharmacology 62, 1564–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson M, Harvey PD, Powchik P and Parrella M (1995) Severity of symptoms in chronically institutionalized geriatric schizophrenic patients. The American journal of psychiatry 152, 197. [DOI] [PubMed] [Google Scholar]
- Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z and Mark M (1999) Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. American Journal of Psychiatry 156, 1328–1335. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Corley J, Gow AJ, Harris SE, Houlihan LM, Marioni RE, Penke L, Rafnsson SB and Starr JM (2009) Age-associated cognitive decline. British medical bulletin 92, 135–152. [DOI] [PubMed] [Google Scholar]
- Fett AK, Viechtbauer W, Dominguez MD, Penn DL, van Os J and Krabbendam L (2011) The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neuroscience & Biobehavioral Reviews 35, 573–588. [DOI] [PubMed] [Google Scholar]
- Fioravanti M, Carlone O, Vitale B, Cinti ME and Clare L (2005) A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychology Review 15, 73–95. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M and Williams JB (2002) Structured clinical interview for DSM-IV-TR axis I disorders, research version Patient Edition SCID-I/P. [Google Scholar]
- Fleming SK, Blasey C and Schatzberg AF (2004) Neuropsychological correlates of psychotic features in major depressive disorders: a review and meta-analysis. Journal of Psychiatric Research 38, 27–35. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, White L, Adler D and Davis KL (2001) Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: a comparison with Alzheimer’s disease and normal aging. American Journal of Psychiatry 158, 1441–1448. [DOI] [PubMed] [Google Scholar]
- Fucetola R, Seidman LJ, Kremen WS, Faraone SV, Goldstein JM and Tsuang MT (2000) Age and neuropsychologic function in schizophrenia: a decline in executive abilities beyond that observed in healthy volunteers. Biological Psychiatry 48, 137–146. [DOI] [PubMed] [Google Scholar]
- Gallego JA, Bonetti J, Zhang J, Kane JM and Correll CU (2012) Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophrenia Research 138, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Cakir S, Barrett JA, Najt P, Serap Monkul E, Maples N, Velligan DI and Soares JC (2006) Differential working memory impairment in bipolar disorder and schizophrenia: effects of lifetime history of psychosis. Bipolar Disorders 8, 117–123. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Bearden CE, Barguil M, Barrett J, Reichenberg A, Bowden CL, Soares JC and Velligan DI (2007) The neurocognitive signature of psychotic bipolar disorder. Biological Psychiatry 62, 910–916. [DOI] [PubMed] [Google Scholar]
- Gooding DC and Tallent KA (2002) Spatial working memory performance in patients with schizoaffective psychosis versus schizophrenia: a tale of two disorders? Schizophrenia Research 53, 209–218. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL and Mintz J (2000) Neurocognitive deficits and functional outcome in schizophrenia. Schizophrenia Bulletin 26, 119–136. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W and Gur RC (1998) A follow-up magnetic resonance imaging study of schizophrenia: relationship of neuroanatomical changes to clinical and neurobehavioral measures. Archives of General Psychiatry 55, 145–152. [DOI] [PubMed] [Google Scholar]
- Gur RC, Calkins ME, Satterthwaite TD, Ruparel K, Bilker WB, Moore TM, Savitt AP, Hakonarson H and Gur RE (2014) Neurocognitive growth charting in psychosis spectrum youths. JAMA Psychiatry 71, 366–374. [DOI] [PubMed] [Google Scholar]
- Hall RC (1995) Global assessment of functioning: a modified scale. Psychosomatics 36, 267–275. [DOI] [PubMed] [Google Scholar]
- Hartshorne JK and Germine LT (2015) When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychological Science 26, 433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Silverman JM, Mohs RC, Parrella M, White L, Powchik P, Davidson M and Davis KL (1999) Cognitive decline in late-life schizophrenia: a longitudinal study of geriatric chronically hospitalized patients. Biological Psychiatry 45, 32–40. [DOI] [PubMed] [Google Scholar]
- Heilbronner U, Samara M, Leucht S, Falkai P and Schulze TG (2016) The longitudinal course of schizophrenia across the lifespan: clinical, cognitive, and neurobiological aspects. Harvard Review of Psychiatry 24, 118–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SK, Schuepbach D, Herbener ES, Keshavan MS and Sweeney JA (2004) Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naıve patients with schizophrenia. Schizophrenia Research 68, 49–63. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Nawroz S, Goldberg TE, Bigelow LB, Strong D, Ostrem JL, Weinberger DR and Kleinman JE (1994) Is there cognitive decline in schizophrenia? A cross-sectional study. The British Journal of Psychiatry 164, 494–500. [DOI] [PubMed] [Google Scholar]
- Jones P, Murray R, Jones P, Rodgers B and Marmot M (1994) Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort. The Lancet 344, 1398–1402. [DOI] [PubMed] [Google Scholar]
- Levinson DF, Mowry BJ, Escamilla MA and Faraone SV (2002) The Lifetime Dimensions of Psychosis Scale (LDPS): description and interrater reliability. Schizophrenia Bulletin 28, 683–695. [DOI] [PubMed] [Google Scholar]
- Lin X and Zhang D (1999) Inference in generalized additive mixed models by using smoothing splines. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 61, 381–400. [Google Scholar]
- Løberg E-M and Hugdahl K (2009) Cannabis use and cognition in schizophrenia. Frontiers in Human Neuroscience 3, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacCabe JH, Wicks S, Lofving S, David AS, Berndtsson A, Gustafsson JE, Allebeck P and Dalman C (2013) Decline in cognitive performance between ages 13 and 18 years and the risk for psychosis in adulthood: a Swedish longitudinal cohort study in males. JAMA Psychiatry 70, 261–270. [DOI] [PubMed] [Google Scholar]
- Mathias SR, Knowles EEM, Barrett J, Leach O, Buccheri S, Beetham T, Blangero J, Poldrack RA and Glahn DC (2017) The processing-speed impairment in psychosis is more than just accelerated aging. Schizophrenia Bulletin 43, 814–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias SR, Knowles EEM, Barrett J, Beetham T, Leach O, Buccheri S, Aberizk K, Blangero J, Poldrack RA and Glahn DC (2018) Deficits in visual working-memory capacity and general cognition in African Americans with psychosis. Schizophrenia Research 193, 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk SR, Mueser KT, DeRosa TJ and Wolfe R (2009) Work, recovery, and comorbidity in schizophrenia: a randomized controlled trial of cognitive remediation. Schizophrenia Bulletin 35, 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Reichenberg A, Keefe RS, Fisher HL, Harrington H, Houts R, Poulton R and Moffitt TE (2013) Neuropsychological decline in schizophrenia from the premorbid to the postonset period: evidence from a population-representative longitudinal study. American Journal of Psychiatry 171, 91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockler D, Riordan J and Sharma T (1997) Memory and intellectual deficits do not decline with age in schizophrenia. Schizophrenia Research 26, 1–7. [DOI] [PubMed] [Google Scholar]
- Mohamed S, Paulsen JS, O’leary D, Arndt S and Andreasen N (1999) Generalized cognitive deficits in schizophrenia: a study of first-episode patients. Archives of General Psychiatry 56, 749–754. [DOI] [PubMed] [Google Scholar]
- Mojtabai R, Bromet EJ, Harvey PD, Carlson GA, Craig TJ and Fennig S (2000) Neuropsychological differences between first-admission schizophrenia and psychotic affective disorders. American Journal of Psychiatry 157, 1453–1460. [DOI] [PubMed] [Google Scholar]
- Mollon J, David AS, Zammit S, Lewis G and Reichenberg A (2018) Course of cognitive development from infancy to early adulthood in the psychosis spectrum. JAMA Psychiatry 75, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueser KT, Bellack AS and Blanchard JJ (1992) Comorbidity of schizophrenia and substance abuse: Implications for treatment. Journal of Consulting and Clinical Psychology 60, 845. [DOI] [PubMed] [Google Scholar]
- O’Leary DS, Flaum M, Kesler ML, Flashman LA, Arndt S and Andreasen NC (2000) Cognitive correlates of the negative, disorganized, and psychotic symptom dimensions of schizophrenia. The Journal of Neuropsychiatry and Clinical Neurosciences 12, 4–15. [DOI] [PubMed] [Google Scholar]
- Pencer A and Addington J (2003) Substance use and cognition in early psychosis. Journal of Psychiatry and Neuroscience 28, 48. [PMC free article] [PubMed] [Google Scholar]
- Pirkola T, Tuulio-Henriksson A, Glahn D, Kieseppa T, Haukka J, Kaprio J, Lonnqvist J and Cannon TD (2005) Spatial working memory function in twins with schizophrenia and bipolar disorder. Biological Psychiatry 58, 930–936. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2008) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rajji TK and Mulsant BH (2008) Nature and course of cognitive function in late-life schizophrenia: a systematic review. Schizophrenia Research 102, 122–140. [DOI] [PubMed] [Google Scholar]
- Reichenberg A and Harvey PD (2007) Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychological Bulletin 133, 833–858. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Harvey PD, Bowie CR, Mojtabai R, Rabinowitz J, Heaton RK and Bromet E (2009) Neuropsychological function and dysfunction in schizophrenia and psychotic affective disorders. Schizophrenia Bulletin 35, 1022–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2009). When does age-related cognitive decline begin? Neurobiology of Aging 30, 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samamé C, Martino DJ and Strejilevich SA (2014) Longitudinal course of cognitive deficits in bipolar disorder: a meta-analytic study. Journal of Affective Disorders 164, 130–138. [DOI] [PubMed] [Google Scholar]
- Schatzberg AF, Posener JA, DeBattista C, Kalehzan BM, Rothschild AJ and Shear PK (2000) Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. American Journal of Psychiatry 157, 1095–1100. [DOI] [PubMed] [Google Scholar]
- Schwarz G (1978) Estimating the dimension of a model. The Annals ofStatistics 6, 461–464. [Google Scholar]
- Seidman LJ, Buka SL, Goldstein JM and Tsuang MT (2006) Intellectual decline in schizophrenia: evidence from a prospective birth cohort 28 year follow-up study. Journal of Clinical and Experimental Neuropsychology 28, 225–242. [DOI] [PubMed] [Google Scholar]
- Siman-Tov T, Bosak N, Sprecher E, Paz R, Eran A, Aharon-Peretz J and Kahn I (2016) Early age-related functional connectivity decline in high-order cognitive networks. Frontiers in Aging Neuroscience 8, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE and Dugravot A (2012) Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 344, d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL (1979) Splines as a useful and convenient statistical tool. The American Statistician 33, 57–62. [Google Scholar]
- Stahl S and Grady M (2004) A critical review of atypical antipsychotic utilization: comparing monotherapy with polypharmacy and augmentation. Current Medicinal Chemistry 11, 313–327. [DOI] [PubMed] [Google Scholar]
- Szöke A, Trandafir A, Dupont M-E, Méary A, Schürhoff F and Leboyer M (2008) Longitudinal studies of cognition in schizophrenia: meta-analysis. The British Journal of Psychiatry 192, 248–257. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, Armando M, Vicari S, Shashi V, Hooper SR, Chow EW, Fung WL, Butcher NJ, Young DA, McDonald-McGinn DM, Vogels A, van Amelsvoort T, Gothelf D, Weinberger R, Weizman A, Klaassen PW, Koops S, Kates WR, Antshel KM, Simon TJ, Ousley OY, Swillen A, Gur RE, Bearden CE, Kahn RS and Bassett AS and for the International Consortium on, B. & Behavior in 22q11.2 Deletion, S (2015) Cognitive decline preceding the onset of psychosis in patients with 22q11.2 deletion syndrome. JAMA Psychiatry 72, 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Bora E, Lubman DI, Solowij N, Brewer WJ, Cotton SM, Conus P, Takagi MJ, Fornito A, Wood SJ, McGorry PD and Pantelis C (2012) The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophrenia Bulletin 38, 316–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelli J, Reichenberg A, Morgan K, Fearon P, Kravariti E, Dazzan P, Morgan C, Zanelli C, Demjaha A and Jones PB (2010) Specific and generalized neuropsychological deficits: a comparison of patients with various first-episode psychosis presentations. American Journal of Psychiatry 167, 78–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


