Abstract
A 59-year-old man who was receiving lenvatinib as a third-line tyrosine kinase inhibitor to treat hepatocellular carcinoma and multiple bone metastases complained of general fatigue four months after starting lenvatinib. A blood examination showed unexpectedly elevated serum C-reactive protein (CRP) levels. Computed tomography (CT) revealed rupture of the gallbladder wall, indicating gallbladder perforation. After conservative treatment, the patient received lenvatinib again under informed consent; however, one month later, CT revealed repeated rupture of the gallbladder wall. Gallbladder perforation had again been induced by lenvatinib. For this reason, lenvatinib is strongly considered a causative drug for gallbladder perforation.
Keywords: hepatocellular carcinoma, lenvatinib, bone metastasis, gallbladder perforation
Introduction
For the past decade, sorafenib has been the only first-line systemic therapy with proven survival benefits for unresectable hepatocellular carcinoma (uHCC) patients (1,2). Only regorafenib and ramucirumab are approved in Japan as second-line systemic therapies for patients who do not respond to sorafenib (3,4). A recent phase III trial comparing the overall survival in patients treated with lenvatinib versus sorafenib as the first-line therapy for uHCC revealed that lenvatinib was associated with a noninferior overall survival and significant improvements in the progression-free survival, time to progression, and objective response rate compared to sorafenib (5). In March 2018, lenvatinib was approved for patients with uHCC as the first-line systemic therapy in Japan.
The phase III trial and real-world studies have shown that common adverse events (AEs) are fatigue, hypertension, proteinuria, decreased appetite, diarrhea, hypothyroidism, and hand-foot syndrome (5-7). The incidence of serious AEs, such as hepatic failure, cerebral hemorrhaging, and respiratory failure, was 2% in the lenvatinib group, which was roughly the same as that in the sorafenib group (5). Thus, lenvatinib, like sorafenib, is considered safe and tolerable for uHCC patients.
However, a rare but serious AE of acalculous cholecystitis has been reported in patients with thyroid cancer and those with uHCC treated with lenvatinib in post-marketing surveillance and a phase III trial (5), respectively. Unfortunately, the causal relationship between lenvatinib and cholecystitis remains unclear.
We herein report a case of repeated acalculous gallbladder perforation in a patient with uHCC receiving lenvatinib. Gallbladder perforation was reproduced by treatment with lenvatinib, strongly suggesting that lenvatinib is a causative drug for gallbladder perforation.
Case Report
A 59-year-old man with alcoholic liver cirrhosis developed HCC at liver segment 8 (Fig. 1). Hepatectomy or radiofrequency ablation (RFA) was considered, and the patient decided to receive RFA under informed consent.
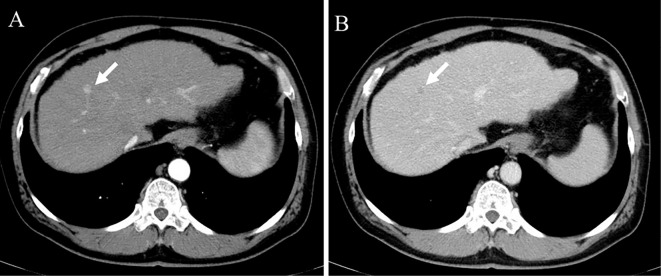
Figure 1.
Computed tomography imaging. CT in the arterial (A) and portal (B) phases revealed a small hepatocellular carcinoma at liver segment 8 (arrow). CT: computed tomography
One year after receiving RFA, the patient complained of numbness in the left leg. Magnetic resonance imaging revealed metastatic bone tumors at the Th-8, Th-11, and L-3 vertebrae (Fig. 2A), and a bone biopsy revealed metastatic HCC (Fig. 2B, C). After radiation therapy, sorafenib was administered. Three months after starting sorafenib, the patient developed acute myocardial infarction (AMI), and percutaneous coronary intervention was performed. Although the patient resumed sorafenib, bone metastases progressed at the sternum, sacrum, skull base, and C-3 vertebra. Thirteen months after starting sorafenib, the patient received regorafenib as a second-line therapy. Due to the progression of HCC, seven months later, lenvatinib was started at a dose of 4 mg/day because of thrombocytopenia, and one month later, the dose was increased to 8 mg/day.
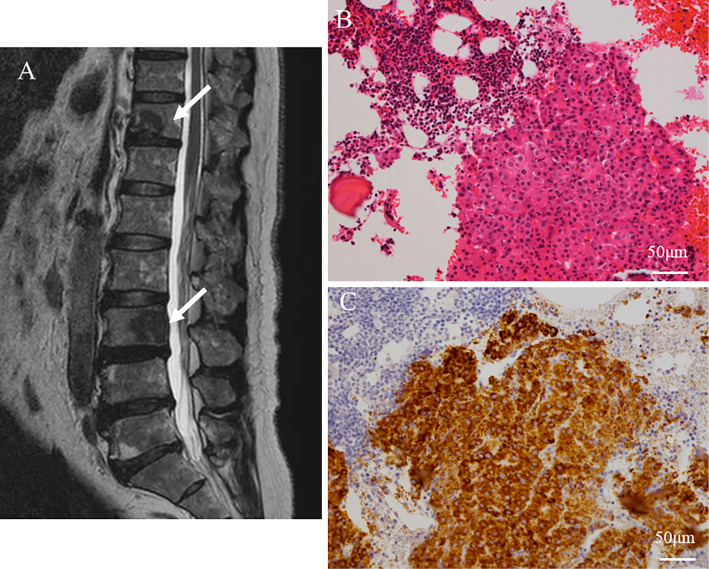
Figure 2.
Bone metastases of hepatocellular carcinoma. Magnetic resonance imaging revealed metastatic bone tumors at the Th-11 and L-3 vertebrae (arrows) (A), and then a bone biopsy was performed at the L-3 vertebra. Histopathological examinations of the bone biopsy specimens showed a thick trabecular pattern of tumor cells (Hematoxylin and Eosin staining) (B). Immunohistochemical staining was positive for Hep Par 1, suggesting metastatic hepatocellular carcinoma (Hep Par 1) (C).
However, three months later, the patient complained of general fatigue without abdominal pain. Blood examinations revealed marked elevation of the serum C-reactive protein (CRP) levels (Fig. 3, Table 1). Although previous computed tomography (CT) scans had not shown gallbladder stones, bile duct stones or tumors, and gallbladder metastasis of HCC (Fig. 4A), CT and ultrasonography (US) now revealed rupture of the gallbladder wall and ascites around the gallbladder, indicating gallbladder perforation (Fig. 4B, C).
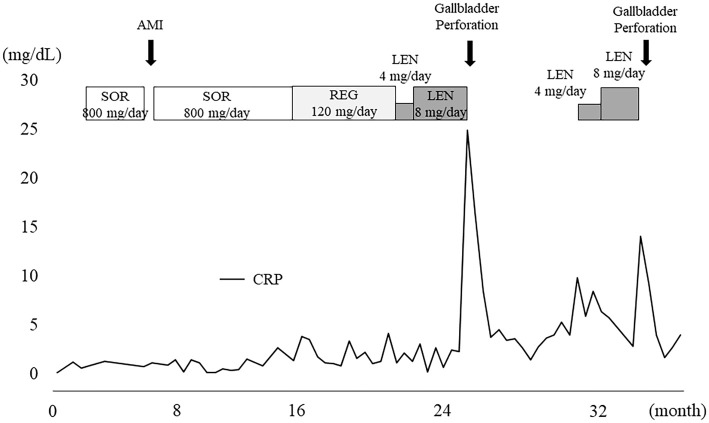
Figure 3.
Clinical course. After the administration of LEN, the serum CRP levels suddenly increased. The first gallbladder perforation developed. After resuming LEN, the serum CRP levels increased and the gallbladder perforation was reproduced. SOR: sorafenib, AMI: acute myocardial infarction, REG: regorafenib, LEN: lenvatinib, CRP: C-reactive protein, CT: computed tomography
Table 1.
Laboratory Data on First Gallbladder Perforation.
| WBC | 7,600 | /µL | LDH | 197 | mg/dL | |||
| Neut | 82.0 | % | γGTP | 112 | U/L | |||
| Lymp | 10.5 | % | ChE | 118 | U/L | |||
| Mono | 7.5 | % | BUN | 37.9 | mg/dL | |||
| Eos | 1.5 | % | Cre | 0.92 | mg/dL | |||
| Baso | 0.0 | % | Na | 129 | mEq/L | |||
| RBC | 2.47 | ×106/µL | K | 4.4 | mEq/L | |||
| Hb | 7.8 | g/dL | Cl | 97 | mEq/L | |||
| PLT | 9.7 | ×106/µL | CRP | 24.97 | mg/dL | |||
| AST | 41 | U/L | AFP | 8.5 | ng/mL | |||
| ALT | 33 | U/L | PIVKA-II | 26,430 | mAU/mL | |||
| ALP | 983 | mg/dL |
AFP: α-fetoprotein, PIVKA-II: protein induced by vitamin K absence or antagonist-II
Figure 4.
Imaging of the first gallbladder perforation. CT revealed no gallbladder stones or gallbladder metastases of HCC (A). CT showed rupture of the gallbladder wall (arrow) and ascites around the gallbladder, suggesting gallbladder perforation (B). US also indicated rupture of the gallbladder wall (arrow) (C). CT: computed tomography, HCC: hepatocellular carcinoma, US: ultrasonography
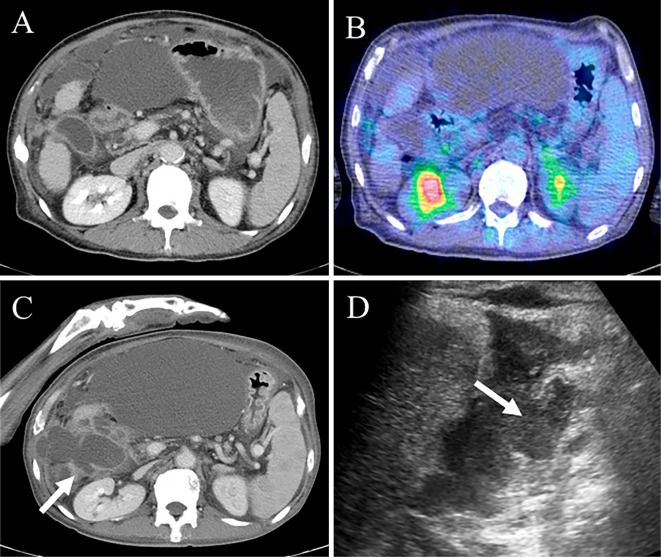
Because the general condition of the patient was poor, he received conservative treatment and did not undergo cholecystectomy. Fortunately, CT revealed improvement in the gallbladder perforation with conservative treatment alone (Fig. 5A); however, he was unable to receive cholecystectomy because his general condition was still poor, including massive ascites after improvement in the gallbladder perforation (Fig. 5A). The 18F-fluorodeoxy glucose-positron emission tomography/CT (18F-FDG PET/CT) findings did not show any FDG uptake in the gallbladder or biliary tract, indicating the absence of gallbladder metastases of HCC and other biliary diseases (Fig. 5B).
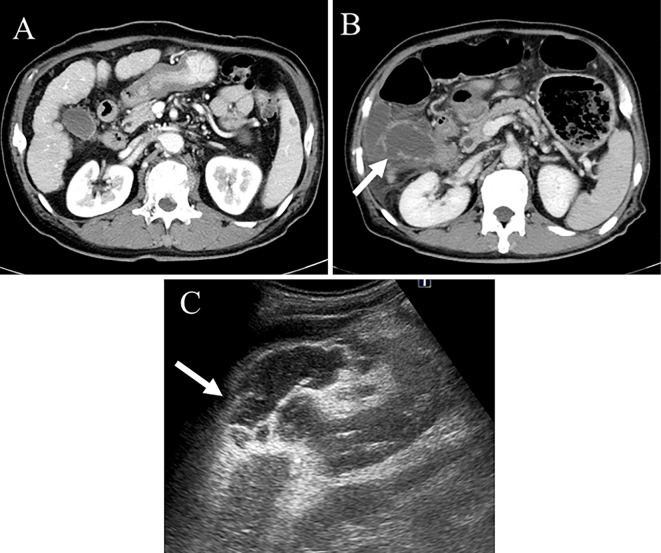
Figure 5.
Imaging of the second gallbladder perforation. CT showed improvement in the gallbladder perforation, but massive ascites remained (A). The 18F-FDG PET/CT findings did not show any FDG uptake in the gallbladder (B). CT and US showed rupture of the gallbladder wall (arrow) (C, D). CT: computed tomography, 18F-FDG PET/CT: 18F-fluorodeoxy glucose-positron emission tomography/computed tomography, US: ultrasonography
During treatment, the tumor markers were increased, and bone metastases progressed, so lenvatinib was resumed five months later under informed consent, including awareness of the possibility of gallbladder perforation. The patient was retreated with lenvatinib at a dose of 4 mg/day, and subsequently, the dose was increased to 8 mg/day. However, one month later, the patient complained of general fatigue with elevated serum CRP levels (Fig. 3, Table 2). CT and US revealed gallbladder perforation similar to the previous occurrence (Fig. 5C, D). Ascites around the gallbladder fluid was bloody, and the cytology indicated marked inflammatory cells without atypical cells. After gallbladder perforation, lenvatinib was immediately discontinued. Although gallbladder perforation was improved by conservative treatment again, the patient died 48 days after the second incident of gallbladder perforation because of progression of HCC.
Table 2.
Laboratory Data on Second Gallbladder Perforation.
| WBC | 6,500 | /µL | LDH | 190 | mg/dL | |||
| Neut | 82.4 | % | γGTP | 48 | U/L | |||
| Lymp | 10.2 | % | ChE | 81 | U/L | |||
| Mono | 7.2 | % | BUN | 12.3 | mg/dL | |||
| Eos | 0.6 | % | Cre | 0.47 | mg/dL | |||
| Baso | 0.2 | % | Na | 128 | mEq/L | |||
| RBC | 2.30 | ×106/µL | K | 4.1 | mEq/L | |||
| Hb | 7.8 | g/dL | Cl | 96 | mEq/L | |||
| PLT | 6.3 | ×106/µL | CRP | 14.10 | mg/dL | |||
| AST | 39 | U/L | AFP | 1,554.8 | ng/mL | |||
| ALT | 16 | U/L | PIVKA-II | 65,234 | mAU/mL | |||
| ALP | 494 | mg/dL |
AFP: α-fetoprotein, PIVKA-II: protein induced by vitamin K absence or antagonist-II
Discussion
In the phase III trial, only 1 case (0.2%) of grade ≥ 3 acute cholecystitis was reported (5). In patients with thyroid cancer treated with lenvatinib, five cases of acalculous cholecystitis were reported in post-marketing surveillance from 2015 to 2017 in Japan. In contrast, five cases of acute acalculous cholecystitis in patients treated with other tyrosine kinase inhibitors (TKIs) were reported, including two patients with sorafenib and three with sunitinib (8-12).
We herein report the first case of repeated perforation of the gallbladder in a patient with HCC who was treated with lenvatinib. In our case, retreatment with lenvatinib caused gallbladder perforation again, strongly suggesting lenvatinib-related gallbladder perforation. There are some possible reasons why lenvatinib causes gallbladder perforation. One possible reason is the shrinkage of metastatic gallbladder tumors. A case of lenvatinib-related gastrointestinal perforation was previously reported in a patient with thyroid cancer (13). The pathological findings from the surgical resection of the perforated sites revealed perforations at the primary cancer, anastomotic site, and abdominal carcinomatosis (14,15). These findings indicate that one mechanism of gastrointestinal perforation may be related to the rapid tumor shrinkage induced by lenvatinib. Similarly, gallbladder perforation might develop because of the rapid shrinkage of metastatic gallbladder tumors. The incidence of HCC metastasis to the gallbladder is 2.8-5.8% of the extrahepatic metastases in autopsy studies (16,17). However, in the present case, CT and 18F-FDG PET/CT findings did not indicate the presence of HCC at the gallbladder. In addition, after gallbladder perforation, the cytology around the gallbladder fluid did not indicate positive HCC, suggesting that gallbladder metastasis was likely absent. The second possible reason is the strong anti-angiogenic effect of this drug. Lenvatinib, which is a TKI that targets vascular endothelial growth factor receptors (VEGFRs) 1-3 and fibroblast growth factors receptors (FGFRs) 1-4, can inhibit VEGF- and FGF-driven angiogenesis (18-20). The homeostasis between the vascular endothelium and platelets may be disturbed by lenvatinib-mediated damage to vascular endothelial cells, allowing platelets to aggregate more readily on the surface of the vascular endothelium and inducing ischemic damage (21). Although the relationship between TKIs and cholecystitis is unclear, previous reports suggest that this TKI-related ischemic damage is likely the pathologic mechanism of sorafenib and sunitinib-related cholecystitis (8-11). In fact, in the present case, AMI developed during sorafenib treatment; thus, the vascular endothelial cells may be susceptible to damage by TKIs. Furthermore, the patient had suffered from diabetes mellitus for six years, which might have promoted ischemic damage to the vascular endothelial cells. Finally, VEGF plays a role in modulating cholangiocyte proliferation in response to cholestasis and cholangiocytes express VEGFRs (22). Inhibiting VEGFRs in the biliary tract cells can induce an imbalance in stress adaption, causing biliary disease, including cholecystitis (11).
Of note, the patient had received treatment with multiple TKIs for a long time, so long-term treatment with TKIs might have induced the gallbladder perforation. However, previous reports (8-12) showed that cholecystitis developed about four weeks after initiating TKIs. These findings suggest that TKI-related cholecystitis developed after a relatively short treatment duration. In the present case, gallbladder perforation developed four months after lenvatinib and was reproduced one month after resuming lenvatinib. Although we cannot exclude the possibility that long-term treatment with TKIs might have induced the gallbladder perforation, lenvatinib was strongly considered a causative drug for gallbladder perforation.
The patient in the present case did not complain of abdominal pain even when gallbladder perforation developed. Since the patient had been treated with opioids because of cancer pain from bone metastases, the abdominal pain might have been masked by the opioid effects. Bone is a common metastatic site of HCC (25.4-38.5%) (23,24); therefore, patients treated with lenvatinib are often administered opioids because of cancer pain from bone metastases. Thus, even if patients do not complain of abdominal pain, radiological examinations should be performed when blood examinations show unexpectedly high serum CRP levels.
In summary, we described a case of repeated gallbladder perforation in a patient with metastatic HCC receiving lenvatinib. Lenvatinib was strongly considered a causative drug for gallbladder perforation. Hepatologists and oncologists should be aware of the risk of gallbladder perforation, which is a rare but serious adverse effect, when patients are treated with lenvatinib.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378-390, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 25-34, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389: 55-66, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Chau I, Peck-Radosavljevic M, Borg C, et al. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib: patient-focused outcome results from the randomised phase III REACH study. Eur J Cancer 81: 17-25, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 39: 1163-1173, 2018. [DOI] [PubMed] [Google Scholar]
- 6.Obi S, Sato T, Sato S, et al. The efficacy and safety of lenvatinib for advanced hepatocellular carcinoma in a real-world setting. Hepatol Int 13: 199-204, 2019. [DOI] [PubMed] [Google Scholar]
- 7.Hiraoka A, Kumada T, Kariyama K, et al. Therapeutic potential of lenvatinib for unresectable hepatocellular carcinoma in clinical practice: multicenter analysis. Hepatol Res 49: 111-117, 2019. [DOI] [PubMed] [Google Scholar]
- 8.Aihara Y, Yoshiji H, Yamazaki M, et al. A case of severe acalculous cholecystitis associated with sorafenib treatment for advanced hepatocellular carcinoma. World J Gastrointest Oncol 4: 115-118, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanda M, Tamai H, Deguchi H, et al. Acalculous cholecystitis in a patient with hepatocellular carcinoma on sorafenib. ISRN Gastroenterol 2011: 201529, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Lima Lopes G Jr, Rocha Lima CM. Emphysematous cholecystitis in a patient with gastrointestinal stromal tumor treated with sunitinib. Pharmacotherapy 27: 775-777, 2007. [DOI] [PubMed] [Google Scholar]
- 11.da Fonseca LG, Barroso-Sousa R, Sabbaga J, Hoff PM. Acute acalculous cholecystitis in a patient with metastatic renal cell carcinoma treated with sunitinib. Clin Pract 4: 635, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gomez-Abuin G, Karam AA, Mezzadri NA, Bas CA. Acalculous cholecystitis in a patient with metastatic renal cell carcinoma treated with sunitinib. Clin Genitourin Cancer 7: 62-63, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Date E, Okamoto K, Fumita S, Kaneda H. Gastrointestinal perforation related to lenvatinib, an anti-angiogenic inhibitor that targets multiple receptor tyrosine kinases, in a patient with metastatic thyroid cancer. Invest New Drugs 36: 350-353, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Saif MW, Elfiky A, Salem RR. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann Surg Oncol 14: 1860-1869, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Badgwell BD, Camp ER, Feig B, et al. Management of bevacizumab-associated bowel perforation: a case series and review of the literature. Ann Oncol 19: 577-582, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer 7: 462-503, 1954. [DOI] [PubMed] [Google Scholar]
- 17.Nakashima T, Okuda K, Kojiro M, et al. Pathology of hepatocellular carcinoma in Japan. 232 consecutive cases autopsied in ten years. Cancer 51: 863-877, 1983. [DOI] [PubMed] [Google Scholar]
- 18.Matsui J, Funahashi Y, Uenaka T, et al. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res 14: 5459-5465, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): an angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res 2014: 638747, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto Y, Matsui J, Matsushima T, et al. Lenvatinib, an angiogenesis inhibitor targeting VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft models associated with microvessel density and pericyte coverage. Vasc Cell 6: 18, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elice F, Rodeghiero F, Falanga A, Rickles FR. Thrombosis associated with angiogenesis inhibitors. Best Pract Res Clin Haematol 22: 115-128, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 130: 1270-1282, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer 117: 4475-4483, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol 20: 1781-1787, 2005. [DOI] [PubMed] [Google Scholar]