Abstract
INTRODUCTION:
Apolipoprotein E (APOE) status may modify the risk of postoperative delirium conferred by inflammation.
METHODS:
We tested whether APOE modifies the established association between C-reactive protein (CRP) and delirium incidence, severity, and duration in 553 non-cardiac surgical patients age≥70. High postoperative plasma CRP (≥234.12 mg/L) was defined by the highest sample-based quartile. Delirium was determined using the Confusion Assessment Method (CAM) and chart review, and severity by the CAM-Severity score.
RESULTS:
APOE ε4 carrier prevalence was 19%, and postoperative delirium occurred in 24%. The relationship between CRP and delirium incidence, severity, and duration differed by ε4 status. Among ε4 carriers, there was a strong relationship between high CRP (vs. low CRP) and delirium incidence (relative risk [RR] (95% confidence interval [CI]: 3.0(1.4-6.7)); however, no significant association was observed among non-ε4 carriers (RR(95% CI): 1.2(0.8-1.7)).
DISCUSSION:
Our findings raise the possibility that APOE ε4 carrier status may modify the relationship between POD2 CRP levels and postoperative delirium.
Keywords: Delirium, Apolipoprotein E, C-reactive protein, inflammation, gene-protein interaction
INTRODUCTION
Delirium, an acute confusional state, is common, morbid, and costly. It affects 25% of older adults undergoing major elective surgery, and up to 50% of older patients undergoing high-risk procedures (e.g., cardiac surgery and hip fracture repair) [1–3]. Delirium is associated with longer length of hospitalization [4], greater nosocomial complications [5], higher rates of discharge to nursing homes [6], increased risk of cognitive and functional decline [7–9], incident dementia [10], and mortality [11–12]. The estimated annual U.S. health care costs attributable to delirium ranges upwards of $182 billion [13].
Delirium and Alzheimer’s disease (AD) are common causes of late-life cognitive impairment with clear epidemiologic relationships. Although each can occur independently, the two often coexist. AD is a leading risk factor for delirium [14], and following an episode of delirium, there is an increased risk of cognitive decline and incident AD [6,10,15]. Among patients with AD, delirium is associated with an accelerated rate of cognitive decline [16–18], and recent work suggests that in the presence of AD pathology, delirium is associated with cognitive decline beyond that expected for delirium or AD alone [19]. Moreover, preclinical AD has been linked to delirium, such that Alzheimer’s-related cortical atrophy has been associated with postoperative delirium severity in older adults without dementia [20], and individuals with mild cognitive impairment who developed delirium had a synergistically increased risk of developing new impairments in cognitive functioning [21]. This suggests that delirium can lead to faster progression of AD symptoms, resulting in earlier and greater functional disability, and higher healthcare expenditures. Despite these compelling epidemiological relationships, the pathophysiological mechanisms linking delirium and AD remains largely unknown.
Apolipoprotein E (APOE) ε4, the strongest genetic risk factor for late-onset AD, has been the most widely studied genetic risk marker for delirium (a total of 11 publications [22–32]). This work has been largely motivated by posited shared commonalities in pathophysiological mechanisms linking delirium and AD. While initial studies including broad patient samples suggested an association of APOE ε4 with delirium [29, 31], more recent work in older surgical patients free of dementia reported that APOE ε4 does not confer significantly increased risk of delirium [22–25, 32]. Despite this lack of a direct association, APOE may influence risk of delirium indirectly by modifying the relationship of delirium with other risk factors, such as inflammation. Evidence for other indirect genetic influences has been observed in previous examinations of gene-protein interactions with postoperative delirium. For instance, although no direct association between catechol-O-methyltransferase (COMT) genotype, a key regulator of the stress response, and postoperative delirium was observed, COMT genotype was found to modify the previously reported association between inflammatory marker C-reactive protein (CRP) and postoperative delirium [33]. Among older surgical patients with the COMT Val/Val genotype, high CRP measured on postoperative day 2 (POD2) was not associated with delirium. In contrast, patients with COMT Val/Met or Met/Met genotypes and high POD2 CRP had an increased risk of postoperative delirium. Thus, the COMT Val/Val genotype seems to confer reserve against the increased risk of delirium associated with postsurgical inflammation. Such gene-protein interactions have been observed in multiple other conditions, beyond delirium and Alzheimer’s disease (e.g., cancer [34]). This highlights that some genes operate through a moderating effect on stressors or exposures and that examining only their direct influences considers only part of the complexities of gene functional networks [35–37].
To investigate the potential gene-protein interaction of APOE and CRP on postoperative delirium, we examined whether APOE ε4 carrier status modified the previously established relationship between CRP and postoperative delirium incidence, severity, and duration in older surgical patients without dementia [38]. Our Aims were to determine whether the association between POD2 CRP and postoperative delirium incidence, severity, and duration differ among patients with and without an APOE ε4 allele.
METHODS
Study Population
The Successful Aging after Elective Surgery (SAGES) study is an ongoing prospective cohort study focused on investigating risk factors and long-term outcomes of delirium. The SAGES study enrolled patients age ≥70 scheduled for major non-cardiac surgery (N=560), including orthopedic, vascular, or colectomy – under general or spinal anesthesia. Patients with dementia were excluded based on a detailed screening process, which included a complete baseline neuropsychological test battery and a functional status battery (see [39,40] for details). Informed consent for study participation was obtained from all subjects according to procedures approved by the institutional review boards of Beth Israel Deaconess Medical Center and Brigham and Woman’s Hospital, the two surgical sites, and Hebrew SeniorLife, the study coordinating center, all located in Boston, Massachusetts.
Specimen Collection
All patients underwent phlebotomy at 4 time points: preoperatively (PREOP), post-anesthesia care unit (PACU), postoperative day 2 (POD2), and 1 month postoperatively (PO1MO). Based on previous findings, we focused on the POD2 time point for measurement of CRP (described below) to align with the time of the peak stress response following surgery [38]). For the POD2 time point, blood collection was incorporated into clinical blood draws taken on the surgical wards, and usually occurred in the morning between 6-8 AM. Mechanical disruption during phlebotomy was minimized to prevent hemolysis, and blood was stored on ice in heparinized tubes until processing. We used low-speed centrifugation (1500g for 15 minutes at 4°C) to separate plasma and cellular material, and plasma was stored at −80°C until analysis.
Apolipoprotein E.
Phlebotomy was performed PREOP on the entire cohort as described above, usually 1-2 weeks before surgery at the time of preoperative testing. To determine APOE genotype, DNA was extracted from whole blood as previously described [41], which yields high quantities of purified DNA of relatively high molecular weight that can be amplified using polymerase chain reaction (PCR) and restriction enzyme digestion. DNA was extracted, allele-specific PCR assays were conducted in the Brigham Research Assay Core, and APOE genotyping was performed by the Partners Center for Personalized Medicine. The genotype frequencies were in Hardy-Weinberg equilibrium (χ2 = 2.44, df = 3, p ≈ 0.49). To consider the potential effects of APOE ε4 on the relationship between CRP and postoperative delirium, we considered whether patients were ε4 carriers (i.e., ε4ε4, ε4ε3, ε4ε2) versus non-ε4 carriers.
CRP.
CRP on POD2 was measured in the entire SAGES sample using a high-sensitivity enzyme linked immunosorbent assay (ELISA) kit from R&D Systems, with all standards and samples run in duplicate (previously described [38,42]), and coefficients of variation confirmed at ≤5%. ELISA plates were read using a BioTek MX plate reader at Optical Density 450. Since only community-based high-risk cutpoints for CRP have been identified (e.g., [43]) and are not relevant for patients 2 days post-surgery, a cutpoint for ‘high’ CRP on POD2 (i.e., our definition of a heightened stress response) was defined based on the CRP levels observed in the highest quartile of our sample (Q1 ≤116.31 mg/L, Q2 116.32-158.85 mg/L, Q3 158.86-245.83 mg/L, Q4, ≥234.12 mg/L). Additionally, we considered the possible dose-response effect of higher CRP levels by examining each sample based quartile of CRP (Q2-Q4) relative to Q1.
Delirium.
Postoperative delirium was determined by daily interviews during hospitalization, supplemented with a validated chart review method to identify cases missed during daily interviews (e.g., delirious episodes that occurred only at night) [44]. All interviewers underwent training to conduct brief structured cognitive assessments of attention, orientation, and memory. Delirium was assessed using the Confusion Assessment Method (CAM) diagnostic algorithm, which required the patient to have an acute onset of change or fluctuating mental status, inattention, and either disorganized thinking or altered level of consciousness [45]. The presence of delirium by chart review was adjudicated by at least two delirium experts, and discordance was resolved through consensus [46]. Patients were considered delirious if delirium was present on either the CAM or the chart review method on any postoperative day; otherwise, patients were considered non-delirious [47].
Delirium severity and duration.
Delirium severity was quantified using the CAM-Severity long form (CAM-S LF) score [48], which sums the severity ratings of 10 CAM features (range 0-19, 19 most severe), all having possible values of 0 (absent), 1 (present, mild), or 2 (present, marked), with the exception of acute onset or fluctuating course (scored 0 [absent] or 1 [present]). We considered the sum of CAM-S scores across all postoperative hospital days (sum CAM-S), which reflects intensity and duration, thereby capturing the total burden of delirium features throughout the entire hospitalization. Sum CAM-S has been found to be the delirium feature severity measure most strongly associated with clinical outcomes [49]. Delirium duration was defined as the total number of postoperative days the patient was delirious based on the CAM or chart review from the day following surgery until hospital discharge.
Covariates.
We examined covariates associated with APOE and postoperative delirium, including age, sex, and surgery type. Surgical procedures were classified into three types: 1) orthopedic (total knee or hip replacement, lumbar laminectomy, and cervical laminectomy), 2) vascular (lower extremity bypass surgery; abdominal and thoracoabdominal aortic aneurysm repair [open procedure, not endovascular]), and 3) gastrointestinal (open or laparoscopic colectomy).
Statistical Analysis.
We estimated generalized linear models (GLMs) with a log link and binomial error term to assess the association (unadjusted relative risks [RR]) between CRP and postoperative delirium incidence, stratified by APOE ε4 carrier status. GLM models with an identity-link were used to determine the association between CRP and delirium severity and days, stratified by APOE ε4 carrier status. All associations were further examined by adjusting for age, sex, and surgery type (adjusted models). All analyses were conducted using SAS Version 9.4, Cary N.C.
Sensitivity Analyses.
We conducted five sets of sensitivity analyses. First, to determine whether our results were robust to alternate CRP cut points, we considered sample-based tertiles (T1: ≤146.62, T2: 146.63-210.00, T3: ≥210.00 mg/L). Second, to examine the robustness of our findings for the outcome delirium severity, we considered peak CAM-S score as an alternate measure to capture the maximum point of delirium severity that is less dependent on length of stay relative to the sum CAM-S score. Third, to evaluate the influence of medications and conditions associated with inflammation, we conducted separate analyses that excluded patients taking anti-inflammatory medications (nonsteroidal anti-inflammatory drugs, such as cyclooxygenase inhibitors [ibuprofen, naproxen, diclofenac, celecoxib/Celebrex], steroids [prednisone/prednisolone], and other potent immunomodulators [methotrexate, monteleukast, hydroxyurea]) and patients with inflammatory conditions (preoperative connective tissue disease). Fourth, we excluded patients with a major postoperative complication, including unstable arrhythmia, new heart block, non-ST-elevation myocardial infarction (NSTEMI), respiratory failure, pulmonary embolism, pneumonia, sepsis, new renal failure, stroke, and surgical complications (detailed in [50]). Fifth, we excluded patients with the ε4ε2 genotype from our analyses since this genotype includes one risk allele [ε4] and one protective allele [ε2]. Sixth, we additionally adjusted for baseline cognition measured using GCP. We adopted the criteria described for each sensitivity analysis, and ran analytic models as described above.
RESULTS
Table 1 reports the clinical characteristics of our study sample. On average, our total sample was older (mean age 76.7 years) and had a higher than US average preoperative general cognitive performance score (see Table 1 footnote for description). Slightly more than half of the sample was women (58%), and most underwent orthopedic surgery (81%) with fewer colectomies (6%) and vascular surgeries (13%). The clinical characteristics presented in Table 1 were generally similar in APOE ε4 carriers and non-ε4 carriers.
Table 1.
Clinical Characteristics of the Study Sample
| Characteristic | Entire cohort (N=553) | Apolipoprotein ε4 status |
|
|---|---|---|---|
| ε4 (N=106) | No ε4 (N=447) | ||
| Age, M ± SD | 76.7 ± 5.2 | 75.8 ± 4.3 | 77.0 ± 5.3 |
| Female, n (%) | 324 (58) | 63 (58) | 261 (58) |
| Surgery type, n (%) | |||
| Orthopedic | 451 (81) | 81 (75) | 370 (83) |
| Vascular | 35 (6) | 7 (6) | 28 (6) |
| Colectomy | 71 (13) | 20 (19) | 51 (11) |
| Preoperative GCP*, M ± SD | 57.6 ± 7.3 | 57.6 ± 7.9 | 57.6 ± 7.1 |
| CRP postop day 2**, M ± SD | 181.9 ± 81.2 | 167.5 ± 85.7 | 185.3 ± 79.8 |
| Major postop complication, n (%) | 47 (8) | 8 (7) | 39 (9) |
| Delirium incidence, n (%) | 132 (24) | 26 (25) | 106 (24) |
| Delirium severity***, M ± SD | 9.3 ± 11.4 | 9.0 ± 11.2 | 9.3 ± 11.5 |
| Delirium days, n (%) | |||
| 0 | 421 (76) | 80 (75) | 341 (76) |
| 1 | 82 (15) | 17 (16) | 65 (14) |
| 2 | 28 (5) | 6 (6) | 22 (5) |
| 3+ | 22 (4) | 3 (3) | 19 (5) |
abbreviations: CRP=C-reactive protein, GCP =general cognitive performance, M=mean, SD=standard deviation
Apolipoprotein E ε4 carriers defined as ε4ε4 and ε4ε3.
Composite measure of neuropsychological measures reflecting cognitive domains vulnerable to delirium, population mean 50 ± 10, externally scaled to the Health and Retirement Survey of Aging, Demographics, and Memory Study [61]
See Supplementary Figure 2 for distribution of CRP measured preoperatively and on POD2 by Apolipoprotein E ε4 carrier status
Defined as the sum of Confusion Assessment Method (CAM)-S scores, which sums the severity ratings of 10 CAM features (range 0-19, 19 most severe).
Table 2 shows the incidence and adjusted relative risk (RR) of postoperative delirium for each sample based quartile of CRP (i.e., Q2, Q3, and Q4 vs. Q1) and among patients in Q4 compared to patients in Q1-3. In the entire SAGES cohort, patients in the highest quartile (Q4) had an increased risk of postoperative delirium compared to patients in Q1 (RR 1.6, 95% confidence interval [CI] 1.0*-2.7, *actual value 0.96). However, we observed differences in the relationship between CRP POD2 and postoperative delirium by APOE ε4 carrier status. Among ε4 carriers, patients with CRP in Q4 had an increased risk of postoperative delirium relative to patients in Q1-3 (48% vs. 18%; RR 2.8, 95% confidence interval [CI] 1.3-6.1). This association was similarly strong when comparing patients in Q4 to those in Q1 (48% vs. 17%; RR 2.9, 95% CI 1.1-8.1). Of note, both RRs for the APOE ε4 carriers were substantially larger than the RRs observed in the entire SAGES cohort. In contrast among non-ε4 carriers, no significant differences in risk of postoperative delirium by CRP POD2 were observed (Q4 vs Q1-3: 27% vs. 22%; RR 1.2, 95% CI: 0.8-1.8). The p-values for the APOE x CRP interaction on delirium incidence were 0.10 and <.01 for CRP as individual quartiles and CRP as a dichotomous variable (Q4 vs. Q1-3), respectively, confirming that APOE significantly modifies the CRP-delirium relationship.
Table 2.
Association between C-reactive Protein on Postoperative Day 2 (Quartiles) and Delirium by Apolipoprotein E ε4 Carrier Status
| Apolipoprotein E ε4 carrier status |
||||||
|---|---|---|---|---|---|---|
| Entire cohort (N=553) | ε4 carriers (N=106) | ε4 non-carriers ( N=447) | ||||
| CRP level, POD2 (mg/L) | Delirium n(%) | RR (95% CI) | Delirium n(%) | RR (95% CI) | Delirium n(%) | RR (95% CI) |
| Q1 (≤116.31) | 26 (19) | REF | 6(17) | REF | 20(19) | REF |
| Q2 (116.32-158.85) | 27 (20) | 1.1 (0.6-1.9) | 4(15) | 1.0 (0.3-3.6) | 23(20) | 1.1 (0.6-2.0) |
| Q3 (158.86-245.83) | 37 (27) | 1.5 (0.9-2.4) | 5(21) | 1.2 (0.4-3.9) | 32(28) | 1.5 (0.8-2.6) |
| Q4 (≥245.83) | 42 (30) | 1.6 (1.0*-2.7) | 11(48) | 2.9 (1.1-8.1) | 31(27) | 1.4 (0.8-2.5) |
| p-for trend | 0.02 | 0.03 | 0.16 | |||
| Q1-3 (ref) | 90 (22) | 1.4 (1.0**-2.0) | 15(18) | 2.8 (1.3-6.1) | 75(23) | 1.2 (0.8-1.8) |
| Q4 | 42 (30) | 11(48) | 31(27) | |||
abbreviations: CRP=C-reactive protein, CI=confidence interval, POD2=postoperative day 2, Q=quartile, RR=relative risk,
All models adjusted for age, sex, and surgery type
Apolipoprotein E ε4 carriers defined as ε4ε4 and ε4ε3.
p-for interaction: 0.07 (individual quartiles), <.01 (dichotomous: Q4 vs. Q1-3)
Bold indicates significant at p<.05
Actual value 1.004
Actual value 0.96
Table 3 reports the association between CRP POD2 and delirium severity in the entire SAGES cohort and stratified by APOE ε4 carrier status. In the entire cohort and in the APOE ε4 carriers and non-ε4 carriers, we observed significant associations between increasing levels of CRP POD2 and higher mean of the sum CAM-S score, with a larger increase in delirium severity observed among ε4 carriers. Among the APOE ε4 carriers, patients in CRP Q4 had an average of 10.7 more points on their sum CAM-S score than patients in CRP Q1 (p<.01). To put these results into context, prior work indicated that patients with a sum CAM-S score of 7-13 had a statistically significant increased risk of death 30-days post-discharge and to be discharged to a nursing home, compared to patients with a sum CAM-S score of 0-3 (relative risk [RR]: 2.9 and 2.6, respectively) [39]. Among APOE non-ε4 carriers, patients in Q4 had an average of 4.5 more points on their sum CAM-S score than patients in Q1 (p<.01). Prior work found that patients with a sum CAM-S score of 4-6 had a non-significant increased risk of death 30-days post-discharge relative to patients with a sum CAM-S score of 0-3 (RR 2.1) [51]. Both p-values for the APOE x CRP interaction on delirium severity were <.01 for CRP as individual quartiles and CRP as a dichotomous variable.
Table 3.
Generalized Linear Identity-Link Models Predicting Delirium Severity (Sum CAM-S) by C-reactive Protein (Quartiles) on Postoperative Day 2 Stratified by Apolipoprotein E ε4 Carrier Status (N=553)
| Apolipoprotein E ε4 carrier status |
||||||
|---|---|---|---|---|---|---|
| Entire cohort (N=553) |
ε4 carriers (N=106) |
ε4 non-carriers ( N=447) |
||||
| CRP POD2 (mg/L) | Sum CAM-S score* | p | Sum CAM-S score* | p | Sum CAM-S score* | p |
| Q1 (≤116.31) | REF | REF | REF | |||
| Q2 (116.32-158.85) | 1.1 | <.01 | 2.0 | <.01 | 0.8 | 0.03 |
| Q3 (158.86-245.83) | 3.3 | <.01 | 2.1 | <.01 | 3.3 | <.01 |
| Q4 (≥245.83) | 5.6 | <.01 | 10.7 | <.01 | 4.5 | <.01 |
| p-for trend | <.01 | <.01 | <.01 | |||
| Q4 vs. Q1-3 (ref) | 4.2 | <.01 | 9.5 | <.01 | 3.2 | <.01 |
abbreviations: CAM-S=Confusion Assessment Method-Severity, CI=confidence interval, CRP=C-reactive protein, POD2=postoperative day 2, RR=relative risk, Q=quartile
All models adjusted for age, sex, and surgery type
Apolipoprotein E ε4 carriers defined as ε4ε4 and ε4ε3.
p-for interaction<.01 (for individual quartiles and dichotomous [Q4 vs Q1-3])
Bold indicates significant at p<.05
Mean of the differences in the sum CAM-S score
Table 4 presents findings on the relationship between CRP and delirium days in the patients who developed postoperative delirium. In the entire cohort and in APOE ε4 carriers, we observed a significant association between increasing levels of CRP POD2 and increasing number of delirium days. Among APOE ε4 carriers, patients in Q4 of CRP POD2 had on average almost two more delirium days than patients in the lowest quartile (Q1), p<.01. In contrast, this relationship was non-significant among non-ε4 carriers. P-values for the APOE x CRP interaction on delirium days were 0.05 and <.01 for CRP as individual quartiles and CRP as a dichotomous variable, respectively.
Table 4.
Generalized Linear Identity-Link Models Predicting Delirium Duration (Total Number of Delirium Days) by C-reactive Protein (Quartiles) on Postoperative Day 2 Stratified by Apolipoprotein E ε4 Carrier Status (N=553)
| Apolipoprotein E ε4 Carrier Status |
||||||
|---|---|---|---|---|---|---|
| Entire cohort (N=132) |
ε4 carriers (N=106) |
ε4 non-carriers (N=447) |
||||
| CRP level, POD2 (mg/L) | Days | p | Days | p | Days | p |
| Q1 (≤116.31) | REF | REF | REF | |||
| Q2 (116.32-158.85) | 0.1 | 0.19 | 0.1 | 0.87 | 0.1 | 0.62 |
| Q3 (158.86-245.83) | 0.3 | <.01 | 0.1 | 0.92 | 0.4 | 0.06 |
| Q4 (≥245.83) | 0.4 | <.01 | 1.8 | 0.01 | 0.2 | 0.24 |
| p-for trend | <.01 | 0.03 | 0.13 | |||
| Q4 vs. Q1-3 (ref) | 0.3 | <.01 | 1.7 | <.01 | 0.1 | 0.67 |
abbreviations: CI=confidence interval, CRP=C-reactive protein, POD2=postoperative day 2, RR=relative risk, Q=quartile
All models adjusted for age, sex, and surgery type
Apolipoprotein E ε4 carriers defined as ε4ε4 and ε4ε3.
p-for interaction: 0.05 (individual quartiles), <.01 (Q4 vs. Q-1)
Bold indicates significant at p<.05
Mean of the differences in total number of delirium days
Sensitivity Analyses.
When analyzing CRP based on tertiles, the general conclusions of our study findings remained: the association between CRP and delirium incidence, severity, and duration differs by APOE ε4 carrier status (Supplementary Figure), such that these associations were most pronounced in APOE ε4 carriers relative to non-ε4 carriers.
When we considered peak CAM-S score as an alternate measure of delirium severity, our overall study conclusions remained (Supplementary Table 1).
Exclusion of patients with preoperative connective tissue disease (n=42), of patients taking drugs that might influence CRP levels (n=127), and patients with a major postoperative complication (n=47) did not substantially alter the conclusions of our findings (Supplementary Table 2a, 2b, and 3, respectively).
When we excluded patients with the ε4ε2 genotype (n=9), our study conclusions remained (Supplementary Table 4).
When baseline cognitive function measured by GCP was added to our analytic models, the overall study conclusions remained similar (Supplementary Table 5).
DISCUSSION
In this study of older adults without dementia undergoing major non-cardiac surgery, the association between inflammatory marker CRP and postoperative delirium incidence, severity, and duration differed by APOE ε4 carrier status. Among APOE ε4 carriers, we observed a strong and significant association between high POD2 CRP and delirium incidence; however, this association was much weaker and non-significant among APOE ε4 non-carriers. Moreover, we found high POD2 CRP was significantly associated with greater delirium severity and duration in APOE ε4 carriers, with a pronounced increase in delirium severity among ε4 carriers with high CRP. This suggests that APOE ε4 may be an indicator of brain vulnerability, which could be interpreted as either a proxy for an increased risk of preclinical AD pathology or highly correlated with preclinical AD pathology. Our results demonstrate the importance of examining gene-protein interactions in understanding delirium pathophysiology and underscore one potential shared pathophysiologic mechanism underlying the delirium-AD relationship.
Our previous work did not find that APOE genotype directly affected risk of delirium in this surgical sample free of dementia [32]. However, the current study sheds light on a potential more nuanced role that APOE genotype plays in delirium pathophysiology. Our current findings suggest that some patients (APOE ε4 carriers) may be at greater risk for postoperative delirium under specific circumstances (high postoperative inflammation) than others (APOE non-ε4 carriers), and these patients also experience delirium of greater duration and severity. Our findings further align with the growing literature that patients with more severe delirium are at highest risk for cognitive decline [51], and it adds to the expanding knowledge of the complexity of gene functional networks, including gene-protein interactions [52–54].
Our results align with previous work on gene-protein interactions in delirium and AD. These findings (e.g., [33]) support the notion that delirium pathophysiology is complex and warrants examination beyond direct gene association studies. Separately, work in AD pathophysiology has uncovered complex biological mechanisms of AD by the identification of gene and protein networks contributing to an AD-specific immune-endocrine-neuronal regulatory network [52]. More specifically, in a study examining several serum proteins as potential mediators of the association between APOE and dementia, Royall et al. [53] found CRP to be the only mediator of this relationship following correction for multiple comparisons. Similarly, an APOE x CRP interaction has been reported for cognitive function among post-menopausal women [54] and 4-year decline in cognitive function in community-dwelling older adults [55]. Taken together, our finding of a gene-protein interaction between APOE and CRP on postoperative delirium fit well with the studies of gene-protein interactions on AD.
Importantly and distinctly from previous work, our study examined the indirect effects of APOE by examining gene-protein interactions associated with postoperative delirium, an innovative approach to understanding delirium pathophysiology. More specifically, we explored the role of APOE genotype on delirium within the context of a brain reserve model under conditions of heightened stress/inflammation (as measured by CRP). This model is particularly illuminating as it may provide a means to understand our current findings, as well as previous findings on APOE and delirium. Under conditions of acute stress (surgery) marked by a heightened inflammatory response (high CRP on POD2), older patients with enhanced reserve (APOE non-ε4 carriers) are less susceptible as manifested by lower rates of postoperative delirium. In comparison, older adults with greater vulnerability (APOE ε4 carriers) under these same conditions experience greater delirium incidence, duration, and severity (illustrated in Figure 1).
Figure 1.
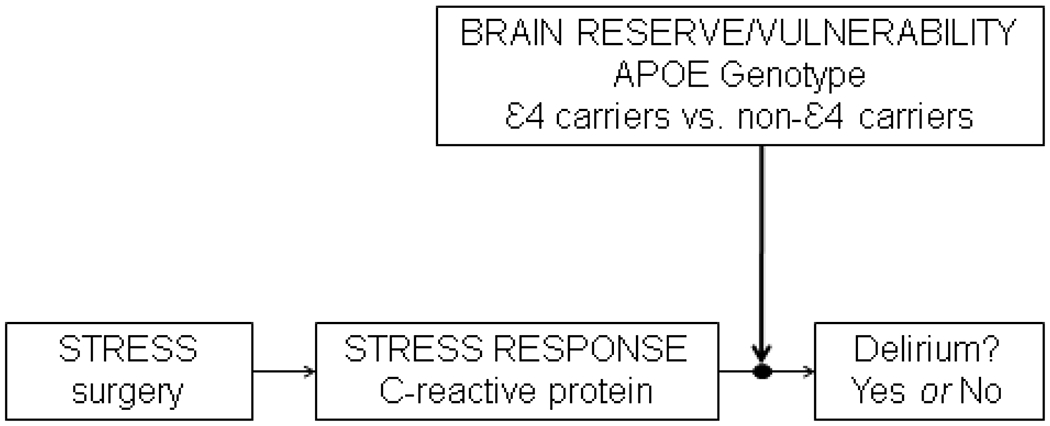
Conceptual model of relationship between Apolipoprotein E, C-reactive protein, and postoperative delirium
APOE ε4 genotype may increase risk of preclinical Alzheimer’s Disease (AD) pathology; however, we are unable to directly test this possibility given the absence of AD biomarkers in the Successful Aging after Elective Surgery (SAGES) Study.
Our finding of lower CRP in APOE ε4 carriers (Supplementary Figure 2) is consistent with reporting across multiple studies of varying populations, including population-based samples in Finnish nonagenarians [56], Germans [57], Icelanders [58], Taiwanese [59], Bolivians [60], and in the U.S. (participants of the Texas Alzheimer’s Research and Care Consortium [53]). Although there are no definitive explanations for this relationship, various mechanisms have been hypothesized. One possible explanation may be that lower CRP levels among APOE ε4 carriers are not causally linked with inflammation, but are attributable to hepatic clearance of CRP with involvement of the mevalonate pathway [56], an important cellular metabolic pathway responsible for a range of functions including the production of cholesterol and growth control.
We highlight several study strengths. SAGES is the first study of postoperative delirium conducted in a large patient sample free of dementia at baseline. Enrollment of patients scheduled for major non-cardiac surgery allowed for rigorous screening of baseline cognitive status in order to exclude patients with evidence of dementia, and to thereby clearly distinguish risk factors for delirium independent of dementia. Another strength includes our use of state-of-the-art delirium measures of incidence, severity, and duration. Additionally, we obtained APOE genotypes and CRP values on >95% of our SAGES sample. Our consistent finding of APOE x CRP interactions for delirium incidence, severity, and duration highlights the robustness of our findings and strengthens our conclusion that the indirect effect of APOE (as opposed to its direct effect) warrants attention and further investigation.
Some study limitations warrant mention. First, our use of a single measure of inflammation (CRP) may not completely capture the entire postoperative inflammatory load experienced following surgery. Nonetheless, we believe CRP is a representative general marker of inflammation based on its widespread clinical use, and our previous identification of CRP as the protein most strongly and consistently associated with postoperative delirium [38,42]. In future work, we intend to explore whether APOE modifies the synergistic effects of multiple inflammatory markers on delirium incidence, severity, and duration. Second, our restricted enrollment of patients without dementia may not be generalizable to the entire older adult population. Although we acknowledge this threat to generalizability, this restriction enabled us to conduct a pristine analysis of how the relationship between postoperative CRP and delirium differs by APOE genotype in the absence of dementia. Third, the unavailability of AD biomarkers limited our ability to identify patients with more brain amyloid pathology, who potentially have an increased vulnerability to the effects of systemic inflammation. It is possible that APOE ε4 carrier status is a proxy for greater likelihood of preclinical AD pathology, an intriguing explanation for our findings that is not directly testable in the SAGES cohort. Our future work will address this limitation with the collection of AD biomarkers in a separate cohort of patients. Fourth, the current work uses a candidate gene approach to evaluate one possible gene-protein interaction in the complex network of delirium pathophysiologic mechanisms. There are thousands of genetic loci not currently considered, which may differ by APOE ε4 carrier status or postoperative delirium status, and may in-part explain our findings. Further work investigating other genetic loci in larger, more diverse study samples is critical to determining the robustness and reproducibility of these initial, preliminary findings. Finally, future studies may benefit from consideration of the added benefit of considering genetic factors, along with demographic variables, in predictive models of postoperative delirium.
In summary, we found that the relationship between CRP and postoperative delirium differs by APOE genotype. Specifically, among APOE ε4 carriers, high CRP was associated with a significantly increased delirium incidence, severity, and duration; however, no such associations were observed among APOE non-ε4 carriers. This suggests that the APOE ε4 allele may be associated with less reserve in the setting of high postoperative inflammation, and thereby increasing risk for delirium. Within the context of delirium and its association with AD, this work is innovative in its expansion from examining direct genetic effects toward examining indirect, gene-protein interactions, which may be more informative of the shared pathophysiology linking delirium and AD. Importantly, this work may inform the targeting of future interventions, such as anti-inflammatory treatments, to those with genetic susceptibility (ε4 carrier status) for prevention of postoperative delirium and its associated adverse long-term cognitive outcomes, including AD.
Supplementary Material
Acknowledgments
Funding:
This research was supported by the National Institute on Aging grants (K01AG057836 [SMV], R03AG061582 [SMV], P01AG031720 [SKI], R24AG054259 [SKI], K07AG041835 [SKI], R21AG057955 [TGF], R01AG041274 [ZX], R21AG048600 [ZX], R01AG051658 [ERM, TAL], and K24AG035075 [ERM]), the Charles A. King Trust Postdoctoral Research Fellowship Program, Bank of America, N.A., Co-Trustee [SMV], and the Alzheimer’s Association (AARF-18-560786 [SMV]). Dr. Inouye holds the Milton and Shirley F. Levy Family Chair. The authors gratefully acknowledge the contributions of the patients, family members, nurses, physicians, staff members, and members of the Executive Committee who participated in the Successful Aging after Elective Surgery (SAGES) Study.
REFERENCES
- [1].Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA 2012;308:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med 2017;377:145–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med 2007;120:807–813. [DOI] [PubMed] [Google Scholar]
- [5].Martin BJ, Buth KJ, Arora RC, Baskett RJ. Delirium as a predictor of sepsis in post-coronary artery bypass grafting patients: a retrospective cohort study. Crit Care 2010;14:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010;304:443–451. [DOI] [PubMed] [Google Scholar]
- [7].Koster S, Hensens AG, van der Palen J. The long-term cognitive and functional outcomes of postoperative delirium after cardiac surgery. Ann Thorac Surg 2009;87:1469–1474. [DOI] [PubMed] [Google Scholar]
- [8].Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc 2000;48:618–624. [DOI] [PubMed] [Google Scholar]
- [9].Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med 2012;367:30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Davis DH, Muniz Terrera G, Keage H, Rahkonen T, Olinas M, Matthews FE, et al. Delirium is a strong risk factor for dementia in the oldest-old: A population-based cohort study. Brain 2012;135:2809–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Marcantonio ER, Goldman L, Mangione CM, Ludwig LE, Muraca B, Haslauer CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA 1994;271:134–139. [PubMed] [Google Scholar]
- [12].Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg 2009;249:173–178. [DOI] [PubMed] [Google Scholar]
- [13].Leslie DL, Inouye SK. The importance of delirium: Economic and societal costs. J Am Geriatr Soc 2011;59:241–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Elie M, Cole MG, Primeau FJ, Bellavance F. Delirium risk factors in elderly hospitalized patients. J Gen Intern Med 1998;13:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Inouye SK, Marcantonio ER, Kosar CM, Tommet D, Schmitt EM, Travison TG, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dementia 2016;12:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fong TG, Jones RN, Shi P, Marcantonio ER, Yap L, Rudolph JL, Yang FM, Kiely DK, Inouye SK. Delirium accelerates cognitive decline in Alzheimer’s disease. Neurology 2009;72:1570–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurol 2015;14:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Weiner MF. Impact of delirium on the course of Alzheimer disease, Arch Neurol 2012;69:1639–1640. [DOI] [PubMed] [Google Scholar]
- [19].Davis DH, Muniz-Terrera G, Keage HA, Stephan BC, Fleming J, Ince PG, et al. Association of delirium with cognitive decline in late life: A neuropathologic study of 3 population-based cohort studies. JAMA Psychiatry 2017;74:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Racine AM, Fong TG, Travison TG, Jones RN, Gou Y, Vasunilashorn SM, Marcantonio ER, Alsop DC, Inouye SK, Dickerson BC. Alzheimer’s-related cortical atrophy is associated with postoperative delirium severity in persons without dementia. Neurobiol Aging 2017;59:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Racine AM, Fong TG, Gou Y, Travison TG, Tommet D, Erickson K, Jones RN, Dickerson BC, Metzger E, Marcantonio ER, Schmitt EM, Inouye SK. Clinical outcomes in older surgical patients with mild cognitive impairment. Alzheimers Dement 2018;14:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Abelha FJ, Fernandes V, Botelho M, Santos P, Santos A, Machado JC, et al. Apolipoprotein E e4 allele does not increase the risk of early postoperative delirium after Major surgery. J Anesth 2012;26:412–421. [DOI] [PubMed] [Google Scholar]
- [23].Adamis D, Treloar A, Martin FC, Gregson N, Hamilton G, Macdonald AJ. APOE and cytokines as biological markers for recovery of prevalent delirium in elderly medical inpatients. Int J Geriatr Psychiatry 2007;22:688–694. [DOI] [PubMed] [Google Scholar]
- [24].Alexander SA, Ren D, Gunn SR, Kochanek PM, Tate J, Ikonomovic M et al. Interleukin 6 and apolipoprotein E as predictors of acute brain dysfunction and survival in critical care patients. Am J Crit Care 2014;23:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bryson GL, Wyand A, Wonzny D, Rees L, Taliaard M, Nathan H. A prospective Cohort study evaluating associations among delirium, postoperative cognitive dysfunction, and apolipoprotein E following open aortic repair. Can J Anaesth 2011;58:246–255. [DOI] [PubMed] [Google Scholar]
- [26].Ely EW, Girard TD, Shintani AK, Jackson JC, Gordon SM, Thomason JW, et al. Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med 2007;35:112–117. [DOI] [PubMed] [Google Scholar]
- [27].Oldenbeuving AW, de Kort PL, Kappelle LF, van Duijn CM, Roks G. Delirium in the acute phase after stroke and the role of the apolipoprotein E gene. Am J Geriatr Psychiatry 2013;21:935–937. [DOI] [PubMed] [Google Scholar]
- [28].Tagarakis GI, Tsolak-Tagaraki F, Tsolaki M, Diegeler A, Tsilimingas NB, Papassotiropoulous A. The role of apolipoprotein E in cognitive decline and delirium after bypass heart operations. Am J Alzheimers Dis Other Demen 2007;22:223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Van Munster BC, Korevaar JC, de Rooij SE, Levi M, Zwinderman AH. The association between delirium and the apolipoprotein E epsilon4 allele in the elderly. Psychiatr Genet 2007;17:261–266. [DOI] [PubMed] [Google Scholar]
- [30].van Munster BC, Korevaar JC, Zwinderman AH, Leeflang MM, de Rooij SE. The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta-analysis. Am J Geriatr Psychiatry 2009;17:856–862. [DOI] [PubMed] [Google Scholar]
- [31].Leung JM, Sands LP, Wang Y, et al. Apolipoprotein E e4 allele increases the risk of early postoperative delirium in older patients undergoing noncardiac surgery. Anesthesiology 2007;107:406–411. [DOI] [PubMed] [Google Scholar]
- [32].Vasunilashorn SM, Ngo L, Kosar CM, Fong TG, Jones RN, Inouye SK, Marcantonio ER. Does Apolipoprotein E genotype increase risk of postoperative delirium? Am J Geriatr Psychiatry 2015;23:1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vasunilashorn SM, Ngo LH, Jones RN, Inouye SK, Hall KT, Gallagher J, et al. The association between C-reactive protein and postoperative delirium differs by catechol-O-methyltransferase genotype. Am J Geriatr Psychiatry 2019;27:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Zhang L, Athale CA, Deisboeck TS. Development of a three-dimensional multiscale agent-based tumor model: Simulating gene-protein interaction profiles, cell phenotypes and multicellular patterns in brain cancer. J Theoretical Biol 2007;244:96–107. [DOI] [PubMed] [Google Scholar]
- [35].Adams J The complexity of gene expression, protein interaction, and cell differentiation. Nature Educ 2008;1:110.Z [Google Scholar]
- [36].Barabási A- L, Oltvai ZN. Network biology: understanding the cell’s functional organization. Nature Rev Genet 2004;5:101–113. [DOI] [PubMed] [Google Scholar]
- [37].Polo A, Marchese S, De Petro G, Montella M, Ciliberto G, Budillon A, Costantini S. Identifying a panel of genes/proteins/miRNAs modeulated by arsenicals in bladder, prostate, kidney cancers, Sci Rep 2018;8:10395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vasunilashorn SM, Dillon ST, Inouye SK, Ngo LH, Fong TG, Jones RN, Travison TG, Schmitt EM, Alsop DC, Freedman SD, Arnold SE, Metzger ED, Libermann TA, Marcantonio ER. High C-reactive protein predicts delirium incidence, duration, and severity after major non-cardiac surgery. J Am Geriatr Soc 2017;65:e109–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schmitt EM, Marcantonio ER, Alsop DC, Jones RN, Rogers SO Jr, Fong TG, et al. Novel risk markers and long-term outcomes of delirium: The Successful Aging after Elective Surgery (SAGES) Study Design and Methods. J Am Med Dir Assoc 2012;13:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schmitt EM, Saczynski JS, Kosar CM, Jones RN, Alsop DC, Fong TG, et al. The Successful Aging after Elective Surgery Study: Cohort description and data quality procedures. J Am Geriatr Soc 2015;63:2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Ciulla TA, Sklar RM, Hauser S. A simple method for DNA purification from periopheral blood. Anal Biochem 1988;174:485–488. [DOI] [PubMed] [Google Scholar]
- [42].Dillon ST, Vasunilashorn SM, Ngo L, Out HH, Inouye SK, Jones RN, et al. Higher C-reactive protein levels predict postoperative delirium in older patients undergoing major elective surgery: A longitudinal nested case-control study. Biol Psychiatry 2017;15:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ridker PM. High-sensitivity C-reactive protein and cardiovascular risk: Rational for screening and primary prevention. Am J Cardiol 2003;92:17K–22K. [DOI] [PubMed] [Google Scholar]
- [44].Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: Validation compared with interviewer ratings using the Confusion Assessment Method. J Am Geriatr Soc 2005; 53:312–318. [DOI] [PubMed] [Google Scholar]
- [45].Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. [DOI] [PubMed] [Google Scholar]
- [46].Kimchi EY, Hshieh TT, Guo R, Wong B, O’Connor M, Marcanotnio ER, et al. Consensus approaches to identify incident dementia in cohort studies: Systematic review and approach in the Successful Aging after Elective Surgery Study. J Am Med Dir Assoc. 2017;18:1010–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Saczynski JS, Kosar CM, Xu G, Puelle MR, Schmitt E, Jones RN, et al. A tale of two methods: chart and interview methods for identifying delirium. J Am Geriatr Soc 2014; 62:518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Inouye SK, Kosar CM, Tommet D, Schmitt EM, Puelle MR, Saczynski JS, et al. The CAM-S: development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med 2014;160:526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Vasunilashorn SM, Marcantonio ER, Gou Y, Pisani MA, Travison TG, Schmitt EM, et al. Quantifying the severity of a delirium episode throughout hospitalization: the combined importance of intensity and duration. J Gen Intern Med 2016;31:1164–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gleason LJ, Schmitt EM, Kosar CM, Tabloski P, Saczynski JS, Robinson T, et al. Effect of delirium and other major complications after elective surgery in older adults. JAMA Surg 2015;150:1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vasunilashorn SM, Fong TG, Albuquerque A, Marcantonio ER, Schmitt EM, Tommet D, Gou Y, Travison TG, Jones RN, Inouye SK. Delirium severity and its relationship with long-term cognitive decline. J Alzheimers Dis 2018;61:347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hu Y- S, Xin J, Hug Y, Zhang L, Wang J. Analyzing the genes related to Alzheimer’s disease via a network and pathway-based approach. Alzheimers Res Therapy 2017;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Royall DR, Al-Rubaye S, Bishnoi R, Palmer RF. Few serum proteins mediate APOE’s association with dementia. PLOS One 2017;12:e0172268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bojar I, Gujski M, Pinkas J, Raczhiewicz D, Owoc A, Humeniuk E. Interaction between C-reactive protein and cognitive functions according to APOE gene polymorphism in post-menopausal women. Arch Med Sci 2016;12:1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lima TAS, Adler AL, Minett T, Matthews FE, Brayne C, Marioni RE. C-reactive protein, APOE genotype and longitudinal cognitive change in an older population. Age Ageing 2014;43:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Rontu R, Ojala P, Hervonen A, et al. Apolipoprotein E genotype is related to plasma levels of C-reactive protein and lipids and to longevity in nonagenarians. Clin Endocrinol 2006;64:265–70. [DOI] [PubMed] [Google Scholar]
- [57].Rontu R, Ojala P, Hervonen A, et al. Apolipoprotein E genotype is related to plasma levels of C-reactive protein and lipids and to longevity in nonagenarians. Clin Endocrinol 2006;64:265–70. [DOI] [PubMed] [Google Scholar]
- [58].Eiriksdottir G, Aspelund T, Bjarnadottir K, et al. Apolipoprotein E genotype and statins affect CRP levels through independent and different mechanisms: AGES Reykjavik Study. Atherosclerosis 2006;186:222–4. [DOI] [PubMed] [Google Scholar]
- [59].Vasunilashorn S, Glei DA, Lan CY, Brookmeyer R, Weinstein M, Goldman N. Apolipoprotein E is associated with blood lipids and inflammation in Taiwanese older adults. Atherosclerosis 2011;219:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Vasunilashorn S, Finch CE, Crimmins EM, Vikman SA, Stieglitz J, Gurven M, et al. Inflammatory gene variants in the Tsimane, an indigenous Bolivian population with a high infectious laod. Biodemog Soc Biol 2011;57:33–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jones RN, Rudolph JL, Inouye SK, Yang FM, Fong TG, Milberg WP, et al. Development of a unidimensional composite measure of neuropsychological functioning in older cardiac surgery patients with good measurement precision. J Clin Exp Neuropsych 2010;32:1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


