Abstract
Adult-onset Still's disease (AOSD) usually affects young adults. Some cases of elderly-onset Still's disease (EOSD) have been reported, but its clinical features are unclear. We herein report a 74-year-old woman who developed AOSD with macrophage activation syndrome (MAS). We also reviewed 24 previous EOSD cases in patients over 70 years old and compared the findings with overall AOSD. While the clinical features were similar between the two, including the presence of MAS, disseminated intravascular coagulation was more frequent in EOSD than in AOSD. Furthermore, despite a similar frequency of glucocorticoid use, immunosuppressants and biologics were less frequently administered in EOSD than in AOSD. This report highlights the fact that typical AOSD can develop in elderly patients with some characteristic features.
Keywords: Adult onset Still's disease, elderly, macrophage activation syndrome, interleukin-18
Introduction
Adult-onset Still's disease (AOSD) is a rare systemic disease of unknown etiology. The signs are nonspecific, including a fever, arthralgia and rash. The diagnosis of AOSD is sometimes difficult because of the lack of specific diagnostic markers. While Yamaguchi's classification criteria (1) are widely used for the diagnosis, clinicians must exclude infection, malignancy and other rheumatic diseases. The serum ferritin and interleukin (IL)-18 levels are useful for the diagnosis and evaluation of the disease activity of AOSD (2,3).
Macrophage activation syndrome (MAS) and disseminated intravascular coagulation (DIC) are severe complications that adversely affect the prognosis (4). In the treatment of AOSD, glucocorticoid and immunosuppressants, such as cyclosporine (CyA) and methotrexate (MTX), are frequently used. The efficacy of IL-6 inhibitor has been reported recently as well (5).
Although AOSD most commonly affects young persons, a few patients develop it at an advanced age. The mean age of typical AOSD is about 36 years (6), with peak ages of 15-25 and 36-46 years (7). However, some cases of elderly-onset Still's disease (EOSD) have also been reported (8-31). The oldest such case was 88 years old at the disease onset (8). The frequency of EOSD is so low that the literature is limited to only a few case reports, with no reviews available, so the overall clinical features of EOSD remain unclear.
We herein report a 74-year-old woman who developed AOSD complicated by MAS. She showed typical signs, such as a fever, rash, sore throat and lymphadenopathy. Laboratory tests showed liver dysfunction and elevated serum ferritin and IL-18 levels. She developed MAS during high-dose glucocorticoid therapy, but this improved after starting glucocorticoid pulse therapy with CyA. The cytokine profile was useful for the early diagnosis of MAS. In addition, we conducted a literature review of patients who developed EOSD after 70 years of age and compared them with the findings of overall AOSD cases in order to identify the clinical features specific to EOSD.
Case Report
A 74-year-old woman was admitted to our hospital because of an antimicrobial-resistant fever, rash and sore throat. Nineteen days before admission, itchy erythema had developed on her back, buttocks and femur. Fourteen days previously, a remittent fever over 39℃ and sore throat had developed. She was admitted to another hospital and treated with antibiotics without improvement.
On admission to our hospital for further treatment, her orientation was clear. Her body temperature was 38.6℃, blood pressure 129/59 mmHg and heart rate 85 beats per minute. She had taken azilsartan and atorvastatin regularly for hypertension and dyslipidemia for several years. Her family had no known history of rheumatic diseases. She had an allergy to contrast agents. On a physical examination, she had itchy erythema on her back and left upper arm with Koebner phenomenon, which worsened as the fever increased (Fig. 1). She had no swollen superficial lymph nodes. Her pharynx was normal. She had no arthritis or purpura. Laboratory tests showed leukocytosis with neutrophil predominance and elevation of serum C-reactive protein (CRP), ferritin and IL-18 levels (Table 1). Antinuclear antibodies, rheumatoid factors and anti-neutrophil cytoplasmic antibodies were negative. Although an interferon-gamma release assay was positive, both a smear and polymerase chain reaction test of sputum for Mycobacterium tuberculosis were negative (three-fold repetition).
Figure 1.
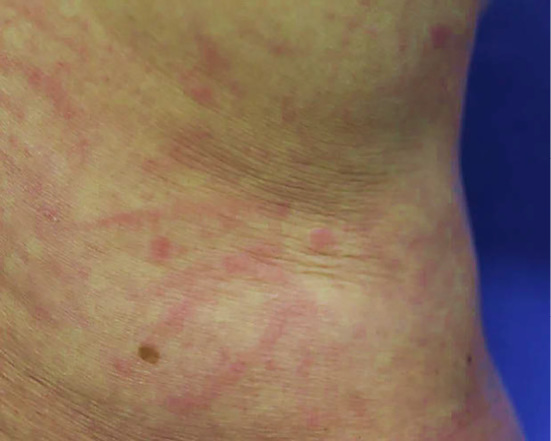
Skin findings. The itchy rash with Koebner phenomenon worsened when the patient had a fever.
Table 1.
Laboratory Data on Admission and at Time of MAS Development.
| On admission | Complicated by MAS | Normal range | ||||
|---|---|---|---|---|---|---|
| Urinalysis | ||||||
| Protein | - | - | ||||
| Occult blood | - | - | ||||
| Blood count | ||||||
| White blood cells (/μL) | 11,970 | 20,240 | 3,300-8,800 | |||
| Neutrophil (%) | 88.2 | 97.0 | 42.0-74.0 | |||
| Hb (g/dL) | 9.6 | 9.3 | 13.5-17.0 | |||
| Plt (/μL) | 39.0 | 28.5 | 13.0-35.0 | |||
| ESR (mm/hr) | 140 | 56 | ||||
| Serum chemistry | ||||||
| BUN (mg/dL) | 13.3 | 21.9 | 8-22 | |||
| Cr (mg/dL) | 0.75 | 0.73 | 0.60-1.00 | |||
| Na (mEq/L) | 134 | 132 | 135-149 | |||
| K (mEq/L) | 4.3 | 5.1 | 3.5-4.9 | |||
| Cl (mEq/L) | 98 | 98 | 96-108 | |||
| AST (IU/L) | 41 | 1,989 | 13-33 | |||
| ALT (IU/L) | 22 | 821 | 8-42 | |||
| LDH (IU/L) | 357 | 2,643 | 119-229 | |||
| TP (g/dL) | 7.4 | 6.0 | 6.7-8.3 | |||
| Alb (g/dL) | 2.5 | 2.4 | 4.0-5.0 | |||
| TG (mg/dL) | 78 | 139 | 30-150 | |||
| Ferritin (ng/mL) | 8,412 | 34,160 | 6.9-323 | |||
| FDP-DD (μg/mL) | 6.2 | 33.8 | <1.0 | |||
| Immunological findings | ||||||
| CRP (mg/dL) | 15.7 | 2.9 | 0.0-0.3 | |||
| IgG (mg/dL) | 1,775 | 870-1,700 | ||||
| IgA (mg/dL) | 283 | 110-410 | ||||
| IgM (mg/dL) | 89.5 | 33-190 | ||||
| C3 (mg/dL) | 184.7 | 65-135 | ||||
| C4 (mg/dL) | 41.7 | 13-35 | ||||
| Anti-nuclear antibody | ×40 | - | ||||
| RF (IU/mL) | 2.0 | <20 | ||||
| MPO-ANCA | - | - | ||||
| PR3-ANCA | - | - | ||||
| sIL-2R (U/mL) | 1,490 | 2,870 | 220-530 | |||
| Cytokine | ||||||
| IL-18 (pg/mL) | 33,500 | 210,000 | <500 | |||
| IL-6 (pg/mL) | 42 | 18 | <5 | |||
| neopterin (nmol/L) | 21.8 | 113 | <5 | |||
| sTNF-R I (pg/mL) | 4,240 | 7,000 | 484-1,407 | |||
| sTNF-R II (pg/mL) | 6,900 | 46,500 | 892-2,262 | |||
| sTNF-R II/I | 1.6 | 6.6 | ||||
MAS: macrophage activation syndrome
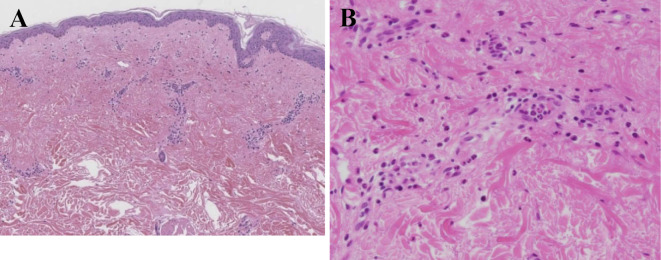
Plain computed tomography (CT) revealed only swelling of the bilateral axillary lymph nodes, without any other abnormal findings, such as hepatosplenomegaly or signs of tuberculosis infection. Contrast-enhanced CT was not performed because of her allergy to contrast agents. Four sets of blood cultures were negative. Laboratory tests also showed past Epstein-Barr virus and cytomegalovirus (CMV) infections and negative results for hepatitis B virus (HBV) surface antigen, HBV surface antibodies, HBV core antibodies and hepatitis C virus antibodies. Upper endoscopy revealed no abnormalities. A fecal occult blood test was negative. A skin biopsy of the erythema on her back showed infiltration of inflammatory cells, including neutrophils and eosinophils, around the vessels and interstitium of the superficial dermic layer without any signs of malignant lymphoma or other malignancy (Fig. 2). After excluding malignancy and infection, AOSD was diagnosed according to Yamaguchi's classification criteria (1).
Figure 2.
A skin biopsy. Infiltration of inflammatory cells, including neutrophils and eosinophils, around vessels and the interstitium of the superficial dermic layer (Hematoxylin and Eosin staining. A ×40, B ×400).
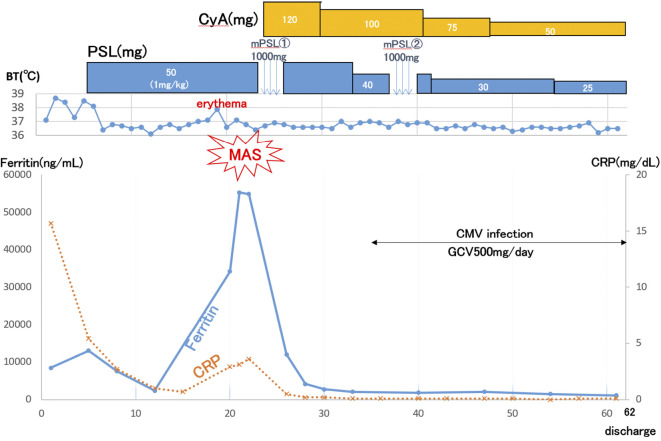
On the 6th hospital day, 50 mg/day (1 mg/kg/day) of prednisolone with isoniazid was administered. Her symptoms showed improvement on the following day. The serum CRP and ferritin levels decreased to 0.7 mg/dL and 2,351 ng/mL, respectively. However, on the 19th hospital day, a fever over 38℃ and erythema developed transiently. Laboratory findings showed increased serum CRP, ferritin, liver enzyme, D-dimer and IL-18 levels without cytopenia or increased serum triglyceride levels (Table 1). Abdominal ultrasound did not reveal hepatosplenomegaly. The serum cytokine profile showed typical findings of AOSD (32-34). MAS was diagnosed clinically.
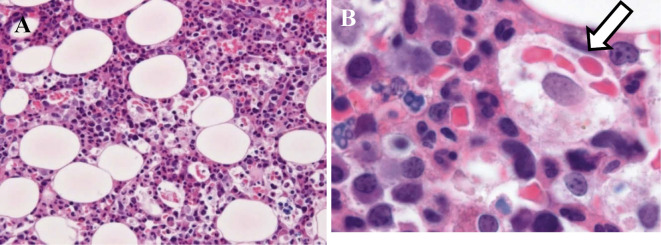
Before the results of a bone marrow biopsy became available, combined intravenous methylprednisolone pulse therapy (1,000 mg/day for 3 days) with CyA was administered, resulting in the immediate improvement of her signs and normalization of serum CRP level. After that, the bone marrow biopsy findings confirmed the presence of hemophagocytosis without any signs of malignancy (Fig. 3). On the 36th hospital day, CMV reactivation developed and was successfully treated with ganciclovir. She was discharged on the 62nd hospital day (Fig. 4). She has remained in remission with a normal serum ferritin level for two years.
Figure 3.
A bone marrow biopsy. Phagocytosis of granulocytes and RBC by macrophage cells (Hematoxylin and Eosin staining. A ×100, B ×1000).
Figure 4.
Clinical course. MAS: macrophage activation syndrome, PSL: prednisolone, CyA: cyclosporine, CMV: cytomegalovirus, GCV: ganciclovir, CRP: C-reactive protein
Literature review
We searched Pubmed for publications on EOSD in English that included sufficient clinical information for the purposes of this study, including background factors, laboratory data, treatments and the clinical course. We defined EOSD here as AOSD developing in patients over 70 years old, in view of the lack of any agreed-upon definition of EOSD in the literature. Our search thus identified 24 EOSD cases (8-31).
First, we compared the clinical features between the 25 EOSD cases (the present case and the 24 cases from the literature review) and the overall AOSD group (7,35-45) (Table 2). The mean onset age was 76.6±4.9 and 24-46 years old in the EOSD and AOSD groups, respectively. EOSD showed similar clinical signs and laboratory findings to overall AOSD. Regarding complications, the frequency of MAS was similar. However, DIC occurred more frequently in the EOSD group than in the AOSD group (20.0% vs. 1.8-6.3%). Regarding treatment, we compared the 25 EOSD cases with 166 Japanese AOSD cases (44) (Table 3). Glucocorticoid use was common, and methylprednisolone therapy was used in about one-third of cases. EOSD cases received immunosuppressants and biologics less frequently than did AOSD cases (24.0% vs. 80.7% and 8.0% vs. 19.9%, respectively). MTX was often used in overall AOSD (41.0%). Regarding the clinical outcome, the rates of remission, relapse and death were similar, but the observation period was longer in the overall AOSD group.
Table 2.
Clinical Features of EOSD and AOSD.
| EOSD n=25 |
AOSD [Ref. 7, 35-45] |
|||
|---|---|---|---|---|
| Japanese patients, n (%) | 17/25 (68) | 168/940 (18) | ||
| Epidemiology | ||||
| Mean age, years old | 76.6±4.9 | 24-46 | ||
| Female, n (%) | 18/25 (74) | 45-75% | ||
| Clinical features | ||||
| Fever, n (%) | 25/25 (100) | 85-100% | ||
| Arthritis, n (%) | 19/25 (76) | 72-100% | ||
| Typical rash, n (%) | 17/25 (68) | 58-94% | ||
| Leukocytes ≥10,000/μL, n (%) | 21/25 (84) | 72-98% | ||
| Neutrophils ≥80%, n (%) | 16/21 (76) | 55-89% | ||
| Sore throat, n (%) | 16/25 (64) | 27-92% | ||
| Lymphadenopathy, n (%) | 9/25 (36) | 28-74% | ||
| Splenomegaly, n (%) | 7/25 (28) | 29-79% | ||
| Elevated liver enzymes, n (%) | 21/25 (84) | 21-76% | ||
| Negative for rheumatoid factor, n (%) | 21/22 (95) | 93-100% | ||
| Negative for antinuclear antibodies, n (%) | 21/21 (100) | 89-100% | ||
| Complications | ||||
| MAS, n (%) | 5/25 (20) | 12-21% | ||
| DIC, n (%) | 5/25 (20) | 1.8-6.3% | ||
| Infection, n (%) | PCP 2/25 (8) CMV 4/25 (16) |
NA | ||
| ARDS, n (%) | 1/25 (4) | NA | ||
| Interstitial pneumonia, n (%) | 1/25 (4) | NA | ||
| Multi-organ dysfunction, n (%) | 1/25 (4) | NA | ||
| Cerebellar hemorrhage, n (%) | 1/25 (4) | NA | ||
| Subacute hepatitis, n (%) | 1/25 (4) | NA | ||
| Malignancy, n (%) | 1/25 (4) | NA | ||
| Inflammatory myopathy, n (%) | 1/25 (4) | NA |
EOSD: elderly-onset Still’s disease, AOSD: adult-onset Still’s disease, MAS: macrophage activation syndrome, DIC: disseminated intravascular coagulation, ARDS: acute respiratory distress syndrome, PCP: pneumocystis pneumonia, CMV: cytomegalovirus, NA: not available
Table 3.
Medication and Clinical Outcome of EOSD and AOSD.
| EOSD n=25 |
AOSD n=166 [Ref. 44] |
|||
|---|---|---|---|---|
| Japanese patients, n (%) | 17 (68) | 166 (100) | ||
| Medication | ||||
| Glucocorticoid, n (%) | 23 (92) | 160 (96.4) | ||
| Pulse glucocorticoid therapy, n (%) | 7 (28) | 52 (31.3) | ||
| NSAIDs, n (%) | 9 (36) | 73 (44.0) | ||
| Immunosuppressants, n (%) | 6 (24) | 134 (80.7) | ||
| Biologics, n (%) | 2 (8) | 33 (19.9) | ||
| IVIG, n (%) | 2 (8) | - | ||
| Others, n (%) | - | 8 (4.8) | ||
| Clinical outcome | ||||
| Remission, n (%) | 23 (92) | 145/164 (88.4) | ||
| Flare, n (%) | 11 (44) | 66/169 (39.1) | ||
| AOSD-related death, n (%) | 0 (0) | 0 (0) | ||
| Follow up time, months | 23.7 | 58.8 |
EOSD: elderly-onset Still’s disease, AOSD: adult-onset Still’s disease, NSAIDs: non-steroidal anti-inflammatory drugs, IVIG: intravenous immunoglobulin
Next, we identified 5 EOSD cases complicated by MAS, including the present case (8,15,28,29), and compared the findings with AOSD cases complicated by MAS from the literature review (46) (Table 4). The mean onset age of MAS was 76.8±7.3 and 40.2±16.0 years old in the EOSD and AOSD groups, respectively. With respect to treatment, glucocorticoids and CyA were the most commonly used agents. EOSD patients received intravenous immunoglobulin (IVIG) and MTX less frequently than did AOSD patients. Although EOSD may show less mortality due to MAS than AOSD, a bias due to the small sample size could not be excluded.
Table 4.
Comparison between EOSD and AOSD Cases Complicated with MAS.
| EOSD with MAS n=5 |
AOSD with MAS n=48 [Ref. 46] |
|||
|---|---|---|---|---|
| Epidemiology | ||||
| Mean age, years old | 76.9±8.4 | 40.2±16.0 | ||
| Females, n (%) | 5 (100) | 35 (72.9) | ||
| Treatment for MAS | ||||
| Glucocorticoids, n (%) | 4 (80) | 43 (90) | ||
| Cyclosporine, n (%) | 2 (40) | 14 (29) | ||
| Anti- IL-6, n (%) | 1 (20) | - | ||
| IVIG, n (%) | - | 19 (40) | ||
| Methotrexate, n (%) | - | 11 (23) | ||
| Cyclosphosphamide, n (%) | - | 6 (13) | ||
| Anakinra, n (%) | - | 1 (2) | ||
| Anti-TNF, n (%) | - | 1 (2) | ||
| Immediate outcome | ||||
| Alive, n (%) | 5 (100) | 42 (88) | ||
| Deceased, n (%) | - | 6 (12) | ||
| Follow up time, months | 14.2±6.5 | 17.5±32.3 |
EOSD: elderly-onset Still’s disease, AOSD: adult-onset Still’s disease, MAS: macrophage activation syndrome, IL-6: interleukin-6, IVIG: intravenous immunoglobulin, TNF: tumor necrosis factor
In this literature review, the overall AOSD group included some EOSD cases because reports of AOSD included all ages. However, as the rate of EOSD cases in the overall AOSD group was considered to be less than 10-20% (4), we did not consider this to have substantially influenced the total trend mentioned above. Furthermore, we did not perform a statistical analysis because of the different cohorts used here and the small sample sizes.
Discussion
We described a 74-year-old woman who developed AOSD complicated by MAS. Although EOSD is rare, elderly patients can develop AOSD, and the number of such cases is expected to increase because of the aging of society. The literature review showed that cases of EOSD had almost the same clinical signs, including MAS, as overall AOSD, although they showed a tendency to develop DIC more frequently than the overall AOSD group. Immunosuppressants were noted to be less frequently used in EOSD patients than in AOSD patients. As elderly patients can develop typical AOSD, clinicians should not exclude AOSD from the differential diagnosis in this group.
The age at the onset of AOSD has been increasing. An investigation from Japan reported that the average age at the onset of AOSD was 38.1±14.3 years old in 1994 (47), in contrast to 46±19 years old in 2010 (44). Another report in 2016 from Japan stated that the mean onset age was 53.1±19.6 years old (4). About 23% of cases developed it at ≥65 years old and about 16% at ≥75 years old (4). This has been attributed to the marked aging of the population now occurring in Japan (4). For this reason, it can be surmised that, as the population continues to age, the age at the onset of AOSD will also continue to increase.
The literature review showed EOSD to be associated with almost the same clinical signs, including MAS, as typical AOSD, although DIC appeared to be more frequent in EOSD patients than in AOSD patients. With respect to complications, while the total prevalence of MAS and DIC has been reported not to differ appreciably across patient age groups (4), we found that DIC was more frequent in EOSD than in AOSD, despite a similar frequency of MAS. MAS is a severe complication related to mortality (48,49), and an older age was shown to be associated with increased mortality in AOSD (50). We could not determine the prognosis statistically because a different cohort was used. In addition, we were unable to determine the prevalence of malignant diseases, infection or other co-morbidities throughout the total clinical course in typical AOSD cases because of a lack of such data in the literature; therefore, whether or not these conditions are more prevalent in EOSD than in AOSD remains unclear.
EOSD is associated with various complications, including opportunistic infections, such as CMV infection (15,17,29) and pneumocystis pneumonia (11,29), acute respiratory distress syndrome (30), interstitial pneumonia (IP) (23), multi-organ dysfunction (23), subacute hepatitis (24), cerebellar hemorrhaging (22), malignancy (16) and inflammatory myopathy with abundant macrophages (28). Two patients with EOSD died of either IP and multi-organ failure (23) or malignancy (16). In AOSD, the mortality was reported to increase with age (4). In addition, as the prevalence of malignancy is increased in older persons, and given that a case of paraneoplastic syndrome with clinical features similar to those of AOSD was reported (51), clinicians should consider the possibility of malignancy when diagnosing EOSD. Cases of AOSD associated with malignancy tend to be older at the onset of symptoms than typical AOSD cases (52). Therefore, although the clinical features of EOSD are similar to those of typical AOSD, it is important to exclude malignant disease and be alert for the possible development of complications, such as MAS and DIC, during the clinical course of EOSD.
Although glucocorticoid was commonly used in both EOSD and AOSD, immunosuppressants such as MTX and CyA were used less often in cases of EOSD. This is similar to the situation with systemic lupus erythematosus, in which immunosuppressants are also less frequently used in elderly patients than in younger ones (53,54). Considering the rates of remission and relapse in EOSD, the response to glucocorticoids might be better in EOSD than in AOSD. In contrast, immunosuppressants and biologics may tend to be avoided in EOSD patients due to concerns about infection. A patient with EOSD who discontinued IL-6 inhibitor due to severe infection has been reported (19). Immunosuppressants and biologics need to be prescribed particularly carefully in EOSD cases.
Several limitations associated with the present study warrant mention. First, the race-related difference between the EOSD and overall AOSD groups may have influenced the clinical features of each group. Japanese patients, who have lower frequencies of chronic pattern (6,44,55) and MTX use (4,35,36), comprised 68% and 18% of the EOSD and overall AOSD groups, respectively. Second, the EOSD group comprised rare case reports, suggesting possible selection bias. Special attention should be paid to these points when comparing these two groups.
In conclusion, AOSD can develop even in persons over 70 years old with typical clinical features, including severe complications, such as MAS. EOSD may be complicated by DIC more frequently than AOSD. The diagnosis of EOSD can sometimes be delayed because clinicians are less likely to consider it in elderly patients (56). Including EOSD properly as a differential diagnosis can help prevent expensive and/or excessive examinations (20,31). This report draws attention to EOSD to make clinicians aware that elderly patients can develop typical AOSD and that such cases can be expected to increase in number with the general aging of society.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank John Gelblum for his critical reading of the manuscript.
References
- 1. Yamaguchi M, Ohta A, Tsunematsu T, et al. Preliminary criteria for classification adult Still's disease. J Rheumatol 19: 424-430, 1992. [PubMed] [Google Scholar]
- 2. Kawaguchi Y, Terajima H, Harigai M, Hara M, Kamatani N. Interleukin-18 as a novel diagnostic marker and indicator of disease severity in adult-onset Still's disease. Arthritis Rheum 44: 1716-1717, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Kawashima M, Yamamura M, Tanial M, et al. Levels of interleukin-18 and its biding inhibitor in the blood circulation of patients with adult-onset Still's disease. Arthritis Rheum 44: 550-560, 2001. [DOI] [PubMed] [Google Scholar]
- 4. Sakata N, Shimizu S, Hirano F, Fushimi K. Epidemiological study of adult-onset Still's disease using a Japanese administrative database. Rheumatol Int 36: 1399-1405, 2016. [DOI] [PubMed] [Google Scholar]
- 5. Castañeda S, Martínez-Quintanilla D, Martín-Varillas JL, García-Castañeda N, Atienza-Mateo B, Gonzáez-Gay MA. Tocilizumab for the treatment of adult-onset Still's disease. Expert Opin Biol Ther 19: 273-286, 2019. [DOI] [PubMed] [Google Scholar]
- 6. Gerfaud-Valentin M, Jamilloux Y, Iwaz J, Sève P. Adult-onset Still's disease. Autoimmun Rev 13: 708-722, 2014. [DOI] [PubMed] [Google Scholar]
- 7. Giacomelli R, Ruscitti P, Shoenfeld Y. A comprehensive review on adult onset Still's disease. J Autoimmun 93: 24-36, 2018. [DOI] [PubMed] [Google Scholar]
- 8. Yamashita S, Furukawa NE, Matsunaga T, Hirakawa Y, Tago M, Yamashita SI. Extremely high serum ferritin: an instrumental marker of masquerading adult-onset Still's disease with hemophagocytic syndrome. Am J Case Rep 18: 1296-1301, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kiyonaga Y, Maeshima K, Imada C, Haranaka M, Ishii K, Shibata H. Steroid-sparing effects of etanercept in a patient with steroid-dependent adult-onset Still's disease. Intern Med 53: 1209-1213, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Ichiki H, Shisido M, Nishiyama S. Two cases of adult onset of Still's disease in the elderly. Nihon Ronen Igakukai Zasshi 29: 960-964, 1992(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 11. Kurasawa M, Kotani K, Kurasawa G, Shida K, Yamada S, Tago T. Adult-onset Still's disease in a patient over 80 years old successfully treated with low-dose methotrexate therapy. Age Aging 36: 104-106, 2007. [DOI] [PubMed] [Google Scholar]
- 12. Ertuqlul BM, Gencer P, Ozturk B, Saylak Ersoy OM, Sakarya S. Adult-onset Still's disease in 83-year-old. J Am Geriatr Soc 60: 162-164, 2012. [DOI] [PubMed] [Google Scholar]
- 13. Sanada I, Kawano F, Tsukamoto A, Kiyokawa T, Shido T, Koga S. Disseminated intravascular coagulation in a case of adult onset Still's disease. Rinsho Ketsueki 38: 1194-1198, 1997(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 14. Hamidou MA, Denis M, Barbarot S, Boutoille D, Belizna C, Le Moël G. Usefulness of glycosylated ferritin in atypical presentations of adult onset Still's disease. Ann Rheum Dis 63: 605, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amenomori M, Migita K, Yoshida S, et al. Cytomegalovirus-associated hemophagocystic syndrome in a patient with adult onset Still's disease. Clin Exp Rheumatol 23: 100-102, 2005. [PubMed] [Google Scholar]
- 16. Shibuya Y, Matuo K, Kawada T, Kosugi T, Gomi T. Adult onset Still's disease associated esophageal cancer: a case report. Ryumachi 43: 577-582, 2003(in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 17. Izumikawa K, Morinaga Y, Kondo A, et al. Adult-Still's disease associated cytomegalovirus infection. J Infect Chemother 13: 114-117, 2007. [DOI] [PubMed] [Google Scholar]
- 18. Vila LM, Molina MJ. Chronic anemia and thrombocytosis as the initial presentation of Still's disease in an elderly patient. Gerontology 53: 289-292, 2007. [DOI] [PubMed] [Google Scholar]
- 19. Kato T, Noguchi K, Uehara M, et al. Angioedema of periorbital region that developed during treatment with etanercept in a case of refractory adult-onset Still's disease. Intern Med 51: 2801-2804, 2012. [DOI] [PubMed] [Google Scholar]
- 20. Rubenstein EJ. Arkfeld DG. Adult Still's disease in a 75-year-old patient. J Am Geriatr Soc 52: 2144-2145, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Tamura K, Kubota K, Kurabayashi H, Take H, Shirakura T. Elderly onset of adult Still's disease: report of a case. Clin Rheumatol 13: 117-118, 1994. [DOI] [PubMed] [Google Scholar]
- 22. Kurabayashi H, Kubota K, Tamura K, Shirakura T. Cerebral haemorrhage complicating adult-onset Still's disease: a case report. J Int Med Res 24: 492-494, 1996. [DOI] [PubMed] [Google Scholar]
- 23. Stoica GS, Cohen RI, Rossoff LJ. Adult Still's disease and respiratory failure in a 74 year old woman. Postgrad Med J 78: 97-98, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takami A, Nakao S, Miyamori H, et al. Adult-onset Still's disease with submassive hepatic necrosis. Intern Med 34: 89-91, 1995. [DOI] [PubMed] [Google Scholar]
- 25. Rech J, Ronneberger M, Englbrecht M, et al. Successful treatment of adult-onset Still's disease refractory to TNF and IL-1 blockade by IL-6 receptor blockade. Ann Rheum Dis 70: 390-392, 2011. [DOI] [PubMed] [Google Scholar]
- 26. Uson J, Pena JM, del Arco A, et al. Still's disease in a 72-year-old man. J Rheumatol 20: 1608-1609, 1993. [PubMed] [Google Scholar]
- 27. Koga T, Tokunaga N, Ichikawa Y, Oizumi K. A 72-year-old female with adult Still's disease. Inter Med 31: 1356-1358, 1992. [DOI] [PubMed] [Google Scholar]
- 28. Umeda M, Origuchi T, Fujikawa K, et al. Hemophagocytic syndrome and inflammatory myopathy with abundant macrophages in a patient with adult-onset Still's disease. Intern Med 53: 2385-2389, 2014. [DOI] [PubMed] [Google Scholar]
- 29. Watanabe E, Sugawara H, Yamashita T, Ishii A, Oda A, Terai C. Successful tocilizumab therapy for macrophage activation syndrome associated with adult-onset Still's disease: a cased review. Case Rep Med 2016: 5656320, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yokoyama M, Suwa A, Shinozawa T, et al. A case of adult onset Still's disease complicated with adult respiratory distress syndrome and disseminated intravascular coagulation. Nihon Rinsho Meneki Gakkai Kaishi 18: 207-214, 1995(in Japanese, Abstract in English). [DOI] [PubMed] [Google Scholar]
- 31. Steffe LA, Cooke CL. Still's disease in a 70-year-old woman. JAMA 249: 2062-2063, 1983. [PubMed] [Google Scholar]
- 32. Inoue N, Shimizu M, Tsunoda S, Kawano M, Matsumura M, Yachie A. Cytokine profile in adult-onset Still's disease: comparison with systemic juvenile idiopathic arthritis. Clin Immunol 169: 8-13, 2016. [DOI] [PubMed] [Google Scholar]
- 33. Ibarra MF, Klein-Gitelman M, Morgan E, et al. Serum neopterin level as a diagnostic marker of hemophagocytic lymphohistiocytosis syndrome. Clin Vaccine Immunol 18: 609-614, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shimizu M, Inoue N, Mizuta M, Nakagishi Y, Yachie A. Characteristic elevation of soluble TNF receptor II:I ratio in macrophage activation syndrome with systemic juvenile idiopathic arthritis. Clin Exp Immunol 191: 349-355, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gerfaud-Valentin M, Maucort-Boulch D, Hot A, et al. Adult-onset still disease: manifestation, treatment, outcome, and prognostic factors in 57 patients. Medicine (Baltimore) 93: 91-99, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cagatay Y, Gul A, Cagatay A, et al. Adult-onset Still's disease. Int J Clin Pract 63: 1050-1055, 2009. [DOI] [PubMed] [Google Scholar]
- 37. Chen PD, Yu SL, Chen S, Weng XH. Retrospective study of 61 patients with adult-onset Still's disease admitted with fever of unknown origin in China. Clin Rheumatol 31: 175-181, 2012. [DOI] [PubMed] [Google Scholar]
- 38. Pouchot J, Sampalis JS, Beaudet F, et al. Adult Still's disease: manifestations, disease course, and outcome in 62 patients. Medicine (Baltimore) 70: 118-136, 1991. [PubMed] [Google Scholar]
- 39. Fautrel B, Zing E, Golmard JL, et al. Proposal for a new set of classification criteria for adult-onset still disease. Medicine (Baltimore) 81: 194-200, 2002. [DOI] [PubMed] [Google Scholar]
- 40. Pay S, Türkçapar N, Kalyoncu M, et al. A multicenter study of patients with adult-onset Still's disease compared with systemic juvenile idiopathic arthritis. Clin Rheumatol 25: 639-644, 2006. [DOI] [PubMed] [Google Scholar]
- 41. Zhu G, Liu G, Liu Y, et al. Liver abnormalities in adult onset Still's disease: a retrospective study of 77 Chinese patients. J Clin Rheumatol 15: 284-288, 2009. [DOI] [PubMed] [Google Scholar]
- 42. Kong XD, Xu D, Zhang W, Shi G. Clinical features and prognosis in adult-onset Still's disease: a study of 104 cases. Clin Rheumatol 29: 1015-1019, 2010. [DOI] [PubMed] [Google Scholar]
- 43. Colina M, Zucchini W, Ciancio G, Orzincolo C, Trotta F, Govoni M. The evolution of adult-onset Still disease: an observational and comparative study in a cohort of 76 Italian patients. Semin Arthritis Rheum 41: 279-285, 2011. [DOI] [PubMed] [Google Scholar]
- 44. Asanuma YF, Mimura T, Tsubo H, et al. Nationwide epidemiological survey of 169 patients with adult Still's disease in Japan. Mod Rheumatol 25: 393-400, 2015. [DOI] [PubMed] [Google Scholar]
- 45. Mimura T, Kondo Y, Ohta A, et al. Evidence-based clinical practice guideline for adult Still's disease. Mod Rheumatol 28: 736-757, 2018. [DOI] [PubMed] [Google Scholar]
- 46. Lenert A, Yao Q. Macrophage activation syndrome complicating adult onset Still's disease: a single center case series and comparison with literature. Semin Arthritis Rheum 45: 711-716, 2016. [DOI] [PubMed] [Google Scholar]
- 47. Wakai K, Ohta A, Tamakoshi A, et al. Estimated prevalence and incidence of adult Still's disease: findings by a nationwide epidemiological survey in Japan. J Epidemiol 7: 221-225, 1997. [DOI] [PubMed] [Google Scholar]
- 48. Ahn SS, Yoo BW, Jung SM, Lee SW, Park YB, Song JJ. Application of the 2016 EULAR/ACR/PRINTO classification criteria for macrophage activation syndrome in patients with adult-onset Still disease. J Rheumatol 44: 996-1003, 2017. [DOI] [PubMed] [Google Scholar]
- 49. Ruscitti P, Rago C, Breda L, et al. Macrophage activation syndrome in Still's disease: analysis of clinical characteristics and survival in paediatric and adult patients. Clin Rheumatol 36: 2839-2845, 2017. [DOI] [PubMed] [Google Scholar]
- 50. Ruscitti P, Cipriani P, Ciccia F, et al. Prognostic factors of macrophage activation syndrome, at the time of diagnosis, in adult patients affected by autoimmune disease: analysis of 41 cases collected in 2 rheumatologic centers. Autoimmun Rev 16: 16-21, 2017. [DOI] [PubMed] [Google Scholar]
- 51. Fukuoka K, Miyamoto A, Ozawa Y, et al. Adult-onset Still's disease-like manifestation accompanied by the cancer recurrence after long-term resting state. Mod Rheumatol 29: 704-707, 2019. [DOI] [PubMed] [Google Scholar]
- 52. Hofheinz K, Schett G, Manger B. Adult onset Still's disease associated with malignancy-cause or coincidence? Semin Arthritis Rheum 45: 621-626, 2016. [DOI] [PubMed] [Google Scholar]
- 53. Boddaert J, Huong DL, Amoura Z, Wechsler B, Godeau P, Piette JC. Late-onset systemic lupus erythematosus: a personal series of 47 patients and pooled analysis of 714 cases in the literature. Medicine (Baltimore) 83: 348-359, 2004. [DOI] [PubMed] [Google Scholar]
- 54. Lalani S, Pope J, de Leon F, Peschken C. Clinical features and prognosis of late-onset systemic lupus erythematosus: results from the 1000 faces of lupus study. J Rheumatol 37: 38-44, 2010. [DOI] [PubMed] [Google Scholar]
- 55. Kadavath S, Efthimiou P. Adult-onset Still's disease-pathogenesis, clinical manifestations, and new treatment options. Ann Med 47: 6-14, 2015. [DOI] [PubMed] [Google Scholar]
- 56. Wouters JM, van Rijswijk MH, van de Putte LB. Adult onset Still's disease in the elderly: a report of two cases. J Rheumatol 12: 791-793, 1985. [PubMed] [Google Scholar]