Abstract
As cells replicate their DNA during mitosis, telomeres are shortened due to the inherent limitations of the DNA replication process. Maintenance of telomere length is critical for cancer cells to overcome cellular senescence induced by telomere shortening. Telomerase reverse transcriptase (TERT) is the rate-limiting catalytic subunit of telomerase, an RNA-dependent DNA polymerase that lengthens telomeric DNA to maintain telomere homeostasis. TERT promoter mutations, which result in the upregulation of TERT transcription, have been identified in several central nervous system (CNS) tumors, including meningiomas, medulloblastomas, and primary glial neoplasms. Furthermore, TERT promoter hypermethylation, which also results in increased TERT transcription, has been observed in ependymomas and pediatric brain tumors. The high frequency of TERT dysregulation observed in a variety of high-grade cancers makes telomerase activity an attractive target for developing novel therapeutics. In this review, we briefly discuss normal telomere biology, as well as the structure, function, and regulation of TERT in normal human cells. We also highlight the role of TERT in cancer biology, focusing on primary CNS tumors. Finally, we summarize the clinical significance of TERT promoter mutations in cancer, the molecular mechanisms through which these mutations promote oncogenesis, and recent advances in cancer therapies targeting TERT.
Keywords: central nervous system tumors, telomerase promoter mutations, TERT
One hallmark of cancer is the ability of cancer cells to replicate indefinitely.1 This replicative potential runs counter to Hayflick’s rule, which imposes a limit on the replicative potential of a normal cell.2 This limitation is a result of the directionality of DNA polymerase, which causes shortening of chromosome ends after each replicative cycle, leading to cellular senescence. The addition of telomeres to the ends of chromosomes allows for successive replicative cycles without chromosomal DNA shortening. Telomeres, which consist of noncoding TTAGGG nucleotide repeats, protect the ends of chromosomes from deterioration and end fusion to other chromosomes.3,4 Telomeres are protected from DNA damage by binding to proteins that create a shelterin complex.4 The telomerase enzyme is an RNA-dependent DNA polymerase that lengthens telomeric DNA to maintain telomere homeostasis.5 Telomerase reverse transcriptase (TERT) is the rate-limiting catalytic subunit of telomerase.6
Telomerase activity is highly regulated. In most somatic cells with finite proliferative potential, telomerase activity is downregulated through epigenetic regulation of the TERT promoter.7,8 Telomeres in these somatic cells shorten with successive mitoses, ultimately triggering cellular senescence.9 In contrast, in stem cells and proliferative cells of self-renewing tissues, telomerase activity is not downregulated and counteracts the shortening of telomeres, allowing for increased replicative potential.
Cancer cells must maintain telomere length to circumvent cellular senescence. Telomerase upregulation can be found in 90% of malignancies, although the mechanism of activation is not always known.9 One well-known mechanism, upregulation of TERT transcription, often occurs through TERT promoter mutations. Such mutations have been found in meningioma, glioblastoma, medulloblastoma, and non-central nervous system (CNS) cancers.9–12 Another well-described mechanism of TERT upregulation is TERT promoter methylation, which paradoxically results in increased TERT expression in glioblastoma, ependymoma, and medulloblastoma, along with several non-CNS cancers.13–17
In this review, we outline normal telomere biology and regulation of TERT. We discuss the mechanisms of TERT upregulation and its role in cancer, focusing on glioblastoma and other CNS tumors. We also describe the clinical and prognostic relevance of TERT mutations in CNS tumors. Finally, we discuss therapies that might target cancers with aberrant TERT upregulation.
Normal Telomere Biology
Telomeres span approximately 10–20 kb at the end of human chromosomes.3,4,18 The noncoding repeats in telomeres bind proteins that form the shelterin complex.3,4,18 Telomeres also consist of a 150–200 nucleotide, G-rich, single-stranded overhang, which ends with a 3′-OH group that is recognized by TERT.3,4 This single-stranded overhang is protected from the DNA double-strand break repair machinery by folding back on itself to form the so-called T-loop and by recruitment of the shelterin complex.3,18 The formation of this nucleoprotein structure protects chromosome ends from nonhomologous end joining and regulates the access of telomerase to telomeres.
Telomeres shorten with DNA replication due to an inability to fill in the gap with complementary DNA on the 5′-end of the DNA strand after the RNA primer is removed during replication, termed the “end replication problem.” 19 The loss of telomeric repeats with successive replication cycles leads to an inability of the telomere to form the T-loop or recruit the shelterin complex. This causes a loss of chromosomal protection that leads to the formation of end-to-end chromosomal fusions and loss of cell viability.3
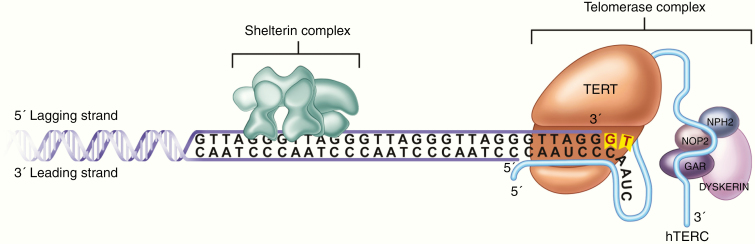
A human telomere contains enough repeats to withstand the loss of length for approximately 50–90 replication cycles in the absence of telomere elongation mechanisms.4 Most cells undergoing continuous division, such as stem cells, overcome the end replication problem by expressing telomerase. The telomerase complex, which is comprised of TERT and an RNA molecule encoded by the telomerase RNA component (TERC) gene, binds to telomeres at the end of the single-telomere overhang.3 Once TERT is bound, its enzymatic activity elongates the telomere, thus allowing the cell to undergo additional DNA replication cycles (Figure 1).
Fig. 1.
Telomere elongation. After recruitment to the telomere by the shelterin complex, TERT catalyzes the addition of the telomere repeats using the template provided by TERC. This counteracts the shortening of chromosomal telomeres in the setting of DNA replication.
Alternative lengthening of telomeres (ALT) is another mechanism of telomere elongation. While most immortal cell lines exhibit telomerase activity, some cell lines, including 10% of cancer lines, exhibit no telomerase activity but still have elongated telomeres as a result of ALT.20 Although the details have not been elucidated, it is postulated that lengthening by ALT occurs by using recombination to copy telomeres from other chromosomes or from the same chromosome.21,22 Whole exome sequencing of glioblastomas with wildtype IDH and TERT promoter identified an ALT positive subgroup of tumors with ATRX or SMARCAL1 mutations. These mutations are mutually exclusive and confer a similar overall survival to TERT promoter mutations, suggesting that ALT plays an important role in glioblastoma.23
More recently, telomeres have been found to have functions other than DNA end protection, such as regulation of gene expression through transcriptional silencing of genes.24 Although the mechanism is poorly understood, the conformation of telomeric DNA, including structures such as the T-loop, is also thought to contribute to telomere function. Additionally, RNA transcribed from telomeric DNA, so-called telomeric repeat-containing RNA, has been implicated in a variety of processes such as regulation of telomerase, heterochromatin organization at telomeres, and regulation of DNA expression.4,24
TERT Structure, Function, and Regulation
The TERT protein is comprised of 4 domains: the telomerase essential N-terminal domain (TEN), the reverse transcriptase domain (RT), the telomerase RNA-binding domain (TRBD), and the C-terminal extension domain.4,25 The TEN domain binds RNA and telomeric DNA and contributes to catalysis.26 The RT domain contains an insertion in the finger domain that distinguishes it from other reverse transcriptases.25,26 TERT binds the TERC-encoded RNA in the TRBD to form the telomerase ribonucleoprotein.
TERT is located at chromosome 5p15.33 in humans, while TERC is located at chromosome 3q26.24 TERC provides the template for synthesis of telomeric repeats, whereas TERT is the catalytic component of telomerase. The presence of TERT and TERC alone is sufficient for telomere elongation in vitro, but in vivo function also requires other components that serve additional roles, such as regulating attachment to telomeres and trafficking of telomerase components into the nucleus.4,24,27
The TERT gene is 40-kb long and consists of 15 introns and 16 exons with a 260-bp promoter core. The TERT promoter region contains GC boxes that bind the zinc finger transcription factor SP1, which increases TERT transcription, and E-boxes that bind both transcriptional enhancers and repressors.4,7,24 The GC-rich areas around the transcription start site are also regions of epigenetic regulation through DNA methylation and chromatin remodeling.4
TERT Dysregulation in Cancer
TERT Promoter Mutations
Analysis of data from metastatic melanoma patients by Huang et al.28 identified 2 recurrent C>T mutations in the TERT promoter region -124 (C228T) and -146 (C250T) base pairs from the transcriptional start site in 71% of patients. Subsequent screening for TERT promoter mutations found varying prevalence among different cancers (Table 1).29TERT promoter mutations are associated with increased TERT expression and telomerase activity.10 Both commonly observed mutations (C228T and C250T) generate identical de novo binding sites for the E-twenty-six (ETS) family of transcription factors.28 Even though there are 27 ETS transcription factors that all bind to similar sequences, only GA Binding Protein Transcription Factor Subunit Alpha (GABPA) selectively binds the mutant TERT promoter and not the wildtype version in several cancers.9,30 GABPA functions as a homodimer or as a heterotetramer with GABPB. The TERT promoter mutant sites cooperate with native ETS sites to recruit GABPA/B as a heterotetramer and activate TERT transcription.30 In contrast, a study using melanoma cell lines found only minimal binding of the GABPA homodimer to the mutant TERT promoter.31
Table 1.
Summary of the Frequency of TERT Promoter Mutations in Central Nervous System Tumors
| Cancer Type | Mutation Frequency (%) |
|---|---|
| CNS cancers | |
| Primary glioblastoma | 70–80%12 |
| Secondary glioblastoma | 30–50%12 |
| Astrocytoma | 30–40%12 |
| Oligoastrocytoma | 35–55%12 |
| Medulloblastoma | 19.8%11 |
| Meningioma | 7.4%11 |
| Ependymoma | 3%12 |
| Skin cancer—non-melanoma | |
| Atypical fibroxanthoma | 93%11 |
| Pleomorphic dermal sarcoma | 76%11 |
| Squamous cell carcinoma | 60%11 |
| Basal cell carcinoma | 47.4%11 |
| Merkel cell carcinoma | 10.2%11 |
| Melanomas | |
| Non-acral melanoma | 35–80%12 |
| Conjunctival melanoma | 30–40%12 |
| Mucosal melanoma | 10–15%12 |
| Acral melanoma | 5–10%12 |
| Uveal melanoma | 0–1%12 |
| Thyroid cancers | |
| Poorly differentiated thyroid carcinoma | 42.9%11 |
| Anaplastic thyroid carcinoma | 39.2%11 |
| Follicular thyroid carcinoma | 17.1%11 |
| Atypical follicular thyroid adenoma | 16.7%11 |
| Hürthle cell carcinoma | 13.1%11 |
| Differentiated thyroid carcinoma | 12.1%11 |
| Papillary thyroid carcinoma | 11.0%11 |
| Gynecological cancers | |
| Ovarian clear cell carcinoma | 15.9%11 |
| Squamous cell carcinoma of the cervix | 9.9%11 |
| Endometrial carcinoma | 6.6%11 |
| Ovarian low-grade serous carcinoma | 4.9%11 |
| Urological cancers | |
| Micropapillary urothelial carcinoma | 100%12 |
| Squamous cell carcinoma of the bladder | 80%12 |
| Infiltrating urothelial carcinoma with glandular differentiation | 72%12 |
| Urothelial carcinoma | 60–70%12 |
| Renal pelvic carcinoma | 45%12 |
| Ureter carcinoma | 19%11 |
| Urethral carcinoma | 15–20%12 |
| Inverted urothelial papilloma | 15%12 |
| Chromophobe renal cell carcinoma | 13%12 |
| Clear cell renal cell carcinoma | 5–10%12 |
| Renal cell carcinoma, unclassified | 8%12 |
| Digestive system cancers | |
| Combined hepatocellular cholangiocarcinoma | 45%12 |
| Hepatocellular carcinoma | 41.2%11 |
| Gallbladder cancer | 4%12 |
| Intrahepatic cholangiocarcinoma | 0–5%12 |
| Esophageal squamous cell carcinoma | 0–2%12 |
| Gastric cancer | 0%11 |
| Pulmonary cancers | |
| Malignant pleural mesothelioma | 15.2%11 |
| Lung and non-small cell lung carcinomas | 0–2%11 |
| Head and neck cancers | |
| Laryngeal carcinoma | 27.2%11 |
| Squamous cell carcinoma of head and neck | 16.3%11 |
| Soft tissue cancers | |
| Myxoid liposarcoma | 69.4%11 |
| Chondrosarcoma | 50%†11 |
| Fibrosarcoma | 33.3%†11 |
| Solitary fibrous tumor | 24.1%11 |
†signifies sample size less than 5.
Increased TERT transcription in the setting of TERT promoter mutation has also been found to occur in an NF-κβ-dependent manner. Unlike the C228T mutation, the C250T mutation creates a binding site for the NF-κβ subunit p52, allowing for the cooperative binding of NF-κβ and ETS1/2 to activate transcription.32 Thus, despite having similar downstream effects, the 2 canonical TERT promoter mutations may distinctly alter TERT expression.
TERT Promoter Mutations in CNS Neoplasms
TERT promoter mutations have been identified in CNS tumors, including primary glial neoplasms (up to 80%), medulloblastomas (20%), and meningiomas (6.4–11%).29,33–40 As with other cancers, the 2 most common promoter mutations are C228T and C250T, which occur in a mutually exclusive fashion. The mutation frequency varies by subtype for each of these tumors.
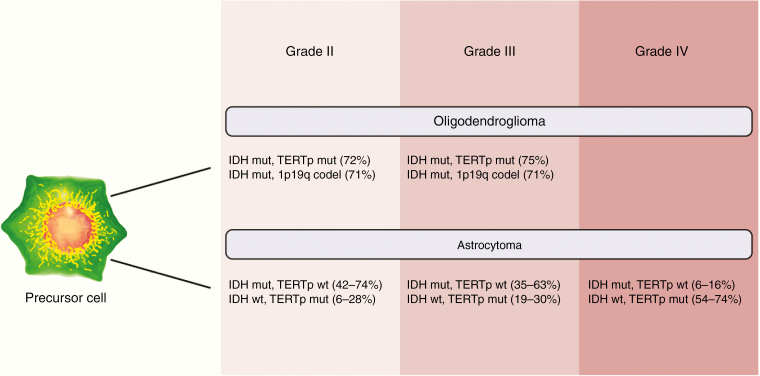
In adult primary glial neoplasms, the frequency of TERT promoter mutations varies by WHO grade (Figure 2). Glioblastoma (WHO grade IV) has a high frequency of mutations (80%). WHO grade II and III lesions, such as oligodendrogliomas, have a relatively lower frequency of TERT mutations (60–70%), and WHO grade I tumors, such as astrocytomas, have the lowest frequency of TERT mutations (30–40%).29,33,37,39 In patients with primary glial neoplasms, particularly glioblastoma, TERT promoter mutations were associated with co-mutations in epidermal growth factor receptor (EGFR), CDKN2A/B, and phosphatase and tensin homolog.40 Data from multi-sector sequencing of glioblastoma tumors revealed TERT promoter mutations to be clonal in all tumor sectors, suggesting that these mutations represent an early oncogenic event in this cancer.41,42
Fig. 2.
Frequency of TERT promoter mutation, IDH mutation, and 1p19q co-deletion in glioma. The frequency of TERT promoter mutations (TERTp mut) increases with increasing grade of primary glial neoplasms. The presence of TERT promoter mutations in combination with other commonly observed genetic abnormalities in gliomas, such as IDH mutation (IDH mut) and chromosome 1p19q co-deletion (1p19q codel), has both diagnostic and prognostic implications. Frequency of selected clinically relevant genomic co-alterations are shown here.33,43,44
In patients with meningiomas, TERT promoter mutations are associated with higher grade lesions (1.7% WHO grade I, 5.7% WHO grade II, and 20% WHO grade III) and with advancing tumor grade upon recurrence.35,38 In patients with medulloblastoma, although the overall mutation frequency is reported as 20%, the frequency of TERT promoter mutations in the wingless (WNT) and sonic hedgehog (SHH) subtypes has been reported to be as high as 31% and 83%, respectively.33,36
TERT Promoter Hypermethylation
TERT promoter hypermethylation has more recently been identified as an additional contributor to TERT dysregulation in cancer cells.13–17 Promoter hypermethylation may represent an alternative means of TERT upregulation or may work in conjunction with TERT promoter mutations to increase TERT expression in cancer cells. Hypermethylation of the promoter region would typically be expected to result in transcriptional silencing. However, in the case of the TERT promoter, a region termed the TERT hypermethylated oncological region (THOR) has been identified as a site of methylation that causes an increase in TERT expression when hypermethylated. THOR is a 433-bp region that contains 52 CpG sites located immediately upstream of the TERT core promoter.17 Importantly, hypermethylation of THOR results in increased TERT expression regardless of TERT promoter mutation status. Furthermore, when THOR is unmethylated, the activating effects of TERT promoter mutations appear to be repressed compared to when THOR is hypermethylated.17 These findings suggest that THOR hypermethylation represents an independent mechanism of increased TERT expression from TERT promoter mutations.
Remarkably, the frequency of THOR hypermethylation may be as high as the frequency of TERT promoter mutations in some cancers. Indeed, more than 70% of prostate, breast, hematological, and colon cancers are characterized by THOR hypermethylation.17 In general, the frequency of THOR hypermethylation appears to be greater in cancers with a low frequency of TERT promoter mutations. Nonetheless, THOR hypermethylation has been observed with relatively high frequency even in cancers with a high frequency of promoter mutations and has been found to be approximately 63% in CNS tumors.17
Impact of TERT Dysregulation in Cancer Cells
All cancer cells acquire molecular aberrations that confer the properties of replicative immortality, sustained proliferative signaling, evasion of growth suppressors, resistance to cell death, angiogenesis, invasion, and metastasis.1,45 Telomere length plays a complex role in oncogenesis. Critical shortening of telomeres results in increased rates of oncogenic chromosomal alterations such as deletions and chromosomal end fusions. At the same time, the shortening of telomeres activates the normal regulatory pathways that result in cellular senescence or even cell death. In 90% of cancers, telomerase upregulation is critical to achieve proliferative immortality.4,20,24 The TERT subunit catalyzes the rate-limiting step of telomerase, making the TERT gene a logical target for upregulation in cancer cells. Indeed, Hahn et al.46 demonstrated that ectopic expression of TERT, in addition to key oncogene expression and tumor suppressor dysfunction, is required for the transformation of human somatic cells into tumorigenic cells.
Analysis of 1230 tumors from 60 cancer types by Killela et al.29 found 2 distinct groups of cancers—those with a high (≥15%) or a low (<15%) frequency of TERT promoter mutations. The 9 cancers in the high-frequency group all originated from tissues with low self-renewal and included tumors such as medulloblastoma and primary gliomas. This finding led to the hypothesis that TERT promoter mutations primarily benefit cancer cells originating from tissues with low self-renewal potential.8 In cancers originating from the hematopoietic system or gastrointestinal tract, for example, TERT promoter mutations would be predicted to have less of an impact on TERT expression since these tissues already express TERT. Accordingly, the frequency of TERT promoter mutations in these cancers is low.8,10,29 In contrast, cancers arising from cells with low replicative potential that do not express TERT, such as astrocytes or melanocytes, would gain a replicative advantage over other cells via aberrant overexpression of TERT resulting from promoter mutations.10,28,29,40,47 Although this explanation is corroborated by the relative frequency of these mutations observed in several cancers, there are some notable exceptions, including non-melanoma skin cancers, which have a high frequency, of TERT promoter mutations.48 These exceptions suggest the possibility that an oncologic advantage may be conferred through alternative functions of the TERT protein or of telomeres themselves.
Indeed, while TERT promoter mutations are associated with increased telomerase expression in some cancers, there is growing evidence that TERT promoter mutations are not always associated with longer telomeres.39 In human fibroblast cell lines that maintain long telomeres through ALT, the expression of oncogenic H-RAS led to inefficient tumorigenicity. However, overexpression of TERT in addition to H-RAS resulted in robust malignant transformation of ALT fibroblasts, suggesting that TERT may have telomere-lengthening-independent roles in tumor initiation and/or maintenance.49 Additionally, Chiba et al.50 found that during malignant transformation in melanoma, TERT promoter mutation-driven telomerase expression does not prevent telomere shortening. Findings such as these have led to the hypothesis that increased telomerase expression may allow for the persistence of genetically unstable clones by maintaining telomeres just above a critically short length, thus avoiding cellular senescence while also promoting rapid disease evolution.
TERT may also have functions entirely independent of telomere biology. For example, TERT is capable of performing RNA-dependent RNA polymerase (RdRP) functions via its association with BRG1, an SWI/SNF-related chromatin remodeling protein, and nucleostemin, a GTP-binding protein, forming the TERT–BRG1–NS complex (TBN complex). The TBN complex synthesizes double-stranded RNAs that are processed into short-interfering RNAs that regulate heterochromatin assembly and mitotic progression.51 Inhibitors targeting these non-canonical functions of TERT serve as promising anticancer agents. One such inhibitor, Eribulin, that targets the RdRP activity of TERT is discussed in a later section.
Additionally, in an in vitro model, TERT knockout somatic cells were reprogrammed into pluripotent stem cells by the introduction of an enzymatically inactive TERT mutant, suggesting that TERT may maintain a stem-like cell state independently of telomere lengthening.52 Potential mechanisms of this function include direct modulation of the Wnt/β-catenin signaling pathway and increased EGFR expression.53,54 Finally, TERT may promote invasion and metastasis of cancer cells by altering the expression of extracellular matrix remodeling and epithelial-to-mesenchymal transition related genes.55–57
Clinical Significance of Aberrant TERT Expression
TERT promoter mutations occur in specific clinical and phenotypic subtypes of various cancers. They are found mainly in adult rather than pediatric tumors and are sometimes associated with more aggressively behaving subtypes of cancers including melanomas, gliomas, meningiomas, and hepatocellular carcinomas.4,33,38,40 Given the normal age-dependent shortening of telomeres, it is unsurprising that TERT promoter mutations are associated with increased age at diagnosis for these cancers. The clinical impact of these mutations has been most extensively studied in melanomas, where TERT promoter mutations were found to be associated with markers of poor prognosis, such as increased Breslow thickness and metastasis, and with poor overall survival and disease-free progression.4,29,47 The clinical impact of these mutations has similarly been studied in primary CNS tumors, including gliomas and meningiomas.
Prognostic Impact of TERT Promoter Mutations on Gliomas
Several genetic aberrations that modify prognosis have been discovered in gliomas. Mutations in isocitrate dehydrogenase 1 (IDH1) are associated with lower histological grade, prolonged progression-free survival, and increased response to chemoradiotherapy.58 These mutations tend to also be associated with secondary gliomas, which progress from an antecedent lower grade to higher grade disease.37,59 Meanwhile, 1p and 19q co-deletions are associated with better survival in oligodendrogliomas and also correlate with IDH1 mutations.60Methylguanine methyltransferase (MGMT) promoter methylation is yet another critical determinant of prognosis and treatment response. Glioblastomas with MGMT promoter methylation have been shown to have a more favorable treatment response to alkylating agents with a resultant survival benefit.61 The various combinations of these genetic abnormalities have identified subtypes of gliomas with clinically distinct behavior. Factoring the presence of TERT promoter mutational status into these combinations may allow for better characterization of gliomas, with implications for precision medicine.
TERT promoter mutation frequency increases with increasing WHO grade in gliomas (Figure 2).29,37 Across all subtypes of glioma, TERT promoter mutations are independently associated with worse overall survival.37,62,63 The frequency of mutations is inversely correlated with IDH1 mutation in all gliomas but positively correlated with 1p and 19q co-deletions in oligodendrogliomas.
The presence of TERT promoter mutations in combination with IDH1 mutations changes overall survival in glioma patients. Gliomas with TERT promoter mutations without IDH1 mutation have worse overall survival than those with wildtype TERT promoter and IDH1 mutation. Meanwhile, gliomas with wildtype TERT promoter and wildtype IDH1 have an intermediate overall survival. Paradoxically, gliomas with both TERT promoter mutation and IDH1 mutation have improved overall survival compared to patients with wildtype TERT promoter and IDH1 mutation.37,62,63 Interestingly, concomitant mutations in TERT and IDH1 occur at nearly the same frequency as concomitant mutations in IDH1 and 1p and 19q co-deletions, providing further insight into the molecular etiology of oligodendrogliomas.64 Therefore, the improved survival in this group of patients is not a result of biological interaction between these mutations but rather identification of a lower grade glioma.37,63 This is corroborated by the fact that the small group of gliomas with co-mutations in TERT promoter and IDH1 without 1p and 19q co-deletions correspond to gliomas with aggressive behavior and survival similar to those with TERT promoter mutations and IDH1 wildtype.37,62,63
The important role of TERT promoter mutations on prognosis and clinical behavior is highlighted by the recently published cIMPACT-NOW update 3, which recommends that IDH wildtype grade II and III infiltrating astrocytomas with TERT promoter mutations, EGFR amplification, and/or chromosome 7 gain and 10 loss be graded as WHO grade IV.65 Although not currently graded as WHO grade IV tumors, these tumors have clinical, histopathological, and radiographic behavior similar to WHO grade IV tumors.63,66 Appropriate stratification of patients based on the genetic profile of their gliomas is thus critically important in informing how aggressively they should be treated. To this end, Shankar et al.67 developed a rapid intraoperative genotyping assay to detect TERT promoter and IDH1 mutational status in WHO grade II and III gliomas. This intraoperative molecular information has the potential to aid in real-time surgical decision making and may allow for precision therapy at the time of surgery.
Prognostic Impact of TERT Promoter Mutations on Other Primary CNS Tumors
TERT promoter mutations are associated with higher grade meningiomas.38 Across all grades of meningioma, the mutation has been associated with a more aggressive natural history. Grade I tumors with the mutation were associated with a higher rate of recurrence with increased risk of progression to a higher grade in recurrent tumors. Analysis of recurrent meningiomas without malignant progression revealed a lack of the TERT mutation compared to recurrent meningiomas with malignant progression.35 Similarly, grade II and III tumors with TERT mutations were associated with higher recurrence rates as well as shorter time to progression and/or recurrence.38,42 In high-grade meningiomas, the TERT promoter mutation was associated with worse overall survival (2.7 years vs 10.8 years).42
The TERT promoter mutation is the most common recurrent somatic point mutation observed in medulloblastomas and occurs primarily in adults with the SHH and WNT subtypes.34,36 The distribution of promoter mutation frequency among the various subtypes of medulloblastoma is not surprising given that the SHH subtype is the most common subtype in adult medulloblastoma patients and, as observed with other cancers, the mutation has a higher frequency among older patients. Interestingly, unlike with other cancers, TERT promoter mutations in SHH medulloblastomas are associated with a more favorable overall survival and with a lower incidence of metastatic disease on presentation. Group 4 medulloblastomas with the TERT promoter mutation follow the pattern observed in gliomas and meningiomas, with worse overall survival than those with wildtype TERT promoter.34 In the WNT and group 3 medulloblastoma subtypes, the TERT promoter mutation does not appear to make a difference in overall survival. While the underlying biology explaining these survival differences is poorly understood, stratification of patients based on mutation status can inform discussions of prognosis and targeted therapeutics as they become available in the future.
TERT as a Therapeutic Target
The high frequency of TERT promoter mutations in cancers makes telomerase activity an attractive target for the development of therapeutic interventions. Additionally, normal cells typically have lower telomerase activity and maintain longer telomeres than cancer cells, allowing cancer cells to be preferentially targeted by telomerase-inhibiting therapies. Despite this, there are challenges to targeting TERT in the treatment of cancers. Telomere shortening to a length that induces cell death requires multiple cell cycles after TERT inhibition is initiated. This results in a lag to the response of these therapies during which time tumor burden increases. Furthermore, since TERT is normally highly expressed in rapidly dividing cells, targeting TERT can have side effects, particularly on the hematopoietic system.24 One strategy to overcome these challenges may involve the use of other, more immediately acting treatments in conjunction with TERT-based therapies.
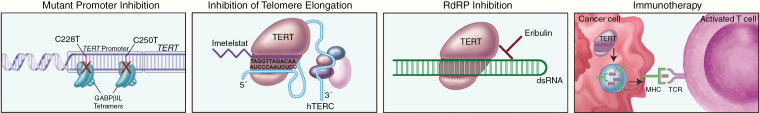
To date, no FDA-approved treatments targeting telomerase are available, but approaches including use of small molecule inhibitors, antisense oligonucleotides, immunotherapy, and G-quadruplex stabilizers are being investigated, with some therapies in various clinical trial phases.24 Promising approaches to TERT targeting include use of the small molecular inhibitor imetelstat (GRN163L), immunotherapy, GABPβ1L inhibition, as well as targeting RdRP activity of TERT using eribulin (Figure 3).
Fig. 3.
Treatment strategies for TERT promoter mutant tumors. Various treatment strategies taking advantage of the presence of TERT promoter mutations have been proposed, including targeting the GABPβ1L tetramers that bind to the mutant promoter sites as transcription factors, blocking the TERT active site using small molecule inhibitors such as Imetelstat that bind to TERC, inhibition of RNA-dependent RNA polymerase (RdRP) activity with small molecule inhibitors such as Eribulin, and vaccine therapy targeting cells with increased TERT expression.
Imetelstat
Imetelstat is the only small molecule inhibitor of TERT to be evaluated in clinical trials.24 Imetelstat is a 13-bp RNA oligonucleotide with a thio-phosphramidate backbone that allows for the formation of RNA duplexes.68 The molecule also contains a lipid moiety that increases cellular uptake and retention.69 Unlike traditional antisense oligonucleotides, imetelstat was not designed to bind to mRNA but rather to bind to TERT.24 In vitro studies demonstrated telomerase inhibition with concomitant telomere shortening in several cell lines including tumors of the bladder, breast, lung, liver, prostate, and pancreas. In vivo studies using mouse xenograft lung cancer models showed similar results, in addition to reduced tumor growth and metastasis and increased sensitivity to chemotherapy.24,70
In vitro studies of a glioblastoma cell line by Marian et al.71 found that imetelstat produced a dose-dependent inhibition of telomerase, with long-term treatment leading to progressive telomere shortening, decreased proliferation, and cell death in glioblastoma tumor-initiating cells. Furthermore, combining treatment with radiation and temozolomide reduced cancer cell survival and enhanced activation of the DNA damage response pathway. In vivo studies using mouse xenograft glioblastoma models revealed that imetelstat is able to cross the blood-brain barrier with 70% inhibition of telomerase activity and reduced growth of subcutaneously implanted tumors. While some imetelstat clinical trials have been completed, some have been suspended due to hematological toxicity.24 A phase 2 clinical trial investigating imetelstat use for recurrent pediatric primary glial neoplasms showed 95% inhibition of intratumoral telomerase activity, but also showed significant hematological toxicity and was halted due to 2 patient deaths related to intratumoral intracranial hemorrhage.72
Immunotherapy
Cancer cells express protein fragments of telomerase as cell surface antigens via the human leukocyte antigen (HLA) class I pathway.73,74 These epitopes can be targeted by cytotoxic T cells to kill cancer cells. The goal of anti-telomerase immunotherapy is to sensitize the immune system to tumor cells expressing TERT epitopes, resulting in the activation of T cells.
One strategy used to achieve this goal is the development of TERT vaccines. Two vaccines have been used to generate an anti-telomerase response in cancer cells: GV1001 and Vx001. GV1001 is a 16-amino acid, HLA class II-restricted peptide that contains a part of the amino acid sequence of the TERT active site. Once injected, the vaccine is processed endogenously to yield an HLA class I peptide as well. This vaccine therefore activates a CD4+ and a CD8+ cellular response. Phase II trials investigating GV1001 alone or in combination with other therapies have been completed for malignant melanoma and hepatocellular carcinoma. Phase III GV1001 trials are ongoing for non-small cell lung carcinoma and metastatic pancreatic cancer. Vx001 is a cryptic peptide vaccine containing a TERT amino acid sequence hidden within a protein structure. Endogenous processing of the peptide results in the HLA class I presentation with a CD8+ T-cell response. A phase 2 trial investigating Vx001 in non-small cell lung cancer is currently ongoing.
Another strategy is to prime antigen-presenting dendritic cells ex vivo and then inject the primed cells back into the patient. GRNVAC1 is a dendritic cell vaccine consisting of autologous dendritic cells transduced with mRNA-encoding TERT and LAMP1, which guides TERT proteins into lysosomes for breakdown and presentation. This results in a polyclonal immune response when the primed dendritic cells are injected back into the patient. One phase 2 trial for GRNVAC1 in acute myelogenous leukemia has been completed. All 3 TERT vaccines have been shown to be well tolerated in cancer patients, with little effect on normal cells and no autoimmunity.24
GABPβ1L Inhibition
Recently, Mancini et al.75 demonstrated that disruption of the β1L isoform of GABP reverses the replicative immortality of glioblastoma cells in a TERT promoter mutation-dependent manner. As previously discussed, the C228T and C250T mutations allow specifically for the binding of the heterotetramer form of the ETS transcription factor GABP.30 GABPβ1L is a tetramer forming isoform of GABP that is not necessary for normal development but binds to the mutant TERT promoter site. The authors knocked down GABPβ1L in TERT promoter mutant glioblastoma cells in vitro, resulting in decreased GABP binding, TERT expression, and cell viability. These effects of GABPβ1L knockdown were not seen in wildtype TERT promoter glioblastoma cells. Concordantly, knocking down GABPβ1L in a mouse xenograft model of glioblastoma impaired tumor growth and increased mouse survival.75 Targeting GABPβ1L rather than TERT itself may represent a means of specifically targeting tumor cells bearing TERT promoter mutations while sparing normal cells, thus avoiding the hematopoietic side effects observed with drugs such as imetelstat.
Eribulin
Eribulin mesylate is a synthetic analog of halichondrin B, a natural isolate from marine sponges and was originally developed as an inhibitor of microtubule dynamics. In the United States, it is approved for the treatment of refractory metastatic breast cancer in patients who have previously received 2 or more chemotherapeutic agents.76 Yamaguchi et al.77 demonstrated that eribulin inhibited the growth of platinum-resistant ovarian cancer cells via inhibition of the RdRP activity, a non-telomere lengthening role of TERT. In a xenotransplantation glioblastoma mouse model, intraperitoneal injection of eribulin led to a significant reduction in intracranial tumor growth demonstrating a promising role for this drug in glioblastoma treatment. A phase II trial in patients with recurrent glioblastoma treated postoperatively with temozolomide, radiation, and bevacizumab is currently ongoing in Japan.78
Conclusions
Telomeres protect the ends of chromosomes from deterioration and end fusion to other chromosomes but shorten with successive mitotic cycles in normal cells. In highly replicative cells, the TERT expression maintains telomere length. TERT expression is otherwise very tightly regulated in normal somatic cells, with most cells silencing expression and entering a senescent state as their telomeres shorten. Two recurrent TERT promoter mutations have been detected in many cancers. In glioblastoma, meningioma, and medulloblastoma, TERT promoter mutation frequency appears to increase with a worsening grade of tumor. In glioblastoma, the mutational frequency is particularly high and the prognostic implications of the TERT mutation vary depending on the presence of other mutations. The underlying biology explaining these survival differences is poorly understood and warrants further investigation. Nevertheless, the clinical significance of TERT mutation highlights the importance of annotating its status in every CNS tumor. Presently, therapies targeting TERT or telomere maintenance are not currently part of standard-of-care regimens, but several candidates are undergoing clinical trials. While promising, targeting TERT is not without challenges. The treatment effect is dependent on telomere shortening that only occurs after several mitotic cycles. Furthermore, rapidly dividing cells, such as hematopoietic cells, depend on TERT activity and are sensitive to its inhibition. Ultimately, many aspects of TERT function and regulation remain unknown. More recently, there is mounting evidence that TERT has functions other than telomere elongation and may play other roles such as regulating gene expression, mitochondrial activity, epithelial–mesenchymal transitions, and DNA damage repair. As our understanding of the role of telomeres and TERT in cancer biology improves, more therapeutic opportunities will likely be identified.
Funding
National Institutes of Health (R25NS090978 to B.P.; K08NS092912 and R01NS112712 to G.P.D.). The Damon Runyon Cancer Research Foundation (to G.P.D.)
Conflict of interest statement. None declared.
References
- 1. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 2. Shay JW, Wright WE. Hayflick, his limit, and cellular ageing. Nat Rev Mol Cell Biol. 2000;1(1):72–76. [DOI] [PubMed] [Google Scholar]
- 3. Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6(8):611–622. [DOI] [PubMed] [Google Scholar]
- 4. Heidenreich B, Kumar R. TERT promoter mutations in telomere biology. Mutat Res. 2017;771:15–31. [DOI] [PubMed] [Google Scholar]
- 5. Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43(2 Pt 1):405–413. [DOI] [PubMed] [Google Scholar]
- 6. Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. [DOI] [PubMed] [Google Scholar]
- 7. Poole JC, Andrews LG, Tollefsbol TO. Activity, function, and gene regulation of the catalytic subunit of telomerase (hTERT). Gene. 2001;269(1–2):1–12. [DOI] [PubMed] [Google Scholar]
- 8. Chiba K, Johnson JZ, Vogan JM, et al. Cancer-associated TERT promoter mutations abrogate telomerase silencing. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bell RJ, Rube HT, Xavier-Magalhães A, et al. Understanding TERT promoter mutations: a common path to immortality. Mol Cancer Res. 2016;14(4):315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vinagre J, Almeida A, Pópulo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4(1):2185. [DOI] [PubMed] [Google Scholar]
- 11. Liu T, Yuan X, Xu D. Cancer-specific telomerase reverse transcriptase (TERT) promoter mutations: biological and clinical implications. Genes (Basel). 2016;7(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heidenreich B, Kumar R. Altered TERT promoter and other genomic regulatory elements: occurrence and impact. Int J Cancer. 2017;141(5):867–876. [DOI] [PubMed] [Google Scholar]
- 13. Guilleret I, Yan P, Grange F, Braunschweig R, Bosman FT, Benhattar J. Hypermethylation of the human telomerase catalytic subunit (hTERT) gene correlates with telomerase activity. Int J Cancer. 2002;101(4):335–341. [DOI] [PubMed] [Google Scholar]
- 14. Zinn RL, Pruitt K, Eguchi S, Baylin SB, Herman JG. hTERT is expressed in cancer cell lines despite promoter DNA methylation by preservation of unmethylated DNA and active chromatin around the transcription start site. Cancer Res. 2007;67(1):194–201. [DOI] [PubMed] [Google Scholar]
- 15. Castelo-Branco P, Choufani S, Mack S, et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: an integrative genomic and molecular study. Lancet Oncol. 2013;14(6):534–542. [DOI] [PubMed] [Google Scholar]
- 16. Lindsey JC, Schwalbe EC, Potluri S, Bailey S, Williamson D, Clifford SC. TERT promoter mutation and aberrant hypermethylation are associated with elevated expression in medulloblastoma and characterise the majority of non-infant SHH subgroup tumours. Acta Neuropathol. 2014;127(2):307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee DD, Leão R, Komosa M, et al. DNA hypermethylation within TERT promoter upregulates TERT expression in cancer. J Clin Invest. 2019;129(1):223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de Lange T. Protection of mammalian telomeres. Oncogene. 2002;21(4):532–540. [DOI] [PubMed] [Google Scholar]
- 19. Watson JD. Origin of concatemeric T7 DNA. Nat New Biol. 1972;239(94):197–201. [DOI] [PubMed] [Google Scholar]
- 20. Bryan TM, Englezou A, Gupta J, Bacchetti S, Reddel RR. Telomere elongation in immortal human cells without detectable telomerase activity. EMBO J. 1995;14(17):4240–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dunham MA, Neumann AA, Fasching CL, Reddel RR. Telomere maintenance by recombination in human cells. Nat Genet. 2000;26(4):447–450. [DOI] [PubMed] [Google Scholar]
- 22. Muntoni A, Neumann AA, Hills M, Reddel RR. Telomere elongation involves intra-molecular DNA replication in cells utilizing alternative lengthening of telomeres. Hum Mol Genet. 2009;18(6):1017–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Diplas BH, He X, Brosnan-Cashman JA, et al. The genomic landscape of TERT promoter wildtype-IDH wildtype glioblastoma. Nat Commun. 2018;9(1):2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jafri MA, Ansari SA, Alqahtani MH, Shay JW. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016;8(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zvereva MI, Shcherbakova DM, Dontsova OA. Telomerase: structure, functions, and activity regulation. Biochemistry (Mosc). 2010;75(13):1563–1583. [DOI] [PubMed] [Google Scholar]
- 26. Lue NF, Lin YC, Mian IS. A conserved telomerase motif within the catalytic domain of telomerase reverse transcriptase is specifically required for repeat addition processivity. Mol Cell Biol. 2003;23(23):8440–8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmidt JC, Cech TR. Human telomerase: biogenesis, trafficking, recruitment, and activation. Genes Dev. 2015;29(11):1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339(6122):957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bell RJ, Rube HT, Kreig A, et al. Cancer. The transcription factor GABP selectively binds and activates the mutant TERT promoter in cancer. Science. 2015;348(6238):1036–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vallarelli AF, Rachakonda PS, André J, et al. TERT promoter mutations in melanoma render TERT expression dependent on MAPK pathway activation. Oncotarget. 2016;7(33):53127–53136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Y, Zhou QL, Sun W, et al. Non-canonical NF-κB signalling and ETS1/2 cooperatively drive C250T mutant TERT promoter activation. Nat Cell Biol. 2015;17(10):1327–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Koelsche C, Sahm F, Capper D, et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol. 2013;126(6):907–915. [DOI] [PubMed] [Google Scholar]
- 34. Remke M, Ramaswamy V, Peacock J, et al. TERT promoter mutations are highly recurrent in SHH subgroup medulloblastoma. Acta Neuropathol. 2013;126(6):917–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goutagny S, Nault JC, Mallet M, Henin D, Rossi JZ, Kalamarides M. High incidence of activating TERT promoter mutations in meningiomas undergoing malignant progression. Brain Pathol. 2014;24(2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kool M, Jones DT, Jäger N, et al. ; ICGC PedBrain Tumor Project Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25(3):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heidenreich B, Rachakonda PS, Hosen I, et al. TERT promoter mutations and telomere length in adult malignant gliomas and recurrences. Oncotarget. 2015;6(12):10617–10633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sahm F, Schrimpf D, Olar A, et al. TERT promoter mutations and risk of recurrence in meningioma. J Natl Cancer Inst. 2016;108(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ceccarelli M, Barthel FP, Malta TM, et al. ; TCGA Research Network Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwaederle M, Krishnamurthy N, Daniels GA, et al. Telomerase reverse transcriptase promoter alterations across cancer types as detected by next-generation sequencing: a clinical and molecular analysis of 423 patients. Cancer. 2018;124(6):1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahlokozera T, Vellimana AK, Li T, et al. Biological and therapeutic implications of multisector sequencing in newly diagnosed glioblastoma. Neuro Oncol. 2018;20(4):472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Juratli TA, Thiede C, Koerner MVA, et al. Intratumoral heterogeneity and TERT promoter mutations in progressive/higher-grade meningiomas. Oncotarget. 2017;8(65):109228–109237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Labussière M, Di Stefano AL, Gleize V, et al. TERT promoter mutations in gliomas, genetic associations and clinico-pathological correlations. Br J Cancer. 2014;111(10):2024–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pekmezci M, Rice T, Molinaro AM, et al. Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathologica. 2017;133(6):1001–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 46. Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400(6743):464–468. [DOI] [PubMed] [Google Scholar]
- 47. Horn S, Figl A, Rachakonda PS, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339(6122):959–961. [DOI] [PubMed] [Google Scholar]
- 48. Denisova E, Heidenreich B, Nagore E, et al. Frequent DPH3 promoter mutations in skin cancers. Oncotarget. 2015;6(34):35922–35930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stewart SA, Hahn WC, O’Connor BF, et al. Telomerase contributes to tumorigenesis by a telomere length-independent mechanism. Proc Natl Acad Sci U S A. 2002;99(20):12606–12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chiba K, Lorbeer FK, Shain AH, et al. Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two-step mechanism. Science. 2017;357(6358):1416–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maida Y, Yasukawa M, Okamoto N, et al. Involvement of telomerase reverse transcriptase in heterochromatin maintenance. Mol Cell Biol. 2014;34(9):1576–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kinoshita T, Nagamatsu G, Saito S, Takubo K, Horimoto K, Suda T. Telomerase reverse transcriptase has an extratelomeric function in somatic cell reprogramming. J Biol Chem. 2014;289(22):15776–15787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park JI, Venteicher AS, Hong JY, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460(7251):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Beck S, Jin X, Sohn YW, et al. Telomerase activity-independent function of TERT allows glioma cells to attain cancer stem cell characteristics by inducing EGFR expression. Mol Cells. 2011;31(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liu Z, Li Q, Li K, et al. Telomerase reverse transcriptase promotes epithelial-mesenchymal transition and stem cell-like traits in cancer cells. Oncogene. 2013;32(36):4203–4213. [DOI] [PubMed] [Google Scholar]
- 56. Tang B, Xie R, Qin Y, et al. Human telomerase reverse transcriptase (hTERT) promotes gastric cancer invasion through cooperating with c-Myc to upregulate heparanase expression. Oncotarget. 2016;7(10):11364–11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Park YJ, Kim EK, Bae JY, Moon S, Kim J. Human telomerase reverse transcriptase (hTERT) promotes cancer invasion by modulating cathepsin D via early growth response (EGR)-1. Cancer Lett. 2016;370(2):222–231. [DOI] [PubMed] [Google Scholar]
- 58. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Okita Y, Narita Y, Miyakita Y, et al. IDH1/2 mutation is a prognostic marker for survival and predicts response to chemotherapy for grade II gliomas concomitantly treated with radiation therapy. Int J Oncol. 2012;41(4):1325–1336. [DOI] [PubMed] [Google Scholar]
- 60. Appin CL, Brat DJ. Molecular genetics of gliomas. Cancer J. 2014;20(1):66–72. [DOI] [PubMed] [Google Scholar]
- 61. Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350–1354. [DOI] [PubMed] [Google Scholar]
- 62. Labussière M, Boisselier B, Mokhtari K, et al. Combined analysis of TERT, EGFR, and IDH status defines distinct prognostic glioblastoma classes. Neurology. 2014;83(13):1200–1206. [DOI] [PubMed] [Google Scholar]
- 63. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Brat DJ, Verhaak RG, Aldape KD, et al. ; Cancer Genome Atlas Research Network Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wijnenga MMJ, Dubbink HJ, French PJ, et al. Molecular and clinical heterogeneity of adult diffuse low-grade IDH wild-type gliomas: assessment of TERT promoter mutation and chromosome 7 and 10 copy number status allows superior prognostic stratification. Acta Neuropathol. 2017;134(6):957–959. [DOI] [PubMed] [Google Scholar]
- 67. Shankar GM, Francis JM, Rinne ML, et al. Rapid intraoperative molecular characterization of Glioma. JAMA Oncol. 2015;1(5):662–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jackson SR, Zhu CH, Paulson V, et al. Antiadhesive effects of GRN163L–an oligonucleotide N3′->P5′ thio-phosphoramidate targeting telomerase. Cancer Res. 2007;67(3):1121–1129. [DOI] [PubMed] [Google Scholar]
- 69. Herbert BS, Gellert GC, Hochreiter A, et al. Lipid modification of GRN163, an N3′–>P5′ thio-phosphoramidate oligonucleotide, enhances the potency of telomerase inhibition. Oncogene. 2005;24(33):5262–5268. [DOI] [PubMed] [Google Scholar]
- 70. Dikmen ZG, Gellert GC, Jackson S, et al. In vivo inhibition of lung cancer by GRN163L: a novel human telomerase inhibitor. Cancer Res. 2005;65(17):7866–7873. [DOI] [PubMed] [Google Scholar]
- 71. Marian CO, Cho SK, McEllin BM, et al. The telomerase antagonist, imetelstat, efficiently targets glioblastoma tumor-initiating cells leading to decreased proliferation and tumor growth. Clin Cancer Res. 2010;16(1):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Salloum R, Hummel TR, Kumar SS, et al. A molecular biology and phase II study of imetelstat (GRN163L) in children with recurrent or refractory central nervous system malignancies: a pediatric brain tumor consortium study. J Neurooncol. 2016;129(3):443–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vonderheide RH, Hahn WC, Schultze JL, Nadler LM. The telomerase catalytic subunit is a widely expressed tumor-associated antigen recognized by cytotoxic T lymphocytes. Immunity. 1999;10(6):673–679. [DOI] [PubMed] [Google Scholar]
- 74. Lev A, Denkberg G, Cohen CJ, et al. Isolation and characterization of human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells directed toward the widely expressed tumor T-cell epitopes of the telomerase catalytic subunit. Cancer Res. 2002;62(11):3184–3194. [PubMed] [Google Scholar]
- 75. Mancini A, Xavier-Magalhães A, Woods WS, et al. Disruption of the β1L isoform of GABP reverses glioblastoma replicative immortality in a TERT promoter mutation-dependent manner. Cancer Cell. 2018;34(3):513–528.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. O’Sullivan Coyne G, Walsh J, Kelly CM. Effectiveness and safety of eribulin mesylate: a new therapeutic option in the treatment of metastatic breast cancer. Expert Opin Drug Saf. 2012;11(4):643–650. [DOI] [PubMed] [Google Scholar]
- 77. Yamaguchi S, Maida Y, Yasukawa M, Kato T, Yoshida M, Masutomi K. Eribulin mesylate targets human telomerase reverse transcriptase in ovarian cancer cells. PLoS One. 2014;9(11):e112438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Takahashi M, Miki S, Fujimoto K, et al. Eribulin penetrates brain tumor tissue and prolongs survival of mice harboring intracerebral glioblastoma xenografts. Cancer Sci. 2019;110(7):2247–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]