Abstract
Background
Surgery is the preferred treatment for resectable oesophageal cancers, and can be performed in different ways. Transhiatal oesophagectomy (oesophagectomy without thoracotomy, with a cervical anastomosis) is one way to resect oesophageal cancers. It can be performed laparoscopically or by open method. With other organs, laparoscopic surgery has been shown to reduce complications and length of hospital stay compared to open surgery. However, concerns remain about the safety of laparoscopic transhiatal oesophagectomy in terms of post‐operative complications and oncological clearance compared with open transhiatal oesophagectomy.
Objectives
To assess the benefits and harms of laparoscopic versus open oesophagectomy for people with oesophageal cancer undergoing transhiatal oesophagectomy.
Search methods
We electronically searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, and trials registers until August 2015. We also searched the references of included trials to identify further trials.
Selection criteria
We considered randomised controlled trials and non‐randomised studies comparing laparoscopic with open transhiatal oesophagectomy in patients with resectable oesophageal cancer, regardless of language, blinding, or publication status for the review.
Data collection and analysis
Three review authors independently identified trials, assessed risk of bias and extracted data. We calculated the risk ratio (RR) or hazard ratio (HR) with 95% confidence intervals (CI), using both fixed‐effect and random‐effects models, with RevMan 5, based on intention‐to‐treat analyses.
Main results
We found no randomised controlled trials on this topic. We included six non‐randomised studies (five retrospective) that compared laparoscopic versus open transhiatal oesophagectomy (334 patients: laparoscopic = 154 patients; open = 180 patients); five studies (326 patients: laparoscopic = 151 patients; open = 175 patients) provided information for one or more outcomes. Most studies included a mixture of adenocarcinoma and squamous cell carcinoma and different stages of oesophageal cancer, without metastases. All the studies were at unclear or high risk of bias; the overall quality of evidence was very low for all the outcomes.
The differences between laparoscopic and open transhiatal oesophagectomy were imprecise for short‐term mortality (laparoscopic = 0/151 (adjusted proportion based on meta‐analysis estimate: 0.5%) versus open = 2/175 (1.1%); RR 0.44; 95% CI 0.05 to 4.09; participants = 326; studies = 5; I² = 0%); long‐term mortality (HR 0.97; 95% CI 0.81 to 1.16; participants = 193; studies = 2; I² = 0%); anastomotic stenosis (laparoscopic = 4/36 (11.1%) versus open = 3/37 (8.1%); RR 1.37; 95% CI 0.33 to 5.70; participants = 73; studies = 1); short‐term recurrence (laparoscopic = 1/16 (6.3%) versus open = 0/4 (0%); RR 0.88; 95% CI 0.04 to 18.47; participants = 20; studies = 1); long‐term recurrence (HR 1.00; 95% CI 0.84 to 1.18; participants = 173; studies = 2); proportion of people who required blood transfusion (laparoscopic = 0/36 (0%) versus open = 6/37 (16.2%); RR 0.08; 95% CI 0.00 to 1.35; participants = 73; studies = 1); proportion of people with positive resection margins (laparoscopic = 15/102 (15.8%) versus open = 27/111 (24.3%); RR 0.65; 95% CI 0.37 to 1.12; participants = 213; studies = 3; I² = 0%); and the number of lymph nodes harvested during surgery (median difference between the groups varied from 12 less to 3 more lymph nodes in the laparoscopic compared to the open group; participants = 326; studies = 5).
The proportion of patients with serious adverse events was lower in the laparoscopic group (10/99, (10.3%) compared to the open group = 24/114 (21.1%); RR 0.49; 95% CI 0.24 to 0.99; participants = 213; studies = 3; I² = 0%); as it was for adverse events in the laparoscopic group = 37/99 (39.9%) versus the open group = 71/114 (62.3%); RR 0.64; 95% CI 0.48 to 0.86; participants = 213; studies = 3; I² = 0%); and the median lengths of hospital stay were significantly less in the laparoscopic group than the open group (three days less in all three studies that reported this outcome; number of participants = 266). There was lack of clarity as to whether the median difference in the quantity of blood transfused was statistically significant favouring laparoscopic oesophagectomy in the only study that reported this information. None of the studies reported post‐operative dysphagia, health‐related quality of life, time‐to‐return to normal activity (return to pre‐operative mobility without caregiver support), or time‐to‐return to work.
Authors' conclusions
There are currently no randomised controlled trials comparing laparoscopic with open transhiatal oesophagectomy for patients with oesophageal cancers. In observational studies, laparoscopic transhiatal oesophagectomy is associated with fewer overall complications and shorter hospital stays than open transhiatal oesophagectomy. However, this association is unlikely to be causal. There is currently no information to determine a causal association in the differences between the two surgical approaches. Randomised controlled trials comparing laparoscopic transhiatal oesophagectomy with other methods of oesophagectomy are required to determine the optimal method of oesophagectomy.
Plain language summary
Key‐hole (laparoscopic) versus standard cut (open) abdominal surgery for people with food‐pipe (oesophageal) cancer
Review question
How does key‐hole (laparoscopic) abdominal surgery compare to standard (open) abdominal surgery for people with food‐pipe (oesophageal) cancer?
Background
The oesophagus (food pipe) is located mainly in the chest; it enters the abdomen (tummy) through an opening in the diaphragm (muscle that separates the chest from the abdomen). Removing tumours by surgery (oesophagectomy) is one of the recommended treatments for cancers that are limited to the oesophagus. The tumour can be removed through an abdominal opening, a chest opening, or a combination. When the tumour is removed through an abdominal opening, it is called transhiatal oesophagectomy (as the oesophagus is separated from its surrounding structures through the opening in the diaphragm). The abdominal surgery can be performed through either a key‐hole or a standard cut. Key‐hole surgery to remove oesophageal cancer (laparoscopic transhiatal oesophagectomy) is a relatively new procedure compared to the well‐established standard cut surgery (open transhiatal oesophagectomy). In operations on other parts of the body, laparoscopic surgery has been shown to reduce complications and length of hospital stays compared to open surgery.
However, concerns remain about the safety of laparoscopic surgery. How do complications after operation (post‐operative complications) compare between the two procedures? Does laparoscopic surgery remove the same amount of cancer and healthy border tissue as open surgery? Do people recovery more quickly after laparoscopic or open surgery? We sought to resolve these issues by searching the medical literature for studies on this topic.
Study characteristics
Randomised controlled trials are the best types of studies to find out whether one treatment is better than another since it ensures that similar types of people receive the new and the old treatment. But we did not find randomised controlled trials; we identified six relevant non‐randomised studies with a total of 334 patients, which compared laparoscopic and open surgeries. Since one of the studies did not provide usable results, five studies, with 326 patients, provided information for this review; laparoscopic surgery = 151 patients and open surgery = 175 patients. In four of these studies, historical information was collected from hospital records. In one study, new information was collected. In general, new information is considered to be more reliable than information from hospital records.
Key results
The differences between laparoscopic and open transhiatal oesophagectomy were imprecise for: deaths during the short‐term and long‐term, the percentage of people with major complications, narrowing of the new junction between the gut, created after removing the oesophagus, cancer returning during the short‐term and long‐term, and the proportion of people who required blood transfusion. The proportion of patients with any complications and the average lengths of hospital stay were less in the key‐hole group than the open cut group. There was lack of clarity about the difference in the amount of blood transfused between the two groups. None of the studies reported difficulty in swallowing after surgery, health‐related quality of life, the amount of time it took to return to normal activity (same mobility as before surgery), or work.
Quality of the evidence
The quality of the evidence was very low. This was mainly because it was not clear whether participants who received laparoscopic surgery were similar to those who had open surgery. This makes the findings unreliable. Well‐designed randomised controlled trials are necessary to obtain high‐quality evidence on the best method to perform oesophagectomy.
Summary of findings
Background
Description of the condition
Oesophageal cancer (squamous cell carcinoma and adenocarcinoma) is the ninth most common cancer and the sixth most common cause of cancer‐related mortality in the world (IARC 2014). In 2012, there were about 455,000 new people diagnosed with oesophageal cancer and 400,000 deaths due to oesophageal cancer globally (IARC 2014). There is global variation in the incidence of oesophageal cancers, with an age‐standardised annual incidence rate of 17 to 24 per 100,000 population in parts of Eastern Africa, such as Malawi and Kenya, parts of Central Asia (Turkmenistan) and East Asia (Mongolia), compared with an age‐standardised annual incidence rate of less than 1 per 100,000 population in parts of Western Africa (Nigeria, Guinea, Guinea‐Bissau) (IARC 2014). The trend in mortality is similar, with an age‐standardised annual mortality rate of 16 to 23 per 100,000 population in the countries with a high incidence and less than 1 per 100,000 population in the countries with a low incidence (IARC 2014). In the UK, there was an increase in the incidence of oesophageal cancer in men and a decrease in the incidence in women from 2001 to 2011 (Cancer Research UK 2014).
The treatment of oesophageal cancer depends upon the stage of cancer. One of the common systems for staging cancer is the 7th edition of the American Joint Committee on Cancer (AJCC) oesophageal cancer staging system (AJCC 2010; Rice 2010). This system is based on TNM classification: tumour (T) involvement of the different layers of the stomach, nodal involvement (N), the presence of metastases (M), plus grade of the tumour (G), and histological type (squamous cell carcinoma or adenocarcinoma; AJCC 2010; Rice 2010; Stahl 2013). The TNM‐G that constitutes the stage of the cancer is dependent upon the histological type (AJCC 2010; Rice 2010). Metastatic oesophageal cancer corresponds to Stage IV of the AJCC oesophageal cancer staging system, regardless of the presence or absence of the other factors. The survival after diagnosis of oesophageal cancer depends upon the stage with five‐year survival ranging from 70% in Stage Ia squamous cell carcinoma and 80% in Stage Ia adenocarcinoma to 15% in Stage IV squamous cell carcinoma or adenocarcinoma (AJCC 2010; Rice 2010). Potentially curative chemoradiotherapy is currently advocated only in people with localised cancer of the oesophagus who are unfit for surgery (Stahl 2013), or in patients with localised squamous cell carcinoma (Allum 2011). Endoscopic resection of squamous cell carcinoma or adenocarcinoma is a viable first‐line treatment option in people with localised T‐1a tumours (Fovos 2012; Stahl 2013). When the person is fit, surgery is the preferred curative option in the treatment of oesophageal cancer, according to the European Society for Medical Oncology guidelines (Stahl 2013). According to the guidelines from the Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology, and the British Association of Surgical Oncology, definitive chemoradiotherapy is the preferred option in the treatment of localised squamous cell carcinoma of the upper third of oesophagus, and an equivalent option to surgery for the treatment of localised squamous cell carcinoma of the middle and lower third of oesophagus (Allum 2011).
Description of the intervention
One of major controversies, and a topic of ongoing debate in oesophagectomy, is whether oesophagectomy should be performed by the transthoracic route or the transhiatal route (Boshier 2011; Colvin 2011; Omloo 2007).
Broadly, transhiatal oesophagectomy involves mobilisation of the lower end of the oesophagus from the abdomen and mobilisation of the cervical oesophagus from the neck. Once the oesophagectomy is competed, restoration of continuity of the gastrointestinal tract is obtained by anastomosing the cervical oesophagus with a tube formed from the stomach or colon through the cervical wound (Orringer 2007).
In open transhiatal oesophagectomy, the surgical access to the abdominal cavity (and hence the lower end of the oesophagus, stomach, and colon) is through an upper midline incision. In laparoscopic transhiatal oesophagectomy, the surgical access to the abdominal cavity (and hence the lower end of the oesophagus, stomach, and colon) is through five small ports (holes) of about 0.5 to 1 cm each, through which laparoscopic instruments can be inserted after the abdomen is distended using carbon‐dioxide pneumoperitoneum (Avital 2005; Cash 2014; Yamamoto 2013). The entire abdominal part of the surgery is performed laparoscopically. Peri‐operative chemotherapy or chemoradiotherapy is administered, depending upon the stage, histological type, and resection margin status after oesophagectomy (Stahl 2013).
Oesophagectomy may also be performed using a combined abdominal, thoracic, and cervical approach (three‐stage approach or McKeown procedure) (McKeown 1976).
Transthoracic oesophagectomy has more postoperative morbidity and mortality compared with transhiatal oesophagectomy, and despite no evidence of a statistically significant difference in the five‐year survival between the two methods, transthoracic oesophagectomy is believed to offer a long‐term survival advantage over transhiatal oesophagectomy (Boshier 2011; Colvin 2011; Omloo 2007).
How the intervention might work
For many surgical procedures, laparoscopic surgery is now preferred over open surgery. This includes surgical procedures such as cholecystectomy (removal of gallbladder), surgery for colon cancer, and hysterectomy. The reason for this preference is decreased pain, decreased blood loss, shorter hospital stay, earlier postoperative recovery, better cosmesis (physical appearance), and decreased costs associated with laparoscopic surgery (Bijen 2009; Keus 2006; Reza 2006; Talseth 2014; Walsh 2009). In addition to these generic advantages of laparoscopic surgery, one of the potential advantages of laparoscopic transhiatal oesophagectomy over open transhiatal oesophagectomy is the direct visualisation of the lower mediastinum without blind dissection (Yamamoto 2013).
Why it is important to do this review
While the smaller incision and earlier postoperative recovery appear to be potential advantages of laparoscopic oesophagectomy, the safety of a laparoscopic approach for a procedure that has a high complication rate, and the rate of cancer clearance has to be ensured before the method can be widely recommended. There are concerns about cancer clearance since port‐site metastases (recurrence of cancer at the laparoscopic port‐site) have been reported after removal of several cancers (e.g. squamous cell carcinoma of the gallbladder (Kais 2014); endometrial cancer (Palomba 2014); renal cancer (Song 2014)). Animal research has shown that the increased intra‐abdominal pressure during laparoscopy (pneumoperitoneum) may drive the malignant cells into the ports, or the malignant cells may adhere to the laparoscopic instruments that are introduced and removed through the ports, resulting in seeding of the port site and port‐site metastases (Hopkins 1999). There is also a concern of the adequacy of cancer clearance in terms of resection margins and the extent of lymph nodes removed with laparoscopy. However, one of the potential advantages of laparoscopic over open transhiatal oesophagectomy, is the direct visualisation of the lower mediastinum without blind dissection, which may facilitate a better nodal clearance (and hence oncological clearance) (Yamamoto 2013). There appear to be ongoing controversies on the best procedure: laparoscopic or open transhiatal oesophagectomy. There is no Cochrane review on this topic.
Objectives
To assess the benefits and harms of laparoscopic versus open oesophagectomy for people with oesophageal cancer undergoing a transhiatal oesophagectomy.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs) for this review. However, there were no randomised controlled trials on this topic. So, we included non‐randomised studies to provide the best available evidence on the topic, along with a critical appraisal of the existing evidence. We included studies reported as full text, studies published as abstract only, and unpublished data.
Types of participants
We included adults undergoing transhiatal oesophagectomy (oesophagectomy without thoracotomy with a cervical anastomosis) for oesophageal cancer (squamous cell carcinoma or adenocarcinoma). While we excluded people undergoing oesophagectomy for oesophageal strictures not amenable for endoscopic treatment or dysplasia whenever possible, we included studies in which no separate outcome data for people undergoing oesophagectomy for oesophageal cancers were available, provided that oesophagectomy for other causes was less than 10% of the participants included in the study.
Types of interventions
We included studies that compared laparoscopic transhiatal oesophagectomy with open transhiatal oesophagectomy. We excluded trials that compared thoracoscopic oesophagectomy with open transthoracic oesophagectomy, or trials that compared minimally invasive approaches with open approaches for McKeown's procedures.
Types of outcome measures
Primary outcomes
-
Mortality.
Short‐term mortality (in‐hospital mortality or mortality within three months).
Long‐term mortality.
-
Serious adverse events (within three months). We accepted the following definitions of serious adverse events.
Clavien‐Dindo classification: Grade III or higher (Clavien 2009; Dindo 2004).
International Conference on Harmonisation ‐ Good Clinical Practice (ICH‐GCP) guideline (ICH‐GCP 1996): we defined serious adverse events as any untoward medical occurrence that resulted in death, was life threatening, required hospitalisation or prolongation of existing hospitalisation, or resulted in persistent or significant disability or incapacity.
Individual complications that could clearly be classified as Grade III or higher with Clavien‐Dindo classification, or as a serious adverse event with ICH‐GCP classification.
Postoperative dysphagia (difficulty in swallowing).
Anastomotic stenosis.
-
Health‐related quality of life (using any validated scale).
Short‐term (four weeks to three months).
Medium‐term (three months to one year).
Secondary outcomes
-
Recurrence (local recurrence, surgical wound recurrence (also called port‐site metastases in the laparoscopic group) or distal metastases).
Short‐term recurrence (within six months).
Long‐term recurrence.
Adverse events (within three months). We accepted all adverse events reported by the study author regardless of the severity of the adverse event.
-
Perioperative blood transfusion requirements (whole blood or red cell transfusion; during surgery or within one week after surgery) .
Proportion of people requiring blood transfusion.
Quantity of blood transfusion.
-
Measures of earlier postoperative recovery.
Length of hospital stay (including the index admission for oesophagectomy and any surgical complication‐related re‐admissions).
Time‐to‐return to normal activity (return to pre‐operative mobility without additional caregiver support).
time‐to‐return to work (in people who were previously employed).
Positive resection margins (presence of macroscopic or microscopic cancer tissue at the plane of resection) at histopathological examination after surgery.
Number of lymph nodes harvested during surgery.
We chose the clinical outcomes to assess whether laparoscopic surgery resulted in adequate cancer clearance, was safe, and was beneficial in terms of decreased blood transfusion requirements; earlier postoperative recovery allowed earlier discharge from hospital, return to normal activity, and return to work; and improvement in health‐related quality of life. We highlight that the positive resection margins at histopathological examination after surgery and the number of harvested lymph nodes during surgery are surrogate outcomes; we included these in order to explore whether these are responsible for any differences in survival or mortality.
Studies that met the inclusions criteria were included, regardless of whether they reported the outcomes of interest.
Search methods for identification of studies
Electronic searches
We conducted a literature search to identify all published and unpublished studies in all languages. We translated any non‐English language papers and fully assessed them for potential inclusion in the review as necessary.
We search the following electronic databases:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 8; Appendix 1);
MEDLINE (1966 to August 2015; Appendix 2);
EMBASE (1988 to August 2015; Appendix 3); and
Science Citation Index (1982 to August 2015; Appendix 4).
On 14 August 2015, we also conducted a search of ClinicalTrials.gov (ClinicalTrials.gov; Appendix 5), and the World Health Organization ‐ International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en/; Appendix 6) .
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We also contacted authors of identified trials and asked them to identify other published and unpublished studies.
On 16 November 2015, we searched for errata or retractions from eligible trials on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Three review authors (KG, EP, SM) independently screened titles and abstracts for inclusion. We coded them as 'retrieve' (eligible or potentially eligible or unclear) or 'do not retrieve'. We retrieved the full‐text study reports for references coded as 'retrieve'. Three review authors (KG, EP, SM) independently screened the full text, identified studies for inclusion, and identified and recorded reasons for those we excluded. We resolved any disagreements through discussion. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table.
Data extraction and management
We used a standard data collection form for study characteristics and outcome data that was piloted on at least one study in the review. Three review authors (KG, EP, SM) extracted study characteristics from included studies and detailed them in a Characteristics of included studies table. We extracted the following study characteristics:
methods: study design, total duration of the study and run‐in, number of study centres and location, study setting, withdrawals, date of study;
participants: number, mean age, age range, gender, tumour stage, tumour location, histological subtype, performance status, American Society of Anesthesiologists (ASA) status (ASA 2014), inclusion criteria, exclusion criteria;
interventions: intervention, comparison, concomitant interventions;
outcomes: primary and secondary outcomes specified and collected, time points reported;
notes: funding for trial, notable conflicts of interest of trial authors.
Three review authors (KG, EP, SM) independently extracted outcome data from included studies. If outcomes were reported multiple times for the same time frame (e.g. short‐term health‐related quality of life reported at six weeks and three months), we had planned to choose the later time point (i.e. three months) for data extraction. For time‐to‐event outcomes where data were censored, we extracted data to calculate the natural logarithm of the hazard ratio (HR) and its standard error using the methods suggested by Parmar, et al. (Parmar 1998).
We included all participants for long‐term outcomes (e.g. mortality or quality of life), which were not conditional upon the short‐term outcomes (e.g. being alive at three months or having a low or high quality‐of‐life index at three months).
We noted under each outcome if outcome data were reported in an unusable way in one or more studies. We resolved disagreements by consensus. One review author (KG) copied the data from the data collection form into the Review Manager 5 (RevMan 2014). We double checked that the data were entered correctly by comparing the study reports with the data in the systematic review.
Assessment of risk of bias in included studies
Three review authors (KG, EP, SM) independently assessed the risk of bias for each study. We had planned to use the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, because of the lack of randomised controlled trials on the topic, we used the relevant risk of bias domains from 'A Cochrane Risk Of Bias Assessment Tool: for Non‐Randomized Studies of Interventions' (ACROBAT‐NRSI; Sterne 2014).
We assessed the risk of bias according to the following domains:
Bias due to confounding
Bias due to the selection of participants
Bias due to departures from intended intervention
Bias in the measurement of outcomes
Bias due to missing data
Bias in selection of the reported findings
We resolved any disagreements by discussion.
We graded each potential source of bias as critical, serious, moderate, low, or no information, and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. Where information on risk of bias relates to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took the risk of bias for the studies that contributed to that outcome into account.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the relevant sections and in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as risk ratio (RR) and continuous data as mean difference (MD) when the outcome was reported, or we converted to the same units in all the trials (e.g. hospital stay, time to return to work); we had planned to calculate the standardised mean difference (SMD) when different scales were used for measuring the outcome (e.g. quality of life). We had planned: to ensure that higher scores for continuous outcomes had the same meaning for the particular outcome, to explain the direction to the reader, and to report where the directions were reversed if this was necessary. We had planned to calculate the rate ratio (RaR) for outcomes such as adverse events and serious adverse events, where it was possible for the same person to develop more than one adverse event (or serious adverse event). If the authors had calculated the RaR of adverse events (or serious adverse events) in the intervention versus control based on Poisson regression, we had planned to use the Poisson regression method to obtain the RaR in preference to calculating the RaR with the number of adverse events (or serious adverse events) during a certain period. We calculated the Hazard Ratio (HR) for time‐to‐event outcomes such as long‐term mortality, long‐term recurrence, and had planned to calculate the HR for time‐to‐first adverse event (or serious adverse event) if the information was reported in this manner.
We undertook meta‐analyses since this was meaningful (i.e. the treatments, participants, and the underlying clinical question were similar enough to pool).
A common way that trialists indicate when they have skewed data is by reporting medians and interquartile ranges. When we encountered this, we noted that the data were skewed by following the rough guide for identifying skewed distribution available in Higgins 2011 and considered the implication of this.
Where multiple trial arms were reported in a single trial, we had planned to include only the relevant arms. If we had entered two comparisons (e.g. laparoscopic oesophagectomy method 1 versus open oesophagectomy and laparoscopic oesophagectomy method 2 versus open oesophagectomy) into the same meta‐analysis, we had planned to halve the control group to avoid double counting. The alternative way of including trials with multiple arms is to pool the results of the laparoscopic oesophagectomy method 1 and laparoscopic oesophagectomy method 2 and compare the pooled results with open oesophagectomy. We had planned to perform a sensitivity analysis to determine if the results of the two methods of dealing with multi‐arm trials lead to different conclusions.
Unit of analysis issues
The unit of analysis was the individual participant undergoing transhiatal oesophagectomy. As expected, we did not find any cluster‐randomised trials for this comparison. If we had identified cluster‐randomised trials, we would have obtained the effect estimate adjusted for the clustering effect. If this was not available, we would have performed a sensitivity analysis excluding the trial from the meta‐analysis, as the variance of the estimate of effect unadjusted for the cluster effect is less than the actual variance that is adjusted for the cluster‐effect, giving inappropriately more weight to the cluster RCT in the meta‐analysis.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). If we were unable to obtain the information from the investigators or study sponsors, we imputed a mean from the median (i.e. considered the median as the mean) and calculated a standard deviation from the standard error, interquartile range, or P values, and assessed the impact of including such studies in a sensitivity analysis, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If we had been unable to calculate the standard deviation from the standard error, interquartile range, or P values, we had planned to impute a standard deviation from the highest standard deviation in the remaining trials included in the outcome, fully aware that this method of imputation would decrease the weight of the studies in the meta‐analysis of MD, and shift the effect towards no effect for SMD.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity as per the Cochrane Handbook for Systematic Reviews of Interventions (greater than 50% to 60%), we explored it by pre‐specified subgroup analyses (Higgins 2011).
Assessment of reporting biases
We attempted to contact study authors to ask them to provide missing outcome data. Had this not been possible, and the missing data were thought to introduce serious bias, we had planned to explore the impact of including such studies in the overall assessment of results, using a sensitivity analysis.
If we had been able to pool more than 10 trials, we had planned to create and examine a funnel plot to explore possible publication biases. We had planned to use Egger's test to determine the statistical significance of the reporting bias (Egger 1997). We would have considered a P value less than 0.05 to be a statistically significant reporting bias.
Data synthesis
We performed analyses using Review Manager 5 (RevMan 2014). We calculated the 95% confidence intervals(CI) for the treatment effect. We used the Mantel‐Haenszel method for dichotomous data, inverse‐variance method for continuous data, and generic‐inverse variance for time‐to‐event data. We had planned to use the inverse‐variance method for count data. We used both the fixed‐effect and random‐effects model for the analyses (Demets 1987; DerSimonian 1986). In the case of discrepancy between the two models, we reported both results; otherwise we reported only the results from the fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table using all the outcomes. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence as it related to the studies that contributed data to the meta‐analyses for the pre‐specified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and using GRADEpro software. We justified all decisions to downgrade or upgrade the quality of the evidence in the footnotes and make comments to aid the reader's understanding of the review where necessary. We considered whether there was additional outcome information that we were unable to incorporate into the meta‐analyses, noted this in the comments, and stated whether it supported or contradicted the information from the meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We had planned to carry out the following subgroup analyses:
different histological types (squamous cell carcinoma and adenocarcinoma);
different cancer stages;
different locations (upper third, middle third, lower third);
people with different anaesthetic risk (ASA I (a healthy person) or II (a person with mild systemic disease) versus ASA III or more (a person with severe systemic disease or worse);
different body mass index (BMI): healthy weight (BMI 18.5 to 25) versus overweight or obese (BMI 25 or greater).
We had planned to use all the primary outcomes in subgroup analyses.
We had planned to use the formal Chi² test for subgroup differences to test for subgroup interactions.
Sensitivity analysis
We had planned to perform sensitivity analyses defined a priori to assess the robustness of our conclusions. These would have involved:
excluding trials at unclear or high risk of bias (one or more of the risk of bias domains (other than blinding of surgeon) classified as unclear or high);
excluding trials in which either mean or standard deviation, or both are imputed;
excluding cluster RCTs in which the adjusted effect estimates are not reported;
different methods of dealing with multi‐arm trials (see Measures of treatment effect).
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice, and our implications for research have given the reader a clear sense of where the focus of any future research in the area should be and what the remaining uncertainties are.
Results
Description of studies
Results of the search
We identified 3069 references through electronic searches of Cochrane Central Register of Controlled Trials (CENTRAL; (Wiley); N = 28), MEDLINE (OvidSP; N = 762), EMBASE (OvidSP; N = 1659), Science Citation Index expanded (N = 604), ClinicalTrials.gov (N = 9) and WHO Trials register (N = 7). After removing duplicate references, there were 1965 remaining. We excluded 1930 clearly irrelevant references through reading abstracts. We retrieved the full publication of 35 references for further detailed assessment. We excluded 24 studies (25 references) for the reasons listed in the Characteristics of excluded studies. Six non‐randomised studies (10 references) fulfilled the inclusion criteria (Characteristics of included studies). The reference flow is shown in Figure 1.
1.
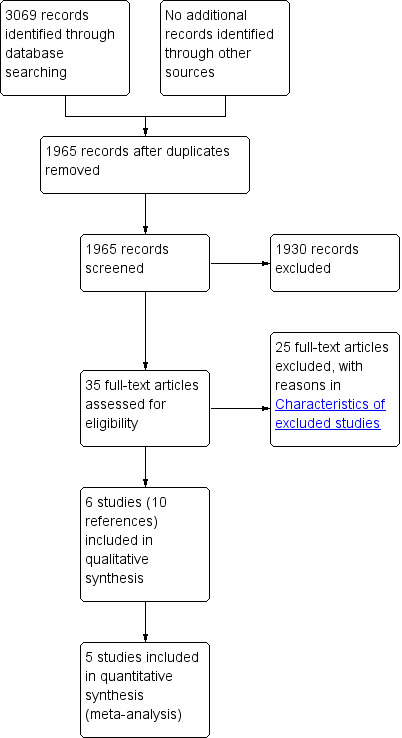
Study flow diagram.
Included studies
We included a total of six non‐randomised studies (Badessi 2003; Cash 2014; Ecker 2015; Maas 2012; Saha 2009; Valenti 2008). Five studies were retrospective studies (Badessi 2003; Cash 2014; Ecker 2015; Maas 2012; Saha 2009), while one of the studies was a prospective study (Valenti 2008). All studies were single institutional studies. Two studies compared laparoscopic oesophagectomy with historical controls who underwent open oesophagectomy (Cash 2014; Maas 2012). Three studies compared laparoscopic oesophagectomy with contemporary controls who underwent open oesophagectomy (Ecker 2015; Saha 2009; Valenti 2008). It was not clear whether one of the studies was a case‐control study or a cohort study (Badessi 2003).
One study included only patients with adenocarcinoma (Saha 2009). Three studies included a mixture of adenocarcinoma and squamous cell carcinoma but did not report the outcome data separately (Ecker 2015; Maas 2012; Valenti 2008). Two studies did not report the histological types of cancer (Badessi 2003; Cash 2014). One study included Stage I cancer only (Saha 2009). Three studies included Stages I to III cancer but did not report the outcome data separately for different stages (Cash 2014; Ecker 2015; Maas 2012). Two studies did not report the stages of cancer (Badessi 2003; Valenti 2008). Three studies indicated that the location of cancer was in the lower third (Maas 2012; Saha 2009; Valenti 2008). Information on the location of the tumours was not available in the remaining three studies. One study included patients with ASA I to III (Maas 2012). One study included patients with ASA I to IV; one patient in each group belonged to ASA IV category, while the remaining patients belonged to ASA I to III (Valenti 2008). Neither study reported outcome data separately for the different ASA stages. Information on ASA was not available in the remaining four studies. There was no restriction based on BMI in any of the studies. None of the studies reported the outcome data separately for healthy weight versus overweight or obese patients.
Five ports were used to perform laparoscopic oesophagectomy in all three studies that provided information on the number of ports (Maas 2012; Saha 2009; Valenti 2008). The remaining studies did not provide this information. A mini‐laparotomy of 7 cm was used to perform the anastomosis and retrieve the specimen in two studies that reported this information (Maas 2012; Valenti 2008). The remaining studies did not provide this information. None of the studies reported the size of incision in the open oesophagectomies. Drain use was not stated in either group, in any of the studies. The proportion of patients that were converted from laparoscopic to open oesophagectomy was 2/33 (6.1%), 4/36 (11.1%), 9/50 (18%), and 0/16 (0%) respectively, in the four studies that reported this information (Cash 2014; Ecker 2015; Maas 2012; Valenti 2008).
A total of 334 patients underwent laparoscopic (154 patients) or open (180 patients) transhiatal oesophagectomy. One study, which included eight patients, did not report any outcomes of interest for this review. Excluding this study, a total of 326 patients, undergoing laparoscopic (151 patients) or open (175 patients) transhiatal oesophagectomy, contributed to one or more outcomes in this review. The mean or median age in the studies ranged from 64 years to 74 years in the five studies that reported this information (Cash 2014; Ecker 2015; Maas 2012; Saha 2009; Valenti 2008). All the studies reported the proportion of females, which ranged from 20% to 37.5%.
The follow‐up period was not stated in three studies (Badessi 2003; Maas 2012; Valenti 2008). The median follow‐up period in the remaining studies were as follows.
Cash 2014: 26 months for the laparoscopic oesophagectomy group and 64 months for the open oesophagectomy group (survival at 24 months was used to calculate proportion survived)
Ecker 2015: 10 months
Saha 2009: 44 months
The outcomes reported in the studies are summarised in Characteristics of included studies.
Excluded studies
Two studies were rejected because they included more than 10% of patients without cancer, but separate outcome data were not available for patients with cancer (Bernabe 2005; Perry 2009). Eight studies were excluded because the patients did not undergo laparoscopic transhiatal oesophagectomy (Blazeby 2011; Burdall 2015; Csendes 2013; Dolan 2013; Parameswaran 2013; Safranek 2010; Schoppmann 2010; Yamasaki 2011). One study was excluded because it was not clear whether patients underwent transhiatal oesophagectomy (Harrison 2013). One study was excluded because there was no control group of open oesophagectomy (Scheepers 2008). Six studies were excluded because separate data were not available for patients who underwent transhiatal oesophagectomy (Bresadola 2006; Fabian 2008; Kang 2013; Mamidanna 2012; Mao 2012; Messenger 2015). The remaining six studies were excluded because they were not primary research (e.g. review, editorial, letter to editor, comment, or cost‐effectiveness study with no primary research data; Cuesta 2012; Ferreira 2004; Lee 2013; Mariette 2012; Rice 2012; Ujiki 2013).
Risk of bias in included studies
Bias due to confounding
There was no information for the risk of bias due to confounding for five studies (Badessi 2003; Ecker 2015; Maas 2012; Saha 2009; Valenti 2008). Although four studies reported that there were no baseline differences between the groups, the studies were not powered to measure the baseline differences, and did not assess the baseline difference for one or more confounding factors (Ecker 2015; Maas 2012; Saha 2009; Valenti 2008). In one study, the tumour size was smaller in patients undergoing laparoscopic surgery, although the proportion of patients who underwent neo‐adjuvant therapy was more in the laparoscopic surgery group (Cash 2014). This is likely to have introduced critical bias to the estimates of effect.
Bias due to the selection of participants
Two studies used historical controls (Cash 2014; Maas 2012). In these two studies, after a certain date, the authors only performed laparoscopic oesophagectomy, and compared the results of laparoscopic oesophagectomy with those of open oesophagectomy before this date. We considered these studies to have moderate risk of bias. In two studies, the decision to perform laparoscopic or open oesophagectomy was based on the surgeon's preference (Saha 2009; Valenti 2008). We classified these studies as providing 'no information'. The criteria used to perform oesophagectomy or open oesophagectomy was not stated in the remaining studies (Badessi 2003; Ecker 2015). We also classified these studies as providing 'no information'.
Bias due to departures from intended intervention
None of the studies reported whether the patient care other than laparoscopic or open procedure was identical in the two groups. We classified all studies as providing 'no information'.
Bias in the measurement of outcomes
Three studies clearly reported that the outcome assessors were not blinded (Maas 2012; Saha 2009; Valenti 2008). This might have introduced bias in the measurement of outcomes other than mortality. We considered these studies to have critical risk of bias. The information on outcome assessor blinding was not reported in the remaining studies (Badessi 2003; Cash 2014; Ecker 2015). We considered these studies as providing 'no information'.
Bias due to missing data
Four studies included all the patients who met the inclusion criteria; we considered them to be at low risk of bias due to missing data (Ecker 2015; Maas 2012; Saha 2009; Valenti 2008). It was not clear whether any patients were excluded from analysis in the remaining two studies (Badessi 2003; Cash 2014). We considered these studies as providing 'no information'.
Bias in selection of the reported findings
Only two studies reported mortality and morbidity adequately and could be considered at low risk of bias due to selective outcome reporting (Cash 2014; Maas 2012). We considered one study to be at critical risk of bias since neither mortality nor morbidity was reported (Badessi 2003). We considered the remaining studies to be at serious risk of bias as morbidity was not reported, since one would expect studies comparing laparoscopic with open oesophagectomy to report the data on mortality and morbidity in a detailed manner (Ecker 2015; Saha 2009; Valenti 2008).
Effects of interventions
Summary of findings for the main comparison. Laparoscopic versus open transhiatal oesophagectomy for oesophageal cancer: primary outcomes.
| Laparoscopic versus open transhiatal oesophagectomy for oesophageal cancer | |||||
|
Patient or population: patients with oesophageal cancer
Settings: upper gastrointestinal surgery unit
Intervention: laparoscopic transhiatal oesophagectomy Control: open transhiatal oesophagectomy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Open transhiatal oesophagectomy | Laparoscopic transhiatal oesophagectomy | ||||
|
Short‐term mortality (in hospital or within 3 months) |
11 per 1000 | 5 per 1000 (1 to 47) | RR 0.44 (0.05 to 4.09) | 326 (5 studies) | ⊕⊝⊝⊝ very low1,2,3 |
| Long‐term mortality Follow‐up: median 2 years | 355 per 1000 | 346 per 1000 (299 to 398) | HR 0.97 (0.81 to 1.16) | 193 (2 studies) | ⊕⊝⊝⊝ very low1,3 |
| Serious adverse events (proportion) | 211 per 1000 | 103 per 1000 (51 to 208) | RR 0.49 (0.24 to 0.99) | 213 (3 studies) | ⊕⊝⊝⊝ very low1,3 |
| Anastomotic stenosis | 81 per 1000 | 111 per 1000 (27 to 462) | RR 1.37 (0.33 to 5.7) | 73 (1 study) | ⊕⊝⊝⊝ very low1,2,3 |
| None of the studies reported post‐operative dysphagia or health‐related quality of life. | |||||
| *The basis for the assumed risk was the mean control group proportion. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Risk of bias was unclear or high in the study/studies. 2 The confidence intervals were wide (overlapped clinically significant effects and no effect). 3 The sample size was small.
Summary of findings 2. Laparoscopic versus open transhiatal oesophagectomy for oesophageal cancer: secondary outcomes.
| Laparoscopic versus open transhiatal oesophagectomy for oesophageal cancer | |||||
|
Patient or population: patients with oesophageal cancer
Settings: upper gastrointestinal surgery unit
Intervention: laparoscopic transhiatal oesophagectomy Control: open transhiatal oesophagectomy | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Open transhiatal oesophagectomy | Laparoscopic transhiatal oesophagectomy | ||||
| Short‐term recurrence (within 6 months) | 1 per 1000 | 1 per 1000 (0 to 18) | RR 0.88 (0.04 to 18.47) | 20 (1 study) | ⊕⊝⊝⊝ very low1,2,3 |
|
Long‐term recurrence Follow‐up: 10 months |
241 per 1000 | 241 per 1000 (207 to 278) | HR 1 (0.84 to 1.18) | 173 (2 studies) | ⊕⊝⊝⊝ very low1,3 |
| Adverse events (proportion) | 623 per 1000 | 399 per 1000 (299 to 536) | RR 0.64 (0.48 to 0.86) | 213 (3 studies) | ⊕⊝⊝⊝ very low1,3 |
| Blood transfusion (proportion) | 162 per 1000 | 13 per 1000 (0 to 219) | RR 0.08 (0 to 1.35) | 73 (1 study) | ⊕⊝⊝⊝ very low1,2,3 |
| Blood transfusion (quantity) | The median blood transfused was 2.5 units | The median blood transfused was 2.5units less (confidence intervals ‐ not available; statistical significance ‐ not known) | 93 (1 study) | ⊕⊝⊝⊝ very low1,3 | |
| Length of hospital stay | The median hospital stay rangedbetween 11 and 16 days | The median hospital stay was 3 days less (confidence intervals ‐ not available; statistically significant) | 266 (3 studies) | ⊕⊝⊝⊝ very low1,3 | |
| Positive resection margins | 243 per 1000 | 158 per 1000 (90 to 272) | RR 0.65 (0.37 to 1.12) | 213 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 |
| Number of harvested lymph nodes | The median number of lymph nodes harvested ranged between 11 and 36 | The median number of lymph nodes was 12 fewer to 3 more (confidence intervals ‐ not available; not statistically significant or statistical significance ‐ not known) | 326 (5 studies) | ⊕⊝⊝⊝ very low1,3,4 | |
| None of the studies reported time‐to‐return to normal activity (return to pre‐operative mobility without additional caregiver support), or time‐to‐return to work. | |||||
| *The basis for the assumed risk is the mean control group proportion except for short‐term recurrence where a control group proportion of 0.1% was used since there was no recurrence in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Risk of bias was unclear or high in the study/studies. 2 The confidence intervals were wide (overlapped clinically significant effects and no effect). 3 The sample size was small.
4 The results were inconsistent across studies.
None of the studies reported post‐operative dysphagia, health‐related quality of life, time‐to‐return to normal activity (return to pre‐operative mobility without additional caregiver support), or time‐to‐return to work. The effects of interventions are summarised in the Table 1 and Table 2.
Mortality
Five studies reported short‐term mortality (Cash 2014; Ecker 2015; Maas 2012; Saha 2009; Valenti 2008). There was no statistically significant difference in the short‐term mortality between the two groups (laparoscopic group: 0/151 (adjusted proportion based on meta‐analysis estimate: 0.5%) versus open group: 2/175 (1.1%); Risk Ratio (RR) 0.44; 95% CI 0.05 to 4.09; participants = 326; studies = 5; I² = 0%; Analysis 1.1). There was no change in results when we used a random‐effects model.
1.1. Analysis.
Comparison 1 Laparoscopic versus open transhiatal oesophagectomy, Outcome 1 Short‐term mortality.
Two studies reported long‐term mortality between the two groups (Cash 2014; Maas 2012). There was no statistically significant difference in the long‐term mortality between the two groups (Hazard Ratio (HR) 0.97; 95% CI 0.81 to 1.16; participants = 193; studies = 2; I² = 0%; Analysis 1.2). The two‐year mortality was 30% and 35% in the laparoscopic and open groups respectively in Cash 2014, while the three‐year mortality was 64% and 62% in the laparoscopic and open groups respectively in Maas 2012. There was no change in results when we used a random‐effects model. In addition to the two studies included in the meta‐analysis, two other studies reported the mortality at the maximum follow‐up (Ecker 2015; Saha 2009). However, we did not include these studies in the meta‐analysis since there were no deaths after a median follow‐up of 10 months in the laparoscopic group (0/36) versus 6% dead in the open group (absolute numbers not available) in Ecker 2015, and no deaths after a median follow‐up of 44 months in the open group (0/4) versus 1/16 (6.3%) dead in laparoscopic group in Saha 2009.
1.2. Analysis.
Comparison 1 Laparoscopic versus open transhiatal oesophagectomy, Outcome 2 Long‐term mortality.
Serious adverse events
Three studies reported the proportion of patients with serious adverse events (Cash 2014; Maas 2012; Saha 2009). The proportion of people with adverse events was statistically significantly lower in the laparoscopic group (10/99; adjusted proportion: 10.3%) compared to the open group (24/114 (21.1%); RR 0.49; 95% CI 0.24 to 0.99; participants = 213; studies = 3; I² = 0%; Analysis 1.3). There was no change in results when we used a random‐effects model.
1.3. Analysis.
Comparison 1 Laparoscopic versus open transhiatal oesophagectomy, Outcome 3 Serious adverse events (proportion).
Details of the serious adverse events were not available in Cash 2014. The serious adverse events in the other studies included complications which required re‐operations, such as re‐inspection of anastomosis, revision of anastomosis, and tracheal repair in Maas 2012, and anastomotic leaks in the Saha 2009.
Ecker 2015 reported anastomotic stenosis. There was no statistically significant difference in the proportion of people with anastomotic stenosis between the two groups (laparoscopic group: 4/36 (11.1%) versus open group: 3/37 (8.1%); RR 1.37; 95% CI 0.33 to 5.70; participants = 73; studies = 1; Analysis 1.4). Since there was only one study for this outcome, the issue of fixed‐effect versus random‐effects model did not arise.
1.4. Analysis.
Comparison 1 Laparoscopic versus open transhiatal oesophagectomy, Outcome 4 Anastomotic stenosis.
None of the studies reported post‐operative dysphagia.
Health‐related quality of life
None of the studies reported health‐related quality of life at any time frame.
Recurrence
Saha 2009 reported short‐term recurrence within six months. There was no statistically significant difference in the proportion of people with short‐term recurrence between the two groups (laparoscopic group: 1/16 (6.3%) versus open group: 0/4 (0%); RR 0.88; 95% CI 0.04 to 18.47; participants = 20; studies = 1; Analysis 1.5). Since there was only one study for this outcome, the issue of fixed‐effect versus random‐effects model did not arise.
1.5. Analysis.
Comparison 1 Laparoscopic versus open transhiatal oesophagectomy, Outcome 5 Short‐term recurrence (within 6 months).
Two studies reported long‐term recurrence (Ecker 2015; Maas 2012). There was no statistically significant difference in the long‐term recurrence between the two groups (HR 1.00; 95% CI 0.84 to 1.18; participants = 173; studies = 2; Analysis 1.6). The 10‐month recurrence was 20% and 24% in the laparoscopic and open groups respectively in Ecker 2015, while the three‐year recurrence was 69% and 70% in the laparoscopic and open groups respectively, and the five‐year recurrence was 77% and 79% in the laparoscopic and open groups respectively in Maas 2012. There was no change in results when we used a random‐effects model. We excluded two other studies from the meta‐analysis (Cash 2014; Saha 2009). In Cash 2014, recurrence at the maximum follow‐up was 8/33 (24.2%) in the laparoscopic group and 15/60 (25%) in the open group. However, the patients in the laparoscopic group were followed for a median period of 26 months, while those in the open group were followed up for a median period of 64 months. So, it was inappropriate to compare the two proportions. In Saha 2009, there were no recurrences after a median follow‐up of 44 months in the open group (0/4) versus 1/16 (6.3%) recurrence in the laparoscopic group.
1.6. Analysis.
Comparison 1 Laparoscopic versus open transhiatal oesophagectomy, Outcome 6 Long‐term recurrence.
Adverse events
Three studies reported the proportion of patients with adverse events (Cash 2014; Maas 2012; Saha 2009). The proportion of people with adverse events was statistically significantly lower in the laparoscopic group (37/99 (39.9%) compared to the open group (71/114 (62.3%); RR 0.64; 95% CI 0.48 to 0.86; participants = 213; studies = 3; I² = 0%; Analysis 1.7). There was no change in results when we used a random‐effects model. One other study reported that the number of complications were fewer in the laparoscopic group compared to the open group, without providing information on the complications or statistical significance (Badessi 2003).
1.7. Analysis.
Comparison 1 Laparoscopic versus open transhiatal oesophagectomy, Outcome 7 Adverse events (proportion).
Perioperative blood transfusion requirements
One study reported the proportion of people who required perioperative transfusion (Ecker 2015). There was no statistically significant difference in the proportion of people who required perioperative transfusion between the two groups (laparoscopic group: 0/36 (0%) versus open group: 6/37 (16.2%); RR 0.08; 95% CI 0.00 to 1.35; participants = 73; studies = 1; Analysis 1.8). Since there was only one study for this outcome, the issue of fixed‐effect versus random‐effects model did not arise.
1.8. Analysis.
Comparison 1 Laparoscopic versus open transhiatal oesophagectomy, Outcome 8 Blood transfusion (proportion).
One study reported the quantity of blood transfused (Cash 2014). The median blood transfused was 0 in the laparoscopic group compared to 2.5 units in the open group (Analysis 1.9). The statistical significance was not clear since the P value presented in this study was for the comparison of three groups (only two of which were eligible for this review).
1.9. Analysis.
Comparison 1 Laparoscopic versus open transhiatal oesophagectomy, Outcome 9 Blood transfusion (quantity).
| Blood transfusion (quantity) | |||
|---|---|---|---|
| Study | Median number of units in laparoscopic oesophagectomy | Median number of units in open oesophagectomy | Statistical significance |
| Cash 2014 | 0 | 2.5 | The statistical significance was not clear since the P value presented in this study was for the comparison of three groups (only two of which were eligible for this review). |
Measures of earlier postoperative recovery
Three studies reported the length of hospital stay (Cash 2014; Ecker 2015; Maas 2012). All three studies reported the median length of hospital stay so we did not perform a meta‐analysis. The median length of hospital stay was statistically significantly lower by three days in the laparoscopic group over the open group in all three studies (Analysis 1.10). One other study reported that the post‐operative hospital stay was shorter in the laparoscopic group compared to the open group without providing information on the length of hospital stay or statistical significance (Badessi 2003).
1.10. Analysis.
Comparison 1 Laparoscopic versus open transhiatal oesophagectomy, Outcome 10 Length of hospital stay.
| Length of hospital stay | ||||
|---|---|---|---|---|
| Study | Median hospital stay in laparoscopic oesophagectomy (days) | Median hospital stay in open oesophagectomy (days) | Difference in median (days) | Statistical significance |
| Badessi 2003 | not reported | not reported | not reported | Authors state that the post‐operative hospital stay was shorter in the laparoscopic group compared to the open group without providing information on the length of hospital stay or statistical significance |
| Cash 2014 | 10 | 13 | ‐3 | Statistically significant |
| Ecker 2015 | 8 | 11 | ‐3 | Statistically significant |
| Maas 2012 | 13 | 16 | ‐3 | Statistically significant |
None of the studies reported time‐to‐return to normal activity or time‐to‐return to work.
Positive resection margins
Three studies reported the proportion of patients with positive resection margins (Ecker 2015; Maas 2012; Valenti 2008). There was no statistically significant difference in the proportion of people with positive resection margins between the two groups (laparoscopic group: 15/102 (adjusted proportion: 15.8%) versus open group: 27/111 (24.3%); RR 0.65; 95% CI 0.37 to 1.12; participants = 213; studies = 3; I² = 0%; Analysis 1.11). There was no change in results when we used the random‐effects model.
1.11. Analysis.
Comparison 1 Laparoscopic versus open transhiatal oesophagectomy, Outcome 11 Positive resection margins.
Number of lymph nodes harvested during surgery
Five studies reported the number of lymph nodes harvested during surgery (Cash 2014; Ecker 2015; Maas 2012; Saha 2009; Valenti 2008). Since four studies reported the median number of lymph nodes harvested during surgery, we did not perform a meta‐analysis (Cash 2014; Ecker 2015; Maas 2012; Saha 2009). Three studies reported that there was no statistically significant difference in the mean or median number of lymph nodes harvested during surgery (Ecker 2015; Maas 2012; Valenti 2008). One study did not report whether the median difference in lymph nodes harvested during surgery was statistically significant (Saha 2009). In the last study, the statistical significance was not clear, since the P value presented in this study was for the comparison of three groups (only two of which were included in this review; Cash 2014; Analysis 1.12).
1.12. Analysis.
Comparison 1 Laparoscopic versus open transhiatal oesophagectomy, Outcome 12 Number of lymph nodes harvested.
| Number of lymph nodes harvested | ||||
|---|---|---|---|---|
| Study | Number of harvested lymph nodes in laparoscopic oesophagectomy (measure) | Number of harvested lymph nodes in open oesophagectomy (measure) | Difference in mean or median | Statistical significance |
| Cash 2014 | 24 (median) | 36 (median) | ‐12 | The statistical significance was not clear since the P value presented in this study was for the comparison of three groups (only two of which were eligible for this review). |
| Ecker 2015 | 14 (median) | 16 (median) | ‐2 | Not statistically significant |
| Maas 2012 | 14 (median) | 11 (median) | 3 | Not statistically significant |
| Saha 2009 | 15 (median) | 16 (median) | ‐1 | Statistical significance was not stated |
| Valenti 2008 | 18 (mean) | 19 (mean) | ‐1 | Not statistically significant |
Assessment of heterogeneity
There was no evidence of heterogeneity demonstrated by the I² statistic, the Chi² test for heterogeneity, or by visual inspection of forest plots to identify overlapping confidence intervals, for any of the outcomes for which we performed a meta‐analysis.
Assessment of reporting biases
We did not assess reporting biases using a funnel plot because we found fewer than 10 studies. There was some evidence of selective outcome reporting, as shown in the Characteristics of included studies.
Subgroup analysis
Different histological types (squamous cell carcinoma and adenocarcinoma)
One study included only patients with adenocarcinoma (Saha 2009). The remaining studies either did not report the histological type of cancer or did not report the outcome data separately for the adenocarcinoma or squamous cell carcinoma. The same trial that included only patients with adenocarcinoma also included patients with Stage I cancer (Saha 2009). The remaining studies either did not report the stage of cancer or did not report the outcome data separately for different stages. So, only one trial (Saha 2009) was included for the subgroup of adenocarcinoma and Stage I cancer. There was no mortality in either group and there was no statistically significant difference in the proportion of people with serious adverse events (RR 0.50; 95% CI 0.06 to 4.23; participants = 20; studies = 1; Analysis 2.2).
2.2. Analysis.
Comparison 2 Laparoscopic versus open transhiatal oesophagectomy (Subgroup analyses), Outcome 2 Short‐term mortality (lower‐third cancer).
Since there were no other subgroups, we did not use the formal Chi² test to test for subgroup interactions.
Different cancer locations
Three studies indicated that the location of cancer was in the lower third (Maas 2012; Saha 2009; Valenti 2008). Information on the location of tumours was not available in the remaining three studies (Badessi 2003; Cash 2014; Ecker 2015). A subgroup analysis of the studies that included lower‐third cancers showed no statistically significant difference between the groups in terms of short‐term mortality, long‐term mortality, or proportion of patients with serious adverse events (Analysis 2.2; Analysis 2.3; Analysis 2.4).
2.3. Analysis.
Comparison 2 Laparoscopic versus open transhiatal oesophagectomy (Subgroup analyses), Outcome 3 Long‐term mortality (lower third cancer).
2.4. Analysis.
Comparison 2 Laparoscopic versus open transhiatal oesophagectomy (Subgroup analyses), Outcome 4 Serious adverse events (proportion) (lower third cancer).
Since lower‐third cancer was the only subgroup, we did not use the formal Chi² test to test for subgroup interactions.
Other subgroup analyses
We were unable to perform a subgroup analysis of different anaesthetic risk or weights, since the studies either did not report this information or did not report the outcome data separately for different categories.
Sensitivity analysis
We did not perform any of the planned sensitivity analyses since none of the studies were at low risk of bias, standard deviation was not imputed for any of the outcomes, there were no cluster RCTs, and we either included studies with only two arms, or included the data from only two arms.
Discussion
Summary of main results
In this systematic review, we compared the benefits and harms of laparoscopic versus open transhiatal oesophagectomy. We found no randomised controlled trials on this topic. We included six observational studies that compared laparoscopic versus open transhiatal oesophagectomy; five studies (326 patients: 151 patients underwent laparoscopic and 175 patients underwent open transhiatal oesophagectomy) provided information for one or more outcomes. There were no statistically significant differences between laparoscopic and open transhiatal oesophagectomy in terms of short‐term mortality, long‐term mortality, anastomotic stenosis, short‐term recurrence, long‐term recurrence, proportion of people who required blood transfusion, proportion of people with positive resection margins, or the number of lymph nodes harvested during surgery. The proportion of patients with serious adverse events, all adverse events, and the median length of hospital stay were significantly less in the laparoscopic group than open oesophagectomy group. There was lack of clarity as to whether the median difference in the quantity of blood transfused was statistically significant, in favour of laparoscopic oesophagectomy. None of the studies reported post‐operative dysphagia, health‐related quality of life, time‐to‐return to normal activity (return to pre‐operative mobility without additional caregiver support), or time‐to‐return to work.
In other surgeries, laparoscopic surgery has been shown to be advantageous over open surgery, with fewer complications, shorter hospital stays, or both (Bijen 2009; Keus 2006; Reza 2006; Walsh 2009). So, the reduction of adverse events and length of hospital stays is not an isolated phenomenon in transhiatal oesophagectomy, and is biologically plausible. Since direct visualisation of the lower mediastinum is possible in laparoscopic rather than in open transhiatal oesophagectomy where the mediastinal dissection is blind, lower morbidity with laparoscopic oesophagectomy is plausible (Yamamoto 2013).
Adverse events, serious adverse events, and length of hospital stays are important patient‐oriented outcomes. There was no statistically significant difference in the long‐term mortality or long‐term recurrence between the two groups. The confidence intervals were relatively narrow, and in the absence of bias, one may be able to conclude that there was no difference in the long‐term mortality or long‐term recurrence between the groups. This could suggest that laparoscopic transhiatal oesophagectomy was superior to open transhiatal oesophagectomy in the short‐term, without affecting the long‐term outcomes. However, our major concerns about the findings are the risks of selection bias, which are discussed further in the Quality of the evidence section, and the relatively small sample sizes, which make the findings unreliable due to both systematic and random errors.
Overall completeness and applicability of evidence
The studies in this review included both adenocarcinoma and squamous cell carcinoma and different stages (I to III) of oesophageal cancer. Hence, the findings of this review are applicable to all oesophageal cancers that are amenable for potentially curative surgery. Two studies clearly mentioned that they included mainly ASA I to III patients (Maas 2012; Valenti 2008). The remaining studies did not state the ASA‐status of patients. In any case, all of the studies only included patients who could withstand major surgery. Hence, the findings of this review are only applicable to this population.
Quality of the evidence
The overall quality of evidence was very low. The major reasons for this were that the studies were observational studies; consequently, the risk of confounding bias was unclear. Studies did not report baseline differences of all the confounding factors and the sample sizes were not sufficient to identify differences in the confounding factors. Even if the sample sizes were large and all the confounding factors were reported, one cannot rule out the problem of residual confounding. It is not clear whether this would have introduced bias in the results. In two studies, the decision to perform laparoscopic or open oesophagectomy was based on the surgeon's preference (Saha 2009; Valenti 2008). It is quite possible that patients with less extensive cancer were operated on laparoscopically while those with more extensive cancer had open surgery. In Cash 2014, the authors performed laparoscopic oesophagectomy after a certain date and compared the results of laparoscopic oesophagectomy with those of open oesophagectomy performed prior to this date. Despite reporting on a consecutive cohort of patients who had undergone open oesophagectomy, the tumour size was smaller in patients who had undergone laparoscopic surgery, and this group also received neo‐adjuvant therapy more often (Cash 2014). This practice either reflects the anxiety of the surgeon about the curative nature of the laparoscopic surgery or an improvement in practice over time (neo‐adjuvant chemotherapy improves survival in patients who undergo oesophagectomy (Sjoquist 2011)). The selection process of patients for oesophagectomy may also have improved over time.
Unless randomised controlled trials are conducted, which ensure that the same type of participants have the opportunity to receive either laparoscopic or open transhiatal oesophagectomy, one cannot draw any reliable conclusions on the safety and effectiveness of laparoscopic versus open transhiatal oesophagectomy, because of residual confounding, i.e. we cannot infer causal association based on the current studies. In terms of other types of bias, many of the outcomes were subjective and the retrospective nature of most of the studies means that blinding of outcome assessors is extremely unlikely, which may also introduce bias. The complications were not reported adequately in most studies, which introduces selective outcome reporting bias.
Small sample sizes resulted in wide confidence intervals for many of the outcomes, and were another factor that decreased the quality of evidence. Future studies should be adequately powered to measure differences in clinically important outcomes.
On a positive note, we found no heterogeneity in the estimates of effect between the studies, despite the differences in study designs.
Potential biases in the review process
We had planned to only include randomised controlled trials in this review. However, in the absence of any randomised controlled trials, we have reported the best available evidence on the topic. We removed the 'randomised controlled trial' term to ensure that observational studies were not removed by the electronic filters. Three authors independently selected studies, without any language restrictions, and extracted data, which decreased the potential errors in study selection and data extraction. However, this is a systematic review of non‐randomised studies. There is no mandatory registration requirement, so studies that show poorer results for laparoscopic oesophagectomy than open oesophagectomy may not have been submitted to the journals since laparoscopic oesophagectomy is a new procedure compared to the established treatment of open oesophagectomy. So, we cannot rule out publication bias.
Agreements and disagreements with other studies or reviews
This is the first systematic review on the topic. Three study authors concluded that laparoscopic transhiatal oesophagectomy was safe, reduced hospital stay, and was the preferable option (Badessi 2003; Cash 2014; Ecker 2015). Four study authors suggested that laparoscopic transhiatal oesophagectomy offered equivalent oncological outcomes (Cash 2014; Ecker 2015; Saha 2009; Valenti 2008). One study author suggested that a randomised controlled trial was necessary to assess the role of laparoscopic transhiatal oesophagectomy in treating oesophageal cancers (Maas 2012). We agree with the last statement that a randomised controlled trial is necessary to assess the role of laparoscopic surgery in people undergoing transhiatal oesophagectomy. However, since transthoracic oesophagectomy is believed to offer a long‐term survival advantage over transhiatal oesophagectomy, despite higher post‐operative morbidity and mortality, and there is a lack of evidence of a difference in the five‐year survival compared with transhiatal oesophagectomy, randomised controlled trials should examine minimally invasive oesophagectomy (thoracoscopic Ivor‐Lewis procedure or combined thoracoscopic and laparoscopic McKneown procedure), and other forms of oesophagectomy to identify the optimal method of oesophagectomy (Boshier 2011; Colvin 2011; Omloo 2007).
We calculated the hazard ratio for long‐term mortality and long‐term recurrence using methods suggested in Parmar 1998. This assumes constant proportional hazards. From the Kaplan‐Meier curves in the studies, the proportional hazards appeared constant for long‐term mortality. We were unable to test this assumption for long‐term recurrence since the Kaplan‐Meier curves were not available.
Authors' conclusions
Implications for practice.
There are currently no randomised controlled trials comparing laparoscopic with open transhiatal oesophagectomy for patients with oesophageal cancers. In observational studies, laparoscopic transhiatal oesophagectomy is associated with fewer complications and shorter hospital stays than open transhiatal oesophagectomy. However, this association is unlikely to be causal. There is currently no information to determine a causal association in the differences between laparoscopic versus open transhiatal oesophagectomy.
Implications for research.
Future studies should try and address as many issues mentioned below as possible. The rationale for the study design is also mentioned alongside.
Study design: Randomised controlled trial (only a randomised controlled trial can establish a causal association in this situation).
Participants: People with potentially resectable oesophageal cancer (Stages I to III adenocarcinoma or squamous cell carcinoma of the oesophagus) and fit to undergo major surgery.
Intervention: Laparoscopic transhiatal oesophagectomy.
Control: Minimally invasive oesophagectomy (thoracoscopic Ivor Lewis procedure or combined thoracoscopic and laparoscopic McKeown's procedure), or open oesophagectomy.
Outcomes: Important patient‐oriented measures, such as short‐term and long‐term mortality (at least two to three years), health‐related quality of life, complications and the sequelae of the complications, measures of earlier post‐operative recovery, such as length of hospital stay, time‐to‐return to normal activity, and time‐to‐return to work (in those who are employed), and recurrence of cancer. In addition, resource use can be collected if the purpose was cost‐effectiveness and clinical effectiveness.
Five‐year follow‐up has been suggested, since oesophageal cancers diagnosed early may have long‐survival periods.
Other aspects of study design:
Observer‐blinded randomised controlled trial: to control for selection and detection bias.
Identical care apart from laparoscopic versus open oesophagectomy: to control for performance bias.
Include all participants in the analysis and perform an intention‐to‐treat analysis: to control for attrition bias.
Acknowledgements
We thank Karin Dearness, Managing Editor, Cochrane Upper Gastrointestinal and Pancreatic Diseases (UGPD) Group for providing administrative and logistical support for the conduct of the current review, and Racquel Simpson, Trials Search Co‐ordinator, Cochrane UGPD Group for developing and executing the search strategies.
We thank the peer reviewers, copy editors and Cochrane Editorial Unit for their comments.
We thank the authors who provided additional information for this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Esophagectomy] explode all trees
#2 (esophagectomy or esophagectomies or oesophagectomy or oesophagectomies or ((esophag* or oesophag*) and (resection or resections or removal or operation or operations)))
#3 #1 or #2
#4 MeSH descriptor: [Laparoscopy] explode all trees
#5 laparoscop*
#6 #4 or #5
#7 MeSH descriptor: [Esophageal Neoplasms] explode all trees
#8 (esophag* near/5 neoplas*)
#9 (oesophag* near/5 neoplas*)
#10 (esophag* near/5 cancer*)
#11 (oesophag* near/5 cancer*)
#12 (esophag* near/5 carcin*)
#13 (oesophag* near/5 carcin*)
#14 (esophag* near/5 tumo*)
#15 (oesophag* near/5 tumo*)
#16 (esophag* near/5 malig*)
#17 (oesophag* near/5 malig*)
#18 MeSH descriptor: [Esophagogastric Junction] explode all trees
#19 #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18
#20 #3 and #6 and #19
Appendix 2. MEDLINE search strategy
1. exp Esophagectomy/
2. (esophagectomy or esophagectomies or oesophagectomy or oesophagectomies or ((esophag* or oesophag*) and (resection or resections or removal or operation or operations))).mp.
3. 1 or 2
4. exp Laparoscopy/
5. laparoscop*.mp.
6. 4 or 5
7. exp esophageal neoplasms/
8. (esophag* adj5 neoplas*).tw.
9. (oesophag* adj5 neoplas*).tw.
10. (esophag* adj5 cancer*).tw.
11. (oesophag* adj5 cancer*).tw.
12. (esophag* adj5 carcin*).tw.
13. (oesophag* adj5 carcin*).tw.
14. (esophag* adj5 tumo*).tw.
15. (oesophag* adj5 tumo*).tw.
16. (esophag* adj5 malig*).tw.
17. (oesophag* adj5 malig*).tw.
18. exp esophagogastric junction/
19. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
20. 3 and 6 and 19
Appendix 3. EMBASE search strategy
1. exp esophagus resection/
2. (esophagectomy or esophagectomies or oesophagectomy or oesophagectomies or ((esophag* or oesophag*) and (resection or resections or removal or operation or operations))).mp.
3. 1 or 2
4. exp Laparoscopy/
5. laparoscop*.mp.
6. 4 or 5
7. exp esophagus tumor/
8. (esophag* adj5 neoplas*).tw.
9. (oesophag* adj5 neoplas*).tw.
10. (esophag* adj5 cancer*).tw.
11. (oesophag* adj5 cancer*).tw.
12. (esophag* adj5 carcin*).tw.
13. (oesophag* adj5 carcin*).tw.
14. (esophag* adj5 tumo*).tw.
15. (oesophag* adj5 tumo*).tw.
16. (esophag* adj5 malig*).tw.
17. (oesophag* adj5 malig*).tw.
18. exp lower esophagus sphincter/
19. 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18
20. 3 and 6 and 19
Appendix 4. Science Citation Index search strategy
#1 TS=(esophagectomy or esophagectomies or oesophagectomy or oesophagectomies or ((esophag* or oesophag*) and (resection or resections or removal or operation or operations)))
#2 TS=(laparoscop*)
#3 TS=(esophag* near/5 neoplas*)
#4 TS=(oesophag* near/5 neoplas*)
#5 TS=(esophag* near/5 cancer*)
#6 TS=(oesophag* near/5 cancer*)
#7 TS=(esophag* near/5 carcin*)
#8 TS=(oesophag* near/5 carcin*)
#9 TS=(esophag* near/5 tumo*)
#10 TS=(oesophag* near/5 tumo*)
#11 TS=(esophag* near/5 malig*)
#12 TS=(oesophag* near/5 malig*)
#13 #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3
#14 #13 AND #2 AND #1
Appendix 5. ClinicalTrials.gov search strategy
Interventional Studies | esophageal cancer | laparoscopic | Phase 2, 3, 4
Appendix 6. WHO ICTRP search strategy
laparoscopic oesophagectomy or laparoscopic esophagectomy or laparoscopic oesophageal resection or laparoscopic esophageal resection
Data and analyses
Comparison 1. Laparoscopic versus open transhiatal oesophagectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term mortality | 5 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.05, 4.09] |
| 2 Long‐term mortality | 2 | 193 | Hazard Ratio (Fixed, 95% CI) | 0.97 [0.81, 1.16] |
| 3 Serious adverse events (proportion) | 3 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.24, 0.99] |
| 4 Anastomotic stenosis | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.33, 5.70] |
| 5 Short‐term recurrence (within 6 months) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.04, 18.47] |
| 6 Long‐term recurrence | 2 | 173 | Hazard Ratio (Fixed, 95% CI) | 1.00 [0.84, 1.18] |
| 7 Adverse events (proportion) | 3 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.48, 0.86] |
| 8 Blood transfusion (proportion) | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.00, 1.35] |
| 9 Blood transfusion (quantity) | Other data | No numeric data | ||
| 10 Length of hospital stay | Other data | No numeric data | ||
| 11 Positive resection margins | 3 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.37, 1.12] |
| 12 Number of lymph nodes harvested | Other data | No numeric data |
Comparison 2. Laparoscopic versus open transhiatal oesophagectomy (Subgroup analyses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events (proportion) (stage I adenocarcinoma) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Short‐term mortality (lower‐third cancer) | 3 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 3 Long‐term mortality (lower third cancer) | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 4 Serious adverse events (proportion) (lower third cancer) | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.17, 1.22] |
2.1. Analysis.
Comparison 2 Laparoscopic versus open transhiatal oesophagectomy (Subgroup analyses), Outcome 1 Serious adverse events (proportion) (stage I adenocarcinoma).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Badessi 2003.
| Methods | Not clear whether this is a cohort study or case‐control study | |
| Participants | Country: Italy
Number eligible: 8
Number excluded: not stated
Number analysed: 8
Average age: not stated
Females: 3 (37.5%)
Stage I: not stated
Stage II: not stated
Stage III: not stated
Stage IV: not stated
Squamous cell carcinoma: not stated
Adenocarcinoma: not stated
Study design: retrospective study (no further details)
Total follow‐up in months: not stated
ASA: not stated
Location: not stated Inclusion criteria Patients undergoing transhiatal oesophagectomy |
|
| Interventions | Group 1: laparoscopic transhiatal oesophagectomy (N = 5). Further details: number of ports ‐ not stated; minilaparotomy incision size ‐ not stated; drain use ‐ not stated. Group 2: open transhiatal oesophagectomy (N = 3). Further details: incision size ‐ not stated; drain use ‐ not stated. | |
| Outcomes | None of the outcomes of interest were reported (adverse events and length of hospital stay were reported but not in sufficient details to allow inclusion for meta‐analysis). | |
| Notes | Proportion of people with cancer: 100% Conversion: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | Unclear risk |
No information Comment: There was no mention about baseline differences. |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: Method of selection of participants to intervention and control was not reported. |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: It was not clear there were other differences in care of the patient apart from the intervention and control. |
| Bias in the measurement of outcomes | Unclear risk |
No information Comment: Information on observer blinding was not available. |
| Bias due to missing data | Unclear risk |
No information Comment: This information was not available. |
| Bias in selection of the reported findings | High risk |
Critical risk of bias Comment: None of the outcomes of interest were reported. |
Cash 2014.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: USA.
Number eligible: 93.
Number excluded: not stated.
Number analysed: 93.
Average age: 74 years.
Females: 20 (21.5%).
Stage I: 37(39.8%).
Stage II: 22 (23.7%).
Stage III: 34 (36.6%).
Stage IV: not stated
Squamous cell carcinoma: not stated
Adenocarcinoma: not stated
Study design: Retrospective cohort study with historical control
Total follow‐up in months: median: 26 months for laparoscopic oesophagectomy group and 64 months for open oesophagectomy group (survival at 24 months was used for calculation of proportion survived)
ASA: not stated
Location: not stated Inclusion criteria Patients undergoing transhiatal oesophagectomy for oesophageal cancer Exclusion criteria People who had undergone major abdominal surgery. |
|
| Interventions | Group 1: laparoscopic transhiatal oesophagectomy (N = 33). Further details: number of ports ‐ not stated; minilaparotomy ‐ incision size not stated; drain use ‐ not stated. Group 2: open transhiatal oesophagectomy (N = 60). Further details: incision size ‐ not stated; drain use ‐ not stated. | |
| Outcomes | The outcomes reported were short‐term and long‐term mortality, morbidity, long‐term recurrence, length of hospital stay, and number of lymph nodes harvested. | |
| Notes | Proportion of people with cancer: 100% Conversion: 2/33 (6.1%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | High risk |
Critical risk of bias Comment: The tumour size was smaller in the laparoscopic group but more patients had neo‐adjuvant therapy in the laparoscopic group. |
| Bias due to selection of participants to intervention and control | Low risk |
Moderate Comment: This was a consecutive series of laparoscopic oesophagectomies where the surgeon performed all transhiatal oesophagectomies laparoscopically other than for those who had undergone major abdominal surgery after July 2008 |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: A historical control was used. It was not clear there were other differences in care of the patient apart from the intervention and control. |
| Bias in the measurement of outcomes | Unclear risk |
No information Comment: Information on observer blinding was not available. |
| Bias due to missing data | Unclear risk |
No information Comment: This information was not available. |
| Bias in selection of the reported findings | Low risk |
Low risk of bias Comment: Mortality and morbidity were reported. |
Ecker 2015.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: USA.
Number eligible: 73.
Post‐randomisation drop‐outs: 0 (0%).
Number analysed: 73.
Average age: 64 years.
Females: 16 (21.9%).
Stage I: 13(17.8%).
Stage II: 19 (26%).
Stage III: 31 (42.5%).
Stage IV: not stated
Squamous cell carcinoma: 4 (5.5%).
Adenocarcinoma: 68 (93.2%).
Study design: Retrospective cohort study with contemporary controls
Total follow‐up in months: median:10 months
ASA: not stated
Location: not stated Inclusion criteria Patients undergoing transhiatal oesophagectomy |
|
| Interventions | Group 1: laparoscopic transhiatal oesophagectomy (N = 36). Further details: number of ports ‐ not stated; minilaparotomy incision size ‐ not stated; drain use ‐ not stated. Group 2: open transhiatal oesophagectomy (N = 37). Further details: incision size ‐ not stated; drain use ‐ not stated. | |
| Outcomes | The outcomes reported were short‐term and long‐term mortality, oesophageal stenosis, short‐term recurrence, blood transfusion, length of hospital stay, positive resection margin, and number of lymph nodes harvested. | |
| Notes | Proportion of people with cancer: 96% to 99% (error in the number of patients with different aetiologies) Conversion: 4/36 (11.1%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | Unclear risk |
No information Comment: There was no evidence of baseline differences between the groups. However, the sample size was not sufficient to identify baseline differences. In addition, not all confounding factors were listed in the baseline differences table (for example, no information was presented on the differences in the size of the tumours) |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: Method of selection of participants to intervention and control was not reported. |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: It was not clear there were other differences in care of the patient apart from the intervention and control. |
| Bias in the measurement of outcomes | Unclear risk |
No information Comment: Information on observer blinding was not available. |
| Bias due to missing data | Low risk |
Low risk of bias Comment: All patients were included in the analysis. |
| Bias in selection of the reported findings | High risk |
Serious risk of bias Comment: Morbidity was not reported. |
Maas 2012.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: Netherlands.
Number eligible: 100.
Post‐randomisation drop‐outs: 0 (0%).
Number analysed: 100.
Average age: 64 years.
Females: 26 (26%).
Stage I: 7(7%).
Stage II: 31 (31%).
Stage III: 62 (62%).
Stage IV: 0(0%).
Squamous cell carcinoma: 28 (28%).
Adenocarcinoma: 69 (69%).
Study design: Retrospective cohort study with historical control
Total follow‐up in months: not stated
ASA: ASA I or II: 38 versus 36; ASA III or IV: 12 versus 14
Location: lower third Inclusion criteria Patients undergoing transhiatal oesophagectomy for distal oesophageal cancer Exclusion criteria Patients with colon interposition |
|
| Interventions | Group 1: laparoscopic transhiatal oesophagectomy (N = 50). Further details: number of ports ‐ 5; minilaparotomy ‐ 7 cm periumbilical incision); drain use ‐ not stated. Group 2: open transhiatal oesophagectomy (N = 50). Further details: incision size ‐ not stated; drain use ‐ not stated. | |
| Outcomes | The outcomes reported were short‐term and long‐term mortality, morbidity, long‐term recurrence, length of hospital stay, positive resection margin, and number of lymph nodes harvested. | |
| Notes | Proportion of people with cancer: 100% Conversion: 9/50 (18%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | Unclear risk |
No information Comment: There was no evidence of baseline differences between the groups. However, the sample size was not sufficient to identify baseline differences. In addition, not all confounding factors were listed in the baseline differences table (for example, no information was presented on the differences in the size of the tumours) |
| Bias due to selection of participants to intervention and control | Unclear risk |
Moderate risk of bias Comment: This was a consecutive series of laparoscopic oesophagectomies where the surgeon performed all transhiatal oesophagectomies laparoscopically after January 2001. |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: A historical control was used. It was not clear there were other differences in care of the patient apart from the intervention and control. |
| Bias in the measurement of outcomes | High risk |
Critical risk of bias Comment: The assessment of the patients was not done blinded (author replies). |
| Bias due to missing data | Low risk |
Low risk of bias Comment: All patients were included in the analysis. |
| Bias in selection of the reported findings | Low risk |
Low risk of bias Comment: Mortality and morbidity were reported adequately. |
Saha 2009.
| Methods | Study design: retrospective cohort study | |
| Participants | Country: UK.
Number eligible: 20.
Number excluded: 0 (0%).
Number analysed: 20.
Average age: 65 years.
Females: 4 (20%).
Stage I: 20(100%).
Stage II: 0 (0%).
Stage III: 0 (0%).
Stage IV: 0(0%).
Squamous cell carcinoma: 0 (0%).
Adenocarcinoma: 20 (100%).
Study design: Retrospective cohort study with contemporary controls
Total follow‐up in months: median: 44 months
ASA: not stated
Location: lower third Inclusion criteria Patients undergoing oesophagectomy for T1 oesophageal adenocarcinoma |
|
| Interventions | Group 1: laparoscopic transhiatal oesophagectomy (N = 16). Further details: Number of ports ‐ 5; minilaparotomy incision size ‐ not stated; drain use ‐ not stated. Group 2: open transhiatal oesophagectomy (N = 4). Further details: incision size ‐ not stated; drain use ‐ not stated. | |
| Outcomes | The outcomes reported were short‐term mortality, long‐term recurrence, and number of lymph nodes harvested. | |
| Notes | Proportion of people with cancer: 100% Conversion: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | Unclear risk |
No information Comment: There was no evidence of baseline differences between the groups. However, the sample size was not sufficient to identify baseline differences. In addition, not all confounding factors were listed in the baseline differences table (for example, no information was presented on the differences in the size of the tumours) |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: Patients were selected for laparoscopic transhiatal or open resection at the discretion of the surgeon. |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: It was not clear there were other differences in care of the patient apart from the intervention and control. |
| Bias in the measurement of outcomes | High risk |
Critical risk of bias Quote: "Outcome assessors were not blinded (author replies)". |
| Bias due to missing data | Low risk |
Low risk of bias Comment: All patients were included in the analysis. |
| Bias in selection of the reported findings | High risk |
Serious risk of bias Comment: Morbidity was not reported. |
Valenti 2008.
| Methods | Study design: prospective cohort study | |
| Participants | Country: UK.
Number eligible: 40.
Number excluded: 0 (0%).
Number analysed: 40.
Average age: 67 years.
Females: 8 (20%).
Stage I: not stated
Stage II: not stated
Stage III: not stated
Stage IV: not stated
Squamous cell carcinoma: 9 (22.5%).
Adenocarcinoma: 28 (70%).
Study design: Prospective cohort study with contemporary controls
Total follow‐up in months: not stated
ASA: ASA I or II: 12 versus 17; ASA III or IV: 4 versus 7
Location: lower third Inclusion criteria Patients undergoing transhiatal oesophagectomy for oesophageal cancer |
|
| Interventions | Group 1: laparoscopic transhiatal oesophagectomy (N = 16). Further details: number of ports ‐ 5; minilaparotomy ‐ 7 cm incision; drain use ‐ not stated. Group 2: open transhiatal oesophagectomy (N = 24). Further details: incision size ‐ not stated; drain used‐ not stated. | |
| Outcomes | The outcomes reported were short‐term mortality and number of lymph nodes harvested. | |
| Notes | Proportion of people with cancer: 93% (remaining patients had high grade dysplasia) Conversion: 0/16 (0%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Bias due to confounding | Unclear risk |
No information Comment: There was no evidence of baseline differences between the groups. However, the sample size was not sufficient to identify baseline differences. In addition, not all confounding factors were listed in the baseline differences table (for example, no information was presented on the differences in the stage of the tumours) |
| Bias due to selection of participants to intervention and control | Unclear risk |
No information Comment: Method of selection of participants to intervention and control was based on surgeon's preference. |
| Bias due to differences in co‐interventions which were different between the groups | Unclear risk |
No information Comment: It was not clear there were other differences in care of the patient apart from the intervention and control. |
| Bias in the measurement of outcomes | High risk |
High risk of bias Quote: "No blinded assessors (author replies)". |
| Bias due to missing data | Low risk |
Low risk of bias Quote: "No patients excluded from analysis (author replies)". |
| Bias in selection of the reported findings | High risk |
Serious risk of bias Comment: Morbidity was not reported. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bernabe 2005 | Transhiatal oesophagectomy was performed for less than 90% of oesophageal cancers and separate data were not available for people with pancreatic cancers |
| Blazeby 2011 | Not transhiatal oesophagectomy |
| Bresadola 2006 | No separate data for transhiatal oesophagectomy |
| Burdall 2015 | Not transhiatal oesophagectomy |
| Csendes 2013 | Not transhiatal oesophagectomy |
| Cuesta 2012 | Editorial |
| Dolan 2013 | Not transhiatal oesophagectomy |
| Fabian 2008 | No separate data for transhiatal oesophagectomy |
| Ferreira 2004 | Review |
| Harrison 2013 | Unclear whether transhiatal oesophagectomies were included |
| Kang 2013 | No separate data for transhiatal oesophagectomy |
| Lee 2013 | Cost‐effectiveness study with no primary research data |
| Mamidanna 2012 | No separate data for transhiatal oesophagectomy |
| Mao 2012 | No separate data for transhiatal oesophagectomy |
| Mariette 2012 | Letter to editor |
| Messenger 2015 | No separate data for transhiatal oesophagectomy |
| Parameswaran 2013 | Not transhiatal oesophagectomy |
| Perry 2009 | Transhiatal oesophagectomy was performed for less than 90% of oesophageal cancers and separate data were not available for people with pancreatic cancers |
| Rice 2012 | Editorial |
| Safranek 2010 | Not transhiatal oesophagectomy |
| Scheepers 2008 | No control group |
| Schoppmann 2010 | Not transhiatal oesophagectomy |
| Ujiki 2013 | Comment on excluded study (Perry 2009) |
| Yamasaki 2011 | Not transhiatal oesophagectomy |
Differences between protocol and review
-
Since no randomised controlled trials were identified, we included non‐randomised studies to provide the current best available evidence. As a result, we made the following modifications to the protocol.
We did not use the filter for randomised controlled trials for the electronic searches of the databases.
We used 'A Cochrane Risk Of Bias Assessment Tool: for Non‐Randomized Studies of Interventions' (ACROBAT‐NRSI) tool for assessment of risk of bias rather than the standard Cochrane risk of bias tool for randomised controlled trials.
We have noted under each outcome if outcome data were reported in an unusable way in one or more studies. In the protocol, we stated that we will do this in the 'Characteristics of included studies' table. However, it is unlikely the readers refer to the Characteristics of included studies' table while reading the results and it appeared more appropriate to provide this information under each outcome.
Contributions of authors
Conceiving of the review: KG
Designing the review: KG
Co‐ordinating the review: KG
Designing search strategies: KG
Data extraction: KG, EP, SM
Data analysis: KG
Writing the review: KG
Providing critical comments on the review: MM
Securing funding for the review: KG
Performing previous work that served as the foundation of the current study: KG
Sources of support
Internal sources
University College London, UK.
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to the Upper Gastro‐intestinal and Pancreatic Diseases group (UGPD) and Cochrane‐Hepato Biliary Group (CHBG). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ Evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service (NHS), the National Institute for Health Research, or the Department of Health.
KG: none known.
EP: none known.
SM: none known.
MM: none known.
New
References
References to studies included in this review
Badessi 2003 {published data only}
- Badessi F, Gusai GP, Fais D, Huscher C. Traditional esophagectomy and esophago‐gastrectomy vs. laparoscopic surgery. Evaluation and results. Tumori 2003;89(4 Suppl):80‐3. [PubMed] [Google Scholar]
Cash 2014 {published data only}
- Cash JC, Zehetner J, Hedayati B, Bildzukewicz NA, Katkhouda N, Mason RJ, et al. Outcomes following laparoscopic transhiatal esophagectomy for esophageal cancer. Surgical Endoscopy 2014;28(2):492‐9. [DOI] [PubMed] [Google Scholar]
- Cash JC, Zehetner J, Heyadati B, Crookes PF, Katkhouda N, Mason RJ, et al. Outcome following laparoscopic trans‐hiatal esophagectomy for esophageal cancer. Surgical Endoscopy 2013;27:S267. [DOI] [PubMed] [Google Scholar]
Ecker 2015 {published data only}
- Ecker B, Savulionyte G, Datta J, Dumon K, Williams N, Kucharczuk J, et al. Laparoscopic transhiatal esophagectomy improves hospital outcomes and reduces cost: A single‐institution cohort analysis of laparoscopic and open techniques. Annals of Surgical Oncology 2015;1:S154. [DOI] [PubMed] [Google Scholar]
Maas 2012 {published data only}
- Maas KW, Biere SS, Scheepers JJ, Gisbertz SS, van‐der‐Peet DL, Cuesta MA. Laparoscopic versus open transhiatal esophagectomy for distal and junction cancer. Revista Española de Enfermedades Digestivas 2012;104(4):197‐202. [DOI] [PubMed] [Google Scholar]
- Makay O, Broek WT, Yuan JZ, Veerman DP, Helfferich DW, Cuesta MA. Anesthesiological hazards during laparoscopic transhiatal esophageal resection: A case control study of the laparoscopic‐assisted vs the conventional approach. Surgical Endoscopy 2004;18(8):1263‐7. [DOI] [PubMed] [Google Scholar]
- Broek WT, Makay O, Berends FJ, Yuan JZ, Houdijk APJ, Meijer S, et al. Erratum: Laparoscopically assisted transhiatal resection for malignancies of the distal esophagus. Surgical Endoscopy 2004;18(8):1292. [DOI] [PubMed] [Google Scholar]
- Broek WT, Makay O, Berends FJ, Yuan JZ, Houdijk AP, Meijer S, et al. Laparoscopically assisted transhiatal resection for malignancies of the distal esophagus. Surgical Endoscopy 2004;18(5):812‐7. [DOI] [PubMed] [Google Scholar]
Saha 2009 {published data only}
- Saha AK, Sutton CD, Sue‐Ling H, Dexter SP, Sarela AI. Comparison of oncological outcomes after laparoscopic transhiatal and open esophagectomy for T1 esophageal adenocarcinoma. Surgical Endoscopy 2009;23(1):119‐24. [DOI] [PubMed] [Google Scholar]
Valenti 2008 {published data only}
- Valenti AV, Fares R, Reynolds N, Cohen P, Theodoro N, Martinez‐Isla A. Open and laparoscopic transhiatal oesophagectomy for cancer of the oesophagus: Analysis of resection margins and lymph nodes. Cirugia Espanola 2008;83(1):24‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bernabe 2005 {published data only}
- Bernabe KQ, Bolton JS, Richardson WS. Laparoscopic hand‐assisted versus open transhiatal esophagectomy: A case‐control study. Surgical Endoscopy 2005;19(3):334‐7. [DOI] [PubMed] [Google Scholar]
Blazeby 2011 {published data only}
- Blazeby JM, Blencowe NS, Titcomb DR, Metcalfe C, Hollowood AD, Barham CP. Demonstration of the ideal recommendations for evaluating and reporting surgical innovation in minimally invasive oesophagectomy. British Journal of Surgery 2011;98(4):544‐51. [DOI] [PubMed] [Google Scholar]
Bresadola 2006 {published data only}
- Bresadola V, Terrosu G, Cojutti A, Benzoni E, Baracchini E, Bresadola F. Laparoscopic versus open gastroplasty in esophagectomy for esophageal cancer: A comparative study. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2006;16(2):63‐7. [DOI] [PubMed] [Google Scholar]
Burdall 2015 {published data only}
- Burdall OC, Boddy AP, Fullick J, Blazeby J, Krysztopik R, Streets C, et al. A comparative study of survival after minimally invasive and open oesophagectomy. Surgical Endoscopy 2015;29(2):431‐7. [DOI] [PubMed] [Google Scholar]
- Burdall OC, Fullick J, Boddy AP, Blazeby J, Krysztopik R, Streets C, et al. Improved survival following minimally invasive oesophagectomy compared to open surgery. Surgical Endoscopy 2014;28:254. [DOI] [PubMed] [Google Scholar]
Csendes 2013 {published data only}
- Csendes A, Braghetto I, Cardemil G, Cortes S, Musleh M. Results of esophagectomy among patients with esophageal cancer and Barrett esophagus. Revista Chilena De Cirugia 2013;65(2):121‐7. [Google Scholar]
Cuesta 2012 {published data only}
- Cuesta MA, Biere S, Berge Henegouwen MI, Peet DL. Randomised trial, minimally invasive oesophagectomy versus open oesophagectomy for patients with resectable oesophageal cancer. Journal of Thoracic Disease 2012;4(5):462‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dolan 2013 {published data only}
- Dolan JP, Kaur T, Diggs BS, Luna RA, Schipper PH, Tieu BH, et al. Impact of comorbidity on outcomes and overall survival after open and minimally invasive esophagectomy for locally advanced esophageal cancer. Surgical Endoscopy 2013;27(11):4094‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fabian 2008 {published data only}
- Fabian T, Martin JT, McKelvey AA, Federico JA. Minimally invasive esophagectomy: A teaching hospital's first year experience. Diseases of the Esophagus 2008;21(3):220‐5. [DOI] [PubMed] [Google Scholar]
Ferreira 2004 {published data only}
- Ferreira EAB. Is there a role for minimally invasive surgery in the treatment of esophageal cancer?. Proceedings of the 9th World Congress of Endoscopic Surgery. 2004:53‐7.
Harrison 2013 {published data only}
- Harrison M, Titcomb D, Sychev I. Do laparoscopically‐assisted and minimally invasive oesophagectomies improve outcomes when compared to the traditional open method?. International Journal of Surgery 2013;11(8):718. [Google Scholar]
Kang 2013 {published data only}
- Kang CH, Hwang YH, Kim HS, Jheon JH, Han KN, Park IK, et al. Long‐term survival after minimally invasive oesophagectomy for oesophageal cancer is comparable to open oesophagectomy: A propensity score‐matched study. Interactive Cardiovascular &Thoracic Surgery 2013;17:S142. [Google Scholar]
Lee 2013 {published data only}
- Lee L, Sudarshan M, Li C, Latimer E, Fried GM, Mulder DS, et al. Cost‐effectiveness of minimally invasive versus open esophagectomy for esophageal cancer. Annals of Surgical Oncology 2013;20(12):3732‐9. [DOI] [PubMed] [Google Scholar]
Mamidanna 2012 {published data only}
- Mamidanna R, Bottle A, Aylin P, Faiz O, Hanna GB. Short‐term outcomes following open versus minimally invasive esophagectomy for cancer in England: A population‐based national study. Annals of Surgery 2012;255(2):197‐203. [DOI] [PubMed] [Google Scholar]
Mao 2012 {published data only}
- Mao T, Fang WT, Gu ZT, Yao F, Guo XF, Chen WH. Comparative study of perioperative complications and lymphadenectomy between minimally invasive esophagectomy and open procedure. Zhonghua Weichang Waike Zazhi 2012;15(9):922‐5. [PubMed] [Google Scholar]
Mariette 2012 {published data only}
- Mariette C, Robb WB. Minimally invasive versus open oesophagectomy for oesophageal cancer. The Lancet 2012;380(9845):883. [DOI] [PubMed] [Google Scholar]
Messenger 2015 {published data only}
- Messenger DE, Higgs SM, Dwerryhouse SJ, Hewin DF, Vipond MN, Barr H, et al. Symptomatic diaphragmatic herniation following open and minimally invasive oesophagectomy: Experience from a UK specialist unit. Surgical Endoscopy 2015;29(2):417‐24. [DOI] [PubMed] [Google Scholar]
Parameswaran 2013 {published data only}
- Parameswaran R, Titcomb DR, Blencowe NS, Berrisford RG, Wajed SA, Streets CG, et al. Assessment and comparison of recovery after open and minimally invasive esophagectomy for cancer: An exploratory study in two centers. Annals of Surgical Oncology 2013;20(6):1970‐7. [DOI] [PubMed] [Google Scholar]
Perry 2009 {published data only}
- Perry KA, Enestvedt CK, Pham T, Welker M, Jobe BA, Hunter JG, et al. Comparison of laparoscopic inversion esophagectomy and open transhiatal esophagectomy for high‐grade dysplasia and Stage I esophageal adenocarcinoma. Archives of Surgery 2009;144(7):679‐84. [DOI] [PubMed] [Google Scholar]
Rice 2012 {published data only}
- Rice TW, Blackstone EH. Minimally invasive versus open esophagectomy for cancer: More questions than answers. Annals of Surgery 2012;255(2):204‐5. [DOI] [PubMed] [Google Scholar]
Safranek 2010 {published data only}
- Safranek PM, Cubitt J, Booth MI, Dehn TC. Review of open and minimal access approaches to oesophagectomy for cancer. British Journal of Surgery 2010;97(12):1845‐53. [DOI] [PubMed] [Google Scholar]
Scheepers 2008 {published data only}
- Scheepers JJ, Veenhof AA, Peet DL, Groeningen C, Mulder C, Meijer S, et al. Laparoscopic transhiatal resection for malignancies of the distal esophagus: Outcome of the first 50 resected patients. Surgery 2008;143(2):278‐85. [DOI] [PubMed] [Google Scholar]
Schoppmann 2010 {published data only}
- Schoppmann SF, Prager G, Langer FB, Riegler FM, Kabon B, Fleischmann E, et al. Open versus minimally invasive esophagectomy: A single‐center case controlled study. Surgical Endoscopy 2010;24(12):3044‐53. [DOI] [PubMed] [Google Scholar]
Ujiki 2013 {published data only}
- Ujiki MB, Perry K, Velasco J, Kim AW. Perioperative outcomes of laparoscopic transhiatal esophagectomy with antegrade esophageal inversion for high‐grade dysplasia and invasive esophageal cancer discussion. Surgery 2013;154(4):907‐8. [DOI] [PubMed] [Google Scholar]
Yamasaki 2011 {published data only}
- Yamasaki M, Miyata H, Fujiwara Y, Takiguchi S, Nakajima K, Kurokawa Y, et al. Minimally invasive esophagectomy for esophageal cancer: Comparative analysis of open and hand‐assisted laparoscopic abdominal lymphadenectomy with gastric conduit reconstruction. Journal of Surgical Oncology 2011;104(6):623‐8. [DOI] [PubMed] [Google Scholar]
Additional references
AJCC 2010
- Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th Edition. New York: Springer, 2010. [Google Scholar]
Allum 2011
- Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Guidelines for the management of oesophageal and gastric cancer. Gut 2011;60(11):1449‐72. [DOI] [PubMed] [Google Scholar]
ASA 2014
- American Society of Anesthesiologists. ASA physical status classification system. Available from: www.asahq.org/Home/For‐Members/Clinical‐Information/ASA‐Physical‐Status‐Classification‐System (accessed 16 November 2014).
Avital 2005
- Avital S, Zundel N, Szomstein S, Rosenthal R. Laparoscopic transhiatal esophagectomy for esophageal cancer. American Journal of Surgery 2005;190(1):69‐74. [DOI] [PubMed] [Google Scholar]
Bijen 2009
- Bijen CB, Vermeulen KM, Mourits MJ, Bock GH. Costs and effects of abdominal versus laparoscopic hysterectomy: systematic review of controlled trials. PLoS One 2009;4(10):e7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boshier 2011
- Boshier PR, Anderson O, Hanna GB. Transthoracic versus transhiatal esophagectomy for the treatment of esophagogastric cancer: a meta‐analysis. Annals of Surgery 2011;254(6):894‐906. [DOI] [PubMed] [Google Scholar]
Cancer Research UK 2014
- Cancer Research UK. Oesophageal cancer incidence statistics. Available from: www.cancerresearchuk.org/cancer‐info/cancerstats/types/oesophagus/incidence/uk‐oesophageal‐cancer‐incidence‐statistics#Trends (accessed 16 November 2014).
Clavien 2009
- Clavien PA, Barkun J, Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Annals of Surgery 2009;250(2):187‐96. [DOI] [PubMed] [Google Scholar]
Colvin 2011
- Colvin H, Dunning J, Khan OA. Transthoracic versus transhiatal esophagectomy for distal esophageal cancer: which is superior?. Interactive Cardiovascular and Thoracic Surgery 2011;12(2):265‐9. [DOI] [PubMed] [Google Scholar]
Demets 1987
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dindo 2004
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of Surgery 2004;240(2):205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fovos 2012
- Fovos A, Jarral O, Panagiotopoulos N, Podas T, Mikhail S, Zacharakis E. Does endoscopic treatment for early oesophageal cancers give equivalent oncological outcomes as compared with oesophagectomy? Best evidence topic (BET). International Journal of Surgery (London, England) 2012;10(9):415‐20. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hopkins 1999
- Hopkins MP, Dulai RM, Occhino A, Holda S. The effects of carbon dioxide pneumoperitoneum on seeding of tumor in port sites in a rat model. American Journal of Obstetrics and Gynecology 1999;181(6):1329‐34. [DOI] [PubMed] [Google Scholar]
IARC 2014
- International Agency for Research on Cancer. GLOBOCAN 2012. Available from: globocan.iarc.fr/Default.aspx (accessed 16 November 2014).
ICH‐GCP 1996
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1996. [Google Scholar]
Kais 2014
- Kais H, Hershkovitz Y, Sandbank J, Halevy A. Port site metastases in squamous cell carcinoma of the gallbladder. Israel Medical Association Journal 2014;16(3):177‐9. [PubMed] [Google Scholar]
Keus 2006
- Keus F, Jong JA, Gooszen HG, Laarhoven CJ. Laparoscopic versus open cholecystectomy for patients with symptomatic cholecystolithiasis. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006231] [DOI] [PubMed] [Google Scholar]
McKeown 1976
- McKeown KC. Total three‐stage oesophagectomy for cancer of the oesophagus. British Journal of Surgery 1976;63(4):259‐62. [DOI] [PubMed] [Google Scholar]
Omloo 2007
- Omloo JM, Lagarde SM, Hulscher JB, Reitsma JB, Fockens P, Dekken H, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five‐year survival of a randomized clinical trial. Annals of Surgery 2007;246(6):992‐1000; discussion 1000‐1. [DOI] [PubMed] [Google Scholar]
Orringer 2007
- Orringer MB, Marshall B, Chang AC, Lee J, Pickens A, Lau CL. Two thousand transhiatal esophagectomies: changing trends, lessons learned. Annals of Surgery 2007;246(3):363‐72; discussion 372‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palomba 2014
- Palomba S, Mandato VD, Sala GB. Isolated port‐site metastasis after robotic hysterectomy for Stage IA endometrial adenocarcinoma. Obstetrics and Gynecology 2014;123(3):664. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reza 2006
- Reza MM, Blasco JA, Andradas E, Cantero R, Mayol J. Systematic review of laparoscopic versus open surgery for colorectal cancer. British Journal of Surgery 2006;93(8):921‐8. [DOI] [PubMed] [Google Scholar]
Rice 2010
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Annals of Surgical Oncology 2010;17(7):1721‐4. [DOI] [PubMed] [Google Scholar]
Sjoquist 2011
- Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta‐analysis. The Lancet Oncology 2011;12(7):681‐92. [DOI] [PubMed] [Google Scholar]
Song 2014
- Song J, Kim E, Mobley J, Vemana G, Tanagho Y, Vetter J, et al. Port site metastasis after surgery for renal cell carcinoma: harbinger of future metastasis. Journal of Urology 2014;192(2):364‐8. [DOI] [PubMed] [Google Scholar]
Stahl 2013
- Stahl M, Mariette C, Haustermans K, Cervantes A, Arnold D, Group EGW. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2013;24(Suppl 6):vi51‐6. [DOI] [PubMed] [Google Scholar]
Sterne 2014
- Sterne JAC, Higgins JPT, Reeves BC on behalf of the development group for ACROBAT‐NRSI. A Cochrane Risk Of Bias Assessment Tool: for Non‐Randomized Studies of Interventions (ACROBAT‐NRSI), Version 1.0.0. Available from http://www.riskofbias.info (accessed on 25th November 2015)..
Talseth 2014
- Talseth A, Lydersen S, Skjedlestad F, Hveem K, Edna TH. Trends in cholecystectomy rates in a defined population during and after the period of transition from open to laparoscopic surgery. Scandinavian Journal of Gastroenterology 2014;49(1):92‐8. [DOI] [PubMed] [Google Scholar]
Walsh 2009
- Walsh CA, Walsh SR, Tang TY, Slack M. Total abdominal hysterectomy versus total laparoscopic hysterectomy for benign disease: a meta‐analysis. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2009;144(1):3‐7. [DOI] [PubMed] [Google Scholar]
Yamamoto 2013
- Yamamoto M, Weber JM, Karl RC, Meredith KL. Minimally invasive surgery for esophageal cancer: review of the literature and institutional experience. Cancer Control 2013;20(2):130‐7. [DOI] [PubMed] [Google Scholar]


