Key Points
The NSG/α-Galnull mouse model mimics the human tissues in lacking α-Gal epitopes.
Humanized NSG/α-Galnull mice support the development of functional human B cells.
Humanized NSG/α-Galnull mice secrete human Abs to α-Gal epitopes.
Visual Abstract
Abstract
Mice have been used as accepted tools for investigating complex human diseases and new drug therapies because of their shared genetics and anatomical characteristics with humans. However, the tissues in mice are different from humans in that human cells have a natural mutation in the α1,3 galactosyltransferase (α1,3GT) gene and lack α-Gal epitopes on glycosylated proteins, whereas mice and other nonprimate mammals express this epitope. The lack of α-Gal epitopes in humans results in the loss of immune tolerance to this epitope and production of abundant natural anti-Gal Abs. These natural anti-Gal Abs can be used as an adjuvant to enhance processing of vaccine epitopes to APCs. However, wild-type mice and all existing humanized mouse models cannot be used to test the efficacy of vaccines expressing α-Gal epitopes because they express α-Gal epitopes and lack anti-Gal Abs. Therefore, in an effort to bridge the gap between the mouse models and humans, we developed a new humanized mouse model that mimics humans in that it lacks α-Gal epitopes and secretes human anti-Gal Abs. The new humanized mouse model (Hu-NSG/α-Galnull) is designed to be used for preclinical evaluations of viral and tumor vaccines based on α-Gal epitopes, human-specific immune responses, xenotransplantation studies, and in vivo biomaterials evaluation. To our knowledge, our new Hu-NSG/α-Galnull is the first available humanized mouse model with such features.
Introduction
The α-Gal epitope is a carbohydrate epitope that is synthesized by the α1,3 galactosyltransferases (α1,3GT) enzyme within the Golgi of the cell (1). Humans have a natural mutation in the α1,3GT gene, and therefore they do not express the enzyme or terminal α-Gal epitopes on glycoproteins (2). In addition, the loss of α-Gal epitopes leads to the loss of immune tolerance in humans and results in production of abundant natural anti-Gal Abs (2). In contrast, mice have an intact α1,3GT gene and active α1,3GT enzyme, therefore they express α-Gal epitopes and lack anti-Gal Abs (3).
Studies in animal models have shown that disrupting or knocking out the α1,3GT gene resulted in complete absence of α-Gal epitopes (4–6). The α1,3GT knockout (GTKO) mice lack the α-Gal epitope by targeted disruption of the α1,3GT gene with the neomycin resistance gene (7). In pigs, disruption of the α1,3GT gene locus, mediated by a pPL657 vector (5), results in an inactive enzyme and lack of the α-Gal epitope. Importantly, the production of anti-Gal Abs was demonstrated in both models (8). Despite the fact that these animals allow for analysis of anti-Gal Abs in mediating enhanced immunogenicity, they are limited to analysis of murine or pig immune responses and cannot be used for HIV-1 studies because they cannot be infected with HIV-1 virus.
Therefore, in an effort to develop a mouse model that is suitable for evaluating human immune responses, produces anti-Gal Abs, and can be infected with HIV-1 virus, this study generated a humanized mouse model that carries the features of both the GTKO (7) and NOD.Cg-PrkdcscidIL2Rγtm1Wjll/SzJ strain, which is commonly known as NOD SCID γc−/− (NSG) (9, 10) mice. The NSG mouse combines the features of the NOD/ShiLtJ background with several deficiencies in innate immunity, the SCID, and an IL-2 receptor γ-chain (IL-2Rγ) knockout (9). As a result, the NSG mice lack mature T cells, B cells, functional NK cells, and are also deficient in cytokine signaling. This strain is among the most immunodeficient described to date (11, 12) and permits the most efficient engraftment of normal human CD34+ hematopoietic stem cells (HSCs) (10–13), resulting in long-term reconstitution and differentiation of a functional human immune system with human T cells, B cells, and dendritic cells (11) as well as sustained HIV infection (14, 15).
It is well documented that the most effective way to enhance poorly immunogenic proteins, such as tumor-associated Ags, is by forming an immune complex with their corresponding IgG Ab, leading to effective uptake and presentation by APC (16). There is a strong body of evidence supporting the use of anti-Gal Abs for enhancing vaccine immunogenicity (17–22) and as an endogenous adjuvant for targeting microbial vaccines to APC. Indeed, in these studies, the enhanced immunogenicity was increase up to 100-fold (19); however, the analysis of the immune response was based on the murine immune cells, and the animal model did not allow investigation of human immune responses when anti-Gal Abs were used as adjuvant.
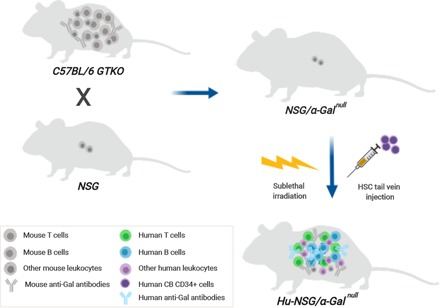
In this study we generated a new humanized mouse model by crossing the GTKO mice with NSG mice. The resulting offspring (following a long breeding regimen) were confirmed to carry the features of both GTKO and NSG mice. This model is of relevance for the investigation of human-specific immune responses when anti-Gal Abs are used as adjuvant. In addition, it is considered to be substantial for HIV-1 studies, as it can be infected with the HIV-1 virus. Furthermore, it can be used in various applications, for example, in preclinical evaluations of human-specific antiviral and antitumor vaccines based on α-Gal epitopes, in xenotransplantation studies, and in in vivo biomaterials evaluation.
Materials and Methods
Generation of NSG GTKO mice
NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (10), commonly known as NOD SCID γc−/− mice), have a mutant Prkdc gene and IL-2Rγ on the NOD background (H-2d, IgHb/IgMb, Ld+) and were obtained from The Jackson Laboratory (005557; Bar Harbor, ME). The GTKO mice were obtained from Colombia University. Animals were maintained in the Tulane Vivarium per Tulane Institutional Animal Care and Use Committee protocol.
Molecular genotyping of mouse strains
Genomic DNA samples were isolated from C57BL/6 GTKO, NSG parental, or NSG/α-Galnull mice splenocytes using the QIAamp DNA Mini Kit (catalog no. 51306; QIAGEN, Germantown, MD) or from ear punches using NaOH lysis buffer (23). The primers for the α1,3GT, Prkdcscid, and IL-2Rγtm1Wjl genes are listed in Table II. For analysis of the α1,3GT gene, the reaction mixture contained 0.5 μM of each primer, 25 μl of TaqMan Universal Master Mix II, no UNG (Life Technologies) and genomic DNA from the ear punch in a final reaction volume of 50 μl. The PCR conditions were 94°C for 3 min, followed by 40 cycles of 94°C for 15 s, 65°C for 15 s, 72°C for 15 s, and a final extension step of 72°C for 10 min. For Prkdcscid and IL-2Rγtm1Wjl amplification, the reaction conditions used 0.2 μM of each primer, 0.2 μM of each probe, 25 μl of TaqMan Universal Master Mix II, no UNG (Life Technologies), and 200 ng of genomic DNA in a final reaction volume of 50 μl. The reaction was initially incubated at 50°C for 2 min, 95°C for 10 min, then cycled 40 times at 95°C for 15 s and 62°C for 1 min. For analysis of the NOD and C57BL/6 genotypes, genomic DNA from NSG/α-Galnull mice (n = 3) was generated from ear punches (23) and was analyzed by 142 single nucleotide polymorphisms (SNPs) to differentiate NOD or C57BL/6 markers (The Jackson Laboratory).
Table II. PCR and real-time PCR primers and probes.
| Name | Sequence |
|---|---|
| α1,3GT gene | |
| GT-NeoR (forward) | 5′-CTATCGCCTTCTTGACGAGTTCTTCTGAGG-3′ |
| GT wild type (reverse) | 5′-CTTGAAAGACTTGATCCACGTCCATGCAG-3′ |
| GT wild type (forward) | 5′-CGTGCACCTGAACCCTCTACATTCCTTAC-3′ |
| Prkdcscid | |
| Forward | 5′-CAGACAATGCTGAGAAAAGGAG-3′ |
| Reverse | 5′-CTGCATTCACAAGTCTTACCAAG-3′ |
| Mutant probe | 5′-[6FAM]TAAAATACGCTAAGCTAAGAGAAAG[BHQ1]-3′ |
| Wild-type probe | 5′-[HEX]TAAAATACGCTATGCTAAGAGAAAG[BHQ1]-3′ |
| IL2Rγ | |
| Forward | 5′-AAGAGATTACTTCTGGCTGTCAG-3′ |
| Mutant reverse | 5′-ATGCTCCAGACTGCCTTG-3′ |
| Mutant probe | 5′-[6FAM]AAAGCGCCTCCCCTACCCG[BHQ1]-3′ |
| Wild-type reverse | 5′-CTCTGGGGTTTCTGGGG-3′ |
| Wild-type probe | 5′-[HEX]TGGAGCTGGACAACAAATGTCTGGT[BHQ1]-3′ |
Human CD34+ cell transplantation
Umbilical cord blood CD34+ cells were either obtained from STEMCELL Technology (Vancouver, BC) or isolated using anti-CD34 IgM, clone 12.8 (a kind gift from Dr. Robert Andrews, Fred Hutchinson Cancer Research Center), and IgM-magnetic beads (Invitrogen). CD34+ cells (1 × 105 cells per mouse) were injected i.v. into sublethally irradiated (350 rad) NSG mice. Blood, spleen, and bone marrow were collected at various times to evaluate human cell engraftment and Ab production in the serum.
Boosting anti-Gal Abs
Production of murine or human anti-Gal Abs was tested before and after human cell engraftment following i.p. immunizations with α-Gal epitopes. Animals were boosted by i.p. immunizations with 50 μl of sheep whole blood lysate (SWBC; Innovative Research, Novi, MI) and 50 μl of IFA (Sigma-Aldrich) mix. These injections were administered in 1-wk intervals.
Immunohistochemistry
For H&E staining, tissues were fixed, embedded in paraffin, and then cut into 10-μm tissue sections. For isolectin immunohistochemical staining, tissue sections were deparaffinized with xylene and rehydrated with ethanol. Ag epitopes were retrieved by incubating in a Dako citrate buffer for 45 s at 123°C, 15 ± 2 pounds per square inch. Endogenous peroxidase activity was inhibited with 1:1 dilution of 3% H2O2 and absolute alcohol for 15 min at room temperature. The slides were washed in running tap water for 5 min and then rinsed in 1× TBS containing 0.1% Tween 20. Sections were then incubated with the Dako Biotin Blocking System (catalog no. X0590). Isolectin IB4 (catalog no. 121414 biotin conjugated; Invitrogen) in a 1:1000 dilution was then added to the tissues for 45 min. Sections were then washed for 10 min with TBS/Tween 20 to remove excess Abs. The sections were then incubated with an avidin–biotin complex (PK-6100; Vector Laboratories, Burlingame, CA) for 30 min and then reincubated with isolectin, as mentioned before, and then treated with Dako’s DAB+ solution for 3–5 min and then washed in running tap water and counter strained with H&E. A confocal laser-scanning imaging system (Leica TCS 4D; Leica Microsystems, Deerfield, IL) was used for capturing the images.
ELISA
The Anti-Alpha-Galactosyl IgG Human ELISA kit (BioVender, Ashville, NC) was used according to manufacture protocol to measure the human α-Gal IgG and was modified to measure mouse α-Gal IgG in sera from humanized NSG mice. Human and murine anti–α-Galactosyl Abs were tested by binding to the terminal β-disaccharide Galα1-3Gal–coated wells with 100 μl of mouse sera or plasma with different dilutions (1:25 up to 1:100). After 1-h incubation followed by multiple washing, 100 μl of the HRP anti-human IgG (conjugated solution) or 100 μl of HRP-conjugated goat anti-mouse IgG (H+L) secondary Ab (Invitrogen) with ratio 1:2000 were added to the wells and incubated for 1 h. After multiple washings, 100 μl of TMB substrate solution was added and incubated for 30 min. The reaction was terminated by adding 100 μl of stop solution, and the absorbance of each well was measured at 450 nm using an Epoch Spectrophotometer from BioTek Instruments (Winoosk, VT). Human plasma (Gulf Coast Regional Blood Center, Houston, TX) and transient supernatant expressing the murine α-Gal Ab M86 VH3-IgG (kindly provided by Dr. James Robinson) were used as the internal standards in each assay.
Immunofluorescence staining and flow cytometry
Single cell suspensions were prepared from the spleen of three different mouse strains: GTKO, NSG parental, and NSG/α-Galnull, and RBCs were lysed by 2 min incubation with ACK lysing buffer (Thermo Fisher Scientific). Splenocytes (at least 1 × 106 cells) were washed with PBS and stained with the corresponding Abs or 0.5 μl of fluorescein-labeled Griffonia simplicifolia lectin I, isolectin B4 (FL-1201; Vector Laboratories) in PBS/BSA for 30 min at 4°C. Cells were then washed twice with PBS and fixed in 300 μl of 1% paraformaldehyde. Relative fluorescence intensity of cells was measured using the LSRFortessa flow cytometer (BD Biosciences, San Jose, CA) and analyzed by using FlowJo software (Tree Star, Ashland, OR).
Abs used for studies
Anti-human Abs used in this study were purchased from BD Horizon and included the following: CD45-allophycocyanin (clone HI30), CD4-BV605 (clone SK3), CD8-BV650 (clone RPA-T8); from Beckman Coulter (Marseille, France) CD20-ECD (clone B9E9) was purchased. Also, we used anti-mouse Abs from BD Horizon, including CD45-PerCP (clone 30-F11), CD3e-V500 (clone 500A2), CD45R/B220-PE (clone RA3-6B2), CD4-BV605 (clone RM4-5), CD8a-BV650 (clone 53-6.7), CD11b-Alexa Fluor 488 (clone M1/70), and IgD-allophycocyanin (clone 11-26c.2a).
Results
Generation of NSG/α-Galnull mouse strain
To mimic the human anti-Gal Ab production, we developed a mouse model that accepts human HSC cell engraftment and is able to secret natural human anti-Gal Abs. Accordingly, we crossed GTKO mice with the immunodeficient NOD, Prkdcscid, and the X-linked IL2Rγnull (NSG) strain. The GTKO mice lack α-Gal epitopes and produce anti-Gal Abs. In vitro fertilization was performed with sperm obtained from GTKO males and ova obtained from NSG females. Offspring were backcrossed to NSG parents. Animals with the Prkdcscid and X-linked IL2Rγnull mutations and that have the disrupted α1,3GT gene were selected for further breeding and genotyping.
Genotyping of the parental mice and the resulting NSG/α-Galnull offspring
To verify mutations in the Prkdcscid, X-linked IL2Rγnull, and α1,3GT genes in the parental and hybrid strains, PCR analysis was performed as described in the Materials and Methods section and in Table I and Table II. We confirmed that the NSG parental mice carry the wild-type α1,3GT gene, whereas the NSG/α-Galnull mice have the mutant Prkdc, IL-2Rγ, and α1,3GT alleles (Table I). Next, we evaluated the relative proportion of C57BL/6 and NOD background present in the genomic DNA by analyzing 142 SNPs in the NSG/α-Galnull mice. We found that the NSG/α-Galnull have an average of 75.5% NSG SNP alleles. Overall, these results demonstrate that the Prkdcscid, X-linked IL2Rγnull, and the mutant allele α1,3GT genes were confirmed in the NSG/α-Galnull mouse and remained fixed in subsequent generations.
Table I. Standard and real-time PCR results for α1,3GT, Prkdcscid, and IL-2Rγtm1Wjl Genes.
| Gene | Allele | C57BL/6 GTKO | NSG Parental | NSG/α-Galnull |
|---|---|---|---|---|
| α1,3GT | NeoR | ++a | −b | ++ |
| Wild-type | − | ++ | − | |
| Prkdcscid | Mutant | − | 25.4 | 25.0 |
| Wild-type | 29.3 | − | − | |
| IL-2Rγtm1Wjl | Mutant | − | 26.8 | 26.6 |
| Wild-type | 27.7 | − | − |
Genomic DNA was isolated from each mouse. Samples were analyzed by standard PCR or real-time PCR for wild-type or mutant alleles. Either the cycle threshold or the amplification efficiency for each reaction is shown.
Samples with detectible PCR amplification of the correct size are shown with “++” sign.
Samples with undetectable signal are shown with a “−” sign.
The NSG/α-Galnull mouse lacks α-Gal epitopes similar to GTKO mouse
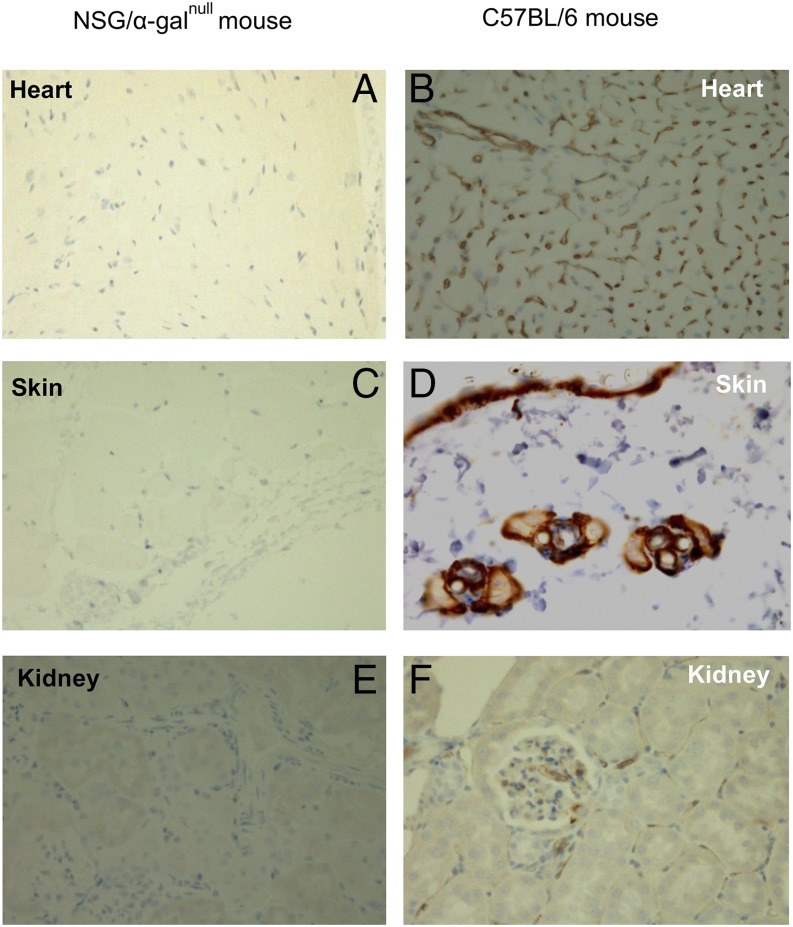
To confirm that the new NSG/α-Galnull mice lack expression of α-Gal epitopes, tissue samples and cells from multiple organs (kidney, skin, and heart) were collected and evaluated for presence or absence of α-Gal epitopes using immunohistochemistry and FACS analysis. Tissue sections from kidney, skin, and heart obtained from NSG/α-Galnull and wild-type mice were embedded in paraffin and then stained with H&E. The samples were also stained with isolectin IB4, which specifically binds α-D-galactosyl residues, and then we examined α-Gal expression using a confocal laser-scanning imaging system. The NSG/α-Galnull mice showed no staining for the α-Gal epitopes (Fig. 1A, 1C, 1E). In contrast, corresponding tissue samples from wild-type C57BL/6 mice clearly showed staining for the α-Gal epitopes (Fig. 1B, 1D, 1F). Similarly, splenocytes were obtained from NSG/α-Galnull, NSG parental, and C57BL/6 GTKO mice for FACS analysis of α-Gal epitope expression. Cells were stained for α-Gal epitopes using a FITC-conjugated lectin Bandeiraea simplicifolia IB4. Only the NSG parental strain expressed the epitope, whereas the NSG/α-Galnull and C57BL/6 GTKO mice lack α-Gal expression (data not shown). These data confirm the absence of α-Gal epitopes in the NSG/α-Galnull mouse.
FIGURE 1.
Immunohistochemical detection of lectin staining of various tissues from NSG/α-Galnull and C57BL/6 mice. Heart tissues (A and B), skin sections (C and D), and kidney sections (E and F) from NSG/α-Galnull and C57BL/6 mice were stained with FITC-conjugated lectin as described in Materials and Methods section. The images were collected at original magnification ×400. All sections from NSG/α-Galnull mice lack α-Gal epitopes.
NSG/α-Galnull mouse lacks murine Gal Ab
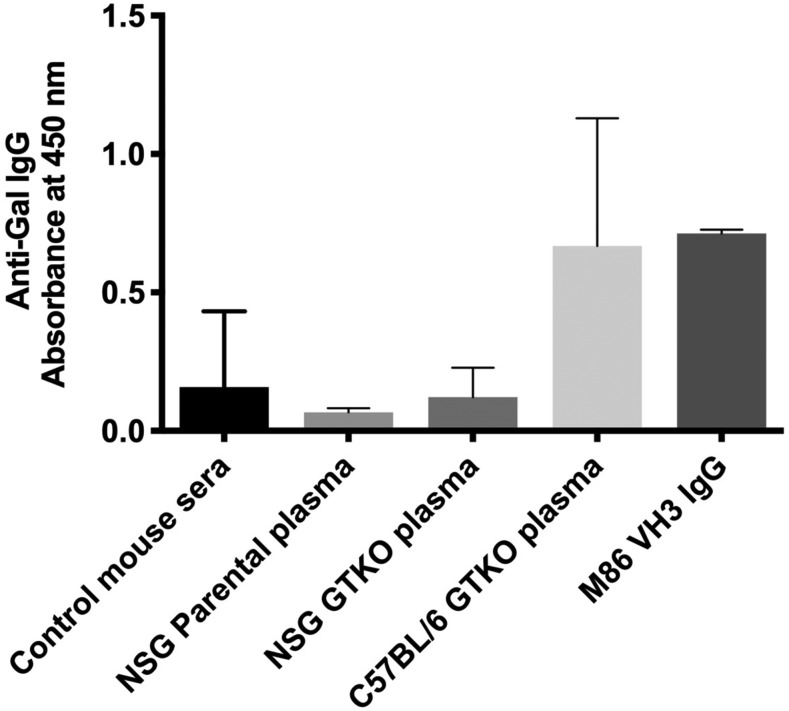
In determining whether the NSG/α-Galnull mice spontaneously produce murine anti-Gal Ab as a result of their nonfunctional α1,3GT gene, blood plasma from C57BL/6 GTKO and NSG/α-Galnull mice were tested for the presence or absence of murine anti-Gal Ab using ELISA. We found that plasma from C57BL/6 GTKO mice displayed modest murine anti-Gal Abs, whereas NSG parental and NSG/α-Galnull mice displayed no anti-Gal Ab (Fig. 2). These data are not surprising because the NSG mice are immunodeficient and have very few B cells.
FIGURE 2.
Production of the murine anti-Gal Abs in NSG parental, C57BL/6 GTKO, and NSG/α-Galnull mice. The production of murine anti-Gal Abs was analyzed by ELISA. Data are represented as mean ± SD of the absorbance.
Boosting effect on murine anti-Gal Ab activity in the NSG parental, C57BL/6 GTKO, and NSG/α-Galnull mouse
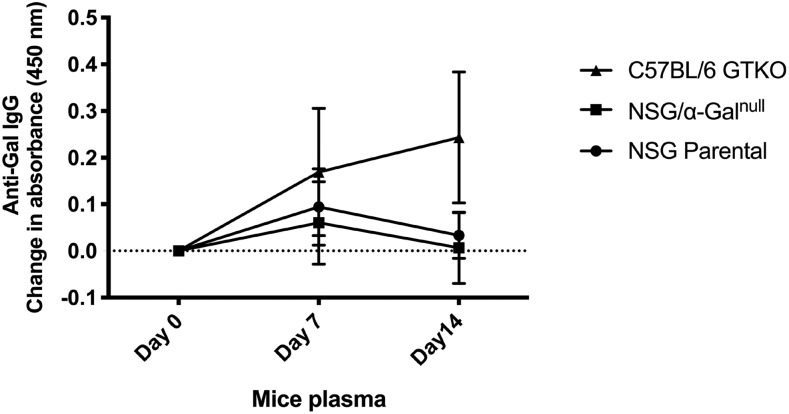
In general, to obtain high levels (similar to that in humans) of anti-Gal Ab secretion in the plasma of GTKO mice, they may require boosting with α-Gal epitopes. Therefore, to determine whether the lack of the murine anti-Gal Ab secretion in NSG/α-Galnull mice was indeed because of the lack of mature B cells and not the level of stimulation, NSG parental, C57BL/6 GTKO, and NSG/α-Galnull mice were immunized three times with SWBC by i.p. injections at multiple intervals. The activity of the murine anti-Gal Ab was monitored by ELISA. As shown in Fig. 3, blood plasma from C57BL/6 GTKO (as positive control) showed a low baseline level of anti-Gal Ab which increased after boosting with SWBC. In contrast, the levels of murine anti-Gal Ab in NSG/α-Galnull mice serum did not increase in response to vaccination as was seen with C57BL/6 GTKO mice. Anti-Gal levels in parental NSG mice remained low both before and after vaccination. These data indicate that an elevated baseline level of murine anti-Gal Ab is seen in the NSG/α-Galnull mice, but the lack of B cells impaired the anti-Gal Ab response to Ag.
FIGURE 3.
Murine anti-Gal Ab levels in serum of C57BL/6 GTKO, NSG parental, and NSG/α-Galnull mice before and after boosting. The production of murine anti-Gal Abs was analyzed by ELISA. Data are represented as mean ± SD of the increase in absorbance 7 and 14 d after boosting with SWBC lysate.
NSG/α-Galnull mice mimic NSG mice lacking both mature B and T cells
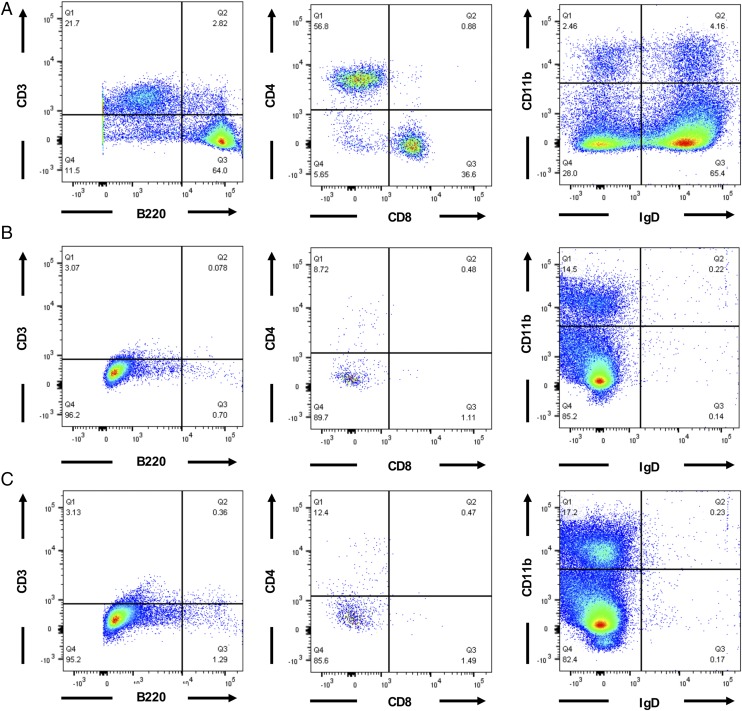
To confirm that the new NSG/α-Galnull mouse strain indeed has impaired immune cells because of the lack of Prkdc and the X-linked IL-2Rγ genes, we analyzed and compared immune cells from NSG parental and NSG/α-Galnull mice to C57BL/6 GTKO mice. Splenocytes were stained with Abs against B220, CD3, CD4, CD8, CD11b, and IgD molecules. Cells were then analyzed using flow cytometry to evaluate the frequency of mature B cells and T cells in our new mouse model compared with the wild-type mice (Fig. 4A). We found that the B cells and CD3+ T cells (both CD4+ and CD8+ cells) were essentially absent in both NSG parental and NSG/α-Galnull mice (Fig. 4B, 4C, respectively). These data explain the impaired ability of the NSG/α-Galnull mice to respond to Ag and to make murine anti-Gal Abs.
FIGURE 4.
Lack of mature IgD+ B cells in NSG/α-Galnull mouse. Flow cytometric analysis of splenocytes from C57BL/6 GTKO (A), NSG parental (B), and NSG/α-Galnull (C) mice. Cells were gated on murine CD45 and stained for murine CD3 and CD20 (left column), gated on CD3+ T cells and stained for CD4 and CD8 (center column), and gated on lymphocytes and stained for CD11b and IgD (right column). The numbers in the quadrants indicate percentages of the respective cell population.
Reconstitution of the NSG/α-Galnull mouse with human stem cells and evaluation of immune cells in the humanized NSG/α-Galnull mouse
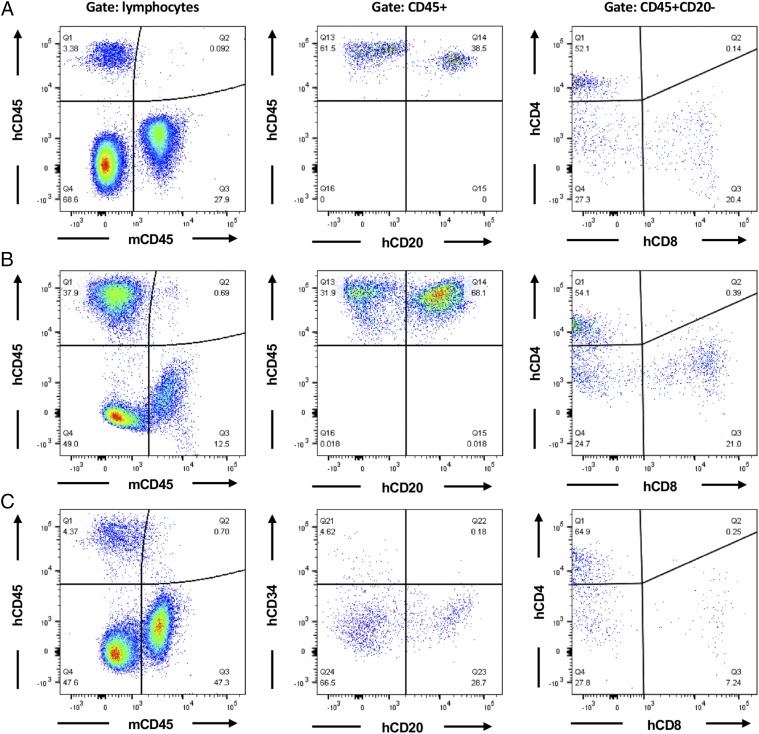
To generate NSG/α-Galnull mice with human immune cells, primary human CD34+ cells (1 × 105 cells) were then injected i.v. into the tail vein of sublethally irradiated NSG/α-Galnull mice at 5–6 wk of age. Engraftment of human immune cells in the blood was followed in NSG/α-Galnull mice at repeated intervals after transplantation. Peripheral blood (PB), spleen, and bone marrow were taken at the final time point for analysis. Reconstitution of immune cells was measured by analyzing the expression of different human immune cell surface markers (CD45, CD4, CD8 and CD20 Abs) in the PB cells (Fig. 5A), spleen (Fig. 5B), and bone marrow (Fig. 5C) by flow cytometry. FACS analysis revealed the presence of human CD45+ lymphocytes, which were composed of human CD20+ B cells and the appropriate ratio (2:1) of CD4/CD8 in the human T cell population up to 30 wk postengraftment (Fig. 5). Even CD34+ HSCs were found in the bone marrow (Fig. 5C). Another interesting observation is that secondary lymphoid organ spleen (Fig. 5B) has much higher engraftment of human CD45+ than is seen in PB (Fig. 5A) or bone marrow (Fig. 5C). These data indicate the successful engraftment of human CD34+ cells and lymphocyte development in the new NSG/α-Galnull mouse model.
FIGURE 5.
Human immune cell reconstitution of NSG/α-Galnull mouse. PBMCs (A), splenocytes (B), and bone marrow (C) were analyzed by FACS staining of the NSG/α-Galnull mouse after engraftment with human CD34+ HSCs. Human CD45 cells versus mouse CD45 cells (left column) and development of human B cells or human CD34+ HSC (center column) and human CD34+ cells (right column) were detected up to 30 wk postengraftment. The numbers in the quadrants indicate percentages of the respective cell population.
Humuanized NSG/α-Galnull mice produce human anti-Gal Abs
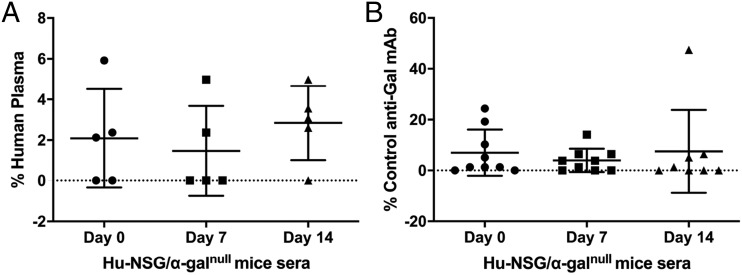
To evaluate the activity of the human anti-Gal Ab in the humanized NSG/α-Galnull mice, sera from Hu-NSG/α-Galnull mice (with demonstrated human cell engraftment) were tested for the presence of human anti-Gal Abs by ELISA. As shown in Fig. 6A, the sera obtained from all humanized NSG/α-Galnull mice were found to contain human anti-Gal Abs; however, they were present at low levels. Next, we boosted the Hu-NSG/α-Galnull mice by i.p. injection of SWBC mixed with IFA to increase the level of the human anti-Gal Ab production. As shown in Fig. 6B, at week 3, one of three mice showed a boosted human anti-Gal response. In contrast, sera from NSG/α-Galnull mice without human cell engraftment did not contain human anti-Gal Abs. These data show proof in principle that human immune reconstitution in NSG/α-Galnull was successful and that human anti-Gal Abs can be produced in the Hu-NSG/α-Galnull mice.
FIGURE 6.
Human anti-Gal Abs were detected in the serum of NSG/α-Galnull mice following human immune reconstitution. Serum from NSG/α-Galnull mice were analyzed for production of human (A) or murine (B) anti-Gal Abs after differentiation of human CD34+ HSCs into T cells and B cells and SWBC boosting. Data are represented as mean ± SD relative to human control serum or the murine α-Gal control Ab M86 VH3 IgG.
Discussion
In this study, we describe the NSG/α-Galnull mouse, which is capable of engrafting human HSCs and can be used for preclinical evaluation of viral and tumor-based α-Gal epitope vaccines. In addition, it could be used in the xenotransplantation studies, in which the binding of anti-Gal Ab to the α-Gal epitope is the main cause of tissue rejection (24–26).
To facilitate analysis of human immune responses to α-Gal epitopes, we crossed the C57BL/6 GTKO mice with the immunodeficient strain NSG mice and verified that the offspring NSG/α-Galnull mice were immunodeficient and lacked the α-Gal epitopes. In this study, we chose the NSG mice because they are known to be excellent hosts for engraftment by human HSCs (13). The NSG mouse combines the features of the NOD/ShiLtJ background with several deficiencies in innate immunity, SCID, and an IL-2Rγ knockout. IL-2Rγ is a cytokine receptor chain shared by multiple cytokines (IL-2, IL-4, IL-7, IL-15, and IL-21) and is required for high-affinity signaling. IL-15 is required for NK cell development and maintenance (27–29), and it has been reported that mutation or deletion of the IL-2Rγ gene results in the absence of NK cells because of the lack of IL-15 signaling (29). NK cells are innate lymphocytes that are specialized in the recognition and elimination of cells that lack expression of MHC class I molecules (30). Human cells are recognized as MHC-negative and eliminated by NK cells. Therefore, the combination of these mutations makes the NSG mouse one of the most permissive hosts for engraftment by human HSCs.
Human cord blood CD34+ HSCs were then engrafted into these mice, and we confirmed successful human HSC engraftment, human T cell and B cell reconstitution, and detected low levels of the human anti-Gal Ab in these humanized NSG/α-Galnull mice. However, the anti–α-Gal response only increased in one of three mice when boosted with a mixture of SWBC with IFA. It is possible that other immunization regimens could be more effective in increasing the human anti-Gal Ab production. For example, using pig kidney membrane for boosting has been reported to be effective (19–22, 31, 32).
Developing a safe and effective adjuvant remains a challenge for vaccine design. Previously, we used the strong binding specificity of the anti-Gal Ab to α-Gal epitope and engineered vaccines that expressed α-Gal epitopes (19–22, 33). Injection of such vaccines into GTKO mice led to strong binding of murine anti-Gal Abs to the vaccines carrying α-Gal epitope and resulted in enhanced Ag processing and presentation. Based on their Fc/FcγR interaction, the anti-Gal–coated immune complexes were effectively internalized by APC, then transported to lymph nodes for enhanced activation of vaccine-specific CD4+ and CD8+ T cells, and thereby increased immunogenicity (21). In addition, we previously reported that intratumoral injection of α-Gal glycolipids induced immune responses that were able to overcome regulatory T cell activity (32), which has recently been reported to be a common feature among several autoimmune diseases (31). In contrast to these murine studies, the new Hu-NSG/α-Galnull mouse model will allow for the evaluation of human-specific humoral and cellular immune responses, including human regulatory T cell development and function, that result from α-Gal epitopes.
Future and ongoing studies may increase engraftment of human HSCs (34) and the subsequent myeloid and lymphoid cells by incorporating the production of human stem cell factor, GM-CSF, and IL-3 present in the NSG-SGM3 mouse strain (35).
In conclusion, by crossing the α1,3GTKO mice with the NSG mice, we have created an immunodeficient mouse strain (NSG/α-Galnull mice) that mimics humans in that it lacks α-Gal epitopes and secretes anti-Gal Abs. In addition, it allows xenografts of components of human cellular immune origin. Genetic and immunohistochemical studies confirmed that the newly generated animal model carries the features of both GTKO and NSG mice. Furthermore, stem cells of human origin transplantation into NSG/α-Galnull mice resulted in human immune cells with both human T and B cells (Hu-NSG/α-Galnull) and production of human anti-Gal Abs. The new Hu-NSG/α-Galnull mouse model can be used in various applications,; for example, it can be used in preclinical evaluations of viral and tumor vaccines based on α-Gal epitopes, in xenotransplantation studies, and in in vivo biomaterials evaluation.
Acknowledgments
We thank Dr. Uri Galili for valuable scientific discussion, Dr. Megan Sykes from Columbia University for the α1,3 galactosyl transferase knockout mice, Drs. Hal E. Broxmeyer and Giao Hangoc (Indiana University School of Medicine) for cord blood samples, and Dr. Robert Andrews (Fred Hutchinson Cancer Research Center) for the anti-CD34 IgM (clone 12.8) antibody. The visual abstract was created using BioRender.
This work was supported by grants from the National Institutes of Health (1R03 AI089352) to U.M.A.-M., the University of Tabuk to F.M.S., the Qatar Foundation, and the Alliance for Cardiovascular Research to S.E.B.
- α1,3GΤ
- α1,3 galactosyltransferase
- GTKO
- 1,3GT knockout
- HSC
- hematopoietic stem cell
- IL-2Rγ
- IL-2 receptor γ-chain
- NSG
- NOD SCID γc−/−
- PB
- peripheral blood
- SNP
- single nucleotide polymorphism
- SWBC
- sheep whole blood lysate.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Taylor S. G., McKenzie I. F., Sandrin M. S. 2003. Characterization of the rat alpha(1,3)galactosyltransferase: evidence for two independent genes encoding glycosyltransferases that synthesize Galalpha(1,3)Gal by two separate glycosylation pathways. Glycobiology 13: 327–337. [DOI] [PubMed] [Google Scholar]
- 2.Galili U. 2015. Significance of the evolutionary α1,3-galactosyltransferase (GGTA1) gene inactivation in preventing extinction of apes and old world monkeys. J. Mol. Evol. 80: 1–9. [DOI] [PubMed] [Google Scholar]
- 3.Koike C., Fung J. J., Geller D. A., Kannagi R., Libert T., Luppi P., Nakashima I., Profozich J., Rudert W., Sharma S. B., et al. 2002. Molecular basis of evolutionary loss of the alpha 1,3-galactosyltransferase gene in higher primates. J. Biol. Chem. 277: 10114–10120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tearle R. G., Tange M. J., Zannettino Z. L., Katerelos M., Shinkel T. A., Van Denderen B. J., Lonie A. J., Lyons I., Nottle M. B., Cox T., et al. 1996. The alpha-1,3-galactosyltransferase knockout mouse. Implications for xenotransplantation. Transplantation 61: 13–19. [DOI] [PubMed] [Google Scholar]
- 5.Dai Y., Vaught T. D., Boone J., Chen S. H., Phelps C. J., Ball S., Monahan J. A., Jobst P. M., McCreath K. J., Lamborn A. E., et al. 2002. Targeted disruption of the alpha1,3-galactosyltransferase gene in cloned pigs. Nat. Biotechnol. 20: 251–255. [DOI] [PubMed] [Google Scholar]
- 6.Phelps C. J., Koike C., Vaught T. D., Boone J., Wells K. D., Chen S. H., Ball S., Specht S. M., Polejaeva I. A., Monahan J. A., et al. 2003. Production of alpha 1,3-galactosyltransferase-deficient pigs. Science 299: 411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thall A. D., Malý P., Lowe J. B. 1995. Oocyte Gal alpha 1,3Gal epitopes implicated in sperm adhesion to the zona pellucida glycoprotein ZP3 are not required for fertilization in the mouse. J. Biol. Chem. 270: 21437–21440. [DOI] [PubMed] [Google Scholar]
- 8.Galili U. 2013. Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology 140: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landel C. P., Dunlap J., Patton J. B., Manser T. 2013. A germline-competent embryonic stem cell line from NOD.Cg-Prkdc ( scid ) Il2rg ( tm1Wjl )/SzJ (NSG) mice. Transgenic Res. 22: 179–185. [DOI] [PubMed] [Google Scholar]
- 10.Shultz L. D., Lyons B. L., Burzenski L. M., Gott B., Chen X., Chaleff S., Kotb M., Gillies S. D., King M., Mangada J., et al. 2005. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J. Immunol. 174: 6477–6489. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa F., Yasukawa M., Lyons B., Yoshida S., Miyamoto T., Yoshimoto G., Watanabe T., Akashi K., Shultz L. D., Harada M. 2005. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor gamma chain(null) mice. Blood 106: 1565–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito M., Hiramatsu H., Kobayashi K., Suzue K., Kawahata M., Hioki K., Ueyama Y., Koyanagi Y., Sugamura K., Tsuji K., et al. 2002. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100: 3175–3182. [DOI] [PubMed] [Google Scholar]
- 13.Shultz L. D., Brehm M. A., Garcia-Martinez J. V., Greiner D. L. 2012. Humanized mice for immune system investigation: progress, promise and challenges. Nat. Rev. Immunol. 12: 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Satheesan S., Li H., Burnett J. C., Takahashi M., Li S., Wu S. X., Synold T. W., Rossi J. J., Zhou J. 2018. HIV replication and latency in a humanized NSG mouse model during suppressive oral combinational antiretroviral therapy. J. Virol. 92: e02118-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim K. C., Choi B. S., Kim K. C., Park K. H., Lee H. J., Cho Y. K., Kim S. I., Kim S. S., Oh Y. K., Kim Y. B. 2016. A simple mouse model for the study of human immunodeficiency virus. AIDS Res. Hum. Retroviruses 32: 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuurhuis D. H., Ioan-Facsinay A., Nagelkerken B., van Schip J. J., Sedlik C., Melief C. J., Verbeek J. S., Ossendorp F. 2002. Antigen-antibody immune complexes empower dendritic cells to efficiently prime specific CD8+ CTL responses in vivo. J. Immunol. 168: 2240–2246. [DOI] [PubMed] [Google Scholar]
- 17.Galili U., LaTemple D. C. 1997. Natural anti-Gal antibody as a universal augmenter of autologous tumor vaccine immunogenicity. Immunol. Today 18: 281–285. [DOI] [PubMed] [Google Scholar]
- 18.LaTemple D. C., Abrams J. T., Zhang S. Y., Galili U. 1999. Increased immunogenicity of tumor vaccines complexed with anti-Gal: studies in knockout mice for alpha1,3galactosyltransferase. Cancer Res. 59: 3417–3423. [PubMed] [Google Scholar]
- 19.Abdel-Motal U., Wang S., Lu S., Wigglesworth K., Galili U. 2006. Increased immunogenicity of human immunodeficiency virus gp120 engineered to express Galalpha1-3Galbeta1-4GlcNAc-R epitopes. J. Virol. 80: 6943–6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdel-Motal U. M., Guay H. M., Wigglesworth K., Welsh R. M., Galili U. 2007. Immunogenicity of influenza virus vaccine is increased by anti-gal-mediated targeting to antigen-presenting cells. J. Virol. 81: 9131–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Motal U. M., Wigglesworth K., Galili U. 2009. Mechanism for increased immunogenicity of vaccines that form in vivo immune complexes with the natural anti-Gal antibody. Vaccine 27: 3072–3082. [DOI] [PubMed] [Google Scholar]
- 22.Abdel-Motal U. M., Wang S., Awad A., Lu S., Wigglesworth K., Galili U. 2010. Increased immunogenicity of HIV-1 p24 and gp120 following immunization with gp120/p24 fusion protein vaccine expressing alpha-gal epitopes. Vaccine 28: 1758–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Truett G. E., Heeger P., Mynatt R. L., Truett A. A., Walker J. A., Warman M. L. 2000. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). Biotechniques 29: 52–54. [DOI] [PubMed] [Google Scholar]
- 24.Jang K. S., Kim Y. G., Adhya M., Park H. M., Kim B. G. 2013. The sweets standing at the borderline between allo- and xenotransplantation. Xenotransplantation 20: 199–208. [DOI] [PubMed] [Google Scholar]
- 25.Sandrin M. S., McKenzie I. F. 1994. Gal alpha (1,3)Gal, the major xenoantigen(s) recognised in pigs by human natural antibodies. Immunol. Rev. 141: 169–190. [DOI] [PubMed] [Google Scholar]
- 26.Oriol R., Ye Y., Koren E., Cooper D. K. 1993. Carbohydrate antigens of pig tissues reacting with human natural antibodies as potential targets for hyperacute vascular rejection in pig-to-man organ xenotransplantation. Transplantation 56: 1433–1442. [DOI] [PubMed] [Google Scholar]
- 27.Lodolce J. P., Boone D. L., Chai S., Swain R. E., Dassopoulos T., Trettin S., Ma A. 1998. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9: 669–676. [DOI] [PubMed] [Google Scholar]
- 28.Carson W. E., Giri J. G., Lindemann M. J., Linett M. L., Ahdieh M., Paxton R., Anderson D., Eisenmann J., Grabstein K., Caligiuri M. A. 1994. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J. Exp. Med. 180: 1395–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kennedy M. K., Glaccum M., Brown S. N., Butz E. A., Viney J. L., Embers M., Matsuki N., Charrier K., Sedger L., Willis C. R., et al. 2000. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191: 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ljunggren H. G., Kärre K. 1990. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol. Today 11: 237–244. [DOI] [PubMed] [Google Scholar]
- 31.Abdel-Motal U. M., Al-Shaibi A., Elawad M., Lo B. 2019. Zero tolerance! A perspective on monogenic disorders with defective regulatory T cells and IBD-like disease. Immunol. Rev. 287: 236–240. [DOI] [PubMed] [Google Scholar]
- 32.Abdel-Motal U. M., Wigglesworth K., Galili U. 2009. Intratumoral injection of alpha-gal glycolipids induces a protective anti-tumor T cell response which overcomes Treg activity. Cancer Immunol. Immunother. 58: 1545–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galili U., Wigglesworth K., Abdel-Motal U. M. 2007. Intratumoral injection of alpha-gal glycolipids induces xenograft-like destruction and conversion of lesions into endogenous vaccines. J. Immunol. 178: 4676–4687. [DOI] [PubMed] [Google Scholar]
- 34.Notta F., Doulatov S., Dick J. E. 2010. Engraftment of human hematopoietic stem cells is more efficient in female NOD/SCID/IL-2Rgc-null recipients. Blood 115: 3704–3707. [DOI] [PubMed] [Google Scholar]
- 35.Coughlan A. M., Harmon C., Whelan S., O’Brien E. C., O’Reilly V. P., Crotty P., Kelly P., Ryan M., Hickey F. B., O’Farrelly C., Little M. A. 2016. Myeloid engraftment in humanized mice: impact of granulocyte-colony stimulating factor treatment and transgenic mouse strain. Stem Cells Dev. 25: 530–541. [DOI] [PubMed] [Google Scholar]