Abstract
The present prospective, double blind, randomized clinical study was designed to evaluate whether dexmedetomidine (Dex) combined with ropivacaine for tranversus abdominis plane (TAP) block could improve analgesic quality and duration, and promote recovery following laparoscopic colectomy. Following induction of anesthesia, ultrasound-guided bilateral TAP block was performed in 60 patients scheduled for elective laparoscopic colectomy with either 20 ml of 0.375% ropivacaine plus 2 ml normal saline 0.9% (R group), or 20 ml of 0.375% ropivacaine plus 2 ml Dex (0.5 µg/kg) (RD group). Visual analogue scale (VAS) score for pain, sedation level, length of hospital stay (LOS), and bowel function recovery time and associated complications were recorded. Overall patient satisfaction with postoperative pain management was also assessed. The hemodynamic variables were not significantly different between the two groups during the surgery. However, the duration of analgesia was significantly longer in the RD group compared with the R group (P<0.05). VAS scores at 1, 2, 6 and 12 h following surgery were significantly decreased in the RD group compared with those in the R group (P<0.05). There was no significant difference in sedation level between the two groups. Notably, postoperative nausea and vomiting in the RD group was significantly decreased compared with those in the R group in the first 24 h (P<0.05). There were no serious adverse events in any group. Furthermore, 90.0 and 66.7% patients were satisfied with the postoperative pain management in the RD group and R group, respectively. The postoperative first bowel movement time was significantly shorter in the RD group compared with the R group (P<0.05). However, the LOS was not significantly different between the two groups. In conlusion, the addition of Dex to ropivacaine could significantly improve the analgesic quality and duration of TAP block, which in turn promotes recovery following laparoscopic colectomy.
Keywords: dexmedetomidine, ropivacaine, transversus abdominis plane, colectomy
Introduction
Satisfactory pain management is important during postoperative treatment as it can increase patient comfort and improve recovery (1). Multimodal analgesia techniques, and techniques combining general and local anesthesia lead to greater pain control, with less reliance on opioids and fewer side effects (2). The optimal regional block should have a rapid onset, a long duration of analgesia and high-quality pain control without adverse side effects. Tranversus abdominis plane (TAP) block is novel peripheral nerve block method, which is used for any surgery technique involving the anterolateral abdominal wall (3). As an important part of multimodal analgesia following abdominal surgery, TAP block can be performed to provide effective analgesia (4). However, a single-shot TAP block is limited in duration and analgesia. Increasing the volume (dose) of local anesthetics (LAs) may provide more effective analgesia duration and long-lasting nerve block, but can also increase the risk of dose-associated systemic toxicity (5). Although continuous catheter-based nerve blocks can improve postoperative analgesia, this procedure is expensive and requires skillful placement techniques (6). Thus far, a range of adjuvants, including dexamethasone, clonidine, fentanyl and midazolam, have been used to prolong the duration of nerve block analgesia with varying degrees of success (7). The roles of adjuvants on peripheral nerve blocks are debated, and previous studies have suggested active and passive impacts (7). Dexmedetomidine (Dex) is a highly selective α2-adrenergic agonist that has sedative, analgesic, sympatholytic and antianxiety effects without respiratory depression (8). Due to its multiple beneficial properties, the systemic administration of Dex during the perioperative period is prevalent as a favorable sedative and analgesic agent (9). Additionally, Dex has been described as an effective adjuvant for regional anesthetic agents. However, clinical trials have produced contradictory results. Some animal and clinical studies have demonstrated that Dex administered as an adjuvant to LAs for peripheral nerve block and plexus block results faster, with a longer duration of block and improved analgesic efficacy without neurologic complications (10-12). Other results have indicated a delay in sensory and motor block onset time, or no effect on sensory and motor block duration (13).
The present study was designed to assess the hypothesis that adding Dex to ropivacaine in an ultrasound-guided TAP block could improve analgesic quality and promote recovery following laparoscopic colectomy.
Materials and methods
Study protocol
This prospective, randomized, double blind clinical study was approved by the Institutional Human Investigations Committee of Yantai Yuhuangding Hospital of Qingdao University and registered at the following clinical trial website: http://www.chictr.org.cn (ChiCTR-IOR-17014122). Written informed consent was obtained from all participants. A total of 64 patients (aged 38-72 years; weighing 52-83 kg) at American Society of Anesthesiologists, with a physical status score (ASA) II-III grade and were scheduled for elective laparoscopic colectomy under general anesthesia, were recruited between February 2017 and March 2018.
Randomization and blinding
Patients were excluded from the present study due to the following reasons: A history of allergic reactions to ropivacaine, treatment with other amino-amide LAs or dexmedetomidine, psychological disorders, infection at the injection site or any other contraindications to TAP block, tolerance to opioids or the use of opioids within 2 days prior to the start of the current study. The patients and all staff involved in patient management and data collection were blind to the group assignment until the end of the study. All TAP blocks were performed by experienced anesthesiologists who were not involved in data collection. Patients agreed to receive TAP blocks while they were under general anesthesia. An anesthesiologist, who did not participate in patient grouping or the research, prepared the therapeutic agents based on randomization. The prepared syringes contained either 20 ml 0.375% ropivacaine (Qilu Pharmaceutical Co., Ltd.) plus 2 ml normal saline for the R group, or 20 ml 0.375% ropivacaine plus 2 ml of Dex (0.5 µg/kg; Jiangsu Hengrui Medicine Co., Ltd.) for the RD group.
Study procedures
One day before surgery, patients were instructed on how to use patient-controlled intravenous analgesia (PCIA), and the Visual Analogue Scale (VAS) scores were determined (0=no pain and 10=the worst possible pain). Patients were assigned to either the R (n=31) or RD (n=31) group on arrival to the operating room using a computer-generated randomization list. Blood pressure (BP), electrocardiograms (ECG), heart rate (HR), blood oxygen saturation (SpO2) and the bispectral index (BIS) were monitored in all patients. A total of 0.2 mg penehyclidine hydrochloride was infused intravenously 5 min prior to the initiation of anesthesia. General anesthesia was induced using midazolam (0.05 mg/kg), sufentanil (0.4 µg/kg), propofol (1.5-2.0 mg/kg) and cisatracurium (0.15 mg/kg). Following tracheal intubation, anesthesia was maintained with 3-12 mg/kg/h propofol and 0.06-0.1 µg/kg/min remifentanil. Cisatracurium (0.03 mg/kg) was administered intermittently to maintain muscle relaxation. The tidal volume was set at 6-8 ml/kg and the respiratory rate at 12 times/min in order to maintain the PETCO2 at 35-45 mmHg. Sufentanil (0.2 µg/kg) was administered prior to skin incision. A BIS value between 45 and 60 was maintained.
Following induction, a bilateral ultrasound-guided TAP block was implemented with an ultrasound machine (Venue 50; GE Healthcare) using the same method outlined in a previous study (14). Following the preparation of the skin with antiseptic solution, a 7-12 MHz high-frequency linear probe was placed transversely on the anterolateral abdominal wall between the costal margin and iliac crest on the mid-axillary line. Optimal images captured the three layers of muscles: The external oblique, the internal oblique and the transversus abdominis. The needle was introduced in the plane of the ultrasound probe directly under the probe and advanced until it reached the plane between the internal oblique and transversus abdominis muscles. Correct positioning of the needle tip was confirmed with an injection of 1 ml normal saline, which led to separation between the internal oblique and transversus abdominis muscle. Subsequently, a total of 22 ml prepared solution was injected using the ultrasound for guidance. From the ultrasound image, expansion of hypoechoic liquid, which reflected the distribution of LAs during injection, indicated a successful outcome. The same procedures were followed on the opposite side.
Propofol and remifentanil were terminated upon completion of skin closure. Dezocine (5 mg) and palonosetron (0.25 mg) were administrated intravenously 30 min prior to termination of the surgical procedure. The infusion of the cisatracurium was stopped ~30 min prior to the end of surgery. Upon completion of surgery, neuromuscular blockade was antagonized via intravenous administration of 1.0 mg neostigmine and 0.5 mg atropine. Propofol and remifentanil infusions were stopped at the end of surgery. Patients were extubated once the extubation criteria (T4/T1 ratio >0.9) were met, and the extubation time was recorded (15). The patients were subsequently sent to the ward, where they received nasal O2 supplementation and had their vital signs continuously monitored. The PCIA pump (WZ-6523C-4 Disposable Infusion Pump; Royal Fornia Medical Equipment Co., Ltd., Zhuhai, China) regimen consisted of 10 mg butorphanol. The PCIA pump was connected to the intravenous line. Settings included a basal infusion rate of 2 ml/h, 15 min lockout and self-controlled doses at 0.5 ml each.
Hemodynamic indexes (HR, BP and SpO2) were recorded at the following time-points: Arrival at the operating room (baseline, T0); induction (T1); intubation (T2); 5 min following TAP block (T3); 60 min following intubation (T4); at extubation (T5); and 1, 2, 6, 12 and 24 h post-surgery (T6-T10). All patients were sent to the ward once they met the recovery room criteria (15). Lactated Ringer's solution was tailored to the requirements of the patients during surgery. Clear fluids were allowed on the day of surgery and free fluids were allowed from the first postoperative day. Ambulation was encouraged on postoperative day 1 and the urinary catheter was removed when the patient was mobile. Patients were discharged from the hospital when the discharge criteria were met (15).
VAS scores were assessed at rest (VASR) and at movement (VASM) at T6-T10 by an investigator blinded to the group allocation. After surgery, the duration of sensory block was assessed via pinprick testing in the area of the TAP block (16). A 3-point scale was applied to evaluate the level of pain by pinprick with a 23 G needle (0, sharp sensation; 1, blunt sensation; 2, no sensation). The duration of sensory block was defined as the time from completion of TAP block to complete recovery of sensation. The duration of analgesia was defined as the time from completion of TAP block to first rescue analgesia (when the VAS score was ≥4 or on patient demand).
The following 5-point scale was applied to assess the level of sedation at T6-T10: Completely awake, 0; drowsy/closed eyes, 1; asleep/easily aroused with light tactile stimulation or a simple verbal command, 2; asleep/arousable only by strong physical stimulation, 3; unarousable, 4. The degree of nausea severity was evaluated using the following 4-point scale: None, 1; mild, 2; moderate, 3; severe, 4. Bradycardia (HR <50 beats/min), hypotension (BP <90/60 mmHg), somnolence (sedation score ≥3) and respiratory depression (SpO2 <90% or respiratory rate <10 bpm lasting 3 min or more) (17) were considered severe adverse events and were treated appropriately. Postoperative nausea and vomiting (PONV) following surgery was also recorded. When the VAS score was ≥4 or the patient demanded rescue analgesia, 5 mg dezocine was intravenously administered as rescue analgesia. Ondansetron (4 mg) was administrated intravenously for PONV when needed. At 48 h following surgery, a 3-point scale was used to evaluate the patient overall satisfaction with the postoperative pain management (1, highly satisfied; 2, satisfied; 3, dissatisfied). The time of bowel function recovery (passage of flatus or stool since the first time of oral fluid and normal diet consumption) post-surgery (15) and the length of hospital stay (LOS) were recorded.
Statistical analysis
All data in the present study were analyzed using SPSS version 16.0 (SPSS, Inc.). Data are presented as the mean ± standard deviation, and count (%). Patient parameters, including age, weight, body mass index (BMI), operation time, blood loss, infusion volume, urine output, duration of sensory block and analgesia, time of bowel function recovery and LOS were compared using an unpaired Student's t-test. HR and mean BP at different time-points were compared between the two groups using a one-way ANOVA, followed by Bonferroni's post hoc test. The male/female ratio, ASA grade, incidence of adverse events and degree of satisfaction were analyzed using the χ2 or Fisher's exact test. The Mann-Whitney U test was performed to compare pain and sedation scores between the groups. The present study considered that block duration differences for ≥60 min revealed the clinical significance between the two groups. To achieve a statistical power of at least 90% using ANOVA with a significance level of 0.05 and a predicted dropout of ~15%, at least 32 subjects were recruited in each group. P<0.05 was considered to indicate a statistically significant difference.
Results
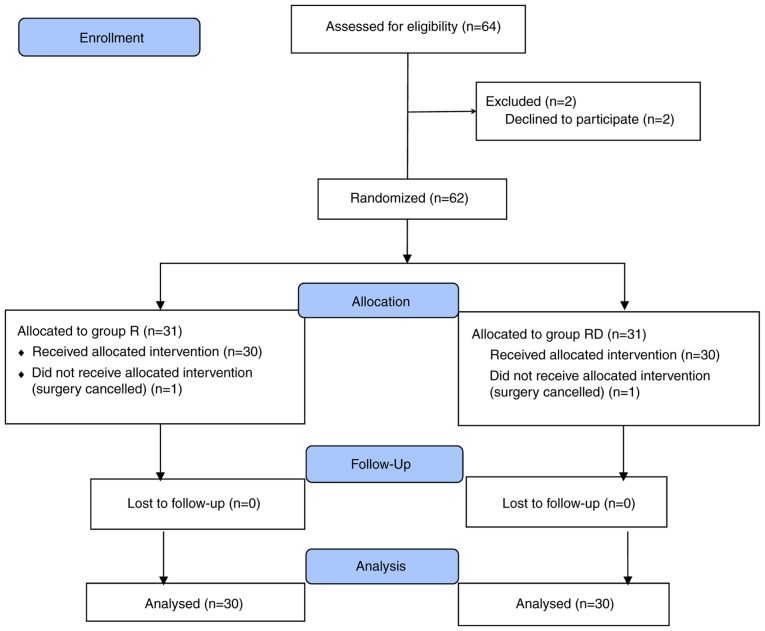
A total of 64 patients were assessed for eligibility. However, a total of 4 patients were excluded from the study as 2 patients refused to participate and 2 surgeries were cancelled. A total of 60 patients completed the trial (n=30 in each group; Fig. 1). TAP block was accurately localized under ultrasound guidance and blocks were successfully completed in all patients. In each group, no patients had any complications that were attributed to TAP block.
Figure 1.
CONSORT flow diagram indicating patient inclusion in the current study.
There were no significant differences between the two groups regarding the clinical characteristics of patients, including age, male/female ratio, ASA grade, weight, BMI and intraoperative data (Table I). In addition, hemodynamic variables were similar in both groups during the surgery. The BP and HR were slightly reduced following anesthesia induction in both groups. Furthermore, decreased HR was indicated in the RD group following TAP block; however, there was no significant difference between the two groups (Fig. 2).
Table I.
Clinical characteristics of patients in the R and RD groups.
| Characteristic | R Group (n=30) | RD Group (n=30) | P-value |
|---|---|---|---|
| Age | 61.5±12.3 | 60.2±13.5 | 0.698 |
| Male/female | 16/14 | 17/13 | 0.795 |
| ASA grade (II/III) | 22/8 | 20/10 | 0.573 |
| Weight (kg) | 69.2±15.6 | 71.3±14.4 | 0.593 |
| Height (cm) | 165.2±10.5 | 163.9±11.8 | 0.654 |
| BMI (kg/m2) | 23.3±4.8 | 23.6±5.1 | 0.815 |
| Operative time (min) | 172.6±52.5 | 178.8±48.6 | 0.637 |
| Blood loss (ml) | 187±69 | 192±94 | 0.814 |
| Infusion volume (ml) | 1738±421 | 1766±384 | 0.789 |
| Urine output (ml) | 596±156 | 622±143 | 0.504 |
Data are expressed as mean ± standard deviation. ASA, American Society of Anesthesiologists physical status score.
Figure 2.
HR and MBP at different time-points. (A) HR and (B) MBP. T0, baseline; T1, induction; T2, intubation; T3, 5 min following TAP block; T4, 60 min following intubation; T5, extubation; T6-T10, 1, 2, 6, 12 and 24 h post-surgery, respectively; MBP, mean blood pressure; HR, heart rate.
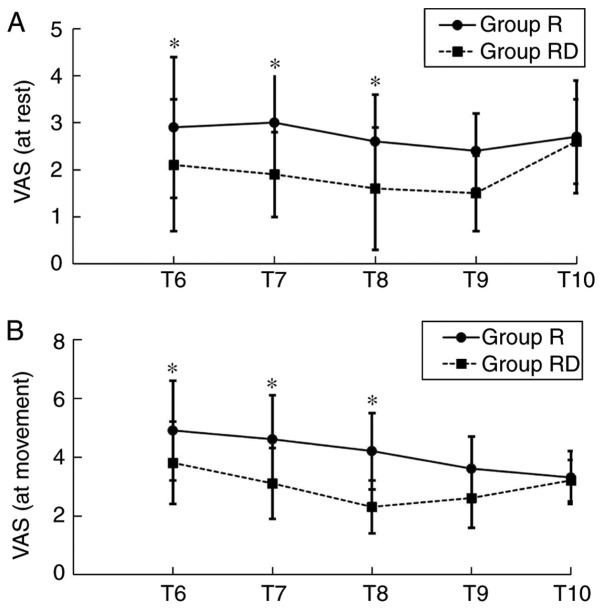
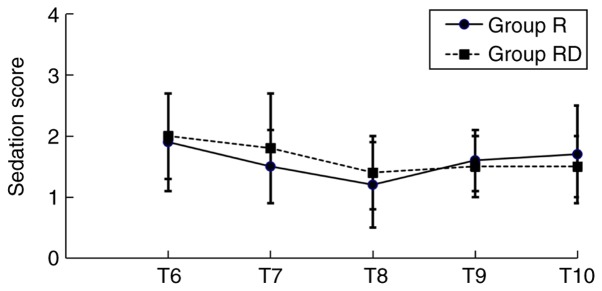
The duration of sensory block and analgesia was significantly increased in the RD group compared with the R group (P<0.05; Table II). However, the highest level of sensory block achieved was similar in both groups. VAS scores at T6-T9 in the RD group, either at rest or movement, were decreased compared with the Group R (Fig. 3). However, no significant difference was observed regarding the levels of sedation between the two groups at T6-T10. The majority of the patients in the present study exhibited a sedation grade ≤3 (Fig. 4).
Table II.
Duration of sensory block and analgesia (h).
| Duration measure | R Group (n=30) | RD group (n=30) | P-value |
|---|---|---|---|
| Sensory block (h) | 9.4±3.5 | 13.5±4.1 | <0.001 |
| Analgesia (h) | 6.4±3.8 | 10.2±4.3 | 0.001 |
Data are expressed as mean ± standard deviation. Duration of sensory block, time from completion of TAP block to complete recovery of sensation; Duration of analgesia, time from completion of TAP block to first rescue analgesia.
Figure 3.
VAS pain scores at different time-points. (A) VAS pain scores at rest and (B) at movement. *P<0.05. T6-T10: 1, 2, 6, 12 and 24 h post-surgery, respectively; VAS, visual analogue scale.
Figure 4.
Sedation scores at different time-points. T6-T10, 1, 2, 6, 12 and 24 h post-surgery, respectively.
In the RD group, bradycardia was observed in 5 patients (4 did not require treatments and 1 patient was treated with atropine). In the R group, bradycardia was observed in 2 patients who did not require treatments. Notably, the 0-24 h rates of PONV in the RD group were significantly decreased compared with the R group (P<0.05; Table III). During the postoperative period, no other side effects, including respiratory depression, hypoxemia, hypotension or somnolence, were indicated in either group.
Table III.
Patients' rates of PONV 0-24 h following surgery.
| Measurement | R Group (n=30) (%) | RD Group (n=30) (%) | P-value |
|---|---|---|---|
| Nausea | |||
| 0-24 h | 18(60) | 5 (16.7) | 0.001 |
| 0-4 h | 8 (26.7) | 2 (6.7) | 0.038 |
| 4-24 h | 10 (33.3) | 3(10) | 0.028 |
| Vomiting | |||
| 0-24 h | 17 (56.7) | 3(10) | 0.001 |
| 0-4 h | 8 (26.7) | 1 (3.3) | 0.026 |
| 4-24 h | 9(30) | 2 (6.7) | 0.023 |
Data are expressed as number (%). PONV, postoperative nausea and vomiting.
There were 90.0 and 66.7% patients who seemed satisfied with the postoperative pain management in the RD and R group, respectively (Table IV). The mean time of first passage of flatus and stool, and time of oral fluid and normal diet consumption was significantly reduced in the RD group compared with the R group (P<0.05). Furthermore, the LOS did not significantly differ between the study groups (Table V).
Table IV.
Patient overall satisfaction with anesthesia.
| Satisfaction | R Group (n=30) (%) | RD Group (n=30) (%) | P-value |
|---|---|---|---|
| Overall satisfied | 20 (66.7) | 27(90) | 0.028 |
| Very satisfied | 6 (20.0) | 10 (33.3) | 0.243 |
| Satisfied | 7 (23.3) | 12(40) | 0.165 |
| Moderately satisfied | 7 (23.3) | 5 (16.7) | 0.519 |
| Not satisfied | 10 (33.3) | 3(10) | 0.028 |
Data are expressed as number (%).
Table V.
Time to recovery of bowel function and hospital LOS.
| Recovery measure | R Group (n=30) | RD Group (n=30) | P-value |
|---|---|---|---|
| Time to first flatus (h) | 2.8±0.4 | 2.2±0.3 | 0.001 |
| Time to first stool (days) | 4.2±1.7 | 3.3±1.4 | 0.029 |
| Time to oral fluids (days) | 1.2±0.2 | 0.9±0.1 | 0.036 |
| Time to normal diet (days) | 3.6±0.8 | 2.9±1.1 | 0.007 |
| Hospital LOS (days) | 9.5±0.9 | 9.2±0.8 | 0.178 |
Data are expressed as mean ± standard deviation. LOS, length of stay.
Discussion
Based on the observations of the current study, it was suggested that adding 0.5 µg/kg Dex to ropivacaine in bilateral TAP block was able to prolong the analgesic duration and improve recovery in patients undergoing laparoscopic colectomy.
TAP block is an ultrasound-guided regional anesthetic technique used for analgesia, which is designed to block the abdominal wall neural afferents (T7-L1) by injection of a local anesthetic between the internal oblique and transversus abdominis muscles. Since first being proposed in 2001 by Rafi (18), TAP block has become increasingly popular. A meta-analysis of 9 studies, which included 413 patients, investigated the efficacy of TAP block. The study revealed that that TAP block reduced the postoperative morphine requirements, opioid associated side-effects and the severity of pain, and enhanced recovery in patients following abdominal surgery (19). Previous research has demonstrated that a single-shot TAP block improved post-cesarean analgesia and the duration of analgesia was limited when compared with intrathecal morphine (20). Many analgesic adjuvants, including opioids, epinephrine and α2 agonists (including clonidine), have been added to LAs in various peripheral nerve blocks to prolong the analgesic duration. A previous study of healthy volunteers revealed that epinephrine, as an adjuvant to TAP block, was effective in decreasing peak plasma concentrations of levobupivacaine, with limited value on block characteristics or duration (21). The addition of dexamethasone (8 mg) to 30 ml levobupivacaine (0.25%) in a TAP block for analgesia following a cesarean delivery prolonged effective analgesia duration, and decreased deep and superficial pain scores (22). In contrast, Bollag et al (23) reported no benefit of a TAP block with or without clonidine following cesarean delivery for a low-risk population receiving a spinal anesthetic with morphine and multimodal analgesia as the analgesic consumption and pain scores were low overall.
Dex is a commonly used sedative for anesthesia and intensive care medicine (24). The clinical effect of Dex as an adjuvant to LAs for peripheral nerve blocks has been investigated in experimental and clinical studies (25-28). The addition of Dex to LAs in various nerve blocks can increase the duration of block and improve analgesia effects, including ulnar nerve block, palatine nerve block, infraorbital nerve block, axillary brachial plexus and cervical plexus, which has been described in scientific literature and applied in the daily clinical practice (12,25-28). Previous studies have indicated that various doses of Dex (20 to 150 µg) can be added to LAs. Dex combined with ropivacaine produces a dose-dependent (50, 100 and 150 µg) prolongation of ulnar nerve sensory block and dose-dependent sedation (29). The addition of 0.75 µg/kg Dex to 0.5% levobupivacaine for supraclavicular plexus block shortens sensory and motor block onset time, and extends sensory block, motor block and analgesia duration (30). A dosage of 0.5 µg/kg Dex in bilateral TAP block was chosen in present study, which was based from data obtained in previous studies and on doses that have been proven safe for sedation (31). Furthermore, this dose was chosen due to concerns regarding potential dose-dependent adverse systemic events that have been associated with Dex. Dex has been broadly applied as an additive to LAs under clinical settings.
To the best of our knowledge, the impact of Dex with ropivacaine in TAP block for postoperative analgesia and recovery has not been reported. The current study, which performed the TAP's block prior to surgery, indicated that this method may be helpful in decreasing opioid dosage and stabilizing BP and HR during operation. Furthermore, patients were not indicated to feel pain under general anesthesia. The present study demonstrated that the addition of Dex to ropivacaine was able to prolong the analgesic duration and enhance analgesic effects. The mechanism of action of Dex in peripheral nerve blocks is not completely understood. A number of mechanisms have been proposed, including central analgesia, vasoconstriction and anti-inflammatory effects (32). Dalle et al (33) indicated that clonidine (an α-2 adrenoreceptor agonist) improves the sensory blockade by blocking the inhibiting hyperpolarization-activated cation (Ih) current, which enhances the level of hyperpolarization and inhibits subsequent action potentials. Other research has suggested that adding Dex to LAs increases the time required for analgesia by decreasing the release of norepinephrine and causing α-2 receptor independent inhibitory effects on nerve fiber action potentials (28). However, further studies are required to investigate the underling effects of Dex in TAP block.
A previous study suggested a balanced anesthesia with Dex decreased PONV following laparoscopic surgery via the addition of Dex to ropivacaine (34). This may be due to the antiemetic properties of Dex. Pain itself is considered a significant risk factor for PONV (34,35). Intense pain may result in PONV and enhanced analgesia in the RD group in turn decreased PONV.
One concern about the use of Dex in TAP block is that Dex may be systemically absorbed and induce bradycardia and excessive sedation (25-28). However, in the current study, the hemodynamic variables that were assessed did not reveal significant differences between two groups during or after surgery. Furthermore, there was no excessive sedation post-surgery and no significant difference in the levels of sedation between the two groups. This may be primarily due to lower doses of Dex used in the present study. Additionally, Dex may be absorbed slowly in the TAP, so the plasma concentration of Dex would be too low to induce bradycardia and excessive sedation.
In the present study, although the HR was not significantly different between the two groups following surgery, bradycardia was observed in 5 patients post-surgery in the RD group, which may be explained by systemic absorption and cannot be ignored. Bradycardia could potentially be life-threatening if not detected and treated in time (26,28). Therefore, when Dex is used as an adjuvant to ropivacaine, patients should be monitored during the operation for potential side effects, including bradycardia, hypotension and sedation. The lack of other side effects, including PONV and respiratory depression, make Dex an appealing adjuvant for the TAP block.
A study by Cheung et al (36) indicated that intraoperative Dex in colorectal surgery did not improve recovery in patients, which was due to inhibition of Dex on intestinal motility via the suppression of acetylcholine release. A few studies have investigated the quality of postoperative recovery following the use of TAP block (37). In the present study, TAP block with Dex and ropivacaine shortened the time to first passage of flatus and first stool, promoted bowel movement and increased patient satisfaction. Multiple factors are considered for recovery from surgery, including pain, and surgery-induced metabolic, endocrine and immune changes, which are also known as ‘stress responses’ (36,37). Acute postoperative pain will reduce movement motivation and restrict patients at a relatively ‘comfortable position’, which impairs the ability of responding to stress physically and mentally, and defer recovery. It has previously been speculated that improvement of recovery in the RD group was primarily associated with promoted analgesia following surgery. However, further investigations are required to verify this hypothesis. No significant difference was observed in the mean LOS between the two groups. It is well know that there are a number of factors (including the surgical floor capacity, discharge criteria and patient- and institution-associated factors) other than the difference in TAP block in the current study that have an influence on the LOS.
The onset time of TAP block was not assessed in the current study, since the procedure was performed when patients were under general anesthesia. Some trials have indicated that adding Dex to LAs reduces the onset time of peripheral nerve or plexus block (25,28). Therefore, it was speculated that adding Dex to ropivacaine could also reduce the onset time of TAP block. However, this requires further study. Additionally, a number of anatomical structures can cause pain following abdominal surgeries, and may be considered superficial and deep, or visceral and somatic. Different analgesic techniques may not indicate a similar pain-relieving effect on different causes of pain. The effect of TAP block on visceral pain that causes deep pain sensations may not be expected to be efficient. In the present study, even though a significant difference in pain score was observed between the two groups, visceral and somatic pain components were not separately assessed. Opioid dosage during and after operation was also not recorded, because this may be an important factor for PONV and bowel function recovery. The majority of postoperative parameters assessment were subjective, however, all parameters were assessed by an investigator blinded to the group allocation. Finally, a relatively low dosage of Dex was used in the present study. Therefore, there is a possibility that a prolongation may be observed if the dosage of Dex had been higher. Therefore, whether a higher dosage of Dex may have better analgesic efficacy of the TAP block should be the focus of future studies. Additional analgesic agents can impact VAS scores. If patients required additional analgesic agents (for example, at 1 h post-surgery), then it would be expected to have an impact on the VAS score at the 2 h time-point.
In conclusion, data from the present study demonstrated that adding Dex to ropivacaine could improve postoperative analgesia and the duration of TAP block, and promote recovery of bowel function following laparoscopic colectomy.
Acknowledgements
Not applicable.
Funding
The current study was financed by the Science and Technology Program Foundation of Yantai, China (grant no. 2019YD022).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
WP and GZL performed the study and also were major contributors in writing the manuscript. MJ and GGL performed the study and collected the data. TL and QS analyzed data. JM and HL designed the study and edited the manuscript. All authors approved the final manuscript.
Ethics approval and consent to participate
Approval was obtained from the Institutional Human Investigations Committee of Yantai Yuhuangding Hospital of Qingdao University. All patients provided written informed consent for participation.
Patient consent for publication
No applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: A meta-analysis. Anesth Analg. 2005;100:757–773. doi: 10.1213/01.ANE.0000144428.98767.0E. table of contents. [DOI] [PubMed] [Google Scholar]
- 2.Buvanendran A, Kroin JS. Multimodal analgesia for controlling acute postoperative pain. Curr Opin Anaesthesiol. 2009;22:588–593. doi: 10.1097/ACO.0b013e328330373a. [DOI] [PubMed] [Google Scholar]
- 3.McDonnell JG, O'Donnell B, Curley G, Heffernan A, Power C, Laffey JG. The analgesic efficacy of transversus abdominis plane block after abdominal surgery: A prospective randomized controlled trial. Anesth Analg. 2007;104:193–197. doi: 10.1213/01.ane.0000250223.49963.0f. [DOI] [PubMed] [Google Scholar]
- 4.Yu N, Long X, Lujan-Hernandez JR, Succar J, Xin X, Wang X. Transversus abdominis-plane block versus local anesthetic wound infiltration in lower abdominal surgery: A systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 2014;14(121) doi: 10.1186/1471-2253-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schoenmakers KP, Wegener JT, Stienstra R. Effect of local anesthetic volume (15 vs. 40 ml) on the duration of ultrasound-guided single shot axillary brachial plexus block: A prospective randomized, observer-blinded trial. Reg Anesth Pain Med. 2012;37:242–247. doi: 10.1097/AAP.0b013e3182405df9. [DOI] [PubMed] [Google Scholar]
- 6.Ilfeld BM. Continuous peripheral nerve blocks: A review of the published evidence. Anesth Analg. 2011;113:904–925. doi: 10.1213/ANE.0b013e3182285e01. [DOI] [PubMed] [Google Scholar]
- 7.Kirksey MA, Haskins SC, Cheng J, Liu SS. Local anesthetic peripheral nerve block adjuvants for prolongation of analgesia: A systematic qualitative review. PLoS One. 2015;10(e0137312) doi: 10.1371/journal.pone.0137312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerlach AT, Dasta JF. Dexmedetomidine: An updated review. Ann Pharmacother. 2007;41:245–252. doi: 10.1345/aph.1H314. [DOI] [PubMed] [Google Scholar]
- 9.Blaudszun G, Lysakowski C, Elia N, Tramer MR. Effect of perioperative systemic α2 agonists on postoperative morphine consumption and pain intensity: Systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2012;116:1312–1322. doi: 10.1097/ALN.0b013e31825681cb. [DOI] [PubMed] [Google Scholar]
- 10.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115:836–843. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–511. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marhofer D, Kettner SC, Marhofer P, Pils S, Weber M, Zeitlinger M. Dexmedetomidine as an adjuvant to ropivacaine prolongs peripheral nerve block: A volunteer study. Br J Anaesth. 2013;110:438–442. doi: 10.1093/bja/aes400. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi R, Shah A, Patel I. Use of dexmedetomidine along with bupivacaine for brachial plexus block. National J Med Res. 2012;2:67–69. [Google Scholar]
- 14.Hebbard P, Fujiwara Y, Shibata Y, Royse C. Ultrasound-guided transversus abdominis plane (TAP) block. Anaesth Intensive Care. 2007;35:616–617. [PubMed] [Google Scholar]
- 15.Ris F, Findlay JM Hompes R, Rashid A, Warwick J, Cunningham C, Jones O, Crabtree N, Lindsey I. Addition of transversus abdominis plane block to patient controlled analgesia for laparoscopic high anterior resection improves analgesia, reduces opioid requirement and expedites recovery of bowel function. Ann R Coll Surg Engl. 2014;96:579–585. doi: 10.1308/003588414X13946184900921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nader A, Doty R Jr, Brodskaia A, Kendall MC, McCarthy RJ. Sensory testing of distal sural and posterior tibial nerves provides early prediction of surgical anesthesia after single-injection infragluteal-parabiceps sciatic nerve block. Anesth Analg. 2010;110:951–957. doi: 10.1213/ANE.0b013e3181ca134b. [DOI] [PubMed] [Google Scholar]
- 17.Overdyk FJ, Carter R, Maddox RR, Callura J, Herrin AE, Henriquez C. Continuous oximetry/capnometry monitoring reveals frequent desaturation and bradypnea during patient-controlled analgesia. Anesth Analg. 2007;105:412–418. doi: 10.1213/01.ane.0000269489.26048.63. [DOI] [PubMed] [Google Scholar]
- 18.Rafi AN. Abdominal field block: A new approach via the lumbar triangle. Anaesthesia. 2001;56:1024–1026. doi: 10.1046/j.1365-2044.2001.02279-40.x. [DOI] [PubMed] [Google Scholar]
- 19.Hain E, Maggiori L, Prost À la Denise J, Panis Y. Transversus abdominis plane (TAP) block in laparoscopic colorectal surgery improves postoperative pain management: A meta-analysis. Colorectal Dis. 2018;20:279–287. doi: 10.1111/codi.14037. [DOI] [PubMed] [Google Scholar]
- 20.Kanazi GE, Aouad MT, Abdallah FW, Khatib MI, Adham AM, Harfoush DW, Siddik-Sayyid SM. The analgesic efficacy of subarachnoid morphine in comparison with ultrasound-guided transversus abdominis plane block after cesarean delivery: A randomized controlled trial. Anesth Analg. 2010;111:475–481. doi: 10.1213/ANE.0b013e3181e30b9f. [DOI] [PubMed] [Google Scholar]
- 21.Corvetto MA, Echevarria GC, De La Fuente N, Mosqueira L, Solari S, Altermatt FR. Comparison of plasma concentrations of levobupivacaine with and without epinephrine for transversus abdominis plane block. Reg Anesth Pain Med. 2012;37:633–637. doi: 10.1097/AAP.0b013e31826c330a. [DOI] [PubMed] [Google Scholar]
- 22.Akkaya A, Yildiz I, Tekelioglu UY, Demirhan A, Bayir H, Ozlu T, Bilgi M, Kocoglu H. Dexamethasone added to levobupivacaine in ultrasound-guided tranversus abdominis plain block increased the duration of postoperative analgesia after caesarean section: a randomized, double blind, controlled trial. Eur Rev Med Pharmacol Sci. 2014;18:717–722. [PubMed] [Google Scholar]
- 23.Bollag L, Richebe P, Siaulys M, Ortner CM, Gofeld M, Landau R. Effect of transversus abdominis plane block with and without clonidine on post-cesarean delivery wound hyperalgesia and pain. Reg Anesth Pain Med. 2012;37:508–514. doi: 10.1097/AAP.0b013e318259ce35. [DOI] [PubMed] [Google Scholar]
- 24.Carollo DS, Nossaman BD, Ramadhyani U. Dexmedetomidine: A review of clinical applications. Curr Opin Anaesthesiol. 2008;21:457–461. doi: 10.1097/ACO.0b013e328305e3ef. [DOI] [PubMed] [Google Scholar]
- 25.Obayah GM, Refaie A, Aboushanab O, Ibraheem N, Abdelazees M. Addition of dexmedetomidine to bupivacaine for greater palatine nerve block prolongs postoperative analgesia after cleft palate repair. Eur J Anaesthesiol. 2010;27:280–284. doi: 10.1097/EJA.0b013e3283347c15. [DOI] [PubMed] [Google Scholar]
- 26.Khandaitkar S, Kolte V, Shenoi SR, Budhraja N. A clinical study to determine the efficacy of 7ppm dexmedetomidine as an adjuvant to 2% lignocaine in infraorbital nerve block. Br J Oral Maxillofac Surg. 2016;54:997–1000. doi: 10.1016/j.bjoms.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Esmaoglu A, Yegenoglu F, Akin A, Turk CY. Dexmedetomidine added to levobupivacaine prolongs axillary brachial plexus block. Anesth Analg. 2010;111:1548–1551. doi: 10.1213/ANE.0b013e3181fa3095. [DOI] [PubMed] [Google Scholar]
- 28.Lin YN, Li Q, Yang RM, Mao ZX, Liu JC. Addition of dexmedetomidine to ropivacaine improves cervical plexus block. Acta Anaesthesiol Taiwan. 2013;51:63–66. doi: 10.1016/j.aat.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Brummett CM, Padda AK, Amodeo FS, Welch KB, Lydic R. Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology. 2009;111:1111–1119. doi: 10.1097/ALN.0b013e3181bbcc26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bisui B, Samanta S, Ghoshmaulik S, Banerjee A, Ghosh TR, Sarkar S. Effect of locally administered dexmedetomidine as adjuvant to levobupivacaine in supraclavicular brachial plexus block: Double-blind controlled Study. Anesth Essays Res. 2017;11:981–986. doi: 10.4103/aer.AER_55_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rancourt MP, Albert NT, Côté M, Létourneau DR, Bernard PM. Posterior tibial nerve sensory blockade duration prolonged by adding dexmedetomidine to ropivacaine. Anesth Analg. 2012;115:958–962. doi: 10.1213/ANE.0b013e318265bab7. [DOI] [PubMed] [Google Scholar]
- 32.Iskandar H, Benard A, Ruel-Raymond J, Cochard G, Manaud B. The analgesic effect of interscalene block using clonidine as an analgesic for shoulder arthroscopy. Anesth Analg. 2003;96:260–262. doi: 10.1097/00000539-200301000-00052. [DOI] [PubMed] [Google Scholar]
- 33.Dalle C, Schneider M, Clergue F, Bretton C, Jirounek P. Inhibition of the I(h) current in isolated peripheral nerve: A novel mode of peripheral antinociception? Muscle Nerve. 2001;24:254–261. doi: 10.1002/1097-4598(200102)24:2<254::aid-mus110>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 34.Massad IM, Mohsen WA, Basha AS, Al-Zaben KR, Al-Mustafa MM, Alghanem SM. A balanced anesthesia with dexmedetomidine decreases postoperative nausea and vomiting after laparoscopic surgery. Saudi Med J. 2009;30:1537–1541. [PubMed] [Google Scholar]
- 35.Schnabel A, Meyer-Frießem CH, Reichl SU, Zahn PK, Pogatzki-Zahn EM. Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta-analysis of randomized controlled trials. Pain. 2013;154:1140–1149. doi: 10.1016/j.pain.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 36.Cheung CW, Qiu Q, Ying AC, Choi SW, Law WL, Irwin MG. The effects of intra-operative dexmedetomidine on postoperative pain, side-effects and recovery in colorectal surgery. Anaesthesia. 2014;69:1214–1221. doi: 10.1111/anae.12759. [DOI] [PubMed] [Google Scholar]
- 37.Bekker A, Haile M, Kline R, Didehvar S, Babu R, Martiniuk F, Urban M. The effect of intraoperative infusion of dexmedetomidine on the quality of recovery after major spinal surgery. J Neurosurg Anesthesiol. 2013;25:16–24. doi: 10.1097/ANA.0b013e31826318af. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.