Abstract
We examined a 22-year-old woman who was admitted to our hospital with abdominal distention. At 19 years of age, the patient presented with hepatosplenomegaly. She was examined several times in another hospital; however, the cause was unidentified. Our evaluation showed severe pancytopenia and a spleen 13×24 cm in size. The serum levels of angiotensin-converting enzyme and lysozyme were elevated. She was diagnosed with liver sarcoidosis based on non-caseating epithelioid granuloma in liver biopsy tissue. To improve the symptoms, splenectomy was performed, and her pancytopenia and symptoms improved. Sarcoidosis should be considered in cases of massive splenomegaly.
Keywords: liver sarcoidosis, massive splenomegaly, and splenectomy
Introduction
Sarcoidosis is a systemic granulomatous disease of unknown etiology involving various organs (1,2). It is diagnosed according to the presence of non-caseating granulomas or the typical clinical manifestations in the pulmonary system, eye, or heart after excluding other conditions with similar findings, such as infections and malignancies (3). Pulmonary manifestations of sarcoidosis are a major factor and are absent in less than 10% of cases (4). Liver involvement is common and is characterized by non-caseating granulomas (5). The severity of hepatic sarcoidosis is variable, ranging from asymptomatic or mild liver enzyme abnormalities to end-stage liver disease requiring liver transplantation (6,7).
Portal hypertension is a rare manifestation of sarcoid liver disease, affecting less than 1% of patients (8,9). Portal hypertension was first reported in 1949 by Mino et al. (10), followed by Klatskin in 1950 (11). As a complication, splenomegaly is reported in 5.6-50.0% of sarcoidosis cases (12-14).
We encountered a case of liver sarcoidosis with massive splenomegaly that was difficult to diagnose due to a lack of typical lung and eye findings. This study was performed in accordance with the principles of the Declaration of Helsinki and the ethical guidelines of Tokyo Women's Medical University Hospital (TWMU, Tokyo, Japan).
Case Report
A 22-year-old woman was admitted to our hospital with abdominal distention, fatigue, and appetite loss (Fig. 1). She had previously been diagnosed with bronchial asthma. At 19 years of age, the patient presented with weight loss (5 kg loss in 6 months) and skin pigmentation of the lower extremities, so she was referred to a clinic. Abdominal ultrasound performed at the clinic revealed hepatosplenomegaly. She was referred to another hospital for a further examination. Two years before visiting our hospital, a complete blood count had already revealed pancytopenia [white blood cell (WBC) count, 1,500 /μL; hemoglobin level, 8.9 mg/dL; platelet count, 7.9×104/μL]. At another hospital, she underwent computed tomography (CT), positron emission tomography-CT (PET-CT), bone marrow aspiration (hypercellular bone marrow), a skin biopsy of the pigmented lesions, and a biopsy of the spleen; no definitive diagnosis was established.
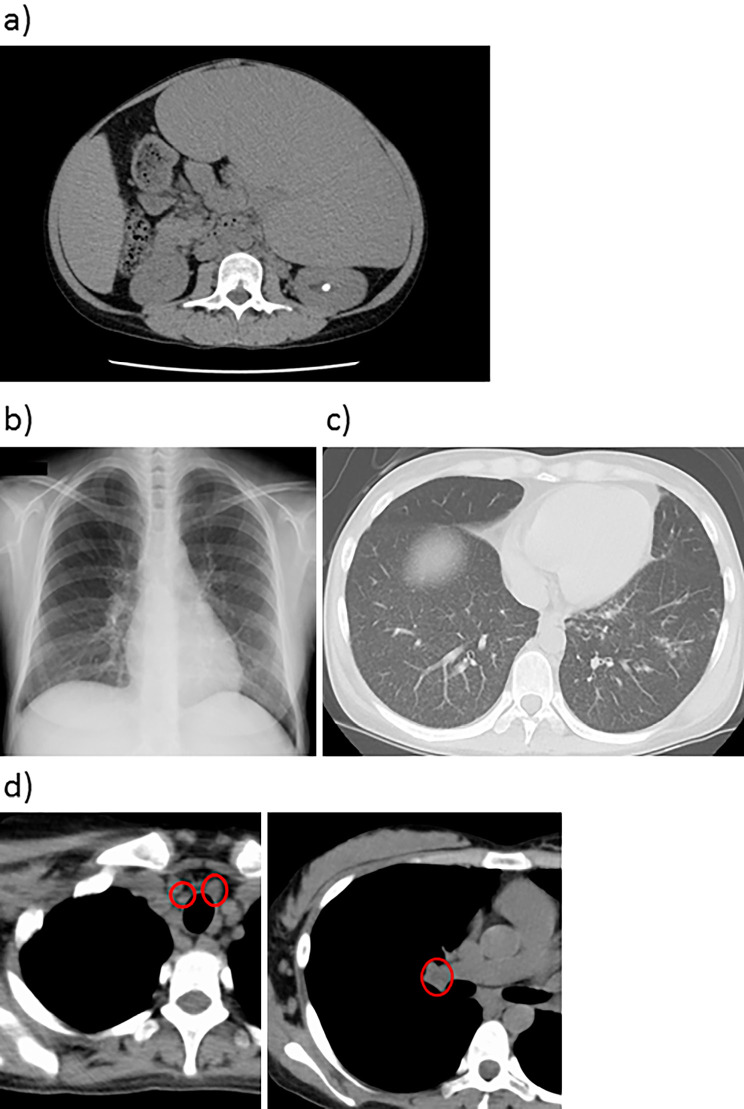
Figure 1.
Findings of abdominal/chest/thorax on CT and chest X-ray. a: Abdominal spleen CT scan, b: chest X-ray, c and d: thorax CT scan. A massive spleen was observed on abdominal CT (a). Typical bilateral hilar lymphadenopathy was absent on chest X-ray (b). Diffuse granular shadow was observed bilaterally on chest CT (c). Swelling of the bilateral hilar and mediastinal lymph nodes was observed on thorax CT (circles) (d). CT: computed tomography
At this point, the splenomegaly had gradually developed and begun to compress the renal artery, thus reducing her renal function. The patient was referred to our hospital at 22 years of age and was admitted for a further analysis. Infection, hemolytic anemia, and collagen disease were excluded. A biochemical examination showed liver disturbance (albumin, 3.9 g/dL; total bilirubin, 0.9 mg/dL; direct bilirubin, 0.1 mg/dL; aspartate aminotransferase, 47 U/L; alanine aminotransferase, 25 U/L; alkaline phosphatase, 586 U/L; gamma-glutamyl transferase, 68 U/L; and prothrombin time %, 66.6%) and pancytopenia [WBC count, 1,750 /μL (58.3% neutrophils and 25.7% lymphocytes); platelet count, 7.5×104/μL] (Table 1). Elevation in the serum levels of soluble interleukin-2 receptor (sIL-2R, 5,990 U/mL), angiotensin-converting enzyme (ACE, 41.5 U/L), lysozymes (43.4 μg/mL), and KL-6 (1,134 IU/mL) was also observed. Hepatomegaly and splenomegaly (13×24 cm) were revealed by an abdominal CT scan (Fig. 1a). A gallium scan showed accumulation in the spleen (Fig. 2a, b). However, massive splenomegaly was negative on PET-CT (Fig. 2c, d). Esophageal varices were not evident. Bilateral hilar lymphadenopathy, considered a typical finding of sarcoidosis, was absent on chest X-ray (Fig. 1b). A bilateral diffuse granular shadow was observed on chest CT (Fig. 1c), in addition to bilateral hilar lymphadenopathy (Fig. 1d). The lymph node of the neck was positive, as shown by PET-CT (Fig. 2e), suggestive of sarcoidosis. A significant decrease in the carbon monoxide diffusing capacity (DLCO; 24.55 mL/min/mmHg) on respiratory function testing and the presence of severe cough suggested exacerbation of pulmonary sarcoidosis. Bronchoalveolar lavage by bronchoscopy showed an increase in small lymphocytes (81.0%) without any increase in the CD4/CD8 ratio, and biopsy results showed epithelial granulomas, both of which are findings consistent with pulmonary sarcoidosis.
Table 1.
Patient Laboratory Data on Admission to Our Hospital.
| Hematology | Coagulation | |||||||
| WBC | 1,750 | /μL | PT-INR | 1.18 | ||||
| Neutrophils | 58.3 | % | PT% | 66.6 | % | |||
| Lymphocytes | 25.7 | % | APTT | 45.9 | s | |||
| Monocytes | 13.1 | % | APTT control | 32.9 | s | |||
| Eosinophils | 2.3 | % | FDP | 3.1 | μg/mL | |||
| RBC | 4.09 | ×106/μL | D-dimmer | 0.9 | μg/mL | |||
| Hb | 10.9 | g/dL | Fibrinogen | 239 | mg/dL | |||
| Ht | 34.2 | % | ||||||
| Plt | 7.5 | ×104/μL | Tumor marker | |||||
| Reticulocytes | 9.7 | ×104/μL | AFP | 2 | U/mL | |||
| CEA | 1.3 | ng/mL | ||||||
| Biochemistry | ||||||||
| TP | 7.3 | g/dL | Hormone | |||||
| ALB | 3.9 | g/dL | ACTH | 18.7 | pg/mL | |||
| T-BIL | 0.9 | mg/dL | Cortisol | 7.3 | μg/mL | |||
| D-BIL | 0.1 | mg/dL | Aldosterone | 314 | ng/mL | |||
| D/T ratio | 0.1 | TSH | 5.47 | μIU/mL | ||||
| AST | 47 | U/L | fT3 | 2.12 | pg/mL | |||
| ALT | 25 | U/L | fT4 | 1.34 | pg/mL | |||
| ALP | 586 | U/L | ||||||
| γ-GTP | 68 | U/L | Serology | |||||
| LDH | 177 | U/L | IgG | 2,123 | mg/dL | |||
| ChE | 114 | U/L | IgM | 74 | mg/dL | |||
| BUN | 18.3 | mg/dL | ACE | 41.5 | U/L | |||
| Cr | 6.6 | mg/dL | s-IL2R | 5,290 | U/mL | |||
| eGFR | 57.8 | mL/min/1.73 m2 | KL-6 | 1,134 | U/mL | |||
| Na | 140 | mEq/L | Lysozyme | 43.4 | μg/mL | |||
| K | 3.5 | mEq/L | ANA | <40 | ||||
| Cl | 109 | mEq/L | AMA | <1.5 | ||||
| Ca | 8.9 | mg/dL | ||||||
| FBS | 108 | Mg/dL | Hepatitis virus | |||||
| HbA1c (NGSP) | 4.8 | % | HBs antigen | (-)<0.02 | IU/mL | |||
| Fe | 37 | μg/mL | HCV antibody | (-) | COI | |||
| Ferritin | 67 | ng/dL | ||||||
| CRP | 0.64 | mg/dL |
WBC: white blood cell, RBC: red blood cell, Hb: hemoglobin, Ht: hematocrit, Plt: platelet, TP: total protein, ALB: albumin, T-BIL: total bilirubin, D-BIL: direct bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, ALP: alkaline phosphatase, γ-GTP: gamma-glutamyltransferase, LDH: lactate dehydrogenase, ChE: Cholinesterase, BUN: blood urea nitrogen, Cr: creatinine, Na: sodium, K: potassium, Cl: chloride, Ca: calcium, FBS: fasting blood sugar, NGSP: National Glycohemoglobin Standardization Program. Fe: iron, CRP: C-reactive protein, PT-INR: international normalized ratio of prothrombin time, PT: prothrombin time, APTT: activated partial thromboplastin time, FDP: fibrin degradation product, AFP: α-fetoprotein, CEA: carcinoembryonic antigen, ACTH: adrenocorticotropic hormone, TSH: thyroid stimulating hormone, fT3: free triiodothyronine, fT4: free thyroxine, IgG: immunoglobulin G, IgM: immunoglobulin M, ACE: angiotensin converting enzyme, s-IL2R: soluble interleukin 2 receptor, KL-6: human sialylated carbohydrate antigen, ANA: antinuclear antigen, AMA anti-mitochondrial antibody, HBs antigen: hepatitis B surface antigen, HCV: hepatitis C virus
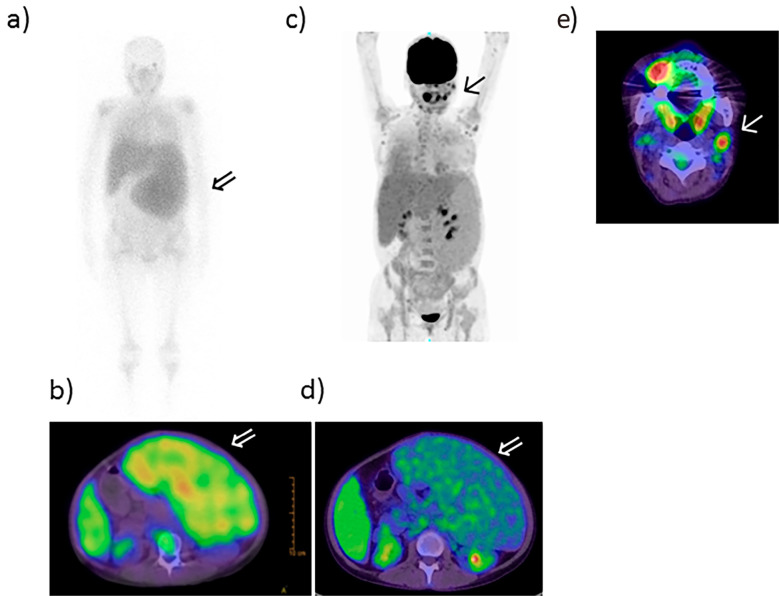
Figure 2.
Gallium and PET-CT scans. a, b: Gallium scan and c-e: PET-CT scan. Gallium scans showed accumulation in the spleen (arrow ⇒) (a, b). The neck lymph node was positive (arrow ↑) (c, e); however, massive splenomegaly was negative (arrow ⇒) (d) on PET-CT. PET-CT: positron emission tomography-computed tomography
To investigate the cause of portal hypertension, a liver biopsy specimen was obtained and revealed non-caseating epithelioid granuloma with multinucleated giant cells; the patient was thus diagnosed with liver sarcoidosis (Fig. 3a, b). The granuloma was observed mainly around the portal area and part of the liver parenchyma. Moderate pleomorphic inflammatory invasion was also noted. The basic architecture of the liver was preserved; however, bridging fibrosis was shown in part of the liver. After 90 days of hospitalization, the patient developed additional dermatological symptoms (purpura on the lower extremities). A skin biopsy revealing non-caseous granuloma indicated sarcoidosis of the skin (Fig. 3c, d). Ultrasound and magnetic resonance imaging (MRI) of the heart revealed no signs of cardiac sarcoidosis. There was no indication of peripheral nerve involvement on the peripheral nerve constriction test nor MRI of the cervical and thoracic spine.
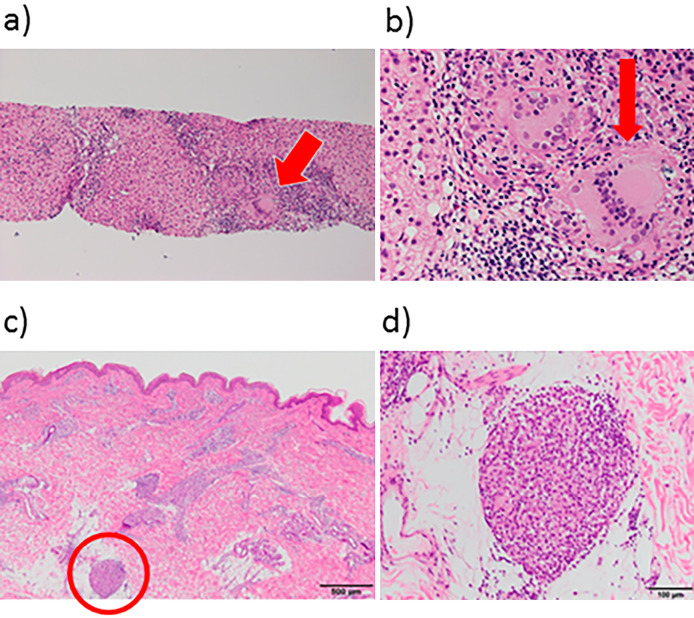
Figure 3.
Representative biopsy specimens of liver and skin. a and b: Liver biopsy specimens; c and d: skin biopsy specimen. The liver biopsy specimen (a, b) and skin biopsy specimen (c, d) showed non-caseating epithelioid granuloma (red arrows and circle) with multinucleated giant cells. The granuloma was observed mainly around the portal area and part of the liver parenchyma. Moderate pleomorphic inflammatory invasion was noted. The basic architecture of the liver was preserved; however, bridging fibrosis was noted in part of the liver.
Although administration of corticosteroids was considered, they carried a risk of inducing splenic rupture; to improve the symptoms associated with splenomegaly, including pancytopenia, we consulted with the surgical team and decided to perform splenectomy prior to steroid treatment. Splenectomy was performed at day 110 of hospitalization, with no surgical complications (Fig. 4a). The removed spleen was 4,300 g in weight and 28×21 cm in size (Fig. 4b). As a gross finding, enlargement of the liver was noted at the operation. The white lesion of the sarcoid nodule was not prominent. Pathological results revealed epithelial granuloma of the spleen (Fig. 4c) with multinucleated giant cells (Fig. 4d). Together, these results were used to diagnose stage II sarcoidosis of the liver, lung, spleen, and skin (13).
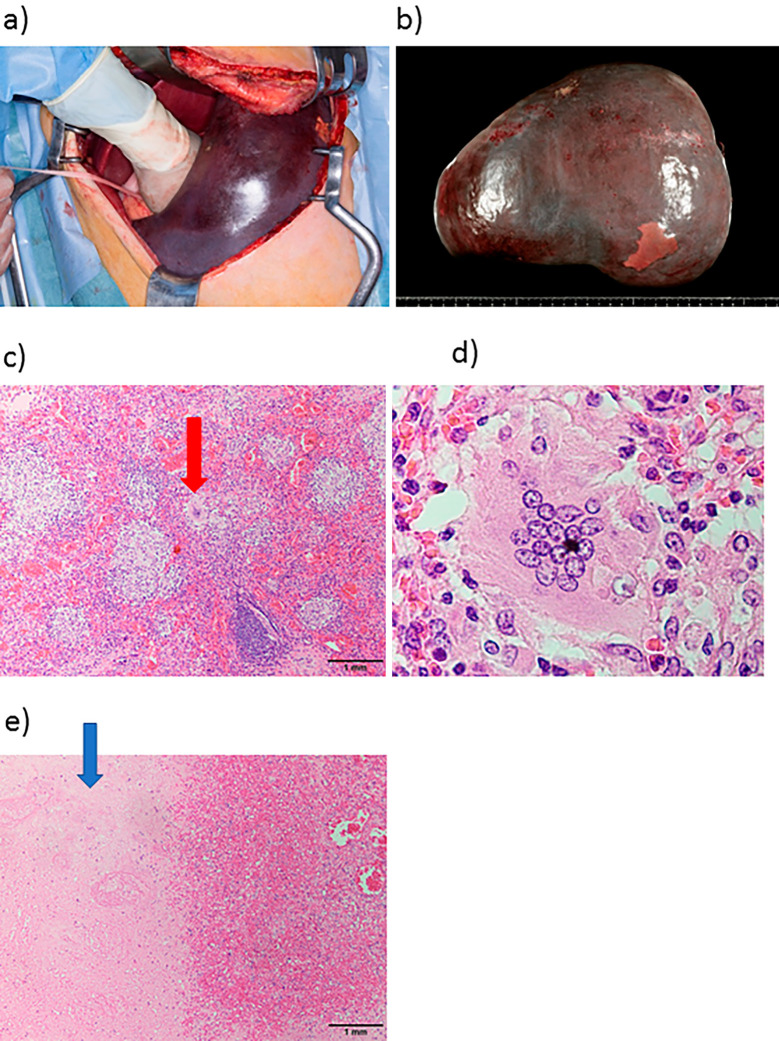
Figure 4.
Splenectomy: macroscopic and pathological findings of the spleen. Splenectomy was performed after ligation of the splenic artery (a). Macroscopic findings included a massively enlarged spleen (4,300 g in weight and 28×21 cm in size) (b). A microscopic specimen of the spleen revealed non-caseating epithelioid granuloma (red arrow: multinucleated giant cells) (c) with multinucleated giant cells (d). Spleen infarction (blue arrow) was also detected in the removed spleen (e).
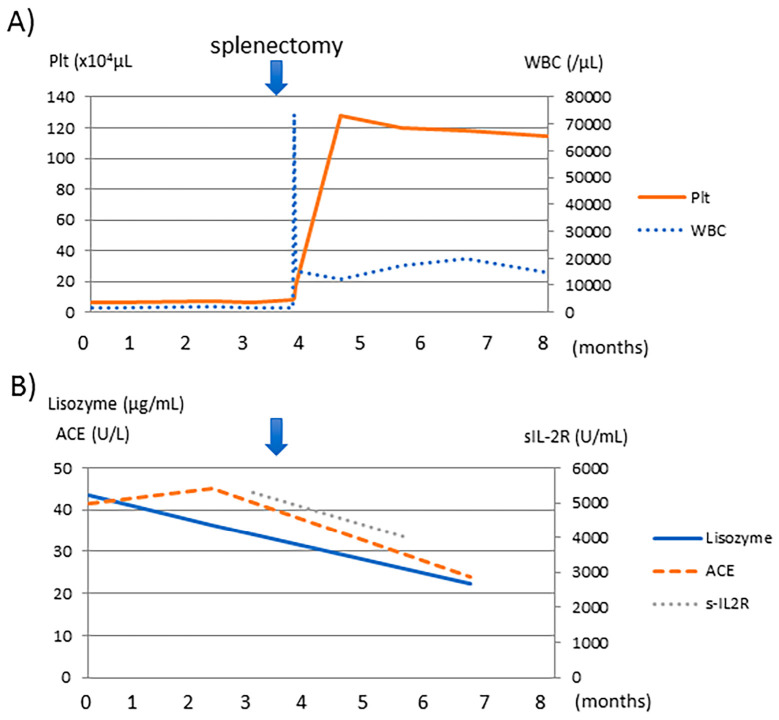
Splenic infarction was also detected in the removed spleen (Fig. 4e). The platelet count increased to 16.9×104/μL on the day after splenectomy (Fig. 5) and then to 100-120×104/μL, due to post-operative inflammation. We administered aspirin and warfarin potassium for prophylaxis and thrombosis. The serum levels of ACE, lysozymes, and sIL-2R decreased slightly. The pulmonary symptoms, including the severe cough, disappeared with the removal of the spleen. The DLCO and liver dysfunction were not affected. Although the patient showed a favorable clinical course without steroid therapy at four months post-operation, we should still consider steroid treatment because some of the markers, including sIL-2R, ACE, and lysozymes, were abnormal, and disturbance of organs, such as the lung and liver, remained.
Figure 5.
The clinical course of our case. Pancytopenia was improved after splenectomy, and the WBC and platelet counts increased as well. At the same time, the serum levels of ACE, lysozymes, and sIL-2R were slightly decreased. Hb: hemoglobin, Plt: platelet, WBC: white blood cell, ACE: angiotensin converting enzyme, s-IL2R: soluble interleukin 2 receptor
Discussion
We herein report a case of sarcoidosis with hepatomegaly, hypersplenism, and massive splenomegaly that was difficult to diagnose. Because the typical findings of sarcoidosis in the lung and eye were absent and hepatosplenomegaly alone as a manifestation of sarcoidosis is rare, sarcoidosis was not initially considered. However, hepatosplenomegaly should be considered as a differential diagnosis of massive splenomegaly, and splenectomy can be considered as a treatment.
The published reports describing splenic involvement in sarcoidosis show varying results (5.6-50.0%) (12-14), mostly likely due to the fact that splenic lesions are not usually clinically problematic, and this phenomenon may therefore influence the reported frequency of splenic involvement. However, the reported incidence of a massive spleen (>4.0 cm) is low (3.0-4.7%) (15,16). The etiology of portal hypertension in sarcoidosis is not completely understood and may involve multiple mechanisms. The portal blood pressure is defined in terms of the portal blood flow and resistance (17). Maddrey et al. suggested that small arterial-venous shunts may form in the region of the granulomas in the liver and spleen, resulting in an elevated portal blood flow that in turn leads to a compensatory increase in intrahepatic resistance (18). Splenomegaly was more commonly observed in patients with a long history of sarcoidosis (38%) than in those with a short history (23%; p<0.05) and patients with extrapulmonary lesions (19). However, the risk factors for splenomegaly were not identified in that study, although a study from Japan observed an association between the human leukocyte antigen (HLA) DQB1 allele and splenomegaly (12).
At our institute, 14 cases of liver sarcoidosis were diagnosed from 1997 to 2019 (Table 2). Eight cases showed splenomegaly; however, only three of these were initially detected based on enlargement of the spleen, including the present case. Another two cases presented with a combination of splenomegaly and typical manifestations of sarcoidosis, including in the lung or eye; however, no significant symptoms of splenomegaly were present.
Table 2.
Cases of Liver Sarcoidosis in Our Hospital
| Case | Age (years) |
Sex | Affected organs | Splenomegaly | Treatment | Efficacy of treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 39 | M | Neuron, Lymph nodes | Mild | PSL | No change | ||||||
| 2 | 23 | M | Eye | Moderate | - | |||||||
| 3 | 74 | F | Skin | Mild | - | |||||||
| 4 | 69 | M | Neuron | - | PSL | No change | ||||||
| 5 | 40 | M | Eye | - | (PSL)* | No change | ||||||
| 6 | 41 | M | Lymph nodes | Marked | - | |||||||
| 7 | 44 | F | Eye | Mild | UDCA | Decreased liver enzymes | ||||||
| 8 | 49 | F | Heart, eye | - | ||||||||
| 9 | 71 | F | Eye | - | ||||||||
| 10 | 44 | M | Lung, eye | Mild | UDCA | No change | ||||||
| 11 | 70 | F | UDCA | Exacerbation | ||||||||
| 12 | 52 | M | Heart, Lung | PSL | Exacerbation | |||||||
| 13 | 41 | M | Eye, skin | Marked | PSL, UDCA | Exacerbation | ||||||
| 14 (Present case) |
22 | F | Lung, skin, spleen | Marked | Splenectomy | Favorable |
*PSL was administered to treat facial nerve palsy. PSL: prednisone, UDCA: ursodeoxycholic acid
The management of splenic enlargement due to sarcoidosis consists of therapy with prednisone, methotrexate, or antimalarial drugs (19). Although long-term treatment with these medications has been shown to improve sarcoidosis (15), the disease commonly progresses after reducing or discontinuing the steroid treatment (20). Splenectomy is a treatment option, but severe bleeding during the operation and thrombosis have been reported (21). Stamou et al. reported that portal vein thrombosis occurred in 7 (4.79%) of 146 patients postsplenectomy (21). Furthermore, no reports have suggested an improved survival rate in sarcoidosis patients, as sarcoidosis is a systemic disease, and the indications for splenectomy are limited. Splenectomy is considered in the following circumstances: [1] massive splenomegaly; [2] severe hypersplenism; [3] the need to exclude lymphoma or malignancy; and [4] as a precautionary measure against splenic rupture (22). In our report, a massive splenomegaly (13×24 cm) was observed, and the risk of rupture was extremely high. Therefore, splenectomy was performed prior to systemic treatment with prednisone. There have been no previous cases of splenomegaly being treated by splenectomy prior to steroid or ursodeoxycholic acid (UDCA) treatment in our hospital (Table 2). In our case, the surgeon compressed the splenic artery in order to reduce the spleen size and ligated the splenic artery/vein to remove the spleen (Fig. 4a). The spleen size decreased, and severe bleeding was thus prevented. After splenectomy, our patient did not present with portal vein thrombosis or other complications and had a favorable clinical course.
Splenomegaly and pancytopenia improved after splenectomy (Fig. 5). The serum levels of ACE, lysozyme, and sIL-2R were slightly decreased, possibly due to decreased sarcoidosis in the targeted organ. Enlargement of the spleen compressed the renal artery, which led to renal dysfunction that subsequently improved. Severe cough and decreased DLCO resulted from an elevated diaphragm, in turn due to splenomegaly, and improved after splenectomy. However, the DLCO did not improve, possibly due to lung sarcoidosis. In addition, liver dysfunction also did not improve, possibly due to hepatic sarcoidosis.
In conclusion, we herein report a case of sarcoidosis lacking typical findings that was diagnosed according to hepatomegaly and massive splenomegaly. Sarcoidosis should be considered as a differential diagnosis of massive splenomegaly, and splenectomy may be applied as a treatment. Splenectomy improved the symptoms of splenomegaly in our case, including pancytopenia.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We would like to thank Dr. Tomoko Yamamo of the Department of Pathology of TWMU for providing advice regarding the pathological diagnosis.
References
- 1. Hutchinson J. Mortimer's malady (A form of lupus pernio). Arch Surg, London 9: 307-315, 1898. [Google Scholar]
- 2. Culver DA. Sarcoidosis. Immunol Allergy Clin North Am 32: 487-511, 2012. [DOI] [PubMed] [Google Scholar]
- 3. Coash M, Forouhar F, Wu CH, Wu GY. Granulomatous liver diseases: a review. J Formos Med Assoc 111: 3-13, 2012. [DOI] [PubMed] [Google Scholar]
- 4. Gavilán F, Pereda T, Sousa JM, et al. Hepatic cirrhosis with sarcoid granulomas. Differential diagnosis and liver transplantation: a case report. Transplant Proc 35: 713-714, 2003. [DOI] [PubMed] [Google Scholar]
- 5. James DG, Vella M. Hepatic granulomas. Sarcoidosis 3: 30-33, 1986. [PubMed] [Google Scholar]
- 6. Blich M, Edoute Y. Clinical manifestations of sarcoid liver disease. J Gastroenterol Hepatol 19: 732-737, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Tadros M, Forouhar F, Wu GY. Hepatic sarcoidosis. J Clin Transl Hepatol 1: 87-93, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Branson JH, Park JH. Sarcoidosis hepatic involvement: Presentation of a case with fatal liver involvement; including autopsy findings and review of the evidence for sarcoid involvement of the liver as found in the literature. Ann Intern Med 40: 111-145, 1954. [DOI] [PubMed] [Google Scholar]
- 9. Devaney K, Goodman ZD, Epstein MS, Zimmerman HJ, Ishak KG. Hepatic sarcoidosis. Clinicopathologic features in 100 patients. Am J Surg Pathol 17: 1272-1280, 1993. [PubMed] [Google Scholar]
- 10. Mino RA, Murphy AI, Livingstone RG. Sarcoidosis producing portal hypertension; treatment by splenectomy and splenorenal shunt. Ann Surg 130: 951-957, 1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klatskin G, Yesner R. Hepatic manifestations of sarcoidosis and other granulomatous diseases; a study based on histological examination of tissue obtained by needle biopsy of the liver. Yale J Biol Med 23: 207-248, 1950. [PMC free article] [PubMed] [Google Scholar]
- 12. Sato H, Nagai S, du Bois RM, et al. HLA-DQB1 0602 allele is associated with splenomegaly in Japanese sarcoidosis. J Intern Med 262: 449-457, 2007. [DOI] [PubMed] [Google Scholar]
- 13. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 160: 736-755, 1999. [DOI] [PubMed] [Google Scholar]
- 14. Kurosaki F, Bando M, Nakayama M, et al. A patient with sarcoidosis who developed heterochronic involvements in different organs from initial organs during 7 years. Respir Investig 52: 71-74, 2014. [DOI] [PubMed] [Google Scholar]
- 15. Salazar A, Mañá J, Corbella X, Albareda JM, Pujol R. Splenomegaly in sarcoidosis: a report of 16 cases. Sarcoidosis 12: 131-134, 1995. [PubMed] [Google Scholar]
- 16. Kataria YP, Whitcomb ME. Splenomegaly in sarcoidosis. Arch Intern Med 140: 35-37, 1980. [PubMed] [Google Scholar]
- 17. Fordice J, Katras T, Jackson RE, et al. Massive splenomegaly in sarcoidosis. South Med J 85: 775-778, 1992. [DOI] [PubMed] [Google Scholar]
- 18. Koh C, Heller T. Approach to the diagnosis of portal hypertension. Clin Liver Dis (Hoboken) 1: 133-135, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maddrey WC, Johns CJ, Boitnott JK, Iber FL. Sarcoidosis and chronic hepatic disease: a clinical and pathologic study of 20 patients. Medicine (Baltimore) 49: 375-395, 1970. [DOI] [PubMed] [Google Scholar]
- 20. Pavlović-Popović , Zarić B, Kosjerina Z, Petrović D. Splenomegaly in sarcoidosis: frequency, treatment, prognosis and long-term follow-up. Srp Arh Celok Lek 143: 279-283, 2015. [DOI] [PubMed] [Google Scholar]
- 21. Webb AK, Mitchell DN, Bradstreet CM, Salsbury AJ. Splenomegaly and splenectomy in sarcoidosis. J Clin Pathol 32: 1050-1053, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stamou KM, Toutouzas KG, Kekis PB, et al. Prospective study of the incidence and risk factors of postsplenectomy thrombosis of the portal, mesenteric, and splenic veins. Arch Surg 141: 663-669, 2006. [DOI] [PubMed] [Google Scholar]
- 23. Sharma OP, Vucinic V, James DG. Splenectomy in sarcoidosis: indications, complications, and long-term follow-up. Sarcoidosis Vasc Diffuse Lung Dis 19: 66-70, 2002. [PubMed] [Google Scholar]