Abstract
Introduction:
Alternative predictive end points for overall survival (OS), such as tumor response and progression-free survival (PFS), are useful in the early detection of drug efficacy; however, they have not been fully investigated in patients with advanced NSCLC treated with anti–programmed death protein 1 (PD-1)/programmed death ligand 1 (PD-L1) antibodies.
Methods:
In a systematic review of the reported prospective clinical trials, data for response rate, median PFS, and median OS were extracted from 12 arms in 10 reported clinical trials using anti–PD-1/PD-L1 antibody, and their correlation was investigated. In a retrospective analysis at our institution, OS was compared according to tumor response on 5- to 9-week computed tomography scans and status of being progression-free at 8, 16, and 24 weeks by landmark analysis in 71 patients with advanced NSCLC treated with anti–PD-1/PD-L1 antibodies between 2013 and 2015.
Results:
In a systematic review, moderate correlations between median OS and median PFS (p = 0.120, r = 0.473) and between median OS and response rate (p = 0.141, r = 0.452) were identified using the Spearman correlation coefficient, although these correlations were not statistically significant. In a retrospective analysis of patients treated at our institution, disease control (partial response [PR]/stable disease versus progressive disease/not evaluable), and progression-free status at 8, 16, and 24 weeks significantly predicted OS (Cox proportional hazards model, PR/stable disease versus progressive disease/not evaluable, p = 0.0104, HR = 3.041; 8-week progression-free yes versus no, p = 0.0183, HR = 2.684; 16-week progression-free yes versus no, p = 0.0036, HR = 4.009; and 24-week progression-free yes versus no, p = 0.0002, HR = 12.726).
Conclusions:
Both disease control (PR plus stable disease status) and landmark progression-free survival were correlated with OS, with the longer interval landmark PFS being the best predictor of survival in patients with NSCLC treated with anti–PD-1/PD-L1 antibodies.
Keywords: Non–small cell lung cancer, Anti–PD-1 antibody, Anti–PD-L1 antibody, Immune checkpoint inhibitor, Alternative end point, Overall survival
Introduction
Lung cancer is the most prevalent cancer worldwide.1 More than half of patients with lung cancer have advanced disease at the time of diagnosis, and these patients are candidates for primary systemic therapy.2-4 Lung cancer has a poor prognosis, and although it is not the most frequently diagnosed cancer in the United States, it is by far the leading cause of cancer-related deaths in the United States and worldwide.1 Therefore, advances in the treatment of lung cancer are urgently needed.
Programmed death protein 1 (PD-1) and its ligands, programmed death ligand 1 (PD-L1) and programmed death ligand 2 (PD-L2), are immune checkpoint proteins that primarily function to limit the effector function of T cells in peripheral tissues during inflammatory responses and limit autoimmunity.5-7 However, when expressed within the tumor microenvironment, this process represents a potent mechanism of tumor-induced immune suppression and evasion. Inhibition of this pathway with antibodies against PD-1 or its ligands has yielded good clinical responses and improved overall survival (OS) in patients with lung cancer.8-11 Currently, nivolumab has been approved by the U.S. Food and Drug Administration and European Medicines Agency for NSCLC, and pembrolizumab has been approved by the U.S. Food and Drug Administration for PD-L1-positive NSCLC.10,12 Several other antibodies against PD-1/PD-L1 and other immunomodulatory targets are also under investigation.13
OS is a precise end point, is easy to measure, and can be documented by the date of death. On the other hand, surrogate end points such as tumor response and progression-free survival (PFS) are also useful end points, especially for phase II oncology clinical trials because they can be measured earlier and more conveniently. To date, several studies have investigated the optimal surrogate marker for OS in patients with advanced or recurrent NSCLC.14-16 However, these studies were performed before the introduction of immune checkpoint inhibitors, and it appears that these parameters might correlate differently in this newer treatment modality. Immune checkpoint inhibitors suppress disease progression for a longer time than do former chemotherapeutic agents in responding patients, and the way patients respond to these inhibitors is different from the way they respond to former chemotherapeutic agents. On the basis of these findings, investigation of the optimal surrogate end points for OS in patients with advanced NSCLC treated with anti–PD-1/PD-L1 antibodies will be important to quickly assess therapeutic efficacy. To date, however, only a few randomized clinical trials using anti–PD-1/PD-L1 antibodies for patients with NSCLC have been reported, and it is hard to evaluate optimal surrogate end points strictly according to the Prentice and Buyse criteria.17,18 On the other hand, because immunotherapy including anti–PD-1/PD-L1 antibodies is rapidly becoming an essential treatment modality for many kinds of cancer, the investigation and discussion of alternative predictive end points for OS in this field are currently quite meaningful for accelerated approval pathways and subsequent development of immunotherapeutic drugs. Therefore, we performed a systematic review of reported prospective clinical trials using anti–PD-1/PD-L1 antibodies, and a retrospective analysis of data from patients treated with these agents at the Ohio State University Comprehensive Cancer Center (OSUCCC) to investigate the relationship between OS and response or progression-free survival.
Materials and Methods
Systematic Review of Reported Prospective Clinical Trials
Search for Trials.
We performed a literature search for trials reported between January 2012 and February 2016. To avoid publication bias, both published and unpublished trials were identified through a computer-based search of the PubMed database and abstracts from the past meetings of the American Society of Clinical Oncology, the European Society for Medical Oncology, and the International Association for the Study of Lung Cancer. To identify the prospective clinical trials in which anti–PD-1/PD-L1 antibodies were used for the treatment of NSCLC, we used lung cancer and PD-1 or lung cancer and PD-L1 as search terms in the PubMed database or reviewed the titles and abstracts of the previously mentioned medical meetings. We also collected clinical trials using docetaxel for NSCLC as a reference. To identify the prospective clinical trials in which docetaxel was used for the treatment of NSCLC, we entered lung cancer and docetaxel as search terms in the PubMed database and reviewed the titles and abstracts of the previously mentioned medical meetings.
Prospective phase I to III clinical trials that included the treatment arm of anti–PD-1/PD-L1 monotherapy or docetaxel monotherapy were eligible. Trials that were designed to assess combined modality treatments, including radiotherapy and surgery, were excluded.
Data Abstraction.
To prevent bias in the data extraction process, two researchers (T.S. and K.M.) independently extracted the data from the trials and subsequently compared their results. The number of patients enrolled and randomized, inclusion criteria for the trial, phase of the trial, systemic treatment regimen, median OS, median PFS, 6-month PFS rate, 1-year PFS rate, response rate (RR), disease control rate, and response evaluation criteria used in the trial were extracted from each report. All data were checked for internal consistency, and disagreements were resolved through discussions among the investigators.
Data Analysis.
To assess the relationship between median OS and median PFS or RR, we used the Spearman correlation coefficient, and to account for the differences in sample size among the trial arms, we weighted all analyses by the number of patients in each arm. The p values for all two-sided tests were reported, and p values less than 0.05 were considered statistically significant. All statistical analyses were performed using JMP version 8.0 for Windows (SAS Institute, Inc., Cary, NC).
Retrospective Analyses of Data Generated at OSUCCC
Patient Selection.
The inclusion criteria for this retrospective study were (1) patients with pathologically confirmed advanced NSCLC and (2) patients treated with single-agent anti–PD-1/PD-L1 antibody.
Seventy-one consecutive patients who met the aforementioned criteria at the OSUCCC between January 2012 and December 2015 were reviewed. The TNM stage was classified using version 7 of the TNM stage classification system.
Immunotherapy.
The anti–PD-1/PD-L1 antibodies administered were nivolumab, atezolizumab, and durvalumab. Nivolumab, atezolizumab, and durvalumab were intravenously administered at the doses of 3 mg/kg every 2 weeks, 1200 mg every 3 weeks, and 10 mg/kg every 2 weeks, respectively. In general, these treatments continued until disease progression, intolerable toxicity, or patient refusal.
Statistical Analysis.
To analyze the OS and PFS, survival curves were plotted using the Kaplan-Meier method. The OS was calculated from the date of initiation of the anti–PD-1/PD-L1 antibody treatment to the date of death and was censored at the date of last visit for patients whose deaths could not be confirmed. The tumor response was evaluated in accordance with the Response Evaluation Criteria in Solid Tumors, version 1.1. The PFS was calculated from the date of initiation of the anti–PD-1/PD-L1 antibody treatment to the date of disease progression or death from any cause. The PFS was censored at the date of the last visit for patients who were still alive without any documented disease progression. Of the 71 patients treated with anti–PD-1/PD-L1 antibodies, 56 underwent computed tomography (CT) to evaluate the lesions 5 to 9 weeks after the initiation of treatment. In these 56 patients, landmark analyses were performed to compare survival according to tumor response at 5 to 9 weeks. Landmark analyses were also performed to compare survival between patients who achieved progression-free status and those who did not achieve progression-free status at 8, 16, and 24 weeks. In the landmark analyses, survival was calculated from the date of CT at 5 to 9 weeks or day 56 (eighth week), day 112 (16th week), or day 168 (24th week) after the initiation of anti–PD-1/PD-L1 antibody. Cox proportional hazards models taking age, sex, smoking history, performance status, histological type, stage, and number of prior systemic treatment into account were used. All p values less than 0.05 were considered statistically significant. All statistical analyses were performed using JMP version 8.0 for Windows (SAS Institute Inc., Cary, NC).
Results
Systematic Review of the Reported Prospective Clinical Trials
Detected Prospective Clinical Trials.
A computer-based literature search of the PubMed database using lung cancer and PD-1 or lung cancer and PD-L1 as search terms to identify the relevant clinical trials reported between January 2012 and February 2016 returned 552 reports. In addition, a computer-based literature search of the PubMed database using lung cancer and docetaxel as search terms to identify the clinical trials reported between January 2012 and February 2016 returned 701 reports. Moreover, titles and abstracts from the past meetings of the American Society of Clinical Oncology, European Society for Medical Oncology, and International Association for the Study of Lung Cancer held between January 2012 and February 2016 were reviewed. In total, 12 trials with 17 cohorts were identified as prospective clinical trials that used anti–PD-l/PD-L1 antibodies, and 28 trials with 29 cohorts were identified as prospective clinical trials that used docetaxel monotherapy (Tables 1 and 2).8-12,19-49 As a result of review of these clinical trial reports, median OS, median PFS, and RR were extracted from 12 arms in 10 prospective clinical trials using anti–PD-1/PD-L1 antibodies and from 23 arms in 22 prospective clinical trials using docetaxel. The 6-month PFS rate and the 1-year PFS rate could be extracted from less than half of the clinical trials.
Table 1.
Clinical Trials Using Anti–PD-1/PD-L1 Antibody Monotherapy for NSCLC
| First Author | Study Name | Line of CTx, Histology, etc |
Phase | N | Agent/Dose/Schedule | OS (mo) |
PFS (mo) |
6-mo PFS (%) |
1-y PFS (%) |
RR (%) |
DCR (%) |
Response Criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gettinger SN19 | CheckMate003 | Second or later | 1 | 129 | Nivolumab 1 mg/kg q2wk 3 mg/kg q2wk 10 mg/kg q2wk |
9.9 | 2.3 | 33 | 22 | 17 | – | RECIST v1.0 |
| Brahmer J8 | CheckMate017 | Sq; second | 3 | 135 | Nivolumab 3 mg/kg q2wk (vs. docetaxel) | 9.2 | 3.5 | – | 21 | 20 | 49 | RECIST v1.1 |
| Rizvi NA20 | CheckMate063 | NonSq; second | 2 | 117 | Nivolumab 3 mg/kg q2wk | 8.2 | 1.9 | 25.9 | 20.0 | 14.5 | 40.2 | RECIST v1.1 |
| Borghaei H9 | CheckMate057 | Second | 3 | 292 | Nivolumab 3 mg/kg q2wk (vs. docetaxel) | 12.2 | 2.3 | – | 19 | 19 | 45 | RECIST v1.1 |
| Fehrenbacher L11 | POPLAR | Second | 2 | 144 | Atezolizumab 1200 mg q3wk (vs. docetaxel) | 12.6 | 2.7 | – | – | 15 | – | RECIST v1.1 |
| Besse B21 | BIRCH | Third | 2 | 139 | Atezolizumab 1200 mg q3wk | – | 2.8 | 31 | – | 17 | – | RECIST v1.1 |
| Besse B21 | BIRCH | Second | 2 | 267 | Atezolizumab 1200 mg q3wk | – | 2.8 | 29 | – | 17 | – | RECIST v1.1 |
| Besse B21 | BIRCH | First | 2 | 253 | Atezolizumab 1200 mg q3wk | – | 5.5 | 46 | – | 19 | – | RECIST v1.1 |
| Garon EB12 | KEYNOTE-001 | Any | 1 | 495 | Pembrolizumab 2 mg/kg q3wk 10 mg/kg q3wk 10 mg/kg q2wk |
12 | 3.7 | – | – | 19.4 | 46.3 | RECIST |
| Herbst RS10 | KEYNOTE-010 | Second | 3 | 344 | Pembrolizumab 2 mg/kg q3wk (vs. docetaxel) | 10.4 | 3.9 | – | – | 18 | – | RECIST v1.1 |
| Herbst RS10 | KEYNOTE-010 | Second | 3 | 346 | Pembrolizumab 10 mg/kg q3wk (vs. docetaxel) | 12.7 | 4.0 | – | – | 18 | – | RECIST v1.1 |
| Horn L22 | Atezolizumab Phase 1 | Any | 1 | 88 | Atezolizumab 0.01-20 mg/kg q3wk | 16 | 4 | – | 31 | 23 | 51 | RECIST v1.1 |
| Spigel DR23 | FIR | First | 2 | 31 | Atezolizumab 1200 mg q3wk | NR | 4.5 | 43 | – | 26 | – | RECIST v1.1 |
| Spigel DR23 | FIR | Second without brain met | 2 | 92 | Atezolizumab 1200 mg q3wk | 10.6 | 2.7 | 39 | – | 16 | – | RECIST v1.1 |
| Spigel DR23 | FIR | Second with brain met | 2 | 13 | Atezolizumab 1200 mg q3wk | 6.8 | 2.5 | 45 | – | 23 | – | RECIST v1.1 |
| Gulley JL24 | Avelumab Phase1b | Second or later | 1b | 184 | Avelumab 10 mg/kg q2wk | 8.4 | 2.7 | – | 18.1 (48 wk)a | 13.6 | 50.5 | RECIST v1.1 |
| Higgs BW25 | Durvalumab Phase1/2 | Any | 1/2 | 200 | Durvalumab 10 mg/kg q2wk | – | – | – | – | 16 | – | RECIST v1.1 |
The 48-week progression-free survival rate.
PD-1, programmed death protein 1; PD-L1, programmed death ligand 1; CTx, chemotherapy; N, number; OS, overall survival; mo, month(s); PFS, progression-free survival; RR, response rate; DCR, disease control rate; Sq, squamous cell lung cancer; nonSq, nonsquamous cell lung cancer; met, metastasis; q2wk, every 2 weeks; RECIST, Response Evaluation Criteria in Solid Tumors; q3wk, every 3 weeks; NR, not reached.
Table 2.
Clinical Trials Using Docetaxel Monotherapy for NSCLC
| First Author | Study Name | Line of CTx, Histology, etc |
Phase | N | Agent/Dose/Schedule | OS (mo) |
PFS (mo) |
6-mo PFS (%) |
1-y PFS (%) |
RR (%) |
DCR (%) |
Response Criteria |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brahmer J8 | CheckMate017 | Sq; second |
3 | 137 | Docetaxel 75 mg/m2 q3wk (vs. nivolumab) | 6.0 | 2.8 | – | 6 | 9 | 43 | RECIST v1.1 |
| Borghaei H9 | CheckMate057 | NonSq; second |
3 | 290 | Docetaxel 75 mg/m2 q3wk (vs. nivolumab) | 9.4 | 4.2 | – | 8 | 12 | 54 | RECIST v1.1 |
| Herbst RS10 | KEYNOTE010 | Second | 3 | 343 | Docetaxel 75 mg/m2 q3wk (vs. pembrolizumab) | 8.5 | 4.0 | – | – | 9 | – | RECIST v1.1 |
| Fehrenbacher L11 | POPLAR | Second | 2 | 143 | Docetaxel 75 mg/m2 q3wk (vs. atezolizumab) | 9.7 | 3.0 | – | – | 15 | – | RECIST v1.1 |
| Kawaguchi T26 | DELTA | Second or third | 3 | 151 | Docetaxel 60 mg/m2 q3wk (vs. erlotinib) | 12.2 | 3.2 | – | – | 17.9 | – | RECIST |
| Murakami H27 | DOC±Zometa | Second or third | 2 | 46 | Docetaxel 60 mg/m2 q3wk (vs. DOC + zometa) | 9.7 | 2.6 | – | – | 4 | 48 | RECIST v1.0 |
| Maemondo M28 | CBDCA + PTX vs. DOC | ≥70 y; first | 2 | 42 | Docetaxel 60 mg/m2 q3wk (vs. CBDCA + PTX) | – | 3.5 | – | – | – | – | – |
| Ramlau R29 | DOC±Aflibercept | NonSq; second |
3 | 457 | Docetaxel 75 mg/m2 q3wk (vs. DOC + aflibercept) | 10.4 | 4.1 | – | – | 8.9 | 54.2 | RECIST v1.0 |
| Lin Q30 | DOC±Cape | Second | 2 | 25 | Docetaxel 35 mg/m2 d 1and d 8 q3wk (vs. DOC + Cape) |
12 | 3a | – | – | 12 | 76 | RECIST v1.1 |
| Ramalingam S31 | GALAXY-1 | Adeno second |
2 | 128 | Docetaxel 75 mg/m2 q3wk (vs. DOC + ganetespib) | 8.4 | 3.2 | – | – | 15 | 70 | RECIST v1.1 |
| Blumenschein GR Jr32 | DOC±trametinib | Second | 2 | 43 | Docetaxel 75 mg/m2 q3wk (vs. DOC + trametinib) | NR | 11wk | – | – | 12 | 53 | RECIST v1.1 |
| Tsukada H33 | JCOG0207 | First | 3 | 63 | Docetaxel 25 mg/m2 d 1, 8, and 15 q3wk (vs. CDDP + DOC) |
10.7 | 3.7 | – | 5.0 | 26.2 | 60.7 | RECIST v1.0 |
| Abe T34 | JCOG0803/WJOG4307L | First | 3 | 134 | Docetaxel 60 mg/m2 q3wk (vs. CDDP + DOC) | 14.8 | 4.4 | – | – | 24.6 | – | RECIST v1.0 |
| Reck M35 | LUME-Lung1 | Second | 3 | 659 | Docetaxel 75 mg/m2 q3wk (vs. DOC + nintedanib) | 9.1 | 2.7 | – | – | 3.3 | 41.3 | RECIST v1.1 |
| Li R36 | Pem vs. DOC in China | Second | 3 ? | 128 | Docetaxel 75 mg/m2 q3wk (vs. Pem) | – | – | – | – | 4.9 | 69.6 | RECIST |
| Garon EB37 | REVEL | Second | 3 | 625 | Docetaxel 75 mg/m2 q3wk (vs. DOC + ramucirumab) | 9.1 | 3.0 | – | – | 14 | 53 | RECIST v1.1 |
| Levy B38 | DOC±PX-866 | Second or third | 2 | 47 | Docetaxel 75 mg/m2 q3wk (vs. DOC + PX-866) | 9.4 | 2.9 | – | – | 0 | 53 | RECIST v1.1 |
| Manegold C39 | DOC±cilengitide | Second | 2 | 34 | Docetaxel 75 mg/m2 q3wk (vs. cilengitide) | 194 days | 67 Days | – | – | 8.8 | 47.1 | RECIST v1.0 |
| Garassino MC40 | TAILOR | Second | 3 | 110 | Docetaxel 75 mg/m2 q3wk or Docetaxel 35 mg/m2 d 1, 8, and 15 q4wk (vs. erlotinib) |
8.2 | 2.9 | 27.3 | – | 15.5 | 44.3 | RECIST v1.1 |
| Jänne PA41 | DOC±selumetinib | Second | 2 | 43 | Docetaxel 75 mg/m2 (vs. DOC + selumetinib) |
5.2 | 2.1 | – | – | 0 | 50.0 | RECIST v1.0 |
| Natale R42 | DOC±LY2181308 | Second | 2 | 60 | Docetaxel 75 mg/m2 (vs. DOC + LY2181308) | 8.8 | 3.35 | – | – | 2.1 | – | RECIST v1.1 |
| Heist RS43 | DOC±Plinabulin | Second or later | 2 | 55 | Docetaxel 75 mg/m2 (vs. DOC + plinabulin 30 mg/m2 d 1 and 8 q3wk) | 7.5 | 3.5 | – | – | 14.5 | – | – |
| Heist RS43 | DOC±Plinabulin | Second or later | 2 | 18 | Docetaxel 75 mg/m2 (vs. DOC + plinabulin 20 mg/m2 d 1 and 8 q3wk) | 10.5 | 3.0 | – | – | 11.1 | – | – |
| Bergqvist M44 | DOC±AXL1717 | Second or third | 2 | 41 | Docetaxel 75 mg/m2 (vs. AXL1717 400 mg twice daily) | 39.6 wk | 12.4 wk | – | – | 12.2 | – | RECIST v1.1 |
| Hosomi Y45 | DOC±Ramucirumab in Japan | Second | 2 | 81 | Docetaxel 60 mg/m2 (vs. DOC + ramucirumab 10 mg/kg q3wk) | 13.93 | 4.21 | – | – | 18.5 | 70.4 | RECIST v1.1 |
| Auliac JB46 | TARSEQ | Second | 2 | 66 | Docetaxel 75 mg/m2 q3wk (vs. DOC + erlotinib) | 8.4 | 2.5 | – | – | – | – | – |
| Hanna N47 | DOC±Vintafolide | Second | 2 | 68 | Docetaxel 75 mg/m2 q3wk (vs. DOC + vintafolide vs. vintafolide) | – | – | – | – | – | – | – |
| Katakami N48 | DOC vs. AMR | Second or third | 3 | 97 | Docetaxel 60 mg/m2 q3wk (vs. AMR 35 mg/m2 d 1–3 q3wk) | 14.6 | 3.6 | – | – | 14.8 | 55.7 | – |
| Kasahara K49 | DOC±Ramucirumab in Japan | Third | 2 | 17 | Docetaxel 60 mg/m2 (vs. DOC + ramucirumab 10 mg/kg q3wk) |
– | 4.4 | – | – | 41.2 | 76.5 | RECIST v1.1 |
Indicating time to progression.
CTx, chemotherapy; N, number; OS, overall survival; PFS, progression-free survival; RR, response rate; DCR, disease control rate; Sq, squamous cell lung cancer; nonSq, nonsquamous cell lung cancer; q3wk, every 3 weeks; RECIST, Response Evaluation Criteria in Solid Tumors; adeno, adenocarcinoma; q4wk, every 4 weeks; DOC, docetaxel; Pem, pemetrexed; CBDCA, carboplatin; PTX, paclitaxel; Cape, capecitabine; CDDP, cisplatin; AMR, amurubicin; NR, not reached.
The Relationship between Median OS and Either Median PFS or RR.
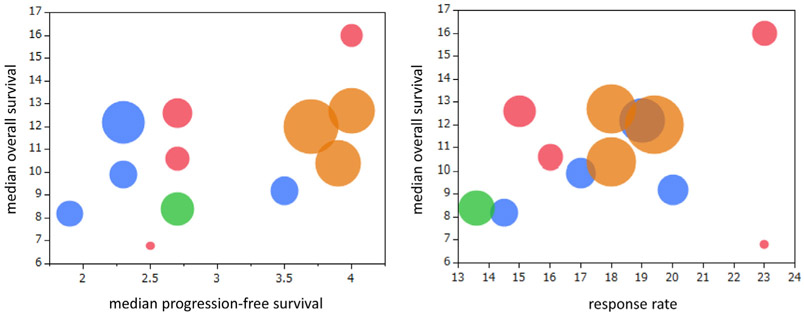
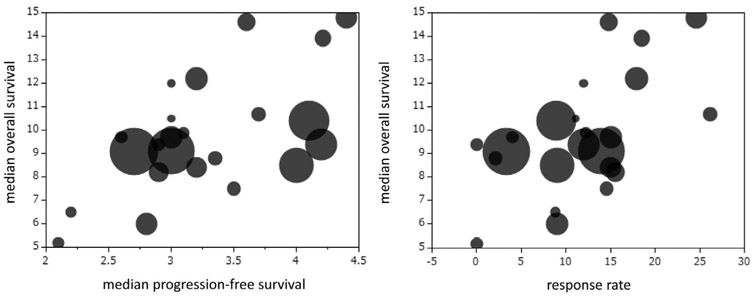
Figure 1 shows the relationship between median OS and either median PFS or RR in prospective clinical trials using anti–PD-1/PD-L1 antibodies in 12 arms. Moderate but not statistically significant correlations between median OS and median PFS (p = 0.120, r = 0.473) and between median OS and RR (p = 0.141, r = 0.452) were identified on the basis of the Spearman correlation coefficient. Figure 2 shows the relationship between median OS and either median PFS or RR in prospective clinical trials using docetaxel monotherapy in 23 arms. Moderate correlations between median OS and median PFS (p = 0.015, r = 0.499) and between median OS and RR (p = 0.053, r = 0.408) were identified on the basis of the Spearman correlation coefficient.
Figure 1.
The relationship between median overall survival and either median progression-free survival or response rate in prospective clinical trials using anti–programmed death protein 1/programmed death ligand 1 antibodies in 12 arms. Blue, nivolumab; pink, atezolizumab; brown, pembrolizumab; green, avelumab.
Figure 2.
The relationship between median overall survival and either median progression-free survival or response rate in prospective clinical trials using docetaxel monotherapy in 23 arms.
Retrospective Analysis of Data Generated at OSUCCC
Patient Characteristics.
Between January 2012 and December 2015, 71 patients with NSCLC received anti–PD-1/PD-L1 antibody treatment. Of the 71 patients, 38 (54%) were men and the median age was 65 years (range 39–86 years). The histological subtypes were adenocarcinoma in 35 patients (49%) and squamous cell carcinoma in 29 patients (41%). Testing for EGFR mutation was performed in 51 patients (72%), and EGFR mutation (exon 19 deletion or exon 21 L858R) was detected in five patients (7%). Most of the patients (85%) had a performance status of 0 or 1. Eight patients (11%) never smoked, and 63 patients (89%) were current or ex-smokers. One patient (1%) had stage IIIA disease, three (4%) had stage IIIB disease, and 67 (94%) had stage IV disease. Fifty-five (77%), 14 (20%), and two patients (3%) were treated with nivolumab, atezolizumab, and durvalumab, respectively. Nineteen patients (27%) received anti–PD-1/PD-L1 antibody as a first line-therapy, 25 (35%) patients received it as second-line therapy, and 27 (38%) received it as third- or later-line therapy (Supplementary Table 1).
Patient Outcomes.
Of the 71 patients treated with anti–PD-1/PD-L1 antibody, 19 showed a partial response (PR), 19 showed stable disease, and 25 showed progressive disease (PD). The response was not evaluable (NE) in eight patients: five patients died before evaluation of the disease and three patients were lost to followup. The RR was 27%, and the disease control rate was 54%. The median follow-up time was 301 days. The median PFS and the median OS were 55 days and 277.5 days, respectively.
OS Analysis according to Tumor Response and Status of Being Progression-Free at 8, 16, or 24 Weeks by Landmark Analyses.
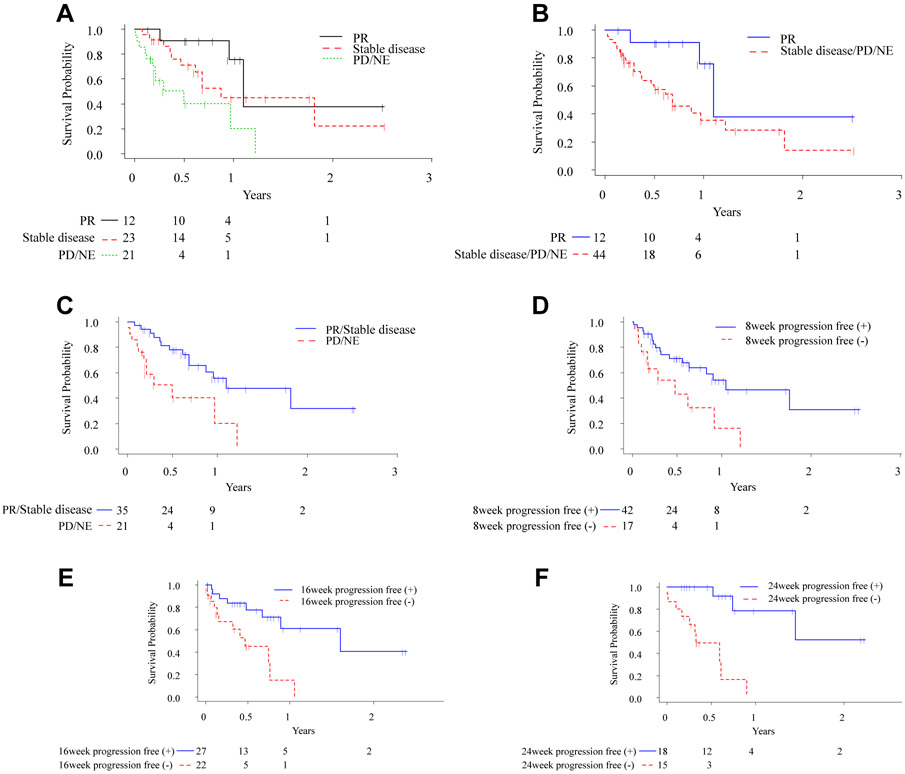
Figure 3 shows the Kaplan–Meier survival curves according to tumor response and status of being progression-free at 8, 16, or 24 weeks. The median survival times from the date of CT at 5 to 9 weeks were 397.5, 287, and 146.5 days for patients with a PR, patients with stable disease, and patients who had PD or were NE, respectively (Fig. 3A). For reference, the Kaplan-Meier curves of OS from the initiation of anti–PD-1/PD-L1 antibody are shown in Supplementary Figure 1, and the shapes of the curves are similar between Figure 3A and Supplementary Figure 1. There was a nearly significant difference in survival from the date of CT between patients with a PR and patients who had stable disease, PD, or were NE (median 397.5 versus 244.5 days; log-rank p = 0.0502) (Fig. 3B). On the other hand, there was a significant difference in survival from the date of CT at 5 to 9 weeks between patients with PR/stable disease and patients who had PD or were NE (median 397.5 versus 146.5 days; log-rank p = 0.0020) (Fig. 3C). The numbers of the patients included in the OS analyses according to status of being progression-free at 8, 16, or 24 weeks were 59, 49, and 33, respectively (Fig. 3D-F). There were also significant differences in survival between patients who did and did not achieve progression-free status at 8, 16, and 24 weeks (8-week progression-free status yes versus no, median 380.5 versus 142.5 days, log-rank p = 0.0065; 16-week progression-free status yes versus no, median 581.5 versus 160 days, log-rank p = 0.0023; and 24 week progression-free status yes versus no, median not reached versus 117.5 days, log-rank p < 0.0001) (Fig. 3D-F).
Figure 3.
(A)–(C) Kaplan–Meier curves of overall survival according to tumor response. (D) Kaplan-Meier curves of overall survival in patients who achieved and did not achieve 8-week progression-free status. (E) Kaplan–Meier curves of overall survival in patients who did and did not achieve 16-week progression-free status. (F) Kaplan–Meier curves of overall survival in patients who achieved and did not achieve 24-week progression-free status.
The results of the univariate and multivariate analyses are shown in Supplementary Tables 2 through 10 and Table 3. The variables used in the multivariate analysis were those that had p values less than 0.20 for OS in univariate analysis. In both univariate and multivariate analyses, disease control (PR/stable disease versus PD/NE), 8-week progression-free status, 16-week progression free status, and 24-week progression-free status significantly predicted OS, whereas tumor response (PR versus stable disease/PD/NE) did not significantly predict OS. The hazard ratio (HR) was the highest when OS was compared between patients who did and did not achieve 24-week progression-free status (Cox proportional hazards model; PR versus stable disease/PD/NE at 5- to 9-week CT, p = 0.0604, HR 2.835, 95% confidence interval (CI): 0.960–12.107; PR/stable disease versus PD/NE at 5- to 9-week CT, p = 0.0104, HR = 3.041, 95% CI: 1.310–6.972; 8-week progression-free status yes versus no, p = 0.0183, HR = 2.684, 95% CI: 1.191–5.839; 16-week progression-free status yes versus no, p = 0.0036, HR = 4.009, 95% CI: 1.574–11.038; and 24-week progression-free status yes versus no, p = 0.0002, HR = 12.726, 95% CI: 3.045–88.359).
Table 3.
Results of Multivariate Landmark Analyses
| Variable | p Value | Hazard Ratio |
95% CI |
|---|---|---|---|
| Tumor response at 5–9 wk CT scan | |||
| PR vs. stable disease/PD/NE | 0.060 | 2.84 | 0.96–12.11 |
| Tumor response at 5-9 wk CT scan | |||
| PR/stable disease vs. PD/NE | 0.010 | 3.04 | 1.31–6.97 |
| 8-wk progression-free | |||
| yes vs. no | 0.018 | 2.68 | 1.19–5.84 |
| 16-wk progression-free | |||
| yes vs. no | 0.004 | 4.01 | 1.57–11.04 |
| 24-wk progression-free | |||
| yes vs. no | <0.001 | 12.73 | 3.05–88.36 |
CI, confidence interval; CT, computed tomography; PR, partial response; PD, progressive disease; NE, not evaluable.
Discussion
The pace of progress in lung cancer therapeutics is accelerating, and to rationally design new trials and get therapies to patients as soon as possible, it is important to have accurate alternative predictive end points for OS to assess therapeutic efficacy. To our knowledge, this is the first study that investigated and evaluated the optimal alternative predictive end point for OS in patients with NSCLC treated with anti–PD-1/PD-L1 antibodies. We conducted a systematic review of the reported prospective clinical trials and a retrospective analysis of consecutive patients treated at a single institution. In the systematic review of the reported clinical trials, neither RR nor PFS was statistically correlated with OS; however, in the retrospective analysis, both response category and landmark PFS were highly correlated with survival, with stronger correlations observed with increasing time to the landmark in patients with NSCLC treated with anti–PD-1/PD-L1 antibodies. The HR for the correlation of 24-week landmark PFS with survival was 12.
In the systematic review performed in 2012 for determining the role of surrogate end points instead of OS, it was concluded that OS is still the best criterion for predicting treatment efficacy in lung cancer, and some intermediate criteria such as time to progression, PFS, and objective response could be early predictors.50 Other groups have performed responder analyses showing that RR with EGFR tyrosine kinase inhibitors was correlated with median survival time in advanced NSCLC and that the tumor size change at week 8 could predict OS and assist in early drug development decisions.51,52 On the other hand, the U.S. Food and Drug Administration performed analyses exploring trial-level and patient-level associations between RR, PFS, and OS in advanced NSCLC trials submitted to the U.S. Food and Drug Administration.16 On the trial level, associations between RR and OS and between PFS and OS were not established. The patient-level analysis showed that responders have better PFS and OS compared with nonresponders. In our study, we identified a significant correlation between median OS and median PFS in docetaxel monotherapy studies, whereas no significant correlation between the median OS and either median PFS or RR in anti–PD-1/PD-L1 antibody studies was identified. In patient-level analyses, tumor response (PR versus stable disease/PD/NE) was nearly significantly predictive of OS; however, PR/stable disease versus PD/NE and landmark PFS at 8, 16, or 24 weeks significantly predicted OS. The longer the landmark, the stronger the correlation, with the 24-week PFS predicting survival better than all of the other end points in patients with NSCLC treated with anti–PD-1/PD-L1 antibodies.
Immunotherapy including anti–PD-1/PD-L1 antibodies has the potential for long-term disease control through the activation of the patients’ own immune system against cancer cells. The comparison of Kaplan-Meier curves of PFS among patients treated with anti–PD-1/PD-L1 antibodies, molecular-targeted agents, and cytotoxic agents showed that the slope of the curve flattened out after 6 months (i.e., 24 weeks) for patients treated with anti–PD-1/PD-L1 antibodies compared with that of the curves for those patients who received other kinds of agents.8-11,53,54 This could be attributed to the long-term disease control conferred by the anti–PD-1/PD-L1 antibodies, and it underlies the findings of the present study in a sequential cohort of patients treated at a single institution; the status of being progression-free at 24 weeks predicted further survival better than did the other end points in patients with NSCLC treated with anti–PD-1/PD-L1 antibodies.
Because median PFS is mainly affected by early progression (the plateau occurring after the median PFS), this systematic review of clinical trials might not find any correlation between median PFS and median OS. Similarly, because anti–PD-1/PD-L1 antibodies provide more or less the same RR and the same median survival, the range of RRs in the different trials is rather small. Therefore, this systematic review might not find a relationship between RR and median OS for anti–PD-1/PD-L1 single agents.
There are several limitations to this study. For a systemic review, the correlation between OS and either PFS or RR was analyzed using the data of not only prospective randomized phase II or III trials but also single-arm phase I or II trials. In previous review articles that analyzed the optimal surrogate marker for OS, the HRs of OS/PFS in randomized phase III trials were used according to the Prentice and Buyse criteria.17,18 To date, only four randomized trial results have been reported (as shown in Table 1), and it is hard for us to perform enough analyses using HRs of OS/PFS in randomized phase II or III trials. This study using individual patient data was retrospective and included a relatively small number of patients treated at a single institution. We approached each of the major companies manufacturing anti-PD-1/PD-L1 antibodies, but they were unwilling to provide individual patient data for us. Although the use of “real-world” patients is a strength, larger numbers of patients are needed to reach a definitive conclusion. We examined all patients for evaluable lesions approximately every 6 to 8 weeks by CT, magnetic resonance imaging, or positron emission tomography during the treatment period. However, although 39 of the 71 patients in this retrospective study were treated in a clinical trial setting, the intervals between evaluations in the present study (i.e., PFS) were not as accurate as those in a prospective study. Of the 71 patients treated with anti–PD-1/PD-L1 antibodies at our institution, 56 patients underwent CT for the evaluation of lesions at 5 to 9 weeks after the initiation of treatment. Most of the other 15 patients either underwent CT earlier because of suspicion of disease progression or died earlier on account of rapid disease progression. In our cohort, patients treated with the anti–PD-1 antibody nivolumab and those treated with an anti–PD-L1 antibody (atezolizumab and durvalumab) were considered the same population. Although anti–PD-L1 antibody does not block interaction between PD-1 and PD-L2, both anti–PD-1 antibody and anti–PD-L1 antibody are considered to show efficacy mainly through inhibition of the interaction between PD-1 and PD-L1. The shapes of the Kaplan–Meier PFS/OS curves in reported prospective clinical trials are almost the same among these antibodies, and the mode of action seems to be similar as a first-order approximation. Because of the nature of a retrospective study, the patient characteristics were heterogeneous. We were concerned that the prior number of chemotherapeutic regimens would affect OS of the patients. However, the activity of anti–PD-1/PD-L1 agents does not seem to be very dependent on the line of treatment, and actually, in our cohort the survival was poorer in patients treated with anti–PD-1/PD-L1 antibodies in a first-line setting than in those treated in a further-line setting in univariate analysis. Moreover, univariate and multivariate analyses were performed with consideration for patient characteristics, including prior number of chemotherapeutic regimens, and they did not appear to strongly influence our conclusions. Despite these limitations, because immunotherapy including anti–PD-1/PD-L1 antibodies is rapidly becoming an essential treatment modality for many kinds of cancer, the investigation and discussion of alternative predictive end points for OS in this field are currently quite meaningful for accelerated approval pathways and subsequent development of immunotherapeutic drugs.
In conclusion, response category and landmark PFS, especially 24-week progression-free status, accurately predicted survival in patients with NSCLC treated with anti–PD-1/PD-L1 antibodies. Therefore, 24-week progression-free status would be useful as an alternative predictive end point of OS in patients with NSCLC treated with anti–PD-1/PD-L1 antibodies and could be available more quickly than OS. On the other hand, if we want to judge the efficacy of anti–PD-1/PD-L1 treatment earlier, disease control (PR/stable disease versus PD/NE) and 8- or 16- week progression-free status would be considered as alternative predictive end points. Further patient-level analysis with a larger number of more homogeneous patients and trial-level analysis after accumulation of more prospective randomized clinical trials are necessary to decide optimal surrogate end points in patients with NSCLC treated with anti–PD-1/PD-L1 antibodies.
Supplementary Material
Acknowledgments
This work was supported by The Lilly Oncology Fellowship from The Japanese Respiratory Society, an alumni scholarship from the Juntendo University School of Medicine, a research fellowship from Uehara Memorial Foundation (to Dr. Shukuya), and the Blue Beautiful Skies Fund (to Dr. Carbone).
Footnotes
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at http://dx.doi.org/10.1016/j.jtho.2016.07.017.
Disclosure: Dr. Shukuya reports personal fees from Chugai Pharmaceutical, AstraZeneca, Ono Pharmaceutical, and Pfizer outside the submitted work. Dr. Otterson reports grants and research and consultation fees from Genentech and grants from Pfizer, Bristol-Myers Squibb, Clovis, Xcovery, and Ignyta outside the submitted work. Dr. Morita reports personal fees from Ono Pharmaceutical and Bristol-Myers Squibb Japan outside the submitted work. Dr. Carbpme reports personal fees from Genentech/Roche, Bristol-Myers Squibb, and Astra Zeneca outside the submitted work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- 2.Peters S, Adjei AA, Gridelli C, et al. Metastatic nonsmall-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(suppl 7):vii56–vii64. [DOI] [PubMed] [Google Scholar]
- 3.National comprehensive cancer network. NCCN clinical practice guidelines in oncology (NCCN Guidelines) Version 2. 2016. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed December 1, 2015.
- 4.Masters GA, Temin S, Azzoli CG, et al. Systemic therapy for stage IV non-small-cell lung cancer: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2015;33:3488–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimura H, Okazaki T, Tanaka Y, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. [DOI] [PubMed] [Google Scholar]
- 8.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer. (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 11.Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387:1837–1846. [DOI] [PubMed] [Google Scholar]
- 12.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 13.ClinicalTrials.gov. https://clinicaltrials.gov/. Accessed February 13, 2016.
- 14.Hotta K, Fujiwara Y, Matsuo K, et al. Time to progression as a surrogate marker for overall survival in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009;4:311–317. [DOI] [PubMed] [Google Scholar]
- 15.Hotta K, Suzuki E, Di Maio M, et al. Progression-free survival and overall survival in phase III trials of molecular-targeted agents in advanced non-small-cell lung cancer. Lung Cancer. 2013;79:20–26. [DOI] [PubMed] [Google Scholar]
- 16.Blumenthal GM, Karuri SW, Zhang H, et al. Overall response rate, progression-free survival, and overall survival with targeted and standard therapies in advanced non-small-cell lung cancer: US Food and Drug Administration trial-level and patient-level analyses. J Clin Oncol. 2015;33:1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–440. [DOI] [PubMed] [Google Scholar]
- 18.Buyse M, Molenberghs G. Criteria for the validation of surrogate endpoints in randomized experiments. Biometrics. 1998;54:1014–1029. [PubMed] [Google Scholar]
- 19.Gettinger SN, Horn L, Gandhi L, et al. Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol. 2015;33:2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rizvi NA, Mazières J, Planchard D, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol. 2015;16:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besse B, Johnson M, Jänne PA, et al. Phase II single-arm trial (BIRCH) of atezolizumab as first-line or subsequent therapy for locally advanced or metastatic PD-L1-selected non-small cell cancer [abstract 16LBA]. Eur J Cancer. 2015;51:S717–S718. [Google Scholar]
- 22.Horn L, Spigel DR, Gettinger SN, et al. Clinical activity, safety and predictive biomarkers of the engineered antibody MPDL3280A (anti-PDL1) in non-small cell lung cancer (NSCLC): update from a phase Ia study [abstract]. J Clin Oncol. 2015;33(suppl):8029. [Google Scholar]
- 23.Spigel DR, Chaft JE, Gettinger SN, et al. Clinical activity and safety from a phase II study (FIR) of MPDL3280A (anti-PDL1) in PD-L1–selected patients with non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol. 2015;33(suppl):8028. [Google Scholar]
- 24.Gulley JL, Rajan A, Spigel DR, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with metastatic or recurrent non-small-cell lung cancer progressing after platinum-based chemotherapy: A phase Ib trial [abstract 3090]. Eur J Cancer. 2015;51(suppl 3):S629. [Google Scholar]
- 25.Higgs BW, Robbins PB, Blake-Haskins JA, et al. High tumoral IFNγ mRNA, PD-L1 protein, and combined IFNγ mRNA/PD-L1 protein expression associates with response to durvalumab (anti-PD-L1) monotherapy in NSCLC patients [abstract 15LBA]. Eur J Cancer. 2015;51(suppl 3):S717. [Google Scholar]
- 26.Kawaguchi T, Ando M, Asami K, et al. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: Docetaxel and Erlotinib Lung Cancer Trial (DELTA). J Clin Oncol. 2014;32:1902–1908. [DOI] [PubMed] [Google Scholar]
- 27.Murakami H, Yamanaka T, Seto T, et al. Phase II study of zoledronic acid combined with docetaxel for non-small-cell lung cancer: West Japan Oncology Group. Cancer Sci. 2014;105:989–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maemondo M, Inoue A, Sugawara S, et al. Randomized phase II trial comparing carboplatin plus weekly pacli-taxel and docetaxel alone in elderly patients with advanced non-small cell lung cancer: north japan lung cancer group trial 0801. Oncologist. 2014;19:352–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramlau R, Gorbunova V, Ciuleanu TE, et al. Aflibercept and docetaxel versus docetaxel alone after platinum failure in patients with advanced or metastatic non-small-cell lung cancer: a randomized, controlled phase III trial. J Clin Oncol. 2012;30: 3640–3647. [DOI] [PubMed] [Google Scholar]
- 30.Lin Q, Meng FJ, Liu Y, et al. Phase II trial of capecitabine combined with docetaxel in previously treated patients with non-small cell lung cancer: A randomized controlled study. Oncol Lett. 2012;3: 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramalingam S, Goss G, Rosell R, et al. A randomized phase II study of ganetespib, a heat shock protein 90 inhibitor, in combination with docetaxel in second-line therapy of advanced non-small cell lung cancer (GALAXY-1). Ann Oncol. 2015;26:1741–1748. [DOI] [PubMed] [Google Scholar]
- 32.Blumenschein R Jr, Smit EF, Planchard D, et al. A randomized phase II study of the MEK1/MEK2 inhibitor trametinib (GSK1120212) compared with docetaxel in KRAS-mutant advanced non-small-cell lung cancer (NSCLC). Ann Oncol. 2015;26:894–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsukada H, Yokoyama A, Goto K, et al. Randomized controlled trial comparing docetaxel-cisplatin combination with weekly docetaxel alone in elderly patients with advanced non-small-cell lung cancer: Japan Clinical Oncology Group (JCOG) 0207. Jpn J Clin Oncol. 2015;45:88–95. [DOI] [PubMed] [Google Scholar]
- 34.Abe T, Takeda K, Ohe Y, et al. Randomized phase III trial comparing weekly docetaxel plus cisplatin versus docetaxel monotherapy every 3 weeks in elderly patients with advanced non-small-cell lung cancer: the intergroup trial JCOG0803/WJOG4307L. J Clin Oncol. 2015;33:575–581. [DOI] [PubMed] [Google Scholar]
- 35.Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol. 2014;15: 143–155. [DOI] [PubMed] [Google Scholar]
- 36.Li R, Sun L, Wang J, Qian J, Wang Z, Jiao X. Pemetrexed versus docetaxel in second line non-small-cell lung cancer: results and subsets analyses of a multi-center, randomized, exploratory trial in Chinese patients. Pulm Pharmacol Ther. 2012;25:364–370. [DOI] [PubMed] [Google Scholar]
- 37.Garon EB, Ciuleanu TE, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384:665–673. [DOI] [PubMed] [Google Scholar]
- 38.Levy B, Spira A, Becker D, et al. A randomized, phase 2 trial of docetaxel with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic non-small-cell lung cancer. J Thorac Oncol. 2014;9:1031–1035. [DOI] [PubMed] [Google Scholar]
- 39.Manegold C, Vansteenkiste J, Cardenal F, et al. Randomized phase II study of three doses of the integrin inhibitor cilengitide versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer. Invest New Drugs. 2013;31:175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14:981–988. [DOI] [PubMed] [Google Scholar]
- 41.Jänne PA, Shaw AT, Pereira JR, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14:38–47. [DOI] [PubMed] [Google Scholar]
- 42.Natale R, Blackhall F, Kowalski D, et al. Evaluation of antitumor activity using change in tumor size of the survivin antisense oligonucleotide LY2181308 in combination with docetaxel for second-line treatment of patients with non-small-cell lung cancer: a randomized open-label phase II study. J Thorac Oncol. 2014;9: 1704–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heist RS, Aren OR, Mita AC, et al. Randomized phase 2 trial of plinabulin (NPI-2358) plus docetaxel in patients with advanced non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol. 2014;32(suppl):8054. [Google Scholar]
- 44.Bergqvist M, Bondarenko I, Thuresson M, Klockare M, Harmenberg J. Randomized, controlled, multicenter, multinational phase 2 study of docetaxel (DCT) or AXL1717 treatment in patients with squamous cell carcinoma (SCC) or adenocarcinoma (AC) on non-small cell lung cancer (NSCLC) [abstract]. J Clin Oncol. 2014;32(suppl):8091. [Google Scholar]
- 45.Hosomi Y, Yoh K, Kasahara K, et al. Docetaxel + ramucirumab (DR) versus docetaxel + placebo (D) as second-line treatment for advanced non-small cell lung cancer (NSCLC): A randomized, phase II, double-blind, multicenter trial in Japan [abstract]. J Clin Oncol. 2015;33(suppl):8054. [Google Scholar]
- 46.Auliac JB, Chouaid C, Greillier L, et al. Randomized open-label non-comparative multicenter phase II trial of sequential erlotinib and docetaxel versus docetaxel alone in patients with non-small-cell lung cancer after failure of first-line chemotherapy: GFPC 10.02 study. Lung Cancer. 2014;85:415–419. [DOI] [PubMed] [Google Scholar]
- 47.Hanna N, Juhász E, Cainap C, et al. A randomized phase 2 trial of vintafolide and docetaxel in folate-receptor positive (FR+) advanced NSCLC patients: final efficacy results. Oral presentation presented at: 16th World Conference on Lung Cancer. September 6–9, 2015; Denver, CO. [Google Scholar]
- 48.Katakami N, Yoshioka H, Okamoto H, et al. Amrubicin (AMR) versus docetaxel (DTX) as second- or third-line treatment for non-small cell lung cancer (NSCLC): A randomized phase III trial [abstract 1236P]. Ann Oncol. 2014;25(suppl 4):iv426–iv470. [Google Scholar]
- 49.Kasahara K, Kiura K, Nogami N, et al. Phase 2: Docetaxel with or without ramucirumab as therapy for non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) activating mutations after prior EGFR tyrosine kinase inhibitor (TKI) and platinum-based chemotherapy [abstract 3072]. Eur J Cancer. 2015;51(suppl 3):S620. [Google Scholar]
- 50.Berghmans T, Pasleau F, Paesmans M, et al. Surrogate markers predicting overall survival for lung cancer: ELCWP recommendations. Eur Respir J. 2012;39: 9–28. [DOI] [PubMed] [Google Scholar]
- 51.Tsujino K, Kawaguchi T, Kubo A, et al. Response rate is associated with prolonged survival in patients with advanced non-small cell lung cancer treated with gefitinib or erlotinib. J Thorac Oncol. 2009;4: 994–1001. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Sung C, Dartois C, et al. Elucidation of relationship between tumor size and survival in non-small-cell lung cancer patients can aid early decision making in clinical drug development. Clin Pharmacol Ther. 2009;86:167–174. [DOI] [PubMed] [Google Scholar]
- 53.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. [DOI] [PubMed] [Google Scholar]
- 54.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.