Abstract
Objective
In Japan, the aging demographic structure is becoming pronounced, and the full-blown graying of society appears not far off, which indicates an increasing population that will require healthcare contact. Klebsiella spp. are major pathogens in healthcare-associated infections, and their importance is increasing. The aim of this study was to clarify the characteristics of Klebsiella spp. chest infections by evaluating the differences in the characteristics of chest infections caused by Klebsiella spp. and pneumoniae.
Methods
We conducted a retrospective study of consecutive patients hospitalized with pneumonia, lung abscess/necrotizing pneumonia, and empyema due to Klebsiella spp. and S. pneumoniae for 15 years at our institution in Saitama, Japan.
Patients
Patients with chest infections due to Klebsiella spp. (K group, n=76) and S. pneumoniae (S group, n=446) were included.
Results
The K group more frequently was male, older, coinfected by Pseudomonas aeruginosa, and had diabetes mellitus, a history of upper digestive system surgery, alcohol drinking habit, a smoking habit, and an impaired premorbid performance status than the S group. The percentages of lung abscesses or necrotizing pneumonia (31.6% vs. 0.9%) and empyema without pulmonary parenchymal shadow (3.9% vs. 0.7%) were higher in the K group than those in the S group. Severity on admission and mortality did not differ between the groups; however, patients in the K group required a longer duration of antibiotics administration and hospital stay than those in the S group.
Conclusion
Klebsiella spp. chest infections have some marked characteristics when compared with pneumococcal infections, and our results serve to differentiate Klebsiella spp. infection from pneumococcal infection.
Keywords: Streptococcus pneumoniae, Klebsiella pneumoniae, lung abscess, necrotizing pneumonia, pulmonary gangrene
Introduction
Klebsiella spp. are Gram-negative rods that can lead to a wide range of disease states, most notably chest and urinary tract infections, septicemia, meningitis, diarrhea, and soft tissue infections. Major chest infections due to Klebsiella spp. include pneumonia, lung abscess, and empyema. Klebsiella spp. infection is an independent prognostic factor in pneumonia (1,2), the mortality of which was reported to be 41% (7 of 15 patients) within 14 days after diagnosis (3,4).
In Japan, the aging demographic structure is becoming pronounced, and the full-blown graying of society appears to be not far off, which indicates an increasing population that will require healthcare contact. Klebsiella spp. are major pathogens in healthcare-associated infections, and their importance appears to be increasing (5). One clinical report from an Asian tertiary teaching hospital showed that Klebsiella spp. and Streptococcus pneumoniae are two leading pathogens of healthcare-associated pneumonia (6). It has been reported that patients with K. pneumoniae chest infection frequently suffer a rapid, fatal outcome (7), and physicians should understand the clinical characteristics of Klebsiella chest infections; however, there have been no recent reports investigating how chest infections due to Klebsiella spp. differ from those due to other pathogens.
We therefore investigated the characteristics of Klebsiella chest infections by comparing the differences in clinical and radiologic features of chest infections due to Klebsiella spp. with those due to S. pneumoniae, which is a major pathogen of chest infections.
Materials and Methods
We conducted a retrospective study of consecutive patients hospitalized with pneumonia [community-acquired pneumonia (CAP) or healthcare-associated pneumonia (HCAP)], lung abscess/necrotizing pneumonia, and empyema due to Klebsiella spp. and S. pneumoniae from January 2002 through November 2017 at our institution in Saitama, Japan.
Lung abscess/necrotizing pneumonia was diagnosed when chest computed tomography (CT) showed cavitary lesions in areas of pulmonary consolidation. Empyema was diagnosed when the pleural effusion was pus or when it met the criteria of class 4 to 7 pleural effusion (8). Parapneumonic effusion was diagnosed when pleural effusion did not meet the above criteria of empyema or could not be evaluated because of its small amount.
Smoking status was classified into current smoker (someone who has smoked greater than 100 cigarettes in their lifetime and has smoked in the last 28 days), ex-smoker (someone who has smoked greater than 100 cigarettes in their lifetime but has not smoked in the last 28 days), and never-smoker (someone who has not smoked greater than 100 cigarettes in their lifetime and does not currently smoke). Alcohol habit (drinking level) was classified into 3 levels: heavy drinking (consumption of 8 or more drinks per week for women and 15 or more drinks per week for men) (9), moderate drinking (up to 1 drink per day for women and up to 2 drinks per day for men), and slight drinking (fewer than the moderate level) or non-drinking. The performance status (PS) (10) of the patients for performing daily life activities before the development of chest infections was recorded on admission based on anamnesis from the patients and their families. Excluded patients comprised those with tuberculosis or a confirmed alternative diagnosis lasting until the end of the follow-up period.
Diagnosis of causative microorganisms was based on the results from the semiquantitative culture of respiratory samples or blood, paired sera, urinary antigen tests for S. pneumoniae and Legionella pneumophila, and nasopharyngeal rapid diagnostic test and reverse transcription polymerase chain reaction for influenza virus, as reported previously (11,12). Biochemical bacterial identification of the isolated bacteria from patients was performed with an automated bacterial identification system (Vitek 2 GN; Sysmex bioMérieux, Tokyo, Japan). Broth microdilution was carried out according to the Clinical and Laboratory Standards Institute (CLSI) guidelines for antibiotics (13). The production of extended-spectrum beta-lactamase (ESBL) was screened and confirmed in accordance with the standards of the CLSI (12). HCAP was defined according to the criteria of the American Thoracic Society/Infectious Disease Society of America (ATS/IDSA) guidelines (4). Severe disease was defined when at least 1 major criterion (invasive mechanical ventilation, septic shock with the need for vasopressors) or 3 minor criteria (respiratory rate ≥30 breaths/min, partial pressure of arterial oxygen (PaO2)/fraction of inspiratory oxygen (FiO2) ratio ≤250, multilobar infiltrates, confusion/disorientation, uremia, leukopenia, thrombocytopenia, hypothermia, hypotension requiring aggressive fluid resuscitation) of the IDSA/ATS guidelines (14) were present.
Approval for this study was obtained from the institutional clinical research ethics board of Saitama Cardiovascular and Respiratory Center.
Statistical analyses
The results are presented as numbers and percentages, median (range) or means ± standard deviation unless otherwise indicated. Fisher's exact test and Student's t-test were used for categorical and continuous data, respectively. In all instances, a 2-tailed p value of <0.05 was considered to indicate statistical significance. All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, USA).
Results
Patients
From January 2002 to December 2017, 76 patients with Klebsiella spp. (K group) and 446 patients with pneumococcal (S group) chest infections were admitted to our institution. All patients were ≥18 years old. There were no HIV-positive patients, patients with non-resected lung cancer, or those undergoing chemotherapeutic treatment. Three patients had mixed infection of both S. pneumoniae and Klebsiella spp. and were excluded.
Clinical features of each group are listed in Table 1. The K group was older and had more males and patients with an impaired premorbid PS than the S group. Alcohol habit in the K group included heavy drinking in 10, moderate drinking in 18, and slight/no drinking in 46 patients. The frequencies of heavy drinking and moderate drinking were greater in the K group than in the S group. Diabetes mellitus and a history of upper digestive system surgery were more frequent in the K group than in the S group.
Table 1.
Patient Characteristics, Laboratory Data, and Outcomes.
| Characteristic | Klebsiella spp. infection (n=76) | Pneumococcal infection (n=446) | p value | |||
|---|---|---|---|---|---|---|
| Infection type | ||||||
| Pneumonia | 45 (59.2%) | 436 (97.8%) | <0.0001 | |||
| Lung abscess/necrotizing pneumonia | 24 (31.6%) | 4 (0.9%) | <0.0001 | |||
| Pneumonia plus empyema | 2 (2.6%) | 2 (0.4%) | 0.1032 | |||
| Lung abscess/necrotizing pneumonia plus empyema | 2 (2.6%) | 1 (0.2%) | 0.0569 | |||
| Empyema | 3 (3.9%) | 3 (0.7%) | 0.0428 | |||
| Sex, male | 66 (86.8%) | 308 (69.1%) | 0.0014 | |||
| Age, mean years (standard deviation) | 72.3 (10.95) | 68.8 (13.90) | 0.0337 | |||
| Vaccine history, yes | ||||||
| PPV | 2 (2.6%) | 20 (4.5%) | 0.7561 | |||
| Influenza | 3 (3.9%) | 29 (6.5%) | 0.6035 | |||
| Smoking status, current smokers | 61 (80.3%) | 283 (63.5%) | 0.0039 | |||
| Alcohol habit, Heavy drinking/Usual/Slight or no drinking | 10/18/5 | 8/27/411 | <0.0001 | |||
| LTOT | 5 (6.6%) | 35 (7.8%) | 0.8192 | |||
| Premorbid PS, 0/1-2/3-4 | 27/25/11 | 240/114/34 | 0.0158 | |||
| Type of pneumonia, CAP | 47 (61.8%) | 303 (67.9%) | 0.2943 | |||
| Underlying diseases, yes | ||||||
| COPD | 15 (19.7%) | 106 (23.8%) | 0.5563 | |||
| Bronchiectasis | 6 (7.9%) | 20 (4.5%) | 0.2475 | |||
| Pulmonary non-tuberculous mycobacteria | 7 (9.2%) | 19 (4.3%) | 0.0831 | |||
| Old tuberculosis | 4 (5.3%) | 22 (4.9%) | 0.7814 | |||
| Chronic pulmonary aspergillosis | 0 (0.0%) | 7 (1.6%) | 0.6009 | |||
| Interstitial pneumonia | 3 (3.9%) | 31 (7.0%) | 0.4528 | |||
| History of lung cancer surgery | 3 (3.9%) | 11 (2.5%) | 0.4407 | |||
| Pneumoconiosis | 0 (0.0%) | 7 (1.6%) | 0.6009 | |||
| Other pulmonary diseases | 3 (3.9%) | 4 (0.9%) | 0.0671 | |||
| Hypertension | 11 (14.5%) | 45 (10.1%) | 0.3138 | |||
| Congestive heart failure | 4 (5.3%) | 26 (5.8%) | 1.0000 | |||
| Ischemic heart disease | 1 (1.3%) | 21 (4.7%) | 0.2279 | |||
| Diabetes mellitus | 20 (26.3%) | 47 (10.5%) | 0.0006 | |||
| Cerebral vascular disease | 4 (5.3%) | 32 (7.2%) | 0.8058 | |||
| Dementia | 2 (2.6%) | 4 (0.9%) | 0.2126 | |||
| Neuromuscular disease | 4 (5.3%) | 5 (1.1%) | 0.0295 | |||
| History of upper digestive system surgery | 7 (9.2%) | 14 (3.1%) | 0.0222 | |||
| Chronic liver disease | 2 (2.6%) | 12 (2.7%) | 1.0000 | |||
| Connective tissue diseases | 4 (5.3%) | 14 (3.1%) | 0.3150 | |||
| Steroid or immunosuppressant | 3 (3.9%) | 27 (6.1%) | 0.6006 | |||
| Malignant disease | 1 (1.3%) | 13 (2.9%) | 0.7041 | |||
| CKD | 2 (2.6%) | 8 (1.8%) | 0.6448 | |||
| None | 9 (11.8%) | 94 (21.1%) | 0.0628 | |||
| Blood tests, median (range) [number measured] | ||||||
| WBC (/mm3) | 10,600.0 (1,300-240) [76] | 11,300.0 (600-47,100) [446] | 0.1170 | |||
| Platelets (×104/mm3) | 26.9 (0.8-64.8) [62] | 22.9 (4.8-86.8) [117] | 0.0068 | |||
| AST (IU/L) | 24.0 (7-514) [75] | 25.0 (9-431) [442] | 0.3553 | |||
| LDH (IU/L) | 208.0 (41-978) [76] | 218.0 (17-923) [437] | 0.8215 | |||
| CK (IU/L) | 76.5 (13-1,384) [58] | 73.0 (7-20,485) [400] | 0.4981 | |||
| BUN (mg/dL) | 16.0 (7-123) [65] | 18.0 (5-98) [437] | 0.5943 | |||
| Creatinine (mg/dL) | 0.7 (0.3-2.4) [65] | 0.7 (0.3-3.4) [440] | 0.9052 | |||
| Na (mmol/L) | 138.0 (127-153) [60] | 137.0 (116-148) [143] | 0.1019 | |||
| CRP (mg/dL) | 13.3 (0.8-54.6) [65] | 13.5 (0.09-54.3) [445] | 0.6862 | |||
| Duration, days, mean±standard deviation | ||||||
| Antibiotic administration | 13.3±6.2 | 9.5±4.6 | <.0001 | |||
| Hospital stay | 22.6±19.7 | 17.0±19.2 | 0.0179 | |||
| Hospital mortality | 5 (6.6%) | 22 (4.9%) | 0.5731 |
PPV: pneumococcal polysaccharide vaccine, LTOT: long-term oxygen therapy, PS: performance status, CAP: community-acquired pneumonia, COPD: chronic obstructive pulmonary disease, CKD: chronic kidney disease, WBC: white blood cell count (/mm3), AST: aspartate transaminase, LDH: lactate dehydrogenase, CK: creatine kinase, BUN: blood urea nitrogen, CRP: C-reactive protein
Types of chest infections
Pulmonary infection was found in 73 (pneumonia in 45 and lung abscess in 28) of the 76 patients in the K group and in 443 (pneumonia in 438 and lung abscess in 5) of the 446 patients in the S group (Table 1). Empyema was found in 5 of the 76 patients in the K group and in 6 of the 446 patients in the S group. Two of the 5 patients with empyema in the K group were complicated with pulmonary infection, and 3 did not show any pulmonary shadows. Compared with the S group, the K group had a higher frequency of patients with a lung abscess or necrotizing pneumonia alone (31.6% vs. 0.9%) and empyema alone (3.9% vs. 0.7%) but a lower frequency of patients with pneumonia alone (59.2% vs. 99.1%).
Klebsiella subspecies
The Klebsiella subspecies identified included K. pneumoniae (n=72), K. rhinoscleromatis (n=1), K. oxytoca (n=1), and K. ozaenae (n=2).
Etiology of mixed infection
Diagnostic methods used to identify etiologies in both the K and S groups are shown in Table 2. Mixed infection was found on admission in 19 patients (25.0%). The most common microorganism causing mixed infection in the K group was Pseudomonas aeruginosa (n=6), followed by influenza virus (n=4) and other microorganisms (Table 3). Patients in the K group were more frequently coinfected by P. aeruginosa than those in the S group (7.9% vs. 0.4%, p=0.0002).
Table 2.
Diagnostic Methods and Results of the Patients in the Present Study (n=522).
| Method | No. of samples studied | No. of positive diagnostic studies (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall pathogens | Klebsiella spp. infection | Pneumococcal infection | ||||||
| Paired sera | 343 | 36 (10.5) | 4 (10.8) | 32 (10.5) | ||||
| Rapid influenza diagnostic test | 497 | 41 (8.2) | 4 (7.1) | 36 (8.3) | ||||
| Urinary antigen | 484 | 394 (81.4) | 1 (2.0) | 386 (90.4) | ||||
| Culture | ||||||||
| Sputum | 477 | 215 (45.0) | 53 (89.8) | 157 (39.3) | ||||
| Transbronchial aspirate | 23 | 9 (39.1) | 6 (60.0) | 3 (25.0) | ||||
| Protected specimen brush | 15 | 13 (86.7) | 12 (100) | 1 (33.3) | ||||
| Bronchial washing | 18 | 12 (66.7) | 7 (77.8) | 5 (62.5) | ||||
| Bronchoalveolar lavage fluid | 7 | 3 (42.9) | 1 (14.3) | 2 (33.3) | ||||
| Blood | 340 | 26 (7.6) | 4 (9.8) | 22 (7.4) | ||||
| Pleural fluid | 18 | 9 (50.0) | 4 (66.7) | 4 (36.4) | ||||
Table 3.
Etiologies of Mixed Infection.
| Species | Klebsiella spp. infection | Pneumococcal infection | p value | |||
|---|---|---|---|---|---|---|
| Mixed infection | 19 (25.0%) | 104 (23.3%) | 0.7704 | |||
| Haemophilus influe nzae | 1 (1.3%) | 17 (3.8%) | 0.4934 | |||
| Legionella sp. | 0 (0.0%) | 6 (1.3%) | 0.5997 | |||
| Influenza virus | 4 (5.3%) | 53 (11.9%) | 0.1101 | |||
| Pseudomonas aerugino sa | 6 (7.9%) | 2 (0.4%) | 0.0002 | |||
| Moraxella catar rhalis | 0 (0.0%) | 5 (1.1%) | 1.0000 | |||
| Enterobacteriaceae other than Klebsiella spp. | 1 (1.3%) | 1 (0.2%) | 0.2702 | |||
| Streptococcus sp. other than pneumococcus | 2 (2.6%) | 1 (0.2%) | 0.0569 | |||
| Chlamydophila pneu moniae | 2 (2.6%) | 16 (3.6%) | 1.0000 | |||
| Chlamydophi la psittaci | 1 (1.3%) | 1 (0.2%) | 0.2702 | |||
| MSSA | 1 (1.3%) | 5 (1.1%) | 1.0000 | |||
| Others | 1 (1.3%) | 1 (0.2%) | 0.2702 |
MSSA: methicillin-sensitive Staphylococcus aureus
Laboratory data on admission
Laboratory data on admission are shown in Table 1. The platelet count of patients in the K group was significantly higher than that of patients in the S group (median, 26.9×104/mm3 vs. 22.9×104/mm3, p=0.007). The ratios of blood cultures positive for Klebsiella spp. and S. pneumoniae were 4 of 41 (9.8%) and 22 of 294 (7.5%), respectively, in the tested patients.
Antibacterial susceptibility of isolated Klebsiella spp.
The antibacterial susceptibilities of the isolated Klebsiella spp. are listed in Table 4. Susceptibility to penicillins was low; however, β-lactamase inhibitors apparently restored the activities of the penicillins. ESBL-producing Klebsiella spp. were found in one patient.
Table 4.
Antibacterial Susceptibility of Isolated Klebsiella species.
| Number tested | Susceptible | (%) | ||||
|---|---|---|---|---|---|---|
| ABPC | 50 | 0 | 0 | |||
| AMPC/CVA | 16 | 14 | 87.5 | |||
| ABPC/SBT | 28 | 26 | 92.9 | |||
| PIPC | 56 | 29 | 51.8 | |||
| PIPC/TAZ | 9 | 7 | 77.8 | |||
| CAZ | 57 | 56 | 98.2 | |||
| CEZ | 56 | 56 | 100 | |||
| CMZ | 55 | 55 | 100 | |||
| CTRX | 12 | 12 | 100 | |||
| CFPM | 28 | 28 | 100 | |||
| IPM | 56 | 56 | 100 | |||
| MEPM | 26 | 26 | 100 | |||
| GM | 58 | 58 | 100 | |||
| AMK | 58 | 57 | 98.3 | |||
| LVFX | 26 | 26 | 100 | |||
| CPFX | 28 | 27 | 96.4 | |||
| MINO | 57 | 54 | 94.7 | |||
| AZT | 58 | 48 | 85.8 |
ABPC: ampicillin, AMPC: amoxicillin, CVA: clavulanic acid, SBT: sulbactam, PIPC: piperacillin, TAZ: tazobactam, CAZ: ceftazidime, CEZ: cefazolin, CMZ: cefmetazole, CTRX: ceftriaxone, CFPM: cefepime, IPM: imipenem, MEPM: meropenem, GM: gentamicin, AMK: amikacin, LVFX: levofloxacin, CPFX: ciprofloxacin, MINO: minocycline, AZT: aztreonam
Severity on admission
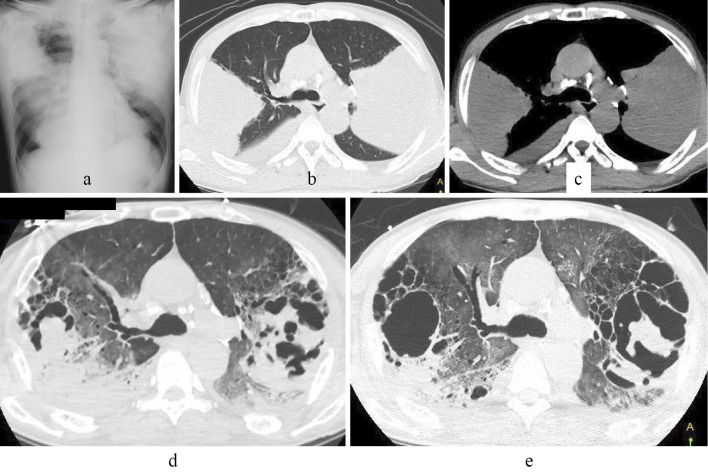
In the K group, 15 patients (19.7%) were in a severe condition on admission (Table 1) (K. pneumoniae, n=12, K. oryzae, n=2, K. rhinoscleromatis, n=1, K. oxytoca, n=1), and 7 (12.3%) patients required mechanical ventilation. The disease in one patient diagnosed with lung abscess/necrotizing pneumonia was compatible with pulmonary gangrene clinically (Figure). The frequency of patients with a severe condition did not differ significantly between the K and S groups (19.7% vs. 18.2%, p=0.7494).
Figure.
Images from chest X-ray and computed tomography (CT) performed at the local hospital of a 64-year-old man with a history of hypertension showed bilateral consolidation (a-c). On the 10th day after referral to our hospital (7th hospital day at our institution), CT showed bilateral consolidation with a hyperlucent area (d), and consolidation was replaced by cavitary lesions (e).
Treatment and outcomes
Antibiotics were administered to all patients following admission (Table 5). When compared with the patients in the S group, those in the K group more frequently received single penicillins, penicillins combined with β-lactamase inhibitors, and single antibiotic therapy as initial (empirical) antibiotics therapy. Piperacillin was administered to 4 patients of the K group who received single penicillins. Susceptibility of the isolated K. pneumoniae to piperacillin was good, and the patients recovered. Patients in the K group required antibiotics for a longer duration and showed a longer hospital stay than those in S group (Table 1). Five patients in the K group died (K. pneumonia, n=4, K. oryzae, n=1), but the in-hospital mortality did not differ significantly between the K and S groups.
Table 5.
Treatment and Outcomes.
| Klebsiella spp. infection (n=76) | Pneumococcal infection (n=446) | p value | |
|---|---|---|---|
| Mechanical ventilation | 7 (9.2%) | 19 (4.3%) | 0.0831 |
| Initial antibiotics | |||
| Carbapenems, 3rd- or 4th-generation cephalosporins plus macrolides | 3 (3.9%) | 62 (13.9%) | 0.0135 |
| Carbapenems, 3rd- or 4th-generation cephalosporins plus fluoroquinolones | 1 (1.3%) | 9 (2.0%) | 1.0000 |
| Carbapenems, 3rd- or 4th-generation cephalosporins plus minocyclines | 1 (1.3%) | 2 (0.4%) | 0.3769 |
| Single carbapenems, 3rd- or 4th-generation cephalosporins | 9 (11.8%) | 41 (9.2%) | 0.5255 |
| Penicillins plus macrolides | 0 (0%) | 2 (0.4%) | 1.0000 |
| Single penicillins | 4 (5.3%) | 4 (0.9%) | 0.0184 |
| Penicillins combined with β-lactamase inhibitors | 27 (35.5%) | 109 (24.4%) | 0.0480 |
| Penicillins combined with β-lactamase inhibitors plus macrolides | 27 (35.5%) | 179 (40.1%) | 0.5258 |
| Penicillins combined with β-lactamase inhibitors plus fluoroquinolones | 1 (1.3%) | 13 (2.9%) | 0.7041 |
| Penicillins combined with β-lactamase inhibitors plus minocyclines | 1 (1.3%) | 3 (0.7%) | 0.4681 |
| Others | 1 (1.3%) | 5 (1.1%) | 1.0000 |
| Number of antibiotics for initial treatment | |||
| Single drug | 40 (52.66%) | 171 (38.3%) | 0.0227 |
| ≥2 drugs | 36 (47.4%) | 275 (61.7%) |
Discussion
To our knowledge, there have been few reports comparing clinical features between Klebsiella and pneumococcal chest infection. Lin et al. compared the characteristics of bacteremic lobar pneumonia due to Klebsiella spp. (mean patient age, 73 years old) and S. pneumoniae (mean patient age, 76 years old), and patients with bacteremic Klebsiella pneumonia showed a lower white blood cell count, higher frequency of bilateral involvement on chest X-ray, greater disease severity on admission, more frequent admission to the intensive-care unit, and higher mortality rate than those with bacteremic pneumococcal pneumonia (15). In that report, the frequencies of male gender, diabetes mellitus, alcoholism, and cavitary lesions on chest X-ray did not differ markedly between the two groups (15).
In the present study, there were significant differences in several patient demographics and laboratory data, but no differences were noted in the frequency of patients with severe conditions or mortality between the two groups. Severity and mortality are affected by several factors, including patient demographics and treatment (11,12). In addition, the prevalence of virulent factors differs across countries and regions (16). For example, the prevalence of K1 isolates is higher in Japan than in the United States or Europe but lower than in Taiwan (16). These differences are likely the cause of the difference in results between the previous studies (15,17) and ours.
Pneumonia caused by Klebsiella spp. has been reported to occur predominantly in men in their 60s, in chronic alcoholics (18), and in patients with conditions such as chronic bronchopulmonary diseases, cardiac diseases, diabetes mellitus, and malignancy (18,19). Our patients in the K group showed impaired premorbid PS, suggesting that debilitated patients may be predisposed to Klebsiella spp. infection (18). The major underlying conditions in patients with Klebsiella spp. infection tend to be chronic diseases that require contact with healthcare providers, and the frequency of Klebsiella spp. infection was reported to be lower in patients with CAP than in those with HCAP (18). An impaired PS is not included in the diagnostic criteria of HCAP but is included in those of nursing- and healthcare-associated pneumonia, which is a Japanese variant of HCAP (20). The higher proportion of patients with an impaired PS in the K group than in the S group suggests that a higher proportion of patients in the K group likely had nursing- and healthcare-associated pneumonia. These characteristics may be one reason for the longer duration of antibiotics treatment and hospital stay in the K group patients, which is compatible with the findings of a previous report (21). The higher proportion of lung abscesses, necrosis, and empyema in the K group may also explain the longer duration of antibiotic treatment, as empyema and lung abscesses frequently require longer antibiotics administration than pneumonia (22).
Although K. pneumoniae remains the primary species causing human diseases such as pneumonia, numerous causative Klebsiella species have been identified, including K. pneumoniae (K. pneumoniae subsp. pneumoniae), K. rhinoscleromatis (K. pneumoniae subsp. rhinoscleromatis), K. ozaenae (K. pneumoniae subsp. ozaenae), K. planticola, and K. ornithinolytica. Klebsiella spp. can colonize or cause chronic upper and lower respiratory tract infections, septicemia, and severe pneumonia (18-21,23-25), and severe cases of infection were also found in our patients with K. rhinoscleromatis, K. ozaenae, and K. oxytoca.
Polymicrobial infection was found in 19 of our 76 (25.0%) patients in the K group, which was similar to the 27% frequency in a previous report of Klebsiella pneumonia (4). P. aeruginosa was the leading pathogen of coinfection with Klebsiella species in our study. The K group patients had an impaired PS more frequently than those in the S group, suggesting that the K group patients may have received nursing and home-care service more frequently than those in the S group (20) with a risk of multidrug-resistant pathogen infection, including P. aeruginosa (20). Previously reported results investigating mixed infection were mainly based on culture results (26). We evaluated the etiology of mixed infection by paired sera and rapid diagnostic tests. As a result, influenza virus was the second-most frequent pathogen found to be coinfecting with K. pneumoniae. Damaged epithelium and an impaired defense mechanism in local and systemic immune systems may induce infections caused by Klebsiella species (27). There have been few reports of pneumonia due to coinfection of influenza and Klebsiella species (28), but the frequency of coinfection with influenza in the K group did not differ markedly from that in the S group. Influenza is a common disease, and such coinfection may be underrecognized. The investigation of polymicrobial infection seems to result in both a better selection of appropriate antibiotics and better infection control, especially in patients with influenza coinfection.
A previous report that investigated chest CT findings of Klebsiella pneumonia in 23 cases showed pleural effusion in 8 cases and necrosis or cavities in all cases. Two cases showed clinical courses compatible with pulmonary gangrene (29). In our study, we defined lung abscess/necrotizing pneumonia when cavities were found in areas of pulmonary consolidation. A cavity is the result of pathological processes, including suppurative necrosis, caseous necrosis, ischemic necrosis, and cystic dilatation of lung structures. K. pneumoniae is associated with extensive pyogenic lung necrosis and frequent cavitation, and this organism is also disproportionately represented among cases of pulmonary gangrene, in which there is extensive pulmonary necrosis and infarction. These findings suggest that the organism possesses pathogenic determinants that lead to pulmonary necrosis and cavitation more frequently than S. pneumoniae (30,31). A previous report of 205 patients with lung abscess included 10 cases (4.9%) due to Klebsiella spp. but only 3 cases (1.5%) due to S. pneumoniae (32). Another study from Taiwan implicated K. pneumoniae as the most common single cause of lung abscess, isolated from 28 of 90 (31%) patients with lung abscesses (33). These results suggest that the detection of cavity formation suggests a Klebsiella spp. infection rather than a S. pneumoniae infection.
Our study showed a higher percentage of empyema alone in the K group than in the S group; however, the number of patients with empyema alone was 3 in both groups. Because S. pneumoniae is a common cause of chest infection, it may cause a significant fraction of empyema cases, even though pneumococcal pneumonia complicated empyema less frequently than Klebsiella pulmonary infection (pneumonia and lung abscess/necrotizing pneumonia). Certainly, the etiology of empyema in a previous study included Klebsiella spp. in 23 (3.5%) and S. pneumonia in 81 (12.5%) of 650 patients (8). Thus, we think it difficult to differentiate Klebsiella spp. infection and pneumococcal infection based on complication of empyema.
S. pneumoniae is generally susceptible to penicillins; however, K. pneumoniae is frequently resistant to penicillins because it produces a penicillin-specific β-lactamase (34), which may result in treatment failure of the initial antibiotics. Results of the antibacterial susceptibility of isolated Klebsiella species in our patients were similar to those reported throughout Japan (35). Our K group patients frequently received single penicillins as initial antibiotics. Fortunately, the single penicillin administered in these patients was piperacillin, to which the isolated Klebsiella spp. were susceptible; however, about half of the isolated Klebsiella spp. showed resistance to piperacillin, which may cause an unfavorable clinical course in other patients. Correct prediction of Klebsiella spp. infection results in better antibiotics selection. Our results indicated that patients' demographics and radiologic findings (cavity formation) may be clues to differentiate between infections caused by these two pathogens.
There are several limitations in the present study. It is a retrospective, single-center study with a limited number of patients, so the results may not be applicable to other settings. In addition, we did not investigate the presence of certain factors (K1 and K2 antigen), genes (rmp A and mag A), or phenotypes (hypermucoviscosity) with increased pathogenicity. It may be best to investigate these factors in future studies when assessing the clinical features of Klebsiella infections. We adopted the 2007 IDSA/ATS severity criteria for CAP to compare the disease severity between the K and S groups. This score can also be applied to HCAP (36); however, our study included a small number of patients (6 cases) with empyema without pulmonary parenchymal shadow, so the application of another severity score might be more appropriate.
In conclusion, the characteristics of Klebsiella chest infections as investigated by comparing them with pneumococcal chest infection included an old age, male predominance, impaired premorbid PS, an alcohol habit, some underlying diseases, a high frequency of developing lung abscess/necrotizing pneumonia and empyema, and a long duration of antibiotics administration and hospital stay. Severe cases of infection were also found in patients with Klebsiella spp. other than K. pneumoniae.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Paganin F, Lilienthal F, Bourdin A, et al. Severe community-acquired pneumonia: assessment of microbial aetiology as mortality factor. Eur Respir J 24: 779-785, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Mycek ST, Kollef KE, Reichley RM, Roubinian N, Kollef MH. Healthcare-associated pneumonia and community-acquired pneumonia: a single-center experience. Antimicrob Agents Chemother 51: 3568-3573, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Holmes RB. Friedländer's pneumonia. Am J Roentgenol Radium Ther Nucl Med 75: 728-747, 1956. [PubMed] [Google Scholar]
- 4. Korvick AJ, Hackett AK, Yu VL, Muder RR. Klebsiella pneumonia in the modern era: clinicoradiographic correlations. South Med J 84: 200-204, 1991. [DOI] [PubMed] [Google Scholar]
- 5. American Thoracic Society; Infectious Disease Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171: 388-416, 2005. [DOI] [PubMed] [Google Scholar]
- 6. Ahn JH, Lee KH, Chung JH, et al. Clinical characteristics and prognostic risk factors of healthcare-associated pneumonia in a Korean tertiary teaching hospital. Medicine (Baltimore) 96: e8243, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jong GM, Hsiue TR, Chen CR, Chang HY, Chen CW. Rapidly fatal outcome of bacteremic Klebsiella pneumoniae pneumonia in alcoholics. Chest 107: 214-217, 1995. [DOI] [PubMed] [Google Scholar]
- 8. Parapneumonic effusions and empyema. . Pleural Diseases. 4th ed. Light RW, Ed. Lippincott Williams & Wilkins, Philadelphia, 2001: 151-181. [Google Scholar]
- 9. Alcohol and Public Health. Centers for Disease Control and Prevention [Internet]. [cited 2018 Mar 27]. Available from: https://www.cdc.gov/alcohol/faqs.htm
- 10. Olken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5: 649-655, 1982. [PubMed] [Google Scholar]
- 11. Ishiguro T, Takayanagi N, Gochi M, et al. Etiology and factors contributing to mortality in healthcare-associated pneumonia: a single-center study. Showa Univ J Med Sci 25: 263-275, 2013. [Google Scholar]
- 12. Ishiguro T, Takayanagi N, Yamaguchi S, et al. Etiology and factors contributing to the severity and mortality of community-acquired pneumonia. Intern Med 52: 317-324, 2013. [DOI] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute (CLSI).. Performance standards for antimicrobial susceptibility testing; 19th informational supplement CLSI documents M100-S19. Wayne, PA, 2009. [Google Scholar]
- 14. Mandell LA, Wunderink RG, Anzueto A, et al. ; Infectious Diseases Society of America, American Thoracic Society . Infectious Disease Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44 (Suppl 2): S27-S72, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin YT, Jeng YY, Chen TL, Fung CP. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: clinical and microbiological characteristics in Taiwan, 2001-2008. BMC Infect Dis 10: 307-313, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harada S, Ishii Y, Saga T, Aoki K, Tateda K. Molecular epidemiology of Klebsiella pneumoniae K1 and K2 isolates. Diag Microbiol Infect Dis 91: 354-359, 2018. [DOI] [PubMed] [Google Scholar]
- 17. Feldman C, Kallenbach JM, Levy H, Thorburn JR, Hurwitz MD, Koornhof HJ. Comparison of bacteraemic community-acquired lobar pneumonia due to Streptococcus pneumoniae and Klebsiella pneumoniae in an intensive care unit. Respiration 58: 265-270, 1991. [DOI] [PubMed] [Google Scholar]
- 18. Pierce AK, Sanford JP. Aerobic gram-negative bacillary pneumonias. Am Rev Resp Dis 110: 647-658, 1974. [DOI] [PubMed] [Google Scholar]
- 19. Miyashita N, Kawai Y, Akaike H, et al. Clinical features and the role of atypical pathogens in nursing and healthcare-associated pneumonia: differences between a teaching university hospital and a community hospital. Intern Med 51: 585-594, 2012. [DOI] [PubMed] [Google Scholar]
- 20. The Japanese Respiratory Society.. The JRS Guidelines for the Management of Pneumonia in Adults. 2017: 34-47(in Japanese). [DOI] [PubMed] [Google Scholar]
- 21. Ishida T, Tachibana H, Ito A, Yoshioka H, Arita M, Hashimoto T. Clinical characteristics of nursing and healthcare-associated pneumonia: a Japanese variant of healthcare-associated pneumonia. Intern Med 51: 2537-2544, 2012. [DOI] [PubMed] [Google Scholar]
- 22. Lorber B. Bacterial lung abscess. In: Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 8th ed. Bennett JE, Dolin R, Blaser MJ, Eds.. Elsevier Saunders, Philadelphia, 2015: 855-859. [Google Scholar]
- 23. Porto R, Hevia O, Hensley GT, Meyer PR. Disseminated Klebsiella rhinoscleromatis infection. Arch Pathol Lab Med 113: 1381-1383, 1989. [PubMed] [Google Scholar]
- 24. Power JT, Calder MA. Pathogenic significance of Klebsiella oxytoca in acute respiratory tract infection. Thorax 38: 205-208, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Carpenter JL. Klebsiella pulmonary infections: occurrence at one medical center and review. Rev Infect Dis 12: 672-682, 1990. [DOI] [PubMed] [Google Scholar]
- 26. Okimoto J, Fujita K, Oba H. A study on Klebsiella pneumoniae pneumonia. Nihon Kyobu Rinsho 55: 940-944, 1996(in Japanese, Abstract in English). [Google Scholar]
- 27. Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis 6: 303-312, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Trinus EK, Rudenko AA, Shapiro AV. Clinical picture of acute pneumonia due to Klebsiella ozaenae in patients with influenza. Vrach Delo 8: 106-108, 1988(in Russian). [PubMed] [Google Scholar]
- 29. Moon WK, Im JG, Yeon KM, Han MC. Complications of Klebsiella pneumonia: CT evaluation. J Comput Assist Tomogr 19: 176-181, 1995. [DOI] [PubMed] [Google Scholar]
- 30. Hammond JM, Lyddell C, Potgieter PD, Odell J. Severe pneumococcal pneumonia complicated by massive pulmonary gangrene. Chest 104: 1610-1612, 1993. [DOI] [PubMed] [Google Scholar]
- 31. Taylor SN, Sanders CV. Unusual manifestations of invasive pneumococcal infection. Am J Med 107: 12S-27S, 1999. [DOI] [PubMed] [Google Scholar]
- 32. Takayanagi N, Kagiyama N, Ishiguro T, Tokunaga D, Sugita Y. Etiology and outcome of community-acquired lung abscess. Respiration 80: 98-105, 2010. [DOI] [PubMed] [Google Scholar]
- 33. Wang JY, Chen KY, Fang CT, Hsueh PR, Yang PC, Chang SC. Changing bacteriology of adult community-acquired lung abscess in Taiwan: Klebsiella pneumoniae versus anaerobes. Clin Infect Dis 40: 915-922, 2005. [DOI] [PubMed] [Google Scholar]
- 34. Yanagihara K, Watanabe A, Aoki N, et al. Nationwide surveillance of bacterial respiratory pathogens conducted by the surveillance committee of Japanese Society of Chemotherapy, the Japanese Association for Infectious Diseases, and the Japanese Society for Clinical Microbiology in 2012; general view of the pathogens' antibacterial susceptibility. J Infect Chemother 23: 587-597, 2017. [DOI] [PubMed] [Google Scholar]
- 35. Carrabba M, Zarantonello M, Bonara P, et al. Severity of healthcare-associated pneumonia and pneumonia in immunosuppression. Eur Respir J 40: 1201-1210, 2012. [DOI] [PubMed] [Google Scholar]
- 36. Boye K, Hansen DS. Sequencing of 16S rDNA of Klebsiella: taxonomic relations within the genus and to other Enterobacteriaceae. Int J Med Microbiol 292: 495-503, 2003. [DOI] [PubMed] [Google Scholar]