Abstract
Background
It is possible that oestrogen deficiency may be an aetiological factor in the development of urinary incontinence in women. This is an update of a Cochrane review first published in 2003 and subsequently updated in 2009.
Objectives
To assess the effects of local and systemic oestrogens used for the treatment of urinary incontinence.
Search methods
We searched the Cochrane Incontinence Group Specialised Register of trials (searched 21 June 2012) which includes searches of MEDLINE, the Cochrane Central Register of Controlled Trials (CENTRAL) and handsearching of journals and conference proceedings, and the reference lists of relevant articles.
Selection criteria
Randomised or quasi‐randomised controlled trials that included oestrogens in at least one arm in women with symptomatic or urodynamic diagnoses of stress, urgency or mixed urinary incontinence or other urinary symptoms post‐menopause.
Data collection and analysis
Trials were evaluated for risk of bias and appropriateness for inclusion by the review authors. Data were extracted by at least two authors and cross checked. Subgroup analyses were performed by grouping participants under local or systemic administration. Where appropriate, meta‐analysis was undertaken.
Main results
Thirty‐four trials were identified which included approximately 19,676 incontinent women of whom 9599 received oestrogen therapy (1464 involved in trials of local vaginal oestrogen administration). Sample sizes of the studies ranged from 16 to 16,117 women. The trials used varying combinations of type of oestrogen, dose, duration of treatment and length of follow up. Outcome data were not reported consistently and were available for only a minority of outcomes.
The combined result of six trials of systemic administration (of oral systemic oestrogens) resulted in worse incontinence than on placebo (risk ratio (RR) 1.32, 95% CI 1.17 to 1.48). This result was heavily weighted by a subgroup of women from the Hendrix trial, which had large numbers of participants and a longer follow up of one year. All of the women had had a hysterectomy and the treatment used was conjugated equine oestrogen. The result for women with an intact uterus where oestrogen and progestogen were combined also showed a statistically significant worsening of incontinence (RR 1.11, 95% CI 1.04 to 1.18).
There was some evidence that oestrogens used locally (for example vaginal creams or pessaries) may improve incontinence (RR 0.74, 95% CI 0.64 to 0.86). Overall, there were around one to two fewer voids in 24 hours amongst women treated with local oestrogen, and there was less frequency and urgency. No serious adverse events were reported although some women experienced vaginal spotting, breast tenderness or nausea.
Women who were continent and received systemic oestrogen replacement, with or without progestogens, for reasons other than urinary incontinence were more likely to report the development of new urinary incontinence in one large study.
One small trial showed that women were more likely to have an improvement in incontinence after pelvic floor muscle training (PFMT) than with local oestrogen therapy (RR 2.30, 95% CI 1.50 to 3.52).
The data were too few to address questions about oestrogens compared with or in combination with other treatments, different types of oestrogen or different modes of delivery.
Authors' conclusions
Urinary incontinence may be improved with the use of local oestrogen treatment. However, there was little evidence from the trials on the period after oestrogen treatment had finished and no information about the long‐term effects of this therapy was given. Conversely, systemic hormone replacement therapy using conjugated equine oestrogen may worsen incontinence. There were too few data to reliably address other aspects of oestrogen therapy, such as oestrogen type and dose, and no direct evidence comparing routes of administration. The risk of endometrial and breast cancer after long‐term use of systemic oestrogen suggests that treatment should be for limited periods, especially in those women with an intact uterus.
Keywords: Female; Humans; Postmenopause; Estrogen Replacement Therapy; Estrogen Replacement Therapy/adverse effects; Estrogens; Estrogens/adverse effects; Estrogens/therapeutic use; Randomized Controlled Trials as Topic; Urinary Incontinence; Urinary Incontinence/chemically induced; Urinary Incontinence/drug therapy; Urinary Incontinence, Stress; Urinary Incontinence, Stress/chemically induced; Urinary Incontinence, Stress/drug therapy
Plain language summary
Oestrogens for urinary incontinence in women
Urinary incontinence is the leakage of urine when coughing or exercising (stress urinary incontinence) or after a strong uncontrollable urge to urinate (urgency urinary incontinence). In women who have gone through the menopause, low oestrogen levels may contribute to urinary incontinence. The review found 34 trials including more than 19,000 women of whom over 9000 received oestrogen. The review found that significantly more women who received local (vaginal) oestrogen for incontinence reported that their symptoms improved compared to placebo. There was no evidence about whether the benefits of local oestrogen continue after stopping treatment but this seems unlikely as women would revert to having naturally low oestrogen levels. Trials investigating systemic (oral) administration, on the other hand, found that women reported worsening of their urinary symptoms. The evidence comes mainly from two very large trials including 17,642 incontinent women. These trials were investigating other effects of hormone replacement therapy as well as incontinence, such as prevention of heart attacks in women with coronary heart disease, bone fractures, breast and colorectal cancer. In addition, in one large trial women who did not have incontinence at first were more likely to develop incontinence. There may be risks from long‐term use of systemic oestrogen, such as heart disease, stroke and cancer of the breast and uterus.
Summary of findings
Summary of findings for the main comparison. Oestrogen compared to placebo or no treatment for urinary incontinence in post‐menopausal women.
| Oestrogen compared to placebo or no treatment for urinary incontinence in post‐menopausal women | ||||||
| Patient or population: patients with urinary incontinence in post‐menopausal women Settings: Intervention: Oestrogen Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment | Oestrogen | |||||
| Incontinence not improved (generic inverse variance) (women's observations) ‐ Systemic administration (any incontinence) | 640 per 1000 | 845 per 1000 (749 to 948) | RR 1.32 (1.17 to 1.48) | 6151 (6 studies) | ⊕⊕⊝⊝ low1 | |
| Incontinence not improved (generic inverse variance) (women's observations) ‐ Local administration (any incontinence) | 888 per 1000 | 657 per 1000 (568 to 764) | RR 0.74 (0.64 to 0.86) | 213 (4 studies) | ⊕⊝⊝⊝ very low2,3 | |
| Incontinent episodes over 24 hours ‐ Systemic administration | The mean incontinent episodes over 24 hours ‐ systemic administration in the intervention groups was 0.54 higher (0.5 lower to 1.57 higher) | 82 (2 studies) | ⊕⊕⊝⊝ low | |||
| Number of women with urgency ‐ Systemic administration | Study population | RR 1.05 (0.83 to 1.33) | 89 (2 studies) | ⊕⊕⊕⊝ moderate4 | ||
| 750 per 1000 | 788 per 1000 (622 to 998) | |||||
| Moderate | ||||||
| 767 per 1000 | 805 per 1000 (637 to 1000) | |||||
| Number of women with urgency ‐ Local administration | Study population | RR 0.38 (0.15 to 0.99) | 90 (2 studies) | ⊕⊕⊕⊝ moderate5 | ||
| 289 per 1000 | 110 per 1000 (43 to 286) | |||||
| Moderate | ||||||
| 290 per 1000 | 110 per 1000 (44 to 287) | |||||
| Number with adverse effects ‐ Systemic administration | Study population | RR 13 (1.87 to 90.21) | 40 (1 study) | ⊕⊕⊝⊝ low6 | ||
| 50 per 1000 | 650 per 1000 (94 to 1000) | |||||
| Moderate | ||||||
| 50 per 1000 | 650 per 1000 (94 to 1000) | |||||
| Number with adverse effects ‐ Local administration | Study population | RR 1.33 (0.32 to 5.61) | 144 (2 studies) | ⊕⊕⊕⊝ moderate7 | ||
| 42 per 1000 | 55 per 1000 (13 to 234) | |||||
| Moderate | ||||||
| 34 per 1000 | 45 per 1000 (11 to 191) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Overall pooled effect is driven by one very large study (Hendrix) 2 High risk of bias in Henalla trial 3 P value for heterogeneity is <0.0003; I2 = 84% 4 P = 0.22 for heterogeneity (women with urgency ‐ systemic) 5 P = 0.09 for heterogeneity , I2 = 64% (women with urgency ‐ local) 6 Wide CI for Rufford trial (adverse effects for systemic) 7 Wide CI for pooled estimate (adverse effects of local)
Summary of findings 2. Oestrogen versus other treatments for urinary incontinence in post‐menopausal women.
| Oestrogen versus other treatments for urinary incontinence in post‐menopausal women | ||||||
| Patient or population: patients with urinary incontinence in post‐menopausal women Settings: Intervention: Oestrogen versus other treatments | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oestrogen versus other treatments | |||||
| Number with incontinence not improved (women's observations) ‐ Oestrogen vs PPA | Study population | RR 0.83 (0.63 to 1.09) | 57 (2 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 833 per 1000 | 692 per 1000 (525 to 908) | |||||
| Moderate | ||||||
| 867 per 1000 | 720 per 1000 (546 to 945) | |||||
| Adverse effects ‐ oestrogen vs PPA | See comment | See comment | Not estimable | 30 (1 study) | ⊕⊕⊝⊝ low2 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Relative risk reduction greater than 25% in pooled estimate 2 One trial (Hilton) measured outcome, confidence interval crosses line of no effect
Summary of findings 3. Oestrogen + other treatments versus placebo for urinary incontinence in post‐menopausal women.
| Oestrogen + other treatments versus placebo for urinary incontinence in post‐menopausal women | ||||||
| Patient or population: patients with urinary incontinence in post‐menopausal women Settings: Intervention: Oestrogen + other treatments versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Oestrogen + other treatments versus placebo | |||||
| Incontinent episodes over 24 hours ‐ oestrogen + progesterone vs placebo | The mean incontinent episodes over 24 hours ‐ oestrogen + progesterone vs placebo in the intervention groups was 0.43 lower (1.17 lower to 0.31 higher) | 83 (1 study) | ⊕⊕⊕⊝ moderate1 | |||
| Incontinence not improved (generic inverse variance) (women's observation) ‐ oestrogen + other treatments versus placebo | 663 per 1000 | 736 per 1000 (689 to 782) | RR 1.11 (1.04 to 1.18) | 10,635 (3 studies) | ⊕⊕⊕⊝ moderate2 | |
| Number with incontinence not improved (women's observations) ‐ oestrogen + progesterone vs placebo (Copy) | Study population | RR 1.08 (1.01 to 1.16) | 1514 (2 studies) | ⊕⊕⊕⊕ high | ||
| 663 per 1000 | 716 per 1000 (669 to 769) | |||||
| Moderate | ||||||
| 608 per 1000 | 657 per 1000 (614 to 705) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Confidence interval crosses line of no effect (‐0.20, 0.48) 2 Confidence interval of Fantl trial crosses line of no effect
Background
There are three major naturally occurring oestrogens in women (oestradiol, oestriol, oestrone). It is possible that oestrogen deficiency, for example after the menopause, may be an aetiological factor in the development of urinary incontinence in women. However, it is not known whether oestrogen replacement will help in the treatment of this condition.
Description of the condition
Urinary incontinence is a common and embarrassing problem which may affect women of all ages. Although not life threatening, incontinence can lead to loss of confidence and in some cases social isolation. Urinary incontinence is the complaint of any involuntary leakage of urine (Haylen 2010). Although treatment may be started based on symptoms alone, investigations may be used to accurately diagnose the underlying cause of the incontinence. The value of urodynamics in the management of urinary incontinence has yet to be established (Glazener 2012).
Stress urinary incontinence (SUI), which is the symptom of involuntary loss of urine associated with coughing, sneezing or physical activity, is the most common type (Haylen 2010). Urgency urinary incontinence (UUI) is the involuntary loss of urine accompanied by or immediately preceded by urgency, which is a sudden compelling desire to pass urine that is difficult to defer. Overactive bladder (OAB) syndrome is diagnosed when women have urinary urgency, usually accompanied by frequency and nocturia, with or without UUI, and in the absence of other pathology such as urinary tract infection (UTI) (Haylen 2010). These symptoms may also occur when the bladder is overactive, known as detrusor overactivity (DO), when it is diagnosed using urodynamics. Urodynamic stress incontinence (USI) is diagnosed when stress incontinence is confirmed in the absence of DO, using urodynamic studies. However, many women have a combination of problems including urinary frequency, urgency, and urgency urinary incontinence. Mixed urinary incontinence (MUI) is diagnosed when SUI and UUI, or SUI and DO, co‐exist.
Early studies of oestrogen therapy for incontinence were performed before the widespread use of urodynamic investigation. Therefore, it is likely that those earlier studies included a heterogeneous group of women, some of whom may have had a number of different types of urinary symptoms such as overactive bladder syndrome (OAB) or voiding difficulties (VD) in addition to incontinence.
Description of the intervention
Oestrogen can be given to post‐menopausal women to prevent osteoporosis as well as treat symptoms of menopause, such as hot flushes, vaginal dryness, fatigue, irritability, sweating and incontinence. Synthetic oestrogen can be given using a variety of different doses, routes and modes of delivery or formulations (oral or vaginal tablet, cream, skin patch, subcutaneous implant). Routes include systemic, such as oral and transdermal; or local, including vaginal and into the bladder (intravesical). Duration of therapy and assessment of effect also vary widely. The situation may be confounded by the concurrent use of progestogens to prevent endometrial hyperplasia in women who have not had a hysterectomy, which may themselves exacerbate both irritative urinary symptoms and incontinence (Benness 1991).
How the intervention might work
The female genital and urinary tracts both arise from the primitive urogenital sinus and develop in close anatomical proximity. Oestrogen receptors have been identified in the tissues of the vagina, bladder, urethra and muscles of the pelvic floor (Blakeman 1996; Iosif 1981; Smith 1993). Sex hormones such as oestrogen have a substantial influence on the female bladder throughout adult life with fluctuations in their levels leading to macroscopic, histological and functional changes. Urinary symptoms may therefore develop during the menstrual cycle, in pregnancy and following the menopause (Barlow 1997; Cutner 1992; Van Geelen 1981).
As the tissues involved in the female continence mechanism are oestrogen sensitive, it is possible that oestrogen deficiency may be an aetiological factor in the development of urinary incontinence. Epidemiological surveys have shown that the peak prevalence of stress incontinence occurs around the time of the natural menopause (Jolleys 1988; Kondo 1990; Thomas 1980). In addition, 70% of incontinent post‐menopausal women have been reported to relate the onset of their incontinence to the time of their final menstrual period (Iosif 1984). On the other hand, many studies show that more pre‐menopausal women are affected by incontinence than post‐menopausal women, with the prevalence of stress incontinence actually falling following the menopause (Hannestad 2000).
Why it is important to do this review
A variety of other options are available for the treatment of incontinence, including pelvic floor muscle training (Dumoulin 2010; Boyle 2012; Herbison 2002; Kovoor 2008; Patel 2008), vaginal ring pessaries (Lipp 2011), a number of different types of medication (Alhasso 2005; Madhuvrata 2012; Nabi 2006; Roxburgh 2007) and surgery (Dean 2006; Glazener 2001; Glazener 2004; Kirchin 2012; Lapitan 2012; Liapis 2010 L; Ogah 2009; Rehman 2011). The female hormone oestrogen has been used to treat incontinence over a number of years, either alone or in combination with some of these other options. However, most trials involve only a small number of women and few have attempted long‐term follow up. Two very large trials of women receiving hormone replacement therapy have been published showing a negative effect of hormones on incontinence (Grady 2001 S; Hendrix 2005 S). This review will consider all of the evidence regarding oestrogen therapy as a treatment for incontinence.
Objectives
To assess the effects (both beneficial and harmful) of oestrogen therapy used for the treatment of urinary incontinence.
The following comparisons were made.
1. Oestrogen therapy versus placebo or no treatment for urinary incontinence.
2. Oestrogen therapy versus other forms of treatment (e.g. physical, drugs, surgery) for urinary incontinence.
3. Oestrogen combined with other therapy versus placebo or no treatment for urinary incontinence.
4. Oestrogen given in combination with another treatment versus oestrogen given alone for urinary incontinence.
5. Oestrogen given in combination with another treatment versus that other treatment given alone for urinary incontinence.
6. One type of oestrogen versus another.
7. One method of administration of oestrogen versus another.
8. A high dose of oestrogen versus a lower dose. Subgroup analysis was performed according to the route of administration (systemic or local).
Methods
Criteria for considering studies for this review
Types of studies
All randomised or quasi‐randomised controlled trials of oestrogen therapy for the treatment of urinary stress, urgency or mixed incontinence.
Types of participants
Post‐menopausal women with urinary incontinence and diagnosed as having urinary stress, urgency or mixed incontinence either by symptom classification or by urodynamic diagnosis, as defined by the trialists.
Types of interventions
a) Oestrogen therapy, which includes different types of oestrogens, different doses and different routes of administration.
b) Comparators: no therapy, placebo, or non‐oestrogen therapies (which include physical, pessary, drugs and surgery).
Types of outcome measures
Primary outcomes
1. Women's observations
Continuing incontinence or lack of improvement in incontinence
Secondary outcomes
2. Quantification of symptoms
Pad changes over 24 hours (self‐reported number of pads used)
Incontinent episodes per 24 hours (as indicated from self‐completed bladder chart)
Frequency (micturitions per 24 hours)
Pad tests of quantified leakage (weight of urine loss)
Urgency
3. Clinician's measures (urodynamics or cystometry)
Maximum urethral closure pressure (MUCP) (cm H²O)
Volume at first urge to void (ml)
Maximum bladder capacity (ml)
4. Quality of life
Severity of incontinence e.g. index score: slight, moderate or severe (Sandvik 1993)
Impact of incontinence on quality of life e.g. Urogenital distress Inventory (Shumaker 1994), Disease Specific Quality of Life Questionnaire (Jackson 1996; Kelleher 1997)
Psychological measures e.g. Crown‐Crisp Experimental Index (Crown 1979)
General health status e.g. Short Form‐36 (Ware 1993)
5. Socioeconomics
Costs of intervention(s)
Resource implications of differences in outcomes
Formal economic analysis (cost effectiveness, cost utility)
6. Adverse outcomes
Vaginal bleeding
Uterine cancer
Cardiovascular disease e.g. stroke, heart disease
Breast cancer
7. Other outcomes
Non‐prespecified outcomes that were judged important when performing the review
Search methods for identification of studies
Limitations such as different languages were not imposed on any of the searches described below.
Electronic searches
This review has drawn on the search strategy developed for the Cochrane Incontinence Review Group. Relevant trials were identified from the Cochrane Incontinence Group Specialised Register of controlled trials, which is described under the Incontinence Group's module in The Cochrane Library. The register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE, CINAHL and handsearching of journals and conference proceedings. The date of the most recent search of the register for this review was 21 June 2012.
The trials in the Cochrane Incontinence Group Specialised Register are also contained in CENTRAL. The terms used to search the Incontinence Group Specialised Register are given below: ({DESIGN.CCT*} OR {DESIGN.RCT*}) AND ({INTVENT.CHEM.HORM*} OR {INTVENT.CHEM.DRUG.ESTROGEN CREAM.} OR {INTVENT.CHEM.DRUG.MESTINON.} OR {INTVENT.CHEM.DRUG.MISOPROSTOL} OR {INTVENT.CHEM.DRUG.NORETHANDROLONE} OR {INTVENT.CHEM.DRUG.TAMOXIFEN.}) (all searches were of the keywords field of Reference Manager 12, Thomson Reuters).
Searching other resources
The review authors also searched the reference lists of relevant articles.
Data collection and analysis
Selection of studies
Trials under consideration were evaluated for risk of bias and appropriateness for inclusion by at least two review authors, without prior consideration of the results. Any disagreements were resolved by discussion or with a third party. Assessment of risk of bias was undertaken by at least two authors using the Cochrane Collaboration's assessment criteria, which include quality of random allocation concealment, description of dropouts and withdrawals, analysis on an intent to treat, and 'blinding' at treatment and outcome assessment. Again, any disagreements were resolved by discussion or with a third party.
Data extraction and management
Data extraction was undertaken independently by all three review authors. Where data may have been collected in a study but was not reported, further clarification was sought from the trialists. Trial data were analysed according to the treatment or type of intervention compared and grouped, if possible, by route of administration (systemic or local). Any differences of opinion related to the data extracted were resolved by discussion or with a third party.
Assessment of risk of bias in included studies
Studies were excluded if they were not randomised or quasi‐randomised controlled trials, or if they made comparisons other than those specified for the review. These studies are listed in the Characteristics of excluded studies table.
Measures of treatment effect
Included trial data were processed as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When appropriate, meta‐analysis was undertaken of the parallel group studies. For categorical outcomes the numbers in each group reporting an outcome were related to the numbers at risk to derive a risk ratio (RR), where sufficient data to calculate an RR were available. The data were combined using the Mantel‐Haenszel method. For continuous variables we used means and standard deviations to derive a mean difference (MD). When summary data were not available from all trials for a particular outcome and only adjusted risk ratios were reported, the generic inverse variance method was used to combine risk ratios. A fixed‐effect model was used for calculation of pooled estimates and their 95% confidence intervals (CIs). Data from these trials were presented in 'Other data' tables only.
Assessment of heterogeneity
Trials were only combined if the interventions were similar enough based on clinical criteria (types of interventions). Differences between trials were investigated if significant heterogeneity was found at the statistical significance level of 10% or by using the I2 statistic (Higgins 2003), or it appeared obvious from visual inspection of the results. If there was no obvious reason for the heterogeneity, use of a random‐effects model was considered. If a reason was found then a judgement was made as to whether it was reasonable to combine the results.
Subgroup analysis and investigation of heterogeneity
Trial data were grouped by route of administration (systemic or local), where possible. The suffix S or L was used to denote systemic or local administration, respectively. Further details on type of oestrogen, dose, length of treatment and population (type of urinary incontinence, menopausal status) are given in Table 4.
1. Summary of study characteristics: type of oestrogen, route of administration, dose, length of treatment, population.
| Study ID | Type of Oestrogen | Route of administration | Dose | Length of treatment | Population | |
| Ahlstrom 1990 S | Oestriol | Systemic (oral) | 4mg | 6 weeks | USI (stress), postmenopausal | |
| Assassa 2003 L | Oestrogen | Local (vaginal ring) | ? | 3 months | UI, postmenopausal | |
| Beisland 1984 L | Oestriol | Local (vaginal) | 1 mg | 8 weeks | UI (stress), postmenopausal | |
| Blom 1995 S | Estradiol | Systemic (transdermal) | 0.05 mg | 8 weeks | UUI (OAB), elderly | |
| Cardozo 1993 S | Oestriol | Systemic (oral) | 3 mg | 3 months | UUI (urge), postmenopausal | |
| Cardozo 2001 L | Oestradiol | Vaginal (pessary, Vagifem) | 25 µgm | 3 months | OAB (urge), postmenopausal | |
| Dessole 2004 L | Oestriol | Local (intravaginal ovules) | 1‐2 mg | 6 months | UI (stress), postmenopausal | |
| Ek 1980 S | Oestradiol | Systemic (oral) | 1 mg | 6 weeks | USI (stress), postmenopausal | |
| Enzelsberger 1990 L | Oestriol | Local (vaginal) | 0.5, 1 or 2 mg | Not stated | UI (urge), OAB | |
| Enzelsberger 1991a L; Enzelsberger 1991b L | Oestriol | Local (vaginal) | 1 mg or 3 mg | 3 weeks | UI (urge), OAB, postmenopausal | |
| Fantl 1996 S | Conjugated equine oestrogens + medroxyprogesterone | Systemic (oral) | 0.625 mg / 10 mg | 3 months | UI (stress), postmenopausal | |
| Grady 2001 S | Conjugated oestrogens (premarin) + medroxyprogesterone | Systemic (oral) | 0.625 mg / 2.5 mg | up to 4 years | UI (unspecified), postmenopausal, heart disease, age <80 years, with uterus | |
| Henalla 1989 L | Conjugated equine oestrogen | Local (vaginal cream) | 1.25 mg | 3 months | USI (stress) | |
| Henalla 1990 L | Conjugated equine oestrogen | Local (vaginal cream) | 2 gm | 6 weeks | USI (stress), postmenopausal | |
| Hendrix (hysterectomy) S | Conjugated equine oestrogen | Systemic (oral) | 0.625 mg | 1 year | UI (SUI, UUI, MUI), postmenopausal, prevention of heart disease and hip fracture Without uterus |
|
| Hendrix (no hysterect) S | Conjugated equine oestrogen + medroxyprogesterone | Systemic (oral) | 0.625 mg / 2.5 mg | 1 year | UI (SUI, UUI, MUI), postmenopausal, prevention of heart disease and hip fracture With uterus |
|
| Hilton 1990 SL | Oestrogen | Local (intravaginal) Systemic (oral) |
2 gm 1.25 mg |
1 month | USI (stress), postmenopausal | |
| Ishiko 2001 S | Estriol | Systemic (oral) | 1 mg | 2 years | SUI (stress), postmenopausal | |
| Jackson 1999 S | Oestradiol | Systemic (oral) | 2 mg | 6 months | USI (stress), postmenopausal | |
| Judge 1969 S | Quinestradiol | Not stated | 1 mg | 1 month | UI (unspecified), postmenopausal, geriatric inpatients | |
| Kinn 1988 S | Oestriol | Systemic (oral) | 4 mg | 1 month | USI (stress), postmenopausal | |
| Kurz 1993 L | Oestriol | Local (intravesical) | 1 mg | 3 weeks | UUI, OAB (urge), postmenopausal | |
| Liapis 2010 L | Oestradiol | Local (intravaginal tablets) | 25 mcg | 6 months | SUI | |
| Lose 2000 L | Oestradiol | Local (intravaginal ring, vaginal pessaries) | ring 7.5mg, pessary 0.5 mg | 6 months | UI (unspecified), LUT symptoms, postmenopausal | |
| Melis 1997 L | Oestriol | Local (intravaginal) | 0.5 mg | 3 months | UI, menopausal symptoms | |
| Ouslander 2001 S | Oestrogen + progesterone | Systemic (oral) | 0.625 mg / 2.5 mg | 6 months | UI (unspecified), nursing home residents | |
| Rufford 2003 S | 17‐beta oestradiol | Systemic (implant) | 25 mg | 6 months | UUI, OAB (urge), postmenopausal | |
| Sacco 1990 L | Oestrogen | Local (cream) | 0.5 mg | 3 months | USI (stress), postmenopausal | |
| Samsioe 1985 S | Oestriol | Systemic (oral) | 3 mg | 3 months | MUI (mixture), postmenopausal | |
| Tinelli 2007 L | Oestradiol | Systemic (oral) | 10 mg | 12 months | SUI | |
| Tseng 2007 L | Oestrogen | Local (vaginal) | 1 gm | 12 weeks | OAB | |
| Walter 1978 S | Oestradiol + oestriol | Systemic (oral) | 2 mg / 1 mg | 4 months (with breaks) | SUI, MUI (stress and mixed), postmenopausal | |
| Walter 1990 S | Oestriol | Systemic (oral) | 4 mg | 1‐2 months | SUI (stress), postmenopausal | |
| Wilson 1987 S | Piperazine oestrone sulphate | Systemic (oral) | 3 mg | 3 months (with breaks) | USI (stress), postmenopausal | |
| Zullo 2005 L | Estriol | Local (intravaginal ovules) | 1 mg | 6 months | SUI (stress), postmenopausal |
LUTs = lower urinary tract symptoms; MUI = mixed urinary incontinence; OAB = overactive bladder syndrome; SUI = stress urinary incontinence; UI = urinary incontinence; USI = urodynamic stress incontinence; UUI = urgency urinary incontinence.
Results
Description of studies
Results of the search
The literature search generated 196 records to assess. A total of 87 studies were considered for this review, of which 53 were excluded for the reasons listed in the table Characteristics of excluded studies. In the first update of the original Cochrane review (Moehrer 2003), an extra six trials were included (Dessole 2004 L; Hendrix 2005 S; Ishiko 2001 S; Tinelli 2007 L; Tseng 2007 L; Zullo 2005 L), three trials were updated with new information (Cardozo 2001 L; Grady 2001 S; Rufford 2003 S) and an extra 10 studies were excluded. Two previously included trials were excluded because some of the women were continent (Rud 1980; Chompootaweep 1998 SL). In total, 34 RCTs are now included (see Characteristics of included studies). One study is ongoing (Sant 2002) and one study is 'Awaiting assessment' (Bergman 1985) as the study methods are unclear. The flow of records through the assessment process can be seen in the PRISMA flow diagram (Figure 1).
1.
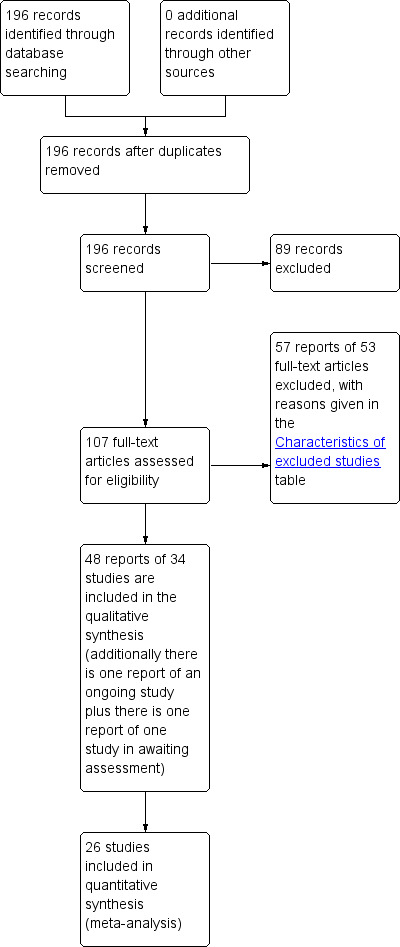
PRISMA study flow diagram.
Included studies
Of the 34 included trials, 18 investigated systemic administration and 17 trials investigated local administration. One trial investigated both local and systemic administration (Hilton 1990 SL). Thirty‐two were full reports and two were published as abstracts only. Three trials were published in German, one in Italian and one in Polish. The remainder of the trials were published in English. Seven trials used a crossover design (Ahlstrom 1990 S; Beisland 1984 L; Blom 1995 S; Ek 1980 S; Judge 1969 S; Kinn 1988 S; Samsioe 1985 S).
Twenty‐six trials had two trial arms, four trials had three trial arms, one trial had four trial arms, and one trial had six trial arms.
The duration of the treatments in the trials also differed: one trial was done over three weeks, five over four weeks, one over five weeks, two over eight weeks, nine over three months, one over four months, nine over six months, one over seven months, two over 12 months, and one over four years (Grady 2001 S). A summary of the types of oestrogens, route of administration, dose of oestrogen, duration of treatment and type of population is given in Table 4.
Sample characteristics
The trials included a total of 19,676 women who had urinary incontinence, of which approximately 9599 received oestrogen therapy (1464 were involved in trials of local oestrogen administration). The sample sizes for each trial ranged from 16 to 16,117 participants. Two large trials were of women receiving systemic hormone replacement therapy for reasons other than incontinence (control of coronary heart disease: Grady 2001 S, N = 1525; prevention of coronary heart disease and hip fracture: Hendrix 2005 S, N = 16,117 incontinent women). Every trial used different inclusion and exclusion criteria.
Type of incontinence
Trials were undertaken on women with urge, stress, mixed or non‐specified incontinence. A range of urodynamic or symptomatic measures were used in the different trials to confirm incontinence. Women were recruited on the basis of symptoms of incontinence or urodynamic diagnosis:
17 trials selected women with stress urinary incontinence (Ahlstrom 1990 S; Beisland 1984 L; Dessole 2004 L; Ek 1980 S; Fantl 1996 S; Henalla 1989 L; Henalla 1990 L; Hilton 1990 SL; Ishiko 2001 S; Jackson 1999 S; Kinn 1988 S; Liapis 2010 L; Sacco 1990 L; Tinelli 2007 L; Walter 1990 S; Wilson 1987 S; Zullo 2005 L);
eight included women with urgency urinary incontinence (Blom 1995 S; Cardozo 1993 S; Cardozo 2001 L; Enzelsberger 1990 L; Enzelsberger 1991a L; Kurz 1993 L; Rufford 2003 S; Tseng 2007 L);
five trials involved a mixed population of women with different types of incontinence or women with mixed incontinence (Assassa 2003 L; Grady 2001 S; Hendrix 2005 S; Samsioe 1985 S; Walter 1978 S);
two trials selected women with urogenital symptoms including incontinence (Lose 2000 L; Melis 1997 L); and
two trials were in elderly women with incontinence and who lived in nursing homes (Judge 1969 S; Ouslander 2001 S).
Menopausal status
All trials examined post‐menopausal women. Most trials excluded women who had used hormone or oestrogen replacement therapy in the preceding two to 12 months, who suffered from a urinary or urogenital infection, or who had contraindications to oestrogen therapy such as oestrogen dependent malignancy, thromboembolic disorders or severe liver disease. Often women with diabetes mellitus or neurological disease were excluded. A major prolapse was an exclusion criterion in some trials, as were symptoms that had started more than three years before the menopause.
Route of administration
Systemic (18 trials)
Oral tablets (Ahlstrom 1990 S; Cardozo 1993 S; Ek 1980 S; Fantl 1996 S; Grady 2001 S; Hendrix 2005 S; Hilton 1990 SL; Ishiko 2001 S; Jackson 1999 S; Judge 1969 S; Kinn 1988 S; Ouslander 2001 S; Samsioe 1985 S; Walter 1978 S; Walter 1990 S; Wilson 1987 S)
Transdermal patches (Blom 1995 S)
Subcutaneous implant (Rufford 2003 S)
Local (17 trials)
Vaginal pessaries or tablets (Cardozo 2001 L; Dessole 2004 L; Liapis 2010 L; Lose 2000 L; Zullo 2005 L)
Vaginal cream (Beisland 1984 L; Enzelsberger 1990 L; Enzelsberger 1991a L; Henalla 1989 L; Henalla 1990 L; Hilton 1990 SL; Melis 1997 L; Sacco 1990 L; Tinelli 2007 L; Tseng 2007 L)
Oestradiol‐releasing vaginal ring (Assassa 2003 L; Lose 2000 L)
Intravesical (into the bladder) (Kurz 1993 L)
Type of oestrogen
1. Oestrogen versus placebo
Systemic
Oestradiol (Blom 1995 S; Jackson 1999 S; Rufford 2003 S)
Oestriol (Cardozo 1993 S; Samsioe 1985 S)
Combination of 2 mg oestradiol and 1 mg oestriol tablets (Walter 1978 S)
Conjugated equine oestrogen (Hendrix 2005 S)
Piperazine oestrone sulfate tablets (Wilson 1987 S)
Quinestradiol (Judge 1969 S)
Local
Oestradiol (Assassa 2003 L; Cardozo 2001 L)
Oestriol (Dessole 2004 L; Enzelsberger 1991a L; Kurz 1993 L; Zullo 2005 L)
Conjugated equine oestrogen (Henalla 1989 L; Henalla 1990 L; Hilton 1990 SL; Sacco 1990 L)
2. Oestrogen versus other treatments
Systemic
Two trials compared oestrogens (oestradiol or oestriol) with phenylpropanolamine (PPA) (Hilton 1990 SL; Walter 1990 S)
Local
Two trials compared oestrogens (oestradiol or oestriol) with phenylpropanolamine (PPA) (Beisland 1984 L; Hilton 1990 SL)
Two trials compared oestrogen (conjugated equine oestrogen cream) with pelvic floor muscle training or electrostimulation (Henalla 1989 L; Henalla 1990 L)
3. Oestrogen plus other treatment versus placebo
Systemic
All four trials comparing oestrogen plus other treatment with placebo used a progestogen (medroxyprogesterone) in combination with conjugated equine oestrogens (Fantl 1996 S; Grady 2001 S; Hendrix 2005 S; Ouslander 2001 S)
4. Oestrogen plus other treatment versus oestrogen
Five trials compared oestrogen plus other treatment with oestrogen alone. The oestrogens were as follows.
Systemic
Oestriol (Ahlstrom 1990 S; Kinn 1988 S)
Oestradiol (Blom 1995 S; Ek 1980 S)
Conjugated equine oestrogen cream (Hilton 1990 SL)
Local
Oestriol (Melis 1997 L)
Conjugated equine oestrogen cream (Hilton 1990 SL)
The other treatment was:
an alpha‐adrenergic drug (phenylpropanolamine (PPA)) (Ahlstrom 1990 S; Ek 1980 S; Hilton 1990 SL; Kinn 1988 );
an anti‐inflammatory and antibacterial drug (benzidamine) (Melis 1997 L);
a non‐steroidal anti‐inflammatory drug (naproxen) (Blom 1995 S).
5. Oestrogen plus other treatment versus other treatment
One trial compared oestrogen (conjugated equine oestrogen cream) plus PPA with PPA alone (Hilton 1990 SL). Another compared oestrogens plus pelvic floor muscle training (PFMT) with PFMT alone (Ishiko 2001 S). Liapis et al (Liapis 2010 L) and Tinelli et al (Tinelli 2007 L) both compared oestrogen plus surgery versus surgery. Liapis et al compared vaginal oestrogen plus tension free vaginal tape‐obturator route (TVT‐O) versus TVT‐O in stress incontinent women. Tinelli et al (Tinelli 2007 L) also compared vaginal oestrogen plus tension free vaginal tape (TVT) versus TVT alone. TVT and TVT‐O are both minimally invasive surgical procedures for stress incontinence. Tseng (Tseng 2007 L) compared vaginal oestrogen plus detrusitol versus detrusitol for overactive bladder.
6. Different types of oestrogen
One trial directly compared different types of oestrogen. The comparisons were:
oestradiol‐releasing vaginal ring versus oestriol pessary (Lose 2000 L).
7. Different routes of administration
One trial directly compared different routes of administration:
vaginal conjugated equine oestrogen cream versus oral conjugated oestrogen (Hilton 1990 SL).
8. High‐dose versus low‐dose oestrogen
Two trials compared high‐dose with low‐dose oestrogen (oestriol) treatment administered locally (one trial is referenced twice for data entry purposes) (Enzelsberger 1990 L; Enzelsberger 1991a L; Enzelsberger 1991b L).
Outcome measures
The different studies used a variety of measures to assess subjective outcomes of treatment including visual analogue scales, a four‐point severity score scale, symptom questionnaires and simple questions. Objective outcomes such as frequency, urgency, nocturia, dysuria and number of incontinent episodes were mainly assessed by urinary diaries. Three trials reported quality of life measurements. Pad tests were performed in seven trials but all the tests were of a different type and duration. Fourteen trials reported urodynamic measurements but put their emphasis on different parameters. No trials reported economic outcome measures.
Duration of follow up
Three trials reported follow up after the trial treatment period (Henalla 1989 L; Kurz 1993 L; Wilson 1987 S). None of the other trials reported outcomes after the end of trial treatment. However, the effect of oestrogen treatment would not be expected to last after treatment stops as women would revert to their post‐menopausal oestrogen‐deficient status.
Excluded studies
Fifty‐three studies were excluded for reasons listed in the table Characteristics of excluded studies.
Risk of bias in included studies
2.
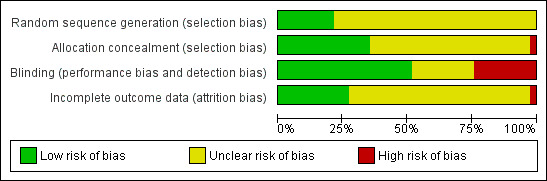
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
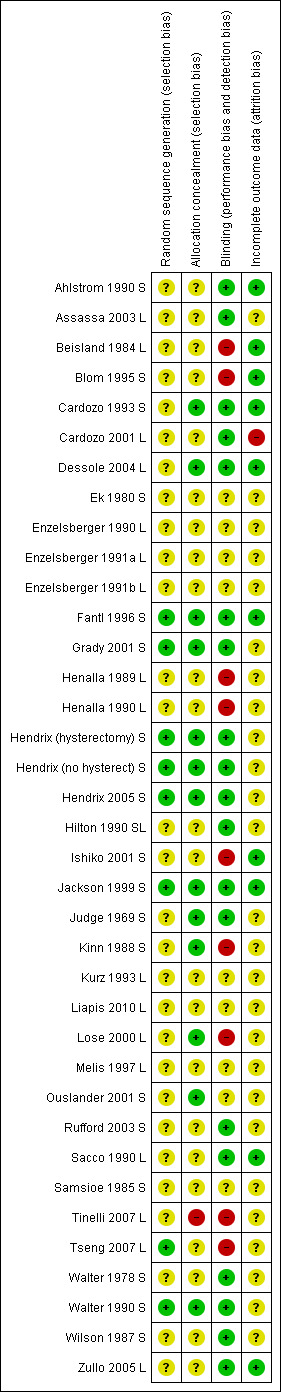
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
A total of 11 trials used an adequate method of allocation concealment (Cardozo 1993 S; Dessole 2004 L; Fantl 1996 S; Grady 2001 S; Hendrix 2005 S; Jackson 1999 S; Judge 1969 S; Kinn 1988 S; Lose 2000 L; Ouslander 2001 S; Walter 1990 S).
In 21 trials, the method of allocation concealment was unclear (Ahlstrom 1990 S; Assassa 2003 L: Beisland 1984 L; Blom 1995 S; Cardozo 2001 L; Ek 1980 S; Enzelsberger 1990 L; Enzelsberger 1991a L; Henalla 1989 L; Henalla 1990 L; Hilton 1990 SL; Ishiko 2001 S; Kurz 1993 L; Liapis 2010 L; Melis 1997 L; Rufford 2003 S; Sacco 1990 L; Samsioe 1985 S; Walter 1978 S; Wilson 1987 S; Zullo 2005 L).
Blinding
Twenty trials were double‐blind (Ahlstrom 1990 S; Assassa 2003 L; Cardozo 1993 S; Cardozo 2001 L; Dessole 2004 L; Ek 1980 S; Fantl 1996 S; Grady 2001 S; Hendrix 2005 S; Hilton 1990 SL; Jackson 1999 S; Judge 1969 S; Kinn 1988 S; Kurz 1993 L; Ouslander 2001 S; Rufford 2003 S; Samsioe 1985 S; Walter 1978 S; Walter 1990 S; Wilson 1987 S). In one trial the women were blinded to their treatment but the assessors were not (Blom 1995 S). In 11 trials neither women nor the assessors were blinded throughout the treatment course. It was not clear whether blinding had not been achieved or whether it was not possible to carry out blinding (Beisland 1984 L; Enzelsberger 1990 L; Enzelsberger 1991a L; Henalla 1989 L; Henalla 1990 L; Ishiko 2001 S; Liapis 2010 L; Lose 2000 L; Melis 1997 L; Sacco 1990 L; Zullo 2005 L).
Incomplete outcome data
Dropsouts or losses to follow up were reported in 19 out of the 35 trials included in the review (Beisland 1984 L; Cardozo 1993 S; Cardozo 2001 L; Ek 1980 S; Enzelsberger 1991a L; Fantl 1996 S; Grady 2001 S; Hendrix 2005 S; Hilton 1990 SL; Ishiko 2001 S; Jackson 1999 S; Judge 1969 S; Liapis 2010 L; Lose 2000 L; Melis 1997 L; Ouslander 2001 S; Rufford 2003 S; Walter 1990 S; Wilson 1987 S). The trials reported between 2% to 10% losses to follow up at varying times.
Effects of interventions
See: Table 1; Table 2; Table 3
Comparison 1. oestrogen therapy versus placebo or no treatment for urinary incontinence
The 20 trials that compared oestrogen with placebo (Hilton 1990 compared systemic and local), 10 systemic oestrogen (Blom 1995 S; Cardozo 1993 S; Hendrix 2005 S; Hilton 1990 SL; Jackson 1999 S; Judge 1969 S; Rufford 2003 S; Samsioe 1985 S; Walter 1978 S; Wilson 1987 S) and 10 local oestrogen (Assassa 2003 L; Cardozo 2001 L; Dessole 2004 L; Enzelsberger 1991a L; Henalla 1989 L; Henalla 1990 L; Hilton 1990 SL; Kurz 1993 L; Sacco 1990 L; Zullo 2005 L), had varying combinations of types of oestrogen, doses, treatment durations and lengths of follow up. A variety of tests were used to measure subjective and objective outcomes. These were not consistent across the studies and so data for individual outcomes were usually available only for a minority of trials.
The three trials (Blom 1995 S; Judge 1969 S; Samsioe 1985 S) which used a crossover design provided no useable data for any of the outcomes in this comparison.
Women's observations
Number with incontinence
Systemic
The results of four small trials showed a statistically significant result favouring oestrogen (RR 0.83, 95% CI 0.70 to 0.98) (Analysis 1.1). However, the larger study (Hendrix (hysterectomy) S), demonstrating contradictory results, did not contribute data to this outcome. There was evidence of statistical heterogeneity and when a random‐effects model was used the result was no longer statistically significant (RR 0.83, 95% CI 0.63 to 1.09). The combined data included studies of women with urgency (Cardozo 1993 S; Rufford 2003 S) and stress symptoms (Jackson 1999 S; Walter 1978 S). Different types of oestrogen were also used: oestriol (Cardozo 1993 S; Dessole 2004 L); oestradiol (Jackson 1999 S) and 17‐beta oestradiol (Walter 1978 S). The Hendrix trial (Hendrix (hysterectomy) S) used conjugated equine oestrogen.
1.1. Analysis.

Comparison 1 Oestrogen versus placebo or no treatment, Outcome 1 Number with incontinence (women's observations).
Local
Three trials (Assassa 2003 L; Dessole 2004 L; Zullo 2005 L) compared local oestrogen against placebo. Two of the studies (Dessole 2004 L; Zullo 2005 L) favoured local administration (RR 0.73, 95% CI 0.62 to 0.87) (Analysis 1.1) with the results being statistically significant, whilst the remaining study (Assassa 2003 L) reported no difference between patients in the two intervention groups. Statistical heterogeneity was shown in this meta analysis and when a random‐effects model was used the result was no longer statistically significant (RR 0.37, 95% CI 0.03 to 5.29). The Zullo study included women after a TVT procedure and the main objective was to investigate the development of urgency. Assassa 2003 L included 220 post‐menopausal women. However, there were no data available in the paper to include in the analysis.
Number with incontinence not improved
Women not cured or improved were considered together.
Systemic
Data from a very large trial (Hendrix 2005 S), which aimed to investigate the effects of systemic hormone replacement treatment for prevention of coronary heart disease and bone fracture, included a subgroup analysis of women who had been incontinent at baseline (Hendrix (hysterectomy) S). When these data were combined (using generic inverse variance) with the results from five other small trials (Cardozo 1993 S; Jackson 1999 S; Rufford 2003 S; Walter 1990 S; Wilson 1987 S) the net effect was to suggest that systemic oestrogen treatment resulted in more urinary incontinence than placebo (RR 1.32, 95% CI 1.17 to 1.48) (Analysis 1.3). The statistical heterogeneity was high and the result was no longer statistically significant if a random‐effects model was used (RR 0.82, 95% CI 0.52 to 1.28). Under this model the much larger Hendrix trial received less weight.
1.3. Analysis.
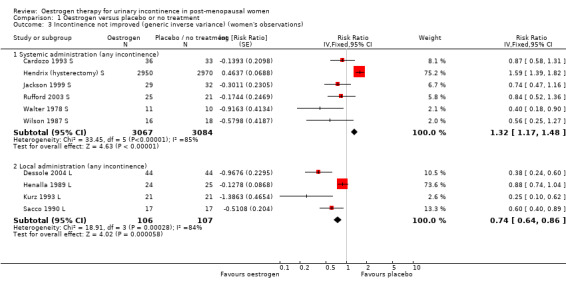
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 3 Incontinence not improved (generic inverse variance) (women's observations).
The women included from the Hendrix study in this meta analysis had all had a hysterectomy and the incontinence was measured at one year (Hendrix (hysterectomy) S). The Jackson and Rufford trials included women who had had a hysterectomy; the other trials made no mention of whether women had hysterectomies. Only Hendrix reported data separately. The result of this meta analysis was based mainly on the Hendrix trial, which received the highest weighting. This is related to the large number of participants (approximately 9000) who were involved. The RR of any urinary incontinence becoming worse was consistently higher in the group treated with oestrogen compared with placebo (RR 1.59, 95% CI 1.39 to 1.82) (Hendrix (hysterectomy) S).
This effect was also seen for increased frequency of incontinence episodes (RR 1.47, 95% CI 1.35 to 1.61) and for limitation of daily activities (RR 1.29, 95% CI 1.15 to 1.45) as given in the trial report (data not shown) (Hendrix (hysterectomy) S).
Local
Combination of data from the four small trials which used local oestrogen administration (Dessole 2004 L; Henalla 1989 L; Kurz 1993 L; Sacco 1990 L) demonstrated a benefit for oestrogen over placebo in terms of a reduction in incontinence (RR 0.74, 95% CI 0.64 to 0.86) (Analysis 1.3). Statistical heterogeneity was demonstrated in this meta‐analysis. The Henalla study seemed to provide different results from the others with a CI which included favouring placebo, and this trial used conjugated equine oestrogen. Kurz used intravesical administration. Only the Kurz trial included women with urgency urinary incontinence; the other three trials were restricted to women with stress urinary incontinence. The result was still statistically significant when a more conservative random‐effects model was used (RR 0.52, 95% CI 0.32 to 0.87).
Quantification of symptoms
Some data describing quantification of symptoms were available from 10 trials (Cardozo 1993 S; Enzelsberger 1991a L; Enzelsberger 1991b L; Henalla 1989 L; Hilton 1990 SL; Jackson 1999 S; Kurz 1993 L; Rufford 2003 S; Sacco 1990 L; Wilson 1987 S) although individual outcomes were not reported consistently.
Systemic
There were too few data to provide evidence of any effect for the outcomes on pad changes (Analysis 1.6), pad tests (Analysis 1.7), incontinent episodes (Analysis 1.8), number of voids in 24 hours (Analysis 1.9), number of nocturnal voids (Analysis 1.10) or number of women with frequency, nocturia or urgency.
1.6. Analysis.
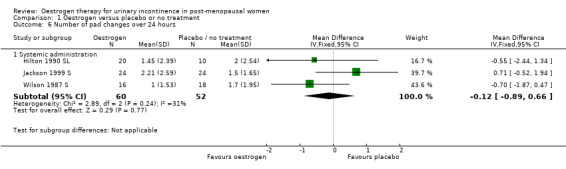
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 6 Number of pad changes over 24 hours.
1.7. Analysis.
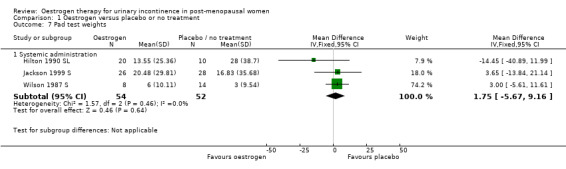
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 7 Pad test weights.
1.8. Analysis.
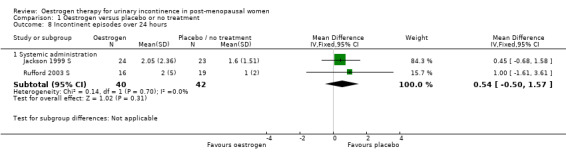
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 8 Incontinent episodes over 24 hours.
1.9. Analysis.
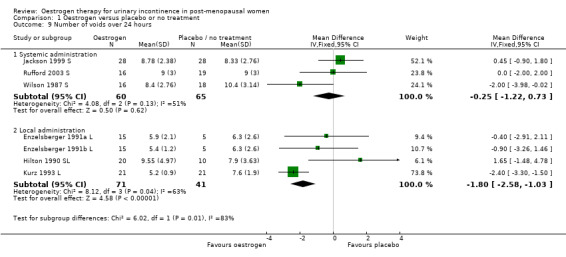
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 9 Number of voids over 24 hours.
1.10. Analysis.
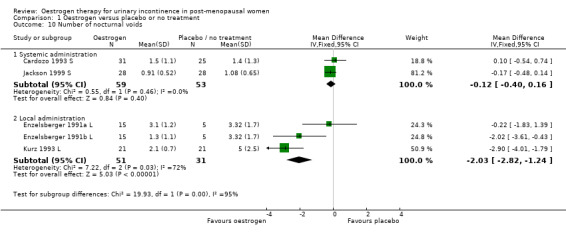
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 10 Number of nocturnal voids.
Local
There were few data describing objective improvements in terms of numbers of women observed to be incontinent (Analysis 1.4), pad changes or pad weights (Analysis 1.6; Analysis 1.7) or incontinent episodes (Analysis 1.8).
1.4. Analysis.
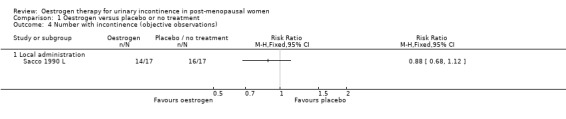
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 4 Number with incontinence (objective observations).
The results of two small trials (Henalla 1990 L; Sacco 1990 L) showed a statistically significant difference favouring local oestrogen in numbers of women whose incontinence had not improved (RR 0.74, 95% CI 0.57 to 0.95) (Analysis 1.5.) There was evidence of a reduction in the number of voids in 24 hours (weighted mean difference (WMD) ‐1.80, 95% CI ‐2.58 to ‐1.03) (Analysis 1.9) and nocturnal voids (WMD ‐2.03, 95% CI ‐2.82 to ‐1.24) (Analysis 1.10) with data favouring oestrogen. Although there was significant heterogeneity the result was still statistically significant when a random‐effects model was used. Overall, there were around one to two fewer voids in 24 hours and nocturnal voids amongst women treated with local oestrogen (Analysis 1.9; Analysis 1.10). The results from two studies (Sacco 1990 L; Zullo 2005 L) showed a statistically significant difference with respect to frequency (RR 0.43, 95% CI 0.19 to 0.98) (Analysis 1.11) and urgency (RR 0.38, 95% CI 0.15 to 0.99) (Analysis 1.13) favouring oestrogen. There were no statistically significant differences in nocturia (Analysis 1.12) but the CIs were wide reflecting the small size of the few trials with data.
1.5. Analysis.
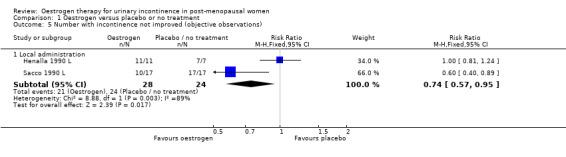
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 5 Number with incontinence not improved (objective observations).
1.11. Analysis.
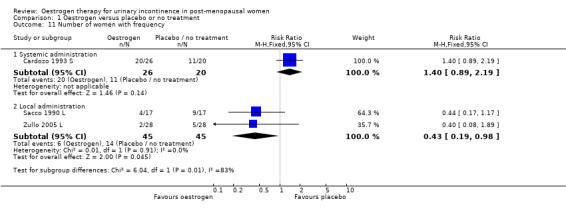
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 11 Number of women with frequency.
1.13. Analysis.
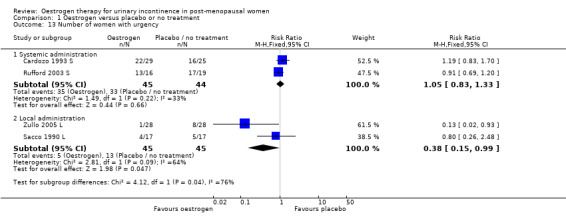
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 13 Number of women with urgency.
1.12. Analysis.
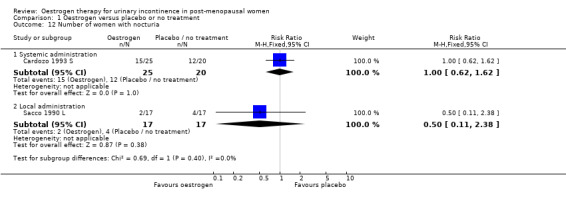
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 12 Number of women with nocturia.
Clinicians' measures
Data on urodynamic measurements (maximum urethral closure pressure, volume at first urge to void and maximum bladder capacity) were available for nine trials (Cardozo 1993 S; Enzelsberger 1991a L; Enzelsberger 1991b L; Henalla 1989 L; Jackson 1999 S; Kurz 1993 L; Rufford 2003 S; Sacco 1990 L; Wilson 1987 S).
Systemic
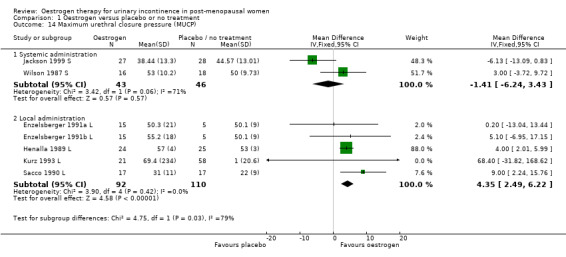
The limited data available for the effects of systemic oestrogen administration versus placebo on maximum urethral closure pressure (MUCP) (Analysis 1.14), volume at first urge to void (Analysis 1.15) and maximum bladder capacity (Analysis 1.16) showed no statistically significant difference between the two interventions.
1.14. Analysis.
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 14 Maximum urethral closure pressure (MUCP).
1.15. Analysis.
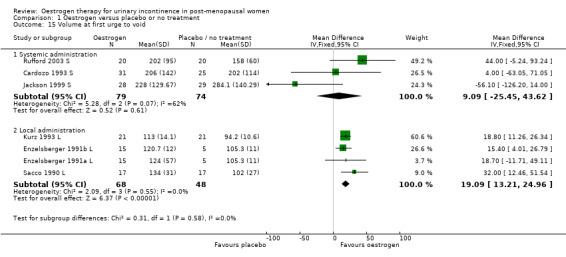
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 15 Volume at first urge to void.
1.16. Analysis.
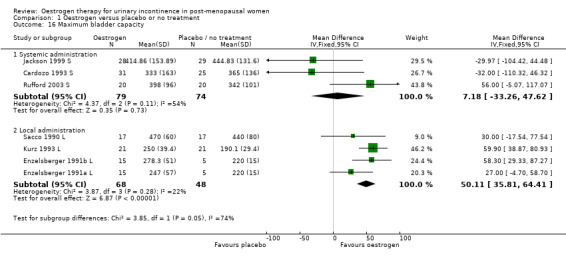
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 16 Maximum bladder capacity.
Local
The data available for maximum urethral closure pressure (MUCP) (RR 4.35, 95% CI 2.49 to 6.22) (Analysis 1.14), volume at first urge to void (RR 19.09, 95% CI 13.21 to 24.96) (Analysis 1.15) and maximum bladder capacity (RR 50.11, 95% CI 35.81 to 64.41) (Analysis 1.16) when comparing local oestrogen to placebo all tended to favour the oestrogen treated groups. The data for all three outcome measures were statistically significant.
Quality of life
One trial provided information about data from the Bristol Female Lower Urinary Tract Symptoms (BFLUTS) questionnaire (Jackson 1999 S) and reported no significant difference between the trial groups. Another trial used the King's Healthcare Quality of Life Questionnaire, but no further information was given (Rufford 2003 S).
Socioeconomic measures
No data were available.
Adverse effects
One trial (Rufford 2003 S) compared systemic oestrogen against placebo and reported more adverse events in the placebo group, with the results being statistically significant (Analysis 1.17) (RR 13.00, 95% CI 1.87 to 90.21). No serious adverse events were reported with women in the trials experiencing mainly vaginal spotting, breast tenderness and nausea. These are detailed in the notes column in the Characteristics of Included studies table.
1.17. Analysis.
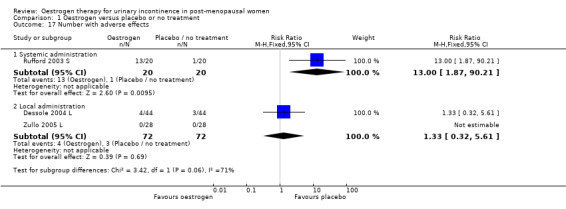
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 17 Number with adverse effects.
Number with bacteriuria
One small trial (Rufford 2003 S) compared systemic oestrogen to placebo and reported a higher incidence of bacteriuria in the oestrogen group.
Comparison 2. oestrogen versus other treatment
Five trials compared oestrogens with other treatment, three with phenylpropanolamine (PPA) (Beisland 1984 L; Hilton 1990 SL; Walter 1990 S) and two with pelvic floor muscle training (PFMT) or electrostimulation (Henalla 1989 L; Henalla 1990 L). All were limited to women with stress incontinence and only one used systemic administration (Walter 1990 S).
Oestrogen versus phenylpropanolamine (PPA)
The three trials that compared oestrogen with PPA included only 79 women in total (Beisland 1984 L; Hilton 1990 SL; Walter 1990 S). PPA is used in prescription and over‐the‐counter nasal decongestants and appetite suppressants, and has an alpha‐adrenergic mode of action. Overall, there were no statistically significant differences in any of the outcomes for which data were available but the CIs were wide. There was some evidence of heterogeneity between the three trials and this may reflect the difference in the periods of active treatment (two versus four weeks). The results for the Beisland crossover trial are presented in the 'Other data' tables only.
Oestrogen versus pelvic floor muscle training (PFMT)
The two trials that compared local oestrogen with PFMT included 69 women (Henalla 1989 L; Henalla 1990 L). Both Henalla trials used conjugated equine oestrogen. Women's incontinence was less likely to improve with oestrogens than with PFMT in the two small trials. The combined data were statistically significant favouring PFMT (RR for lack of improvement in incontinence 2.30, 95% CI 1.50 to 3.52) (Analysis 2.6). There were not enough data for other outcomes or to show if there was a difference in maximum urethral closure pressure as measured by urodynamics.
2.6. Analysis.
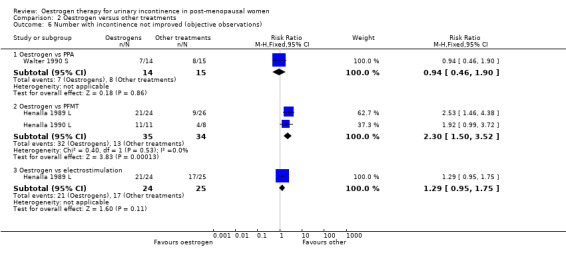
Comparison 2 Oestrogen versus other treatments, Outcome 6 Number with incontinence not improved (objective observations).
Oestrogen versus electrostimulation
A single small trial included 49 women in the comparison of local oestrogen with electrostimulation (Henalla 1990 L). The result favoured electrostimulation over oestrogen in terms of a higher maximum urethral closure pressure as measured by urodynamics (WMD ‐7.00, 95% CI ‐8.97 to ‐5.03) (Analysis 2.11) although there was no statistically significant difference in terms of improvement in objectively observed incontinence (RR 1.29, 95% CI 0.95 to 1.75) (Analysis 2.6).
2.11. Analysis.
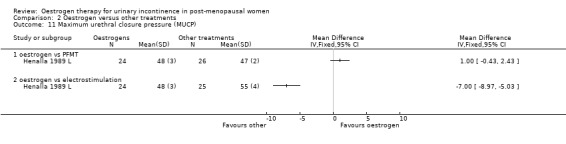
Comparison 2 Oestrogen versus other treatments, Outcome 11 Maximum urethral closure pressure (MUCP).
Comparison 3. oestrogen plus other treatment versus placebo
Progesterone was the only treatment tested as an addition to oestrogen in the four trials identified (Fantl 1996 S; Grady 2001 S; Hendrix 2005 S; Ouslander 2001 S). All four trials used conjugated equine oestrogen.
Oestrogen plus progestogen versus placebo
The four trials that compared the combination of oestrogen and progestogen versus placebo were conducted in contrasting groups of women.
The report by Grady (Grady 2001 S) was based on a subset of post‐menopausal women with urinary incontinence within a large trial of hormone replacement treatment for women with coronary heart disease, all of whom had an intact uterus.
The Hendrix trial included a subset of women with urinary incontinence from a trial where the main objective was to investigate the effects of hormone replacement treatment on prevention of coronary heart disease and bone fracture in post‐menopausal women (Hendrix (no hysterect) S); the women in this subset also had an intact uterus.
The women in the smaller trial reported by Fantl (Fantl 1996 S) were post‐menopausal and had stress incontinence only.
The women in the small trial by Ouslander (Ouslander 2001 S) were incontinent nursing home residents but no data were available for this trial with respect to any of the review outcomes.
When the data on no improvement in incontinence (women's observations) from three of the trials (Fantl 1996 S; Grady 2001 S; Hendrix (no hysterect) S) were combined using generic inverse variance, the net effect was that urinary incontinence in women treated with oestrogen plus progestogen was worse than in those on placebo; the result was statistically significant (RR for no improvement 1.11, 95% CI 1.04 to 1.18) (Analysis 3.2.1). The Grady trial received the highest weighting at 75.3% (Analysis 3.2.1) in the meta‐analysis due to less variance in the results. Both the Grady and Hendrix trials included women with all types of incontinence and all had an intact uterus. Fantl included only women with stress incontinence. There was no significant statistical heterogeneity. All three trials in the meta analysis used systemic conjugated equine oestrogen plus progestogen. No trials were found for this comparison, which addressed the effect of local administration.
3.2. Analysis.
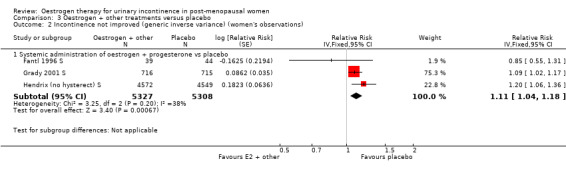
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 2 Incontinence not improved (generic inverse variance) (women's observations).
The report by Hendrix (Hendrix (no hysterect) S) reported that the relative risk of any urinary incontinence becoming worse was consistently higher in the group treated with oestrogen and progesterone compared with placebo (RR 1.20, 95% CI 1.06 to 1.36). This effect was also seen for increased frequency of incontinence episodes (RR 1.38, 95% CI 1.28 to 1.49) and for limitation of daily activities (RR 1.18, 95% CI 1.06 to 1.32).
The Fantl study (Fantl 1996 S) recorded data on incontinent episodes in 24 hours, diurnal and nocturnal voids, and pad weight tests, but the data were too few to show any conclusive findings.
Comparison 4. oestrogen plus other treatment versus oestrogen
Six trials compared oestrogen plus other treatment with oestrogen alone. The other treatments were:
PPA (phenylpropanolamine) (Ahlstrom 1990 S; Ek 1980 S; Hilton 1990 SL; Kinn 1988 S);
benzidamine (Melis 1997 L);
naproxen (Blom 1995 S).
Four trials used systemic administration of oestrogen, one used the local route (Melis 1997 L) and one used different routes in different arms (Hilton 1990 SL) where data from the oestrogen arms have been combined for analysis.
Oestrogen plus PPA versus oestrogen
Usable data were available from only one trial (Hilton 1990 SL) and this involved only 20 women in each group. No statistically significant difference was seen for any outcome except improvement of incontinence, where the addition of PPA to oestrogen was better than oestrogen alone (Analysis 4.1.1) (RR 0.42, 95% CI 0.22 to 0.78) but the CIs were wide. In two small crossover trials (Ahlstrom 1990 S; Kinn 1988 S) involving 53 and 60 participants, respectively, supplementing oestrogen with PPA resulted in marginally fewer incontinent women (Analysis 4.2).
4.1. Analysis.
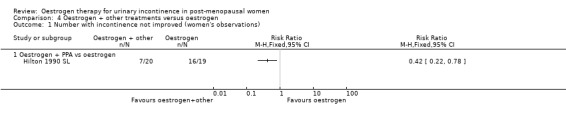
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 1 Number with incontinence not improved (women's observations).
4.2. Analysis.
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 2 Number with incontinence not improved (women's observations) (cross‐over trials).
| Number with incontinence not improved (women's observations) (cross‐over trials) | ||
|---|---|---|
| Study | oestrogen + other drug | oestrogen |
| oestrogen + PPA vs oestrogen | ||
| Ahlstrom 1990 S | 17 out of 27 women | 21 out of 26 women |
| Kinn 1988 S | 14 out of 30 women | 21 out of 30 women |
Oestrogen plus benzidamine versus oestrogen
A single small trial (Melis 1997 L) suggested lower rates of frequency of voids (Analysis 4.6.2) and nocturia (Analysis 4.7.1) if benzidamine was added to oestrogen. However, the CIs were wide and there were no data for other outcomes.
4.6. Analysis.
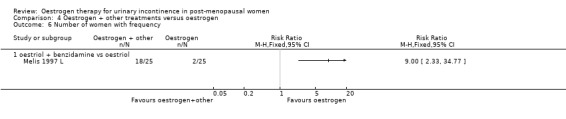
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 6 Number of women with frequency.
4.7. Analysis.
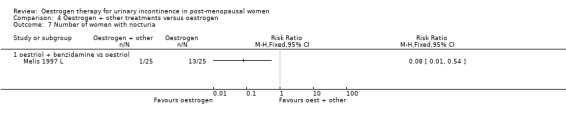
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 7 Number of women with nocturia.
Oestrogen plus naproxen versus oestrogen
In another small crossover trial (Blom 1995 S) the addition of naproxen appeared to decrease the volume at the first urge to void (Analysis 4.9.1) although the decrease in the maximum bladder capacity was modest and not statistically significant (Analysis 4.10.1). There were no data for other outcomes.
4.9. Analysis.
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 9 Volume at first urge to void (cross‐over trials).
| Volume at first urge to void (cross‐over trials) | ||
|---|---|---|
| Study | oestrogen + naproxen | oestrogen |
| oestrogen + PPA vs oestrogen | ||
| Blom 1995 S | 172 ml (SD 12) N=16 | 186 ml (SD 12) N=16 |
4.10. Analysis.
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 10 Maximum bladder capacity (cross‐over trials).
| Maximum bladder capacity (cross‐over trials) | ||
|---|---|---|
| Study | oestrogen + naproxen | oestrogen |
| oestrogen +other vs oestrogen | ||
| Blom 1995 S | 316 ml (SD 17) N=16 | 318 ml (SD 20) N=16 |
Comparison 5. oestrogen plus other treatment versus other treatment
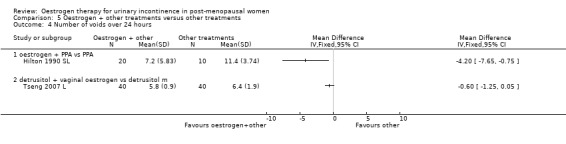
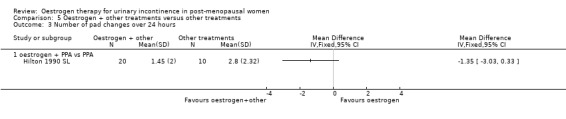
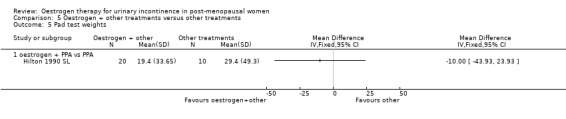
One small trial (Hilton 1990 SL) involving 30 women tested the addition of PPA to oestrogen in comparison with PPA alone. Women who also received oestrogen showed an improvement in incontinence (RR 0.38, 95% CI 0.21 to 0.68) (Analysis 5.2) and around four fewer voids per day (Analysis 5.4) than with PPA alone. The other outcomes measured, number of pad changes (Analysis 5.3) and pad test weights (Analysis 5.5), were not significantly in favour of oestrogen supplementation and had wide CIs.
5.2. Analysis.

Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 2 Number with incontinence not improved (women's observations).
5.4. Analysis.
Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 4 Number of voids over 24 hours.
5.3. Analysis.
Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 3 Number of pad changes over 24 hours.
5.5. Analysis.
Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 5 Pad test weights.
Another trial with 66 women evaluated the benefit of adding oestrogen to PFMT but was too small to demonstrate differences reliably (Ishiko 2001 S).
Tinelli (Tinelli 2007 L) compared tension free vaginal tape (TVT) with TVT plus vaginal oestrogen and reported no statistically significant difference for incontinence rates between the groups in this small study. Liapis (Liapis 2010 L) also compared TVT combined with vaginal oestrogen against TVT alone. The results state that treatment with vaginal oestrogen post‐operatively showed no reduction in urge incontinence when compared to the non‐treated group. Tseng (Tseng 2007 L) compared vaginal oestrogen plus detrusitol with detrusitol for overactive bladder and found that there was a slight increase in the number of voids over 24 hours in the oestrogen plus detrusitol group (RR ‐0.60, 95% CI ‐1.25 to 0.05) (Analysis 5.4).
Comparison 6. different types of oestrogen
One trial directly compared different types of oestrogen. The trial carried out by Lose (Lose 2000 L) compared a vaginal oestradiol ring versus vaginal oestriol pessary.
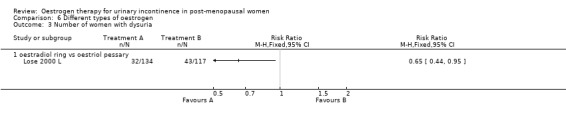
Vaginal oestradiol (ring) versus vaginal oestriol (pessary)
Women preferred the oestradiol ring to the oestriol pessary (women's subjective assessment was markedly in favour of the oestradiol ring: 55% versus 14% grading it as 'excellent') (Lose 2000 L) and fewer women had dysuria (painful urination) (Analysis 6.3), but it was not clear whether this was due to the different type of oestrogen or the different delivery system.
6.3. Analysis.
Comparison 6 Different types of oestrogen, Outcome 3 Number of women with dysuria.
The CIs were wide for all the other outcomes reported (Lose 2000 L).
Comparison 7. different routes of administration
One small trial which directly compared oestrogen vaginal cream with oral tablets involved 20 women (Hilton 1990 SL). Although there were fewer voids and pad changes over 24 hours in the group treated with oestrogen cream, there was not enough evidence to reliably compare the two treatments on number of pad changes (Analysis 7.1), number of voids (Analysis 7.2) or pad test weights (Analysis 7.3).
7.1. Analysis.
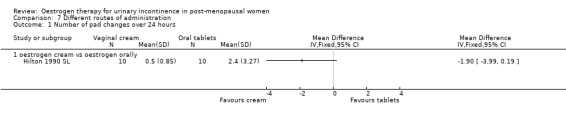
Comparison 7 Different routes of administration, Outcome 1 Number of pad changes over 24 hours.
7.2. Analysis.
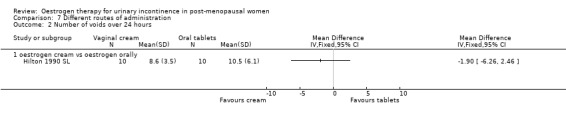
Comparison 7 Different routes of administration, Outcome 2 Number of voids over 24 hours.
7.3. Analysis.
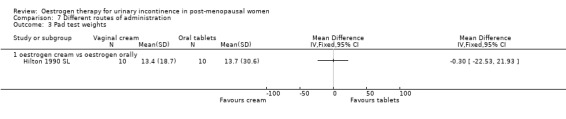
Comparison 7 Different routes of administration, Outcome 3 Pad test weights.
Comparison 8. high‐dose versus low‐dose oestrogen
Two trials compared high‐dose with low‐dose oestrogen treatment for women with urgency urinary incontinence (Enzelsberger 1990 L; Enzelsberger 1991a L).
Women receiving the higher dose had significantly fewer voids per 24 hours (RR ‐1.02, 95% CI ‐1.87 to ‐0.16) (Analysis 8.1) and reduced number of voids at night (RR ‐1.80, 95% CI ‐2.36 to ‐1.24) (Analysis 8.2). Urodynamic measurements were only in favour of high‐dose treatment in terms of a higher maximum bladder capacity (WMD 35 ml, 95% CI 8.35 to 61.45) (Analysis 8.5).
8.1. Analysis.
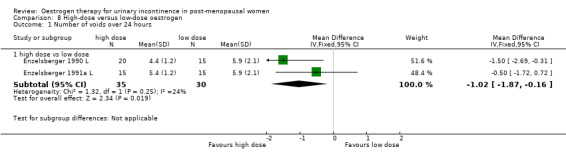
Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 1 Number of voids over 24 hours.
8.2. Analysis.
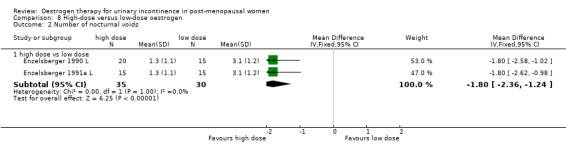
Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 2 Number of nocturnal voids.
8.5. Analysis.
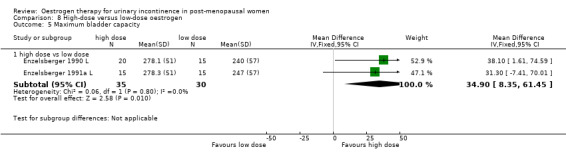
Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 5 Maximum bladder capacity.
Adverse effects
The most common side effects were vaginal bleeding (about one in four women treated) and breast tenderness (about one in five women treated). Other side effects were not consistently reported, or not related to the study arm. In one trial, one women died of a myocardial infarction (Cardozo 2001 L) and in another one woman developed angina (Rufford 2003 S). However, these were not thought to be related to treatment with oestrogens.
Discussion
Summary of main results
The data from the small trials which evaluated local administration of oestrogen suggested that oestrogens may improve urinary incontinence. However, the data regarding systemic administration suggested that hormones (either oestrogen alone or in conjunction with progesterone) made incontinence worse. There was, however, too little evidence from a direct comparison of local versus systemic administration to assess this reliably. The data addressing the other comparisons in the review were relatively few.
The results of the small trials comparing local administration of oestrogen with placebo were generally consistent in suggesting better outcomes associated with oestrogen treatment. However, there was limited evidence from two small trials (Henalla 1989 L; Henalla 1990 L) that pelvic floor muscle training was more effective than local oestrogen treatment in the management of stress incontinence and from one small trial (Hilton 1990 SL) that local oestrogen plus PPA was more effective than local oestrogen alone in stress incontinence. Data from the Hilton trial also showed that oestrogen plus PPA was more effective than PPA alone. PPA is used in prescription and over‐the‐counter cold and cough preparations and appetite suppressants and has an alpha‐adrenergic action, but it has been associated with an increased risk of stroke in younger women (Kernan 2000).
Two very large randomised controlled trials (RCTs) involving systemic hormone replacement therapy (Grady 2001 S; Hendrix 2005 S), where the main objectives were to investigate effects such as cardiac events and bone fractures, reported that both oestrogen treatment alone (Hendrix (hysterectomy) S) and combined with progestogen (Grady 2001 S; Hendrix (no hysterect) S) made incontinence worse. Both studies used conjugated equine oestrogen. The result for oestrogen versus placebo is heavily weighted by the larger Hendrix trial. All the women in this subset had had a hysterectomy. Furthermore, in another subset of one of these trials (Hendrix 2005 S) a group of women who were continent at baseline reported more incontinence after one year of treatment with oestrogen plus progestogen compared with women on placebo treatment (RR 1.39, 95% CI 1.27 to 1.52 for women with a uterus; RR 1.53, 95% CI 1.37 to 1.71 for women after hysterectomy and treated with oestrogen alone). This is consistent with other evidence about the effects of progesterone on urinary symptoms (Benness 1991). Therefore, post‐menopausal women who are considering receiving systemic hormone replacement therapy for reasons other than incontinence should be warned that they may develop urinary incontinence or their urinary symptoms may get worse.
In summary, local rather than oral preparations may reduce the risks of long‐term treatment. There was some evidence from three small trials that local (vaginal) administration might improve continence, whereas evidence from the two large trials suggested that systemic administration made it worse.
Adverse effects
There were no serious adverse effects related to treatment for incontinence reported in the trials; however, the reader should consider longer‐term side effects of hormone therapy presented in other reports (Marjoribanks 2012). The most common side effects were vaginal bleeding (about one in four women treated) and breast tenderness (about one in five women treated). There are, however, concerns about risks of unopposed oestrogens (continuous oestrogens without intermittent progestogen supplementation), particularly amongst women who have an intact uterus. Such women may develop endometrial hyperplasia, which increases the risk of endometrial cancer. In addition, the risk of breast cancer is increased by prolonged use of oestrogens (for example for five years) (Marjoribanks 2012) and women who have predisposing factors for deep venous thrombosis are also advised that oestrogens may increase their risk (BNF 2002). Furthermore, combined continuous hormone replacement therapy (HRT) significantly increases the risk of venous thrombo‐embolism or coronary events (after one year's use), stroke (after three years), breast cancer, gall bladder disease and dementia (Marjoribanks 2012). Long‐term oestrogen‐only HRT significantly increased the risk of venous thrombo‐embolism, stroke and gall bladder disease but did not significantly increase the risk of breast cancer. On the other hand, there is some evidence that oestrogen use before the age of 60 years is relatively safe (Rossouw 2007).
In most of the reviewed trials, oestrogen was not given for prolonged periods and only three trials gave any information about the women after the end of treatment. One risk of unopposed oestrogen (oestrogen alone) is endometrial cancer. Pending further research, women with an intact uterus should receive combined oestrogen and progestogen for limited periods. Women who have had a hysterectomy can receive oestrogen alone but again this should be for limited periods due to the risks already mentioned (Marjoribanks 2012).
PPA has been associated with an increased risk of stroke in younger women, however this adverse effect was not reported in the women involved in the included trials.
Overall completeness and applicability of evidence
While the data from the other comparisons were generally consistent with the findings for oestrogen alone (for example a higher dose of oestrogen appeared to have a greater benefit on incontinence than a lower dose using local administration) they were too few to allow these other questions to be addressed reliably. In particular, there was little direct evidence to address the choice of oestrogen type (oestriol, oestradiol, oestrone, quinestradiol or conjugated equine oestrogens) and the alternative methods of delivery (oral, subcutaneous implant, transdermal cream, gel or patch, vaginal cream, pessary or ring, or intravesical).
The conflicting evidence on the effects of oestrogens while on treatment together with the concerns about longer‐term risks of unopposed oestrogens (oestrogen alone) raise questions about length of treatment. No trials addressed the question of whether the effects of oestrogen therapy were sustained after treatment was stopped but this seems biologically unlikely.
Quality of the evidence
Both the Hendrix (Hendrix 2005 S) and Grady (Grady 2001 S) trials were of good quality and had the longest duration of follow up (one year in the Hendrix trial, four years in the Grady trial). The Grady trial (Grady 2001 S) included only women with heart disease so the results may not be generalisable, although the results from the Hendrix trial concur with the Grady trial. In the Hendrix trial, women on active treatment may have more visits to the doctor for side effects such as vaginal bleeding and hence would have been more likely to report incontinence symptoms, but there is no evidence for this. There was differential withdrawal at one year within the Hendrix trial, with 9.7% of women taking oestrogen plus progesterone and 6.6% taking placebo stopping taking the medication.
The interpretation of the review is complicated by differences between trials. There were marked variations in the type and dose of oestrogen used, route of administration, type of incontinence and the types of populations studied. The risk of bias was generally moderate and most of the trials were small. Also, the fact that data for each of the pre‐chosen measures of outcome were available for only a minority of trials raised the possibility of selective reporting, which causes bias.
The situation may be confounded by the concurrent use of progestogens to prevent endometrial hyperplasia in women who have not had a hysterectomy, which may exacerbate both irritative urinary symptoms and incontinence (Benness 1991).
Authors' conclusions
Implications for practice.
Local oestrogen treatment may improve or cure incontinence. Data from two small trials, however, suggest that pelvic floor muscle training was more effective in the control of stress incontinence than local oestrogen and one trial stated that local oestrogen combined with PPA was more effective than treatment with PPA or oestrogen alone.
Systemic use of combined conjugated equine oestrogen and progestogens or conjugated equine oestrogen alone given to women for reasons other than incontinence appears not to improve incontinence and may in fact make incontinence worse. This should be discussed with women who use HRT for relief of menopausal symptoms, and particularly those who are incontinent. The risks of long‐term treatment with oestrogens (endometrial and breast cancer, thrombosis causing cardiovascular disease and stroke) suggest that treatment should be for limited periods and using local (vaginal) rather than systemic administration, if possible, especially in women with an intact uterus.
It was not possible to clarify the implications for practice of differing types of oestrogen, routes of administration, or combinations of oestrogen with treatments other than progesterone.
Implications for research.
Larger studies comparing different routes of administration of oestrogen are required. While the review suggests that local oestrogen therapy for urinary incontinence may be effective, the currently available evidence may not be convincing to all people because of generally small sample sizes and the different types, dosages and durations of oestrogen treatment. In that case, further well designed randomised controlled trials should be mounted with adequate sample sizes to assess the effectiveness of oestrogens in comparison to placebo, as well as compared to and as supplements to other non‐surgical and surgical management options. These trials should include follow up after oestrogen therapy has stopped to assess whether any benefits are sustained and if so, for how long and any adverse effects. Further research is needed to clarify the advantages and disadvantages of alternative types of oestrogen, the effect of the addition of progesterone in women with an intact uterus, routes of administration and durations of treatment.
Two large RCTs of systemic HRT using conjugated equine oestrogens in women whose primary complaint was not urinary incontinence suggest strongly that existing urinary incontinence may become worse, and women who are initially dry are more likely to become wet. This outcome should therefore be included in future trials involving HRT. It would also be of interest to compare different types of systemic oestrogen (other than conjugated equine oestrogen) and monitor the effects on urinary incontinence.
Future research on incontinence treatments should incorporate standardised, validated, preferably reproducible and simple outcome measures that are relevant to women who have incontinence in order to allow comparisons between trials. In particular, research in this area should include measures of quality of life and formal economic evaluations.
Long‐term follow up is essential for the proper evaluation of incontinence treatments and the evaluation of the risk of adverse effects.
What's new
| Date | Event | Description |
|---|---|---|
| 10 September 2012 | New search has been performed | Added two trials (Assassa 2003 L; Liapis 2010 L). The two trials compared topical oestrogen to other interventions. Removed Chompootaweep (Chompootaweep 1998 SL) as women were not incontinent at baseline; 22 new studies have been excluded. The conclusions are unchanged since the amendment from 2009. |
| 10 September 2012 | New citation required but conclusions have not changed | Added two trials (Assassa 2003 L; Liapis 2010 L). The two trials compared topical oestrogen to other interventions. Removed Chompootaweep (Chompootaweep 1998 SL) as women were not incontinent at baseline; 22 new studies have been excluded. The conclusions are unchanged since the amendment from 2009. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 23 September 2009 | Amended | changed figures for Hendrix |
| 2 April 2009 | New citation required and conclusions have changed | Substantive amendment Issue 4 2009 Substantive update of review. Six new trials have been added, and extra data were added to four existing trials. One previously included trial was excluded and 10 new studies have been excluded. The conclusions have changed as a consequence of information from two large RCTs of hormone replacement therapy, such that urinary incontinence is likely to worsen with systemic conjugated equine oestrogens. However, there may still be benefits from local administration of oestrogens. |
| 21 May 2008 | Amended | Converted to new review format. |
| 1 February 2003 | New search has been performed | First published Issue 2 2003 |
Acknowledgements
Jean Hay‐Smith and Peter Herbison were very helpful with discussions on the form of the review, data interpretation and trial selection in the first version of this review. Peter Dietz and Gaye Ellis kindly helped with the data extraction from some of the included trial reports. Don Wilson and Adrian Grant have given advice and editorial help. Jonathan Cook provided statistical support. Simon Jackson contributed to the first version of the review.
Data and analyses
Comparison 1. Oestrogen versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with incontinence (women's observations) | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Systemic administration | 4 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.98] |
| 1.2 Local administration | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.62, 0.87] |
| 2 Number with incontinence not improved (women's observations) | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Systemic administration | 5 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.93] |
| 2.2 Local administration | 4 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.43, 0.65] |
| 3 Incontinence not improved (generic inverse variance) (women's observations) | 10 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 3.1 Systemic administration (any incontinence) | 6 | 6151 | Risk Ratio (Fixed, 95% CI) | 1.32 [1.17, 1.48] |
| 3.2 Local administration (any incontinence) | 4 | 213 | Risk Ratio (Fixed, 95% CI) | 0.74 [0.64, 0.86] |
| 4 Number with incontinence (objective observations) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Local administration | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number with incontinence not improved (objective observations) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Local administration | 2 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.57, 0.95] |
| 6 Number of pad changes over 24 hours | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Systemic administration | 3 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.89, 0.66] |
| 7 Pad test weights | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Systemic administration | 3 | 106 | Mean Difference (IV, Fixed, 95% CI) | 1.75 [‐5.67, 9.16] |
| 8 Incontinent episodes over 24 hours | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Systemic administration | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.54 [‐0.50, 1.57] |
| 9 Number of voids over 24 hours | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Systemic administration | 3 | 125 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐1.22, 0.73] |
| 9.2 Local administration | 4 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.58, ‐1.03] |
| 10 Number of nocturnal voids | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Systemic administration | 2 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.40, 0.16] |
| 10.2 Local administration | 3 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐2.03 [‐2.82, ‐1.24] |
| 11 Number of women with frequency | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Systemic administration | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.89, 2.19] |
| 11.2 Local administration | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.98] |
| 12 Number of women with nocturia | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Systemic administration | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.62, 1.62] |
| 12.2 Local administration | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.11, 2.38] |
| 13 Number of women with urgency | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Systemic administration | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.83, 1.33] |
| 13.2 Local administration | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.15, 0.99] |
| 14 Maximum urethral closure pressure (MUCP) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 14.1 Systemic administration | 2 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐1.41 [‐6.24, 3.43] |
| 14.2 Local administration | 5 | 202 | Mean Difference (IV, Fixed, 95% CI) | 4.35 [2.49, 6.22] |
| 15 Volume at first urge to void | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 15.1 Systemic administration | 3 | 153 | Mean Difference (IV, Fixed, 95% CI) | 9.09 [‐25.45, 43.62] |
| 15.2 Local administration | 4 | 116 | Mean Difference (IV, Fixed, 95% CI) | 19.09 [13.21, 24.96] |
| 16 Maximum bladder capacity | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 Systemic administration | 3 | 153 | Mean Difference (IV, Fixed, 95% CI) | 7.18 [‐33.26, 47.62] |
| 16.2 Local administration | 4 | 116 | Mean Difference (IV, Fixed, 95% CI) | 50.11 [35.81, 64.41] |
| 17 Number with adverse effects | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 Systemic administration | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.0 [1.87, 90.21] |
| 17.2 Local administration | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.32, 5.61] |
| 18 Number with bacteriuria | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 Systemic administration | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.37, 1.42] |
| 18.2 Local administration | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.3 [0.13, 0.68] |
1.2. Analysis.
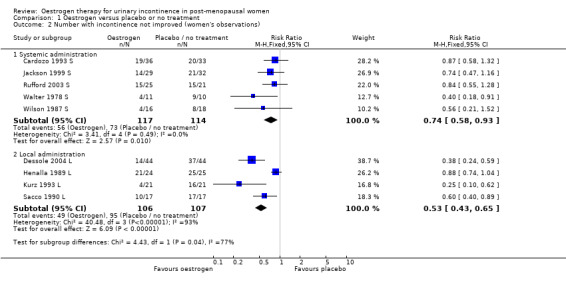
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 2 Number with incontinence not improved (women's observations).
1.18. Analysis.
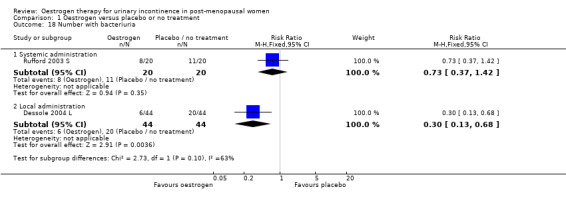
Comparison 1 Oestrogen versus placebo or no treatment, Outcome 18 Number with bacteriuria.
Comparison 2. Oestrogen versus other treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with incontinence (women's observations) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Oestrogen vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number with incontinence, crossover studies (women's observations) | Other data | No numeric data | ||
| 2.1 Oestrogens vs PPA | Other data | No numeric data | ||
| 3 Number with incontinence not improved (women's observations) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oestrogen vs PPA | 2 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.09] |
| 4 Number with incontinence not improved, crossover studies (women's observations) | Other data | No numeric data | ||
| 4.1 Oestrogens vs PPA | Other data | No numeric data | ||
| 5 Number with incontinence (objective observations) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Oestrogen vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number with incontinence not improved (objective observations) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Oestrogen vs PPA | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.46, 1.90] |
| 6.2 Oestrogen vs PFMT | 2 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [1.50, 3.52] |
| 6.3 Oestrogen vs electrostimulation | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.95, 1.75] |
| 7 Number of pad changes over 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Oestrogen vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number of voids over 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Oestrogen vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Pad test weights | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 oestrogen vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 oestrogen vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Maximum urethral closure pressure (MUCP) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 oestrogen vs PFMT | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 oestrogen vs electrostimulation | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Maximum urethral closure pressure (MUCP), crossover studies | Other data | No numeric data | ||
| 12.1 Oetrogens versus PPA | Other data | No numeric data |
2.1. Analysis.
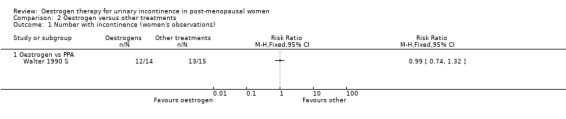
Comparison 2 Oestrogen versus other treatments, Outcome 1 Number with incontinence (women's observations).
2.2. Analysis.
Comparison 2 Oestrogen versus other treatments, Outcome 2 Number with incontinence, crossover studies (women's observations).
| Number with incontinence, crossover studies (women's observations) | ||
|---|---|---|
| Study | Oestrogen | PPA |
| Oestrogens vs PPA | ||
| Beisland 1984 L | 9 out of 10 women | 10 out of10 women |
2.3. Analysis.
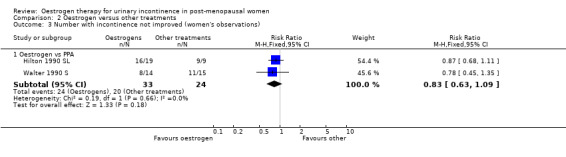
Comparison 2 Oestrogen versus other treatments, Outcome 3 Number with incontinence not improved (women's observations).
2.4. Analysis.
Comparison 2 Oestrogen versus other treatments, Outcome 4 Number with incontinence not improved, crossover studies (women's observations).
| Number with incontinence not improved, crossover studies (women's observations) | ||
|---|---|---|
| Study | Oestrogen | PPA |
| Oestrogens vs PPA | ||
| Beisland 1984 L | 6/10 women | 2/10 women |
2.5. Analysis.
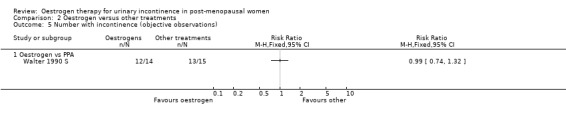
Comparison 2 Oestrogen versus other treatments, Outcome 5 Number with incontinence (objective observations).
2.7. Analysis.
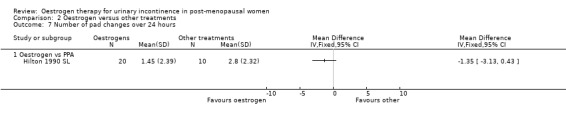
Comparison 2 Oestrogen versus other treatments, Outcome 7 Number of pad changes over 24 hours.
2.8. Analysis.

Comparison 2 Oestrogen versus other treatments, Outcome 8 Number of voids over 24 hours.
2.9. Analysis.
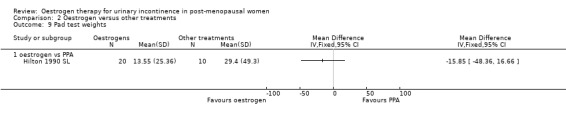
Comparison 2 Oestrogen versus other treatments, Outcome 9 Pad test weights.
2.10. Analysis.
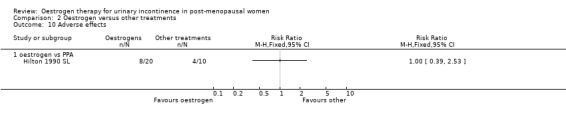
Comparison 2 Oestrogen versus other treatments, Outcome 10 Adverse effects.
2.12. Analysis.
Comparison 2 Oestrogen versus other treatments, Outcome 12 Maximum urethral closure pressure (MUCP), crossover studies.
| Maximum urethral closure pressure (MUCP), crossover studies | ||
|---|---|---|
| Study | Oestrogen | PPA |
| Oetrogens versus PPA | ||
| Beisland 1984 L | Mean=41.6, N=10 | Mean=47.1, N=10 |
Comparison 3. Oestrogen + other treatments versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with incontinence not improved (women's observations) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 oestrogen + progesterone vs placebo | 2 | 1514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [1.01, 1.16] |
| 2 Incontinence not improved (generic inverse variance) (women's observations) | 3 | Relative Risk (Fixed, 95% CI) | Subtotals only | |
| 2.1 Systemic administration of oestrogen + progesterone vs placebo | 3 | 10635 | Relative Risk (Fixed, 95% CI) | 1.11 [1.04, 1.18] |
| 3 Incontinent episodes over 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 oestrogen + progesterone vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of diurnal voids per 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 oestrogen + progesterone vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of nocturnal voids per 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 oestrogen + progesterone vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pad test weights | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 oestrogen + progesterone vs placebo | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Use of drugs for incontinence | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 oestrogen + progesterone vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number of women having incontinence surgery | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 oestrogen + progesterone vs placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
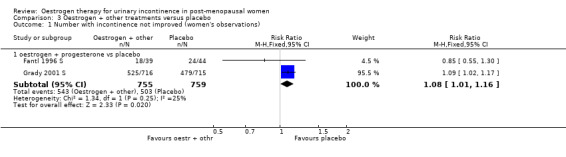
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 1 Number with incontinence not improved (women's observations).
3.3. Analysis.
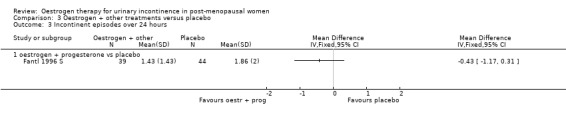
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 3 Incontinent episodes over 24 hours.
3.4. Analysis.
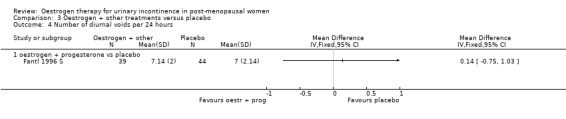
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 4 Number of diurnal voids per 24 hours.
3.5. Analysis.
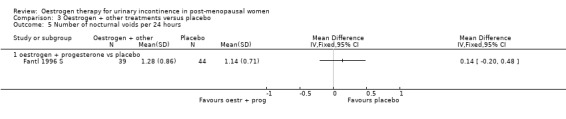
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 5 Number of nocturnal voids per 24 hours.
3.6. Analysis.
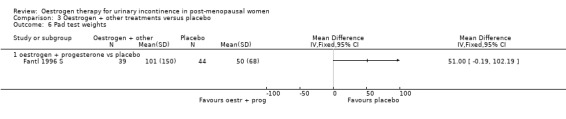
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 6 Pad test weights.
3.7. Analysis.
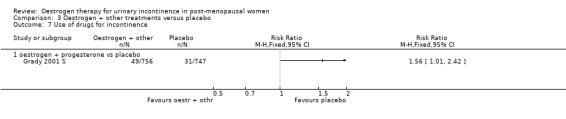
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 7 Use of drugs for incontinence.
3.8. Analysis.
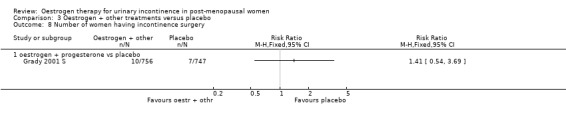
Comparison 3 Oestrogen + other treatments versus placebo, Outcome 8 Number of women having incontinence surgery.
Comparison 4. Oestrogen + other treatments versus oestrogen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with incontinence not improved (women's observations) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Oestrogen + PPA vs oestrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number with incontinence not improved (women's observations) (cross‐over trials) | Other data | No numeric data | ||
| 2.1 oestrogen + PPA vs oestrogen | Other data | No numeric data | ||
| 3 Number of pad changes over 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 oestrogen + PPA vs oestrogen | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of voids over 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 oestrogen + PPA vs oestrogen | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pad test weights | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 oestrogen + PPA vs oestrogen | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of women with frequency | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 oestriol + benzidamine vs oestriol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of women with nocturia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 oestriol + benzidamine vs oestriol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number with adverse effects | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Oestrogen + PPA vs oestrogen | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Volume at first urge to void (cross‐over trials) | Other data | No numeric data | ||
| 9.1 oestrogen + PPA vs oestrogen | Other data | No numeric data | ||
| 10 Maximum bladder capacity (cross‐over trials) | Other data | No numeric data | ||
| 10.1 oestrogen +other vs oestrogen | Other data | No numeric data |
4.3. Analysis.
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 3 Number of pad changes over 24 hours.
4.4. Analysis.
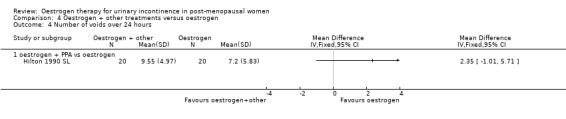
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 4 Number of voids over 24 hours.
4.5. Analysis.
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 5 Pad test weights.
4.8. Analysis.
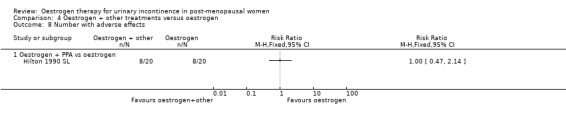
Comparison 4 Oestrogen + other treatments versus oestrogen, Outcome 8 Number with adverse effects.
Comparison 5. Oestrogen + other treatments versus other treatments.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with incontinence (women's observations) | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 oestrogen + PFMT vs PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Vaginal oestrogen + TVT vs TVT | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number with incontinence not improved (women's observations) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 oestrogen + PPA vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of pad changes over 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 oestrogen + PPA vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of voids over 24 hours | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 oestrogen + PPA vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 detrusitol + vaginal oestrogen vs detrusitol m | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Pad test weights | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 oestrogen + PPA vs PPA | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Adverse effects | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 oestrogen + PPA vs PPA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 oestrogen + PFMT vs PFMT | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
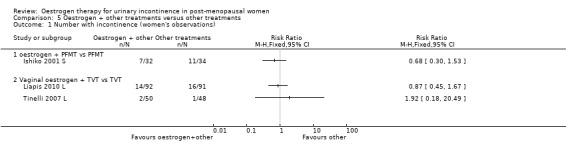
Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 1 Number with incontinence (women's observations).
5.6. Analysis.
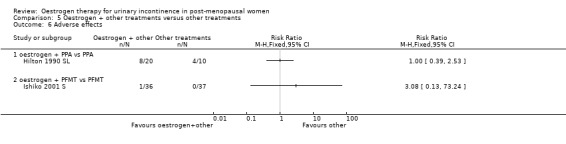
Comparison 5 Oestrogen + other treatments versus other treatments, Outcome 6 Adverse effects.
Comparison 6. Different types of oestrogen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number with incontinence not improved (women's observations) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of women with nocturia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of women with dysuria | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Number of women with urgency | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 oestrogen cream vs vaginal oestrogen + progesterone | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of women with frequency | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 oestrogen cream vs vaginal oestrogen + progesterone | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 oestradiol ring vs oestriol pessary | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
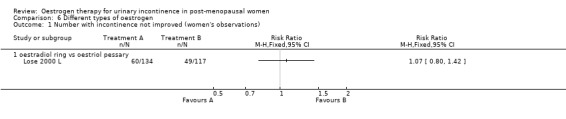
Comparison 6 Different types of oestrogen, Outcome 1 Number with incontinence not improved (women's observations).
6.2. Analysis.
Comparison 6 Different types of oestrogen, Outcome 2 Number of women with nocturia.
6.4. Analysis.
Comparison 6 Different types of oestrogen, Outcome 4 Number of women with urgency.
6.5. Analysis.
Comparison 6 Different types of oestrogen, Outcome 5 Number of women with frequency.
6.6. Analysis.
Comparison 6 Different types of oestrogen, Outcome 6 Number of adverse events.
Comparison 7. Different routes of administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of pad changes over 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 oestrogen cream vs oestrogen orally | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of voids over 24 hours | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 oestrogen cream vs oestrogen orally | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Pad test weights | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 oestrogen cream vs oestrogen orally | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. High‐dose versus low‐dose oestrogen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of voids over 24 hours | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.02 [‐1.87, ‐0.16] |
| 2 Number of nocturnal voids | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐2.36, ‐1.24] |
| 3 Maximum urethral closure pressure (MUCP) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 3.84 [‐5.77, 13.46] |
| 4 Volume at first urge to void | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.56 [‐12.20, 9.08] |
| 5 Maximum bladder capacity | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 high dose vs low dose | 2 | 65 | Mean Difference (IV, Fixed, 95% CI) | 34.90 [8.35, 61.45] |
8.3. Analysis.
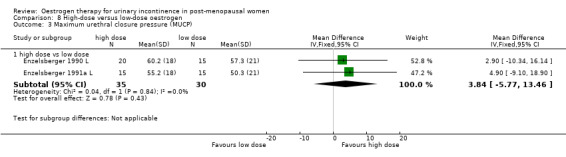
Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 3 Maximum urethral closure pressure (MUCP).
8.4. Analysis.
Comparison 8 High‐dose versus low‐dose oestrogen, Outcome 4 Volume at first urge to void.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahlstrom 1990 S.
| Methods | RCT (double‐blind crossover) ITT | |
| Participants | 29 women Incl: USI, post‐menopausal Excl: hypertension, significant bacteriuria, previous breast or uterine cancer, residual urine, drugs (neuroleptics, sedatives, antihistamines, ephedrine, B‐blockers, gestagens, oestrogens within previous 2 months Mean age 63 (range 51‐73) years Mean weight 71 (range 55‐90) kg | |
| Interventions | Group A (n=29): 4mg oestriol + 50mg PPA twice daily for 6/52 Group B (n=29): 4mg oestriol + placebo for 6/52 | |
| Outcomes | Urodynamics, urinary diary, subjective assessment, assessment of atrophy, urethral + vaginal cytology, feeling of vaginal dryness, pad test | |
| Notes | No useable data; adverse events: group 1: 1 sweating, 1 constipation, 1 insomnia; group 2: 1 gastritis, 1 paraesthesia, 1 tachycardic attack, 1 dizziness, 2 nausea, 1 vomiting, 1 constipation; losses to follow up: group 1:1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization was made in groups of 4" |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "all 29 completed, one woman withdrawn from analyses of second treatment period due to failure in medication" |
Assassa 2003 L.
| Methods | RCT (double‐blind placebo) | |
| Participants | 220 post‐menopausal women Inclusion: Urinary incontinence and storage symptoms |
|
| Interventions | Topical oestrogen (Estring) versus placebo for 3 months Assessment was undertaken using urinary diaries, pad tests and physical examination |
|
| Outcomes | Primary: Symptom severity including urinary incontinence, frequency, urgency and nocturia. Impact on activities, cost effectiveness and patient satisfaction. Secondary: Clinical improvement in incontinence and voiding function. Changes in atrophic vaginitis and sexual function. |
|
| Notes | All women received standard non‐pharmacological therapies by fully trained nurses for storage symptoms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No expansion on "randomisation" |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Beisland 1984 L.
| Methods | Randomised open comparative crossover trial; duration of study: 12/52; assessment after each 4/52 period of treatment | |
| Participants | 20 post‐menopausal women Inclusion criteria: incontinence Exclusion criteria: neurological and gynaecological disorders, urinary tract infections or tumours, general conditions contraindicating oestrogen and phenylpropanolamine therapy | |
| Interventions | Group A (n=20): 1mg oestriol vaginally daily for 4/52, then 50mg phenylpropanolamine (PPA) orally twice daily for 4/52, then combined for 4/52 Group B (n=20): 50 mg phenylpropanolamine twice daily for 4/52, then 1mg oestriol vaginally daily for 4/52, then combined for 4/52 | |
| Outcomes | Subjective assessment of treatment result, urodynamics, atrophy evaluation by clinical picture, cytological investigation by urethral smear Definition of cure: patient described improvement as good, definition of improvement: patient‐reported improved, definition of failure: patient‐reported unchanged or worse | |
| Notes | adverse events: 1 genital bleeding in period of combined treatment, 1 complete insomnia after 3 days with PPA losses to follow up: 2 (same patients as for adverse events) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | "randomised open comparative cross‐over trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18 out of 20 completed the study |
Blom 1995 S.
| Methods | Placebo controlled, single‐blind randomised crossover study Duration of study: 28/52 | |
| Participants | 16 women, Inclusion criteria: ambulant elderly women with established detrusor instability; 13 out of 16 had hysterectomy Exclusion criteria: breast or endometria carcinoma, thromboembolic disorders, severe hypertension, cardiac failure, diabetes mellitus, peptic ulceration Drugs that could interact with effects of study medication were discontinued | |
| Interventions | Crossover study Group A(n=16): oestradiol TTS transdermal system 0.05 mg/day, patch twice weekly for 8/52 Group B (n=16): oestradiol TTS transdermal system 0.05 mg/day, patch twice weekly + naproxen tablets 250 mg bd for 8/52 Group C (n=16): placebo TTS transdermal system, patch twice weekly for 8/52 Washout period of 2/52 followed each medication regimen | |
| Outcomes | Urinary diary, cystometry | |
| Notes | No useable data; adverse events: skin reactions to transdermal system: mild erythema and itching, 2 patients had more severe cutaneous reactions, but continued with treatment, systemic adverse effects: mild breast tenderness, 1 patient with uterus experienced spotting Losses to follow‐up: 3, for reasons not related to study medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label single blind (patients blinded?) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 out of 19 completed. "Drop out not related to study medication" |
Cardozo 1993 S.
| Methods | Multicentre study, 6 centres (10 participated, but 4 did not recruit any patients), countries: UK, NL, D, DK (London, Bristol, Maastricht, Munich, Aalborg, Cheltenham) Double‐blind placebo controlled RCT, randomisation code held centrally Duration of study 3/12, assessments at 1/12 with 3‐day urinary diary and 4‐point severity score + at 3/12 with subjective and objective investigations Definition of cure: absence of a specific symptom at 3/12 that was present at entry obtained from direct symptom questions | |
| Participants | 64 post‐menopausal women with urodynamically confirmed urgency urinary incontinence Baseline characteristics comparable Inclusion criteria: ambulant, postmenopausal >1 year, FSH > 40 IU/l, oestradiol < 220 pmol/l, urgency urinary incontinence Exclusion criteria: symptoms present for > 3 years before menopause, voiding difficulty, pelvic anatomic defect requiring surgery, neurological disease, recent oestrogen usage (< 6/12), concomitant medication that could affect bladder or urethral function, standard contraindications to oestrogen therapy | |
| Interventions | Group A (n=34): oestriol orally 3 mg for 3/12 Group B (n=30): placebo orally for 3/12 | |
| Outcomes | Subjective assessment with doctor‐administered questionnaire concerning presence or absence of specific urinary symptoms (24 questions) and 4‐point severity score scale (9 questions), objective assessment with 3‐day urinary diary, filling and voiding cystometry, urethral pressure profile where available, vaginal smear for maturation index calculated by single pathologist;
Definition of cure: absence of a specific symptom at 3/12 that was present at entry obtained from direct symptom questions Number not cured: urgency urinary incontinence: A, 14/25; B, 16/23; stress incontinence: A, 5/11; B, 4/10 |
|
| Notes | Urgency incontinence stratified into motor and sensory urgency incontinence, motor urgency incontinence defined as uninhibited detrusor contractions exceeding 15 cm of water, sensory urgency incontinence defined as first desire to void during filling cystometry at less than 150 ml and a cystometric capacity of 400 ml in absence of detrusor activity Losses to follow‐up: group A : 3, group B: 5 No adverse outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Low risk | "Randomisation code was held centrally" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 out of 64 women did not complete study (3 reason unknown,1 loss of motivation, 1 lack of efficacy, 1 UTI, 1 exclusion criteria, 1 did not start. Equal dropouts between groups |
Cardozo 2001 L.
| Methods | Double‐blind placebo controlled RCT Duration of study: 12/52, assessments at 4/52 and 12/52 | |
| Participants | 105 post‐menopausal women, Inclusion criteria: at least 1 year since last menstrual period, FSH > 40 U/l, estradiol < 220 pmol/l, complaining of lower urinary tract symptoms of urgency, frequency and/or urgency urinary incontinence with onset not earlier than 3 years prior to menopause; Exclusion criteria: currently or recently (within the last 6/12) on oestrogen therapy, present or past history of oestrogen dependent neoplasia, abnormal genital bleeding of unknown origin, present or past history of thromboembolic disorders, urinary or vaginal infection, recent commencement or change in diuretic treatment | |
| Interventions | Group A: 17B‐oestradiol vaginal pellet (Vagifem) 25 µgm at night for 12/52 Group B: placebo pellet at night for 12/52 | |
| Outcomes | Questionnaire and frequency/volume chart, urethral brushing for cytology, compliance check regarding tablet usage, urodynamics, MSU, FSH, LH, oestradiol, endometrial biopsy, visual analogue score, symptom assessment sheet, cystometry data sheet Key endpoints of interest: symptoms of frequency, urgency, nocturia at final visit | |
| Notes | No useable data; no numbers of women in groups; no adverse events Losses to follow up: 9, of which 2 due to medical reasons (1 intercurrent medical disease, 1 died of myocardial infarction) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In both groups three women failed to commence treatment. Outcome data on 106 out of 110 randomised |
Dessole 2004 L.
| Methods | RCT: placebo controlled, sealed opaque envelopes Duration of study: 6 months Follow up: none after end of treatment | |
| Participants | 88 post‐menopausal women Groups comparable at baseline on age, menopause duration, duration of UI, bacteriuria Inclusion: urodynamic stress incontinence (moderate to severe), urogenital atrophy, recurrent UTI Exclusion: detrusor overactivity, abnormal maximal cystometric capacity, prolapse Grade II or III; systemic disorders, systemic disease, thromboembolic disease, biliary lithiasis, breast or uterine cancer, abnormal uterine bleeding, BMI>25 | |
| Interventions | Group A (44): intravaginal oestriol ovules, 1 mg daily for 2 weeks, 2 mg weekly for 6 months Group B (44): matching placebo Dropout: A: 4/44, B: 3/44 (discomfort, local adverse effects), + B: 4/44 (no benefit) | |
| Outcomes | Incontinent at 6 months: group A: 37/44, group B; 44/44 Not improved at 6 months:group A: 14/44, group B: 37/44 Adverse effects: group A: 4/44,group B: 3/44 (caused dropout) Significant bacteriuria, group A: 6/44, group B: 20/44 | |
| Notes | Participants and outcome assessors blinded to treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Low risk | "sequenced, sealed, opaque envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind "participants and investigators were blinded to the drug being dispensed and to the assigned treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
Ek 1980 S.
| Methods | RCT (double‐blind crossover in 5th and 6th weeks of the study); duration of study: 8/52, F/U at 8/52 | |
| Participants | 16 post‐menopausal women with urodynamically proven GSI | |
| Interventions | All women (n=16): oestradiol valerate 2mg daily for 3/52, then 1mg for 1/52 group A (n=13): oestradiol 1mg + norephedrine 200mg daily for 2/52 after first 4/52 group B (n=13): oestradiol 1mg + placebo daily for 2/52 after first 4/52 after 2 weeks, groups 1 and 2 crossed over to the opposite arm | |
| Outcomes | Residual urine, MSU, urodynamics, clinical stress test, periurethral vaginal biopsies, subjective symptoms | |
| Notes | No useable data; adverse events: "few and acceptable", very small changes in BP, no uterine bleeding Losses to follow up: group 2: 2, group 3: 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Enzelsberger 1990 L.
| Methods | RCT, duration of study 4/52 | |
| Participants | 35 women Inclusion criteria: urgency symptoms, urgency urinary incontinence, frequency, nocturia Exclusion criteria: renal disease, infections in the urogenital area, anticholinergic or hormone therapy | |
| Interventions | Group A (n=15): 0.5 or 1 mg oestriol vaginally Group B (n=20): 2 mg oestriol vaginally | |
| Outcomes | Urinary diary, cystoscopy, urodynamics, urethral and vaginal smear | |
| Notes | No endometrial stimulation or breast tenderness noted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Enzelsberger 1991a L.
| Methods | Placebo controlled RCT, randomisation following randomisation scheme, duration of study: 4/52 | |
| Participants | 40 post‐menopausal women Inclusion criteria: urgency symptoms, urgency urinary incontinence, frequency ( 6 or more micturitions/day), nocturia (3 or more micturitions/ night) Exclusion criteria: neurological bladder problems, renal disease, diabetes, infections in the urogenital area, anticholinergic or hormone‐therapy, malignancy | |
| Interventions | Group A (n=15): 1 mg oestriol vaginally for 3/52 Group B (n=15): 3 mg oestriol vaginally for 3/52 Group C (n=10): placebo vaginally for 3/52 | |
| Outcomes | Cystometry, urethral pressure profile, cystoscopy, urethral smear, urinary diary, MSU, FSH levels, E2 levels | |
| Notes | Adverse outcomes: breast tenderness in 2 patients in group 2 Losses to follow up: group B: 2, group C: 1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Enzelsberger 1991b L.
| Methods | as Enzelsberger A | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Fantl 1996 S.
| Methods | Multicentre trial, 2 centres, double‐blind placebo controlled RCT, patients stratified by baseline severity of symptoms, urodynamics and treatment site; blocked randomisation within each stratum at each site; sequenced sealed opaque envelopes Women and investigators were blinded to treatment allocated Duration of study 3/12 | |
| Participants | 83 post‐menopausal women with UI Inclusion criteria: all patients ambulatory, community‐dwelling residents, age 45 or older, involuntary loss of urine at least once a week, GSI was diagnosed when urine was objectively seen to be lost during exertion in absence of DI; DI defined as involuntary detrusor contractions during retrograde subtracted provocative cystometry; hypooestrogenism = plasma E2 levels 30 pg/ml or less Exclusion criteria: institutionalisation, permanent catheterisation, impaired mental status, functional disability limiting use of toilet, neuropathic or uncontrolled metabolic conditions (e.g. diabetes mellitus), chronic UTI, reversible causes of urinary incontinence (e.g. faecal impaction), major contraindications for use of oestrogens (e.g. breast cancer) |
|
| Interventions | Group A (n=39): conjugated equine oestrogens 0.625 mg for 30 days + medroxyprogesterone 10 mg for 10 days of each cycle Group B (n=44): placebo similar cyclic regimen Women, clinicians and pharmacists were blinded to actual treatment | |
| Outcomes | Data recorded in standardised urinary diaries Primary outcome: number of incontinent episode per week, N, mean (SD): A 39, 10 (10), B 44 13 (14) cure (definition not given), results provided as patient's perception of somewhat or much better: A 54% (21/39), B 45% (20/44), P= 0.435 Secondary outcomes: pad test (volume of urine loss): A 101 (150), B 50 (68) number of voluntary diurnal micturitions per week: A 50 (14), B 49 (15) number of voluntary nocturnal micturitions per week: A 9 (6), B 8 (5) quality of life measurements generic: SF‐36 Health survey + Centre of epidemiological studies depression scale, condition specific QoL: Incontinence Impact Questionnaire‐revised (IIQ‐R): A 97 (87), B 100 (82) Urogenital Distress Inventory (UDI): A 101 (58), B 102 (55) E2 level: A 61 (42), B 10 (16) (placebo unchanged from baseline) vaginal parabasal cell count: A 3±17%, B 49±43% |
|
| Notes | Loss to follow up: 2, and 8 only had diary or QoL data. Groups not given, unclear if these are extra to the 83 with outcome data reported Patients also taking progesterone which may have altered results Power calculation: study had 80% power to detect a difference of 4.5 episodes per week | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Sequenced, sealed, opaque envelopes, each containing the bottle number to be given to an individual patient |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, clinicians and pharmacists blind to drug being dispensed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
Grady 2001 S.
| Methods | RCT, randomisation stratified by clinical centre, within‐strata treatment randomised in fixed size blocks Follow up at 4 months then annually for 4 years |
|
| Participants | 1525 participants age less than 80, post‐menopausal, with intact uterus, and at least 1 episode of UI per week all patients had coronary heart disease Baseline characteristics in both groups similar for age, race, education, menopause age, parity, chronic medical conditions, smoking and alcohol consumption and BMI | |
| Interventions | Group A (n=768) conjugated oestrogen (Premarin) 0.625mg + medroxyprogesterone acetate (Cycrin) 2.5mg Group B (n=757) identical placebo Oral tablets | |
| Outcomes | Worse or unchanged at 1 year: A 525/716, B 479/715
Worse or unchanged at 2 years: A 499/680, B 464/686
Worse or unchanged at 3 years: A 478/640, B 436/639
Worse or unchanged at 4 years: A 541/756, B 506/747 Number of incontinent episodes: A 5.5 per week (increase of 0.7), B 5.6 per week (decrease of 0.1) Using drugs for incontinence by 4 years: A 49/756, B 31/747 Incontinence surgery by 4 years: A 10/756, B 7/747 No change in either group in frequency of stress or urgency urinary incontinence, or in diurnal or nocturnal frequency |
|
| Notes | Compliance at 1st year: A 82%, B 88% still taking the treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes prepared prepared with computer generated random numbers. Stratified by site and performed using randomly permuted blocks of 4 |
| Allocation concealment (selection bias) | Low risk | Eligible participants assigned with equal probability to the two groups by tamper proof randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and investigators blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Henalla 1989 L.
| Methods | RCT, duration of study: 3/12, follow up at 3/12 and 9/12 | |
| Participants | 100 women; Inclusion criteria: urodynamically proven GSI Exclusion criteria: complicated history of incontinence such as fistula or more than one previous incontinence surgery, major prolapse, absolute contraindication to oestrogen treatment | |
| Interventions | Group A (n=24): conjugated equine oestrogen vaginal cream (Premarin) 1.25 mg at night for 12/52 Group B (n=25): no treatment | |
| Outcomes | Pad test, urethral pressure studies at 3/12, symptom questionnaire at 9/12 Definition of cure: negative pad test after previous positive; improvement: more than 50% reduction in pad weights | |
| Notes | Numbers given as "cured or improved" and "unchanged"; recurrences of symptoms at 9/12 in women who had initially improved: group A: 3, group B: 1, group C: 3 (recurrence of symptoms immediately after discontinuing oestrogen treatment) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "allocated at random" |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible, drug compared to PFMT |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 100 out of 104 evaluated no info on dropouts |
Henalla 1990 L.
| Methods | RCT, duration of study: 6/52 | |
| Participants | 26 post‐menopausal women; inclusion criteria: GSI | |
| Interventions | Group A (n=11): conjugated equine oestrogen vaginal cream (Premarin) 2 g at night for 6/52 Group B (n=7): control All failures had surgical repair | |
| Outcomes | Vaginal pH, vaginal cell count from smear, pad test, electromyographic traces to assess urethral sphincter activity; Definition of failure: < 50% reduction from original pad test loss; numbers given as "cured or improved" and "unchanged" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Hendrix (hysterectomy) S.
| Methods | RCT As Hendrix 2005 | |
| Participants | Wet at baseline Population 1: (hysterectomy) N=6528 at baseline, outcome data at one year N=5920 | |
| Interventions | Population 1 (wet at baseline, without uterus): Group A (2950): conjugated equine oestrogen alone Group B (2970): matching placebo | |
| Outcomes | Population 1 (wet at baseline, without uterus)
Likelihood of worsening amount of UI: A versus B: RR = 1.59 (95% CI 1.39 to 1.82)
Likelihood of worsening frequency of UI: A versus B: RR = 1.47 (1.35 to 1.61)
Likelihood of limitation of activities related to UI: A versus B: RR = 1.29 (1.15 to 1.45) Worsening was not related to one type of urinary incontinence alone |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study pill bottles had unique bar codes and computer based selection to enable double blinded dispensing" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed using a study database distributed by the WHI Clinical Coordinating Center to the local centres" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind ‐ clinic staff and participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At 1 year vital status was known for 99.9% of participants in the oestrogen + progesterone trial and 100% for those in oestrogen only trial?? At 1 year 9.7% of women taking oestrogen + progesterone and 6.6% taking placebo stopped taking the pills Oestrogen alone 8.4% and placebo 8% stopped taking the pills |
Hendrix (no hysterect) S.
| Methods | RCT As Hendrix 2005 | |
| Participants | Wet at baseline Population 2: (with uterus) N=9889 at baseline, outcome data at one year N=9121 | |
| Interventions | Population 2 (wet at baseline, with uterus): Group C (n=4572): conjugated equine oestrogen + medroxyprogesterone acetate Group D (n=4549): matching placebo | |
| Outcomes | Population 2 (wet at baseline, with uterus)
Likelihood of worsening amount of UI: C versus D: RR = 1.20 (95% CI 1.06 to 1.36)
Likelihood of worsening frequency of UI: C versus D: RR = 1.38 (1.28 to 1.49)
Likelihood of limitation of activities related to UI: C versus D: RR = 1.18 (1.06 to 1.32) Worsening was not related to one type of urinary incontinence alone |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study pill bottles had unique bar codes and computer based selection to enable double blinded dispensing" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed using a study database distributed by the WHI Clinical Coordinating Center to the local centres" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind ‐ clinic staff and participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At 1 year vital status was known for 99.9% of participants in the oestrogen + progesterone trial and 100% for those in oestrogen only trial?? At 1 year 9.7% of women taking oestrogen + progesterone and 6.6% taking placebo stopped taking the pills Oestrogen alone 8.4% and placebo 8% stopped taking the pills |
Hendrix 2005 S.
| Methods | RCT, 25,597 women Central randomisation, computer‐based selection, double‐blind dispensing Follow up at one year and then a subsample after 3 years Baseline QOL, questionnaire, interview, examination and bloods | |
| Participants | RCT of hormone replacement for prevention of coronary heart disease and hip fracture, with and without symptoms of stress, urgency urinary or mixed incontinence at baseline
Women all aged between 50‐79 years, post‐menopausal Incontinent (wet) women (Populations 1 and 2) Population 1: (hysterectomy) N=6528 at baseline, outcome data at one year N=5920 Population 2: (with uterus) N=9889 at baseline, outcome data at one year N=9121 Continent (dry) women (Populations 3 and 4) Population 3: (hysterectomy) N=3473 at baseline, outcome data at one year N=3073 Population 4: (with uterus) N=5707 at baseline, outcome data at one year N=5182 All women on HRT treatment at baseline had to have 3 month washout Whole sample comparable at baseline on age, education, illness, menopause, parity, breastfeeding history, hormone use, hysterectomy status, physical activity and smoking Exclusion criteria: breast CA, other invasive carcinoma in the last ten years, venous thromboembolism, hypertriglyceridaemia, medical condition which may result in death in the next three years, unwilling or unable to be randomised to placebo, severe menopausal symptoms at washout All women had a four week trial with placebo to which they had to show 80% adherence |
|
| Interventions | Population 1 (wet at baseline, without uterus): group A (2950): conjugated equine oestrogen alone group B (2970): matching placebo Population 2 (wet at baseline, with uterus): group C (n=4572): conjugated equine oestrogen + medroxyprogesterone acetate group D (n=4549): matching placebo Population 3 (continent at baseline, without uterus): group E (1526): conjugated equine oestrogen alone group F (1547): matching placebo Population 4 (continent at baseline, with uterus): group G (2675): conjugated equine oestrogen alone group H (2507): matching placebo | |
| Outcomes | Population 1 (wet at baseline, without uterus)
Likelihood of worsening amount of UI: A versus B: RR = 1.59 (95% CI 1.39 to 1.82)
Likelihood of worsening frequency of UI: A versus B: RR = 1.47 (1.35 to 1.61)
Likelihood of limitation of activities related to UI: A versus B: RR = 1.29 (1.15 to 1.45) Population 2 (wet at baseline, with uterus) Likelihood of worsening amount of UI: C versus D: RR = 1.20 (95% CI 1.06 to 1.36) Likelihood of worsening frequency of UI: C versus D: RR = 1.38 (1.28 to 1.49) Likelihood of limitation of activities related to UI: C versus D: RR = 1.18 (1.06 to 1.32) Worsening was not related to one type of urinary incontinence alone Population 3 (dry at baseline) Any UI at 1 year: E, 557/1526, F, 368/1547 RR=1.53 (1.37 to 1.71) Stress UI at 1 year: E, 266/1526, F, 131/1547 RR=2.15 (1.77 to 2.62) Urgency UI at 1 year: E, 210/1526, F, 184/1547 RR=1.32 (1.10 to 1.58) Mixed UI at 1 year: E, 76/1526, F, 50/1547 RR=1.79 (1.26 to 2.53) Population 4 (dry at baseline) Any UI at 1 year: G, 834/2675; H, 563/2507 RR=1.39 (1.27 to 1.52) Stress UI at 1 year: G, 429/2675; H, 218/2507 RR=1.87 (1.61 to 2.18) Urgency UI at 1 year: G, 304/2675, H, 272/2507 RR=1.15 (0.99 to 1.34) Mixed UI at 1 year: G, 99/2675, H, 69/2507 RR=1.49 (1.10 to 2.01) |
|
| Notes | No data suitable for meta‐analysis were provided in the original report but these have been requested Data used in generic inverse variance analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study pill bottles had unique bar codes and computer based selection to enable double blinded dispensing" |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed using a study database distributed by the WHI Clinical Coordinating Center to the local centres" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, clinic staff and participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At 1 year vital status was known for 99.9% of participants in the oestrogen + progesterone trial and 100% for those in oestrogen only trial?? At 1 year 9.7% of women taking oestrogen + progesterone and 6.6% taking placebo stopped taking the pills Oestrogen alone 8.4% and placebo 8% stopped taking the pills |
Hilton 1990 SL.
| Methods | Double‐blind RCT, patients were randomised into 6 groups; duration of study 4/52, evaluation on day 28 of study | |
| Participants | n= 60 women Inclusion: USI, post‐menopausal Exclusion: Undiagnosed vaginal bleeding, oestrogen therapy within preceding 6 months, malignancy of oestrogen‐dependent tissue, BP >160 mmHg systolic, >100 mmHg diastolic, previous thromboembolic disease, liver disease, patients withholding informed consent Age range 45‐70 years | |
| Interventions | A (10): Intravaginal oestrogen (2 gr nocturnal) + PPA (50 mg twice daily) B(10): Intravaginal oestrogen (2 gr nocturnal) + placebo (twice daily) C (10): Oral oestrogen (1.25 mg daily) + PPA (50 mg twice daily) D (10): Oral oestrogen (1.25 mg daily) + placebo (twice daily) E (10): PPA (50 mg twice daily) F (10): Intravaginal placebo (nocturnal) + placebo (twice daily) Duration of treatment: 4 weeks | |
| Outcomes | Subjective improvement: A: 9/10, B: 1/9, C: 4/10 D: 2/10, E: 0/9, F: 2/11 Mean number of pads/day: A: 0.9, B: 0.3, C: 0.9, D: 1.3, E: 2, F: 1.1 Mean pad weights: A: 8, B: 4, C: 4, D: 4, E: 4, F: 12 Adverse events: A: 3/10, B: 3/10, C: 5/10, D: 5/10, E: 4/10, F: 3/10 (headache, nausea, flushing, sweating, tingling, breast tenderness, ecchymoses) Side effects causing discontinuation: C: 1/10, E: 1/10 | |
| Notes | Data estimated from graphs No SDs Comparisons: alpha adrenergic drug + oestrogen (I and III) versus oestrogen alone (II and IV) versus alpha‐adrenergic drug alone (V) versus placebo (VI) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Ishiko 2001 S.
| Methods | Randomised controlled trial Duration of study 2 years Monthly uterine ultrasound examination and 6‐monthly endometrial PAP smears Setting: Japan |
|
| Participants | 66 post‐menopausal women with stress urinary incontinence, age 54 to 75 years, groups comparable at baseline Dropouts: a further 6 by choice, 1 for adverse drug reaction hepatopathy): A, 4; B, 3 Inclusion criteria: stress urinary incontinence alone (based on questionnaires) Exclusion criteria: urgency or mixed urinary incontinence 10% of women had a previous hysterectomy |
|
| Interventions | A (32): PFMT + oestriol tablet (1 mg/day) B (34): PFMT alone PFMT was taught by a gynaecology specialist, supplemented by videotape. The aim was 15 minutes of exercise a day |
|
| Outcomes | Persisting incontinence: A: 7/32; B, 11/34 (Mild UI: A, 0/12; B, 2/11: Moderate UI: A, 3/14; B, 5/18: Severe UI: A, 4/6; B, 4/5) Adverse effects: A: 1/36; B, 0/37 No report of effect on uterus |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | Randomly assigned |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 out of 36 withdrew from exercise and oestrogen group 3 out of 37 from exercise only group |
Jackson 1999 S.
| Methods | Double‐blind placebo controlled RCT, duration of study 6/12; no definition of cure given, but results of no demonstrable stress incontinence on repeat cystometry (urodynamically 'cured') | |
| Participants | 67 post‐menopausal women with urodynamically proven GSI; inclusion criteria: >12/12 post‐menopausal, not taken HRT in previous 12/12 Exclusion criteria: cancer of endometrium, liver, breast; endometrial thickness > 4mm Included women who had had hysterectomy |
|
| Interventions | Group A (n=33): oestradiol 2 mg orally for 6/12 Group B (n=34): placebo orally similar regime | |
| Outcomes | Urinary diary for 1 week, SF‐36 + B‐FLUTS questionnaires, 1 hour perineal pad test, urodynamics, repeat trans‐vaginal sampling + Pipelle biopsy if endometrial thickness>6mm, compliance, serum oestradiol levels | |
| Notes | Losses to follow up: 3 in group A, 2 in group B; 1 patient in each group left at 3/12 for surgery, both were reassessed and data are included Adverse outcomes: 6 women taking oestradiol had breakthrough bleeding, they started taking cyclical progestogen, repeat investigations in progestogen‐free part of their cycle, no case of endometrial atypia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence generated by computer in block sizes of 10 (5 oestradiol, 5 placebo) |
| Allocation concealment (selection bias) | Low risk | Randomised by hospital pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Women participants and care providers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three out of 33 on oestradiol failed to complete the trial, two out of 34 on placebo. One woman in each arm left trial at three months opting for surgery (included in the analysis) |
Judge 1969 S.
| Methods | Multicentre trial, 2 hospitals; placebo‐controlled double‐blind crossover RCT, duration of study 5 weeks | |
| Participants | 20 post‐menopausal women, patients in two hospitals in geriatric long‐stay beds for at least 1 month, 10 patients confused, 1 demented, 8 mentally normal; 14 had neurological disease Types of incontinence divided into: incontinent and unaware of it; incontinent, aware but not distressed by it; incontinent, aware and distressed Patients divided naturally into two groups (two hospitals), one group mildly incontinent, one group severely incontinent No change of diuretic during trial Excluded if faecally impacted | |
| Interventions | Group A (n=18): quinestradol 0.25 mg qds for 1/12 Group B (n=18) placebo similar regime All 18 patients had quinestradol and placebo, but not stated how many had quinestradol first and then placebo or placebo first and then quinestradol | |
| Outcomes | Observed numbers of incontinent episodes requiring bed change per week | |
| Notes | No useable data; unclear if groups were treated identically as patients had neurological disease or confusion and lived in different geriatric hospitals; losses to follow up: 2 (from initial 20); patients too confused for subjective assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A set of random numbers was used |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacist provided with active ingredient labelled capsule X and capsule Y along with an emergency sealed key for emergency use |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Kinn 1988 S.
| Methods | RCT (randomised double‐blind crossover) | |
| Participants | 36 post‐menopausal women Inclusion criteria: GSI Exclusion criteria: not mentioned Mean age 66 (49‐82) Urgency urinary incontinence n = 7, previous anti‐incontinence operation n = 8, previous gynaecological operations n = 5 | |
| Interventions | Group A (n=36): oestriol 2mg orally + placebo twice daily Group B (n=36): oestriol 2 mg orally + PPA 50 mg twice daily for 4/52 | |
| Outcomes | Subjective improvement: A: 9/30, B: 16/30 Leakage episode/day: A: 2.4, B: 2.4 Mean number of voids/day: A: 7.2, B: 6.9 Mean pad weight/day:A: 34.9, B: 24.9 | |
| Notes | No useable data; adverse events: dryness of the mouth + ? arrhythmia, itch, depression; but not clear on which treatment losses to Follow up: 6 (3 intercurrent diseases, 3 possible drug effects: arrhythmia, itch, depression) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | Crossover design |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no descriptioin |
Kurz 1993 L.
| Methods | Placebo controlled double‐blind RCT, duration of study: 4/52 | |
| Participants | 42 post‐menopausal women Inclusion criteria: urgency urinary incontinence, frequency (6 or more micturitions/day), nocturia (3 or more micturitions/night) Exclusion criteria: endocrine diseases (diabetes mellitus), renal disease, acute or chronic UTI, any other medication for high BP or cardiac failure, previous treatment with parasympatholytics and/or oestrogens | |
| Interventions | Group A (n=21): 1 mg oestriol in 10 ml sesame‐oil intravesically (into the bladder) every other day for 3/52 Group B (n=21): placebo (10 ml sesame‐oil every other day for 3/52) | |
| Outcomes | Urinary diary, MSU, first urge to void, max. bladder capacity, functional urethral length, maximum urethral closure pressure, depression coefficient, cystometry, cystoscopy to assess bladder mucosa, vaginal and urethral smears, hormone levels of LH, FSH, oestradiol, SHBG, urinary diary, subjective measurement with visual analogue scale, assessment of burning at micturition, pain at micturition, bladder spasms, nocturia, frequency | |
| Notes | No adverse outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Liapis 2010 L.
| Methods | Prospective randomised study between February 2004 and January 2009. Intention‐to‐treat analysis |
|
| Participants | 188 randomised, 183 patients completed study Inclusion: Post‐menopausal, stress urinary incontinence Exclusion: Urge or mixed incontinence. Cystocoele > stage II. |
|
| Interventions | Group 1 (n=92): Underwent TVT‐O anti‐incontinence surgery followed by vaginal oestradiol treatment Group 2 (n=91): Underwent TVT‐O surgery only Patients in group 1 took 25 micrograms oestradiol vaginally once daily, nocte, for 2 weeks and then twice weekly for 6 months. |
|
| Outcomes | Patients asked to complete 3 day bladder diary, uroflow, filling and voiding cystometry and urethral profilometry pre‐operatively and at 2 and 6 months follow up. Primary: Post‐operative symptoms (frequency, urgency, nocturia) Secondary: Maximum flow rate, maximum cystometric capacity, maximum urethral closure pressure, maximum urethral length, detrusor pressure at maximum flow, maximum detrusor pressure and post‐void residual. In general, the oestradiol treated group had a statistically significant better outcome compared to the non‐oestradiol one. |
|
| Notes | Subjective cure was considered as the absence of urine leaking with coughing, sneezing, weight lifting as stated by the patient. At the 6 month review, 78 and 75 patients were continent in group 1 and group 2 respectively. One patient in each of the groups did not receive the allocated treatment (either oestradiol or TVT‐O surgery) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" ‐ no further explanation |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 188 participants randomised, 5 lost to follow up (2 lost to follow up in group 2, 3 lost to follow up in group 2) |
Lose 2000 L.
| Methods | Open, randomised, multicentre, parallel‐group controlled trial with an active control, 26 gynaecologists clinics + 1 outpatient clinic at Danish county hospital; duration of study: 24/52 | |
| Participants | 251 post‐menopausal women
Inclusion criteria: at least 1 bothersome lower urinary tract symptom appearing at least 2 years after spontaneous or surgical postmenopause Exclusion criteria: known or suspected oestrogen‐dependent neoplasia, mammary, ovarian, uterine malignancies, vaginal bleeding of unknown origin, clinically significant liver disease, acute or intermittent porphyria, uterovaginal prolapse grade II or III, sex hormone treatment within last 6/12, previous participation in clinical trials within 3/12 prior to inclusion, signs of vaginal irritation other than atrophy derived, signs of vaginal ulceration |
|
| Interventions | Group A (n=134): oestradiol releasing vaginal ring, which constantly releases 7.5 mg oestradiol/24 hours, ring staying in situ for 12/52, then changed, in total treatment for 24/52 Group B (n=117): oestriol vaginal pessaries 0.5 mg every 2nd day for 24/52 | |
| Outcomes | Questionnaire with symptom assessment using visual analogue scale, gynaecological examination including atrophy and pH assessment, assessment of form of administration using 5‐point scale questionnaire, primary outcomes: subjective assessment of urgency, frequency, nocturia, dysuria, stress and urgency urinary incontinence, secondary outcomes: vaginal dryness, dyspareunia Definition of cure: 'symptom‐free': change from 'a minor problem / a problem / a major problem' to 'no problem'; improvement: change from 'a problem' to 'a minor problem' or from 'a major problem' to 'a problem / a minor problem', failure: 'no change': no change from 'a minor problem / a problem / a major problem', 'worse': change from 'no problem' to 'a minor problem / a problem / a major problem' or from 'a minor problem' to 'a problem / a major problem' or from 'a problem' to 'a major problem' | |
| Notes | Losses to follow up: group A: 5, group B: 3 Adverse outcomes: 49 women experienced at least one adverse event: 34 adverse events in group A , 42 adverse events in group B Primary objective was to show equivalence between the two treatments | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Low risk | Central office using numbers sequentially. No number was to be omitted |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 254 randomised, three did not receive treatment 251 eligible for intention‐to‐treat analysis |
Melis 1997 L.
| Methods | Treatment assigned in random fashion; duration of study 3/12 | |
| Participants | 50 post‐menopausal women
Inclusion criteria: physiological menopause of at least 1 year Exclusion criteria: hormone treatment less than 6/12 ago, illness or malignancy that contraindicated oestrogen treatment, BP>140/80, positive MSU |
|
| Interventions | Group A (n=25): oestriol vaginally 0.5 mg / day for 14/7, then alternate days for 3/12 in total Group B (n=25): oestriol vaginally 0.5 mg / day for 14/7, then alternate days for 3/12 in total + benzidamine 140 mg daily | |
| Outcomes | Biochemical markers: azotamine, glucose, GPT, GOT, g‐GT, bilirubin, total cholesterol, triglycerides, HDL‐cholesterol, FSH, oestriol levels
Clinical evaluation of genital symptoms: pruritus, leucorrhoea, sensation of vaginal dryness, dyspareunia)
Evaluation of general climacteric symptoms: psychological, insomnia, headaches, irritability, depression, neurovegetative: hot flushes
Colposcopy
Vaginal cytology, karyopycnotic index Every 15 days women were asked about side effects of treatment and filled in diary with intensity of symptoms |
|
| Notes | No adverse events, no losses to follow up; study designed to look at vaginal changes rather than at incontinence, incontinence forms part of menopausal symptoms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Ouslander 2001 S.
| Methods | Double‐blind placebo controlled RCT, randomisation by table of random numbers, odd and even number envelopes were kept in pharmacy 5 community nursing homes Duration of study: 6/12, assessments at 3/12 and 6/12 | |
| Participants | 32 incontinent female nursing home residents Inclusion criteria: age 65 and older Exclusion criteria: short stay and/or medical instability, terminally ill, (severe cognitive impairment, history of breast or cervical cancer, wet less than once per day, enteral feeding, poor cooperation with screening procedures, severe physical immobility requiring a lift or 3 person transfer) | |
| Interventions | Group A (n=15): oestrogen 0.625 mg + progesterone 2.5 mg, oral tablets, daily for 6/12 Group B (n=17): placebo daily for 6/12 Subjects also received prompted voiding by research aides for 3‐day periods while wet checks were carried out; subjects with bacteriuria on baseline MSU had 7‐day course of norfloxacin | |
| Outcomes | Frequency (percentage of checks at which subjects were found to be wet by research staff during three 8‐hour days of prompted voiding) and volume (reweighing pre‐weighed pad tests) of urinary incontinence, appropriate toileting rate, bladder capacity, cough 'stress test' Vaginal examination focusing on atrophy and inflammatory changes, vaginal pH, maturation indices for vaginal and urethral epithelium, urinalyses and cultures, vaginal cultures, serum levels of oestradiol, oestrone, oestrone sulfate | |
| Notes | No useable data; adverse events: 2 women in group A had single episode of vaginal spotting, ˜10% of women had mild breast discomfort Losses to follow up mainly because of illness which resulted in 2 death, group A: 2 at 3/12, + 4 at 6/12, group B: 1 at 3/12 + 4 at 6/12 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used a table of random numbers |
| Allocation concealment (selection bias) | Low risk | Odd and even numbered envelopes kept in the pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Rufford 2003 S.
| Methods | Double‐blind RCT, placebo controlled Power calculation Duration of study: 6/12 F/U at 1/12, 3/12 and 6/12 | |
| Participants | 40 post‐menopausal women Inclusion criteria: 'urge syndrome', not menstruated for > 12 months or if hysterectomised if oestradiol levels < 150 pmol/L Exclusion criteria: on medication for 'urge syndrome', diabetes mellitus or insipidus, diuretics, HRT within last 3 months or hormone implant or intramuscular hormone injection within previous year, endometrial thickness > 4 mm or abnormal endometrium on histology, UTI or haematuria, pelvic mass, urogenital prolapse, other contra‐indication to oestrogen therapy losses to follow up: A: 2, B: 1 Baseline characteristics: BMI A 26.5 (4.3) B 29.0 (6.72); previous hysterectomy A 6 (30%) B 6 (30%); detrusor instability A 7 (35%) B 15 (75%); age at menopause A 46.55, B 47.75 (6.34) years | |
| Interventions | Group A (n=20): 17 beta oestradiol 25mg implant subcutaneously Group B (n=20): placebo implant subcutaneously | |
| Outcomes | Outcomes measured by frequency/volume chart, King's Healthcare Quality of Life Questionnaire, urinary symptom questionnaire, visual analogue scale of symptom severity, uroflowmetry, video cystourethrography, serum oestradiol levels, endometrial thickness definition of cure: complete absence of a symptom that had been present at the beginning of the study Urgency urinary incontinence not cured: A: 8/15, B: 10/14 Stress incontinence not cured: A: 7/10, B: 5/7 Incontinent episodes in 24 hours (n, mean, SD): A: 16, 2 (5), B: 19, 1 (2) Number of micturitions in 24 hours (n, mean, SD): A: 16, 9 (3), B: 19, 9 (3) Adverse effects: A: 5 hysterectomy, 9 irregular vaginal bleeding, 1 angina (was felt not related to study medication), 4 breast tenderness, 8 UTIs B: 1 breast tenderness, 11 UTIs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 40 women randomised |
Sacco 1990 L.
| Methods | RCT; duration of study: 90 days | |
| Participants | 34 post‐menopausal women Inclusion criteria: USI on urodynamics Exclusion criteria: DO | |
| Interventions | Group A (n=17): oestrogen cream 0.5mg Group B (n=17): placebo cream | |
| Outcomes | Nocturia, frequency, dysuria, urgency urinary incontinence, pad changes, urodynamics, vaginal dryness, vaginal atrophy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor and care giver |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts |
Samsioe 1985 S.
| Methods | Double‐blind placebo controlled crossover RCT, sample of previous population study was randomly selected, of these 34 took part in study | |
| Participants | 34 post‐menopausal women, 11 with stress incontinence, 14 urgency urinary incontinence, 9 mixed incontinence;
Inclusion criteria: women of previous sample with urinary incontinence who lived in catchment area of one of Gothenburg university hospitals Exclusion criteria not stated |
|
| Interventions | Oestriol 3 mg orally or placebo orally, not clear if each treatment has been given for 3/12 and then was crossed over or if the total duration of both treatments was 3/12 | |
| Outcomes | Papanicolau smear to assess percentage of surface cells, MSU, clinical classification of degree of vaginal atrophy, efficacy of bladder control | |
| Notes | No useable data; unclear how many patients had oestrogen first and then placebo or how many had placebo first and the oestrogen; no apparent assessment after each stage of study; methods of assessment not given; no mention of losses to follow up; adverse outcomes: mastodynia, metrorrhagia in 4 patients, subjective side effects | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | no description |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Tinelli 2007 L.
| Methods | Randomised controlled trial, openly randomised | |
| Participants | Post‐menopausal women diagnosed with SUI using clinical and urodynamic evaluation | |
| Interventions | 48 women randomised to 10mg promestriene (oestradiol) as daily suppositories for 21 days before TVT procedure 50 randomised to TVT without preoperative pharmacological therapy |
|
| Outcomes | Postop clinical evaluation at 3, 6, and 12 months At 6 months subjective symptom questionnaire and Kings Health Questionnaire |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no mention |
| Allocation concealment (selection bias) | High risk | Openly randomised |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Tseng 2007 L.
| Methods | Randomised comparative study computer‐generated randomisation list |
|
| Participants | Women with overactive bladder mean age 65.2; range 58‐73, mean parity 2.5; range 1‐5 | |
| Interventions | 40 women 2mg detrusitol; 40 women 2mg detrusitol and vaginal oestrogen 1gm twice a week for a three month period | |
| Outcomes | Clinical exam, bladder diary, UDI questionnaire assessed at 0,6,12, weeks after treatment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Walter 1978 S.
| Methods | Double‐blind RCT, duration of study 4/12 | |
| Participants | 29 post‐menopausal women with stress and mixed incontinence, inclusion criteria: postmenopausal, no detrusor hyperreflexia, neg urine culture | |
| Interventions | Group A (n=15): oestradiol 2 mg + oestriol 1 mg orally for 20 days followed by 8 day break for 4/12 Group B (n=14): placebo orally similar regime |
|
| Outcomes | Interview to differentiate between urgency and stress urinary incontinence, cystoscopy, cystometry, MSU, trigone biopsies during cystoscopy, urethra ‐, vagina ‐ and cervix smears, serum levels of oestradiol, cholesterol and triglyceride | |
| Notes | Tables using different numbers of patients, unclear how they were classified and who had which sort of incontinence, no losses to follow up, no adverse outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Walter 1990 S.
| Methods | Double‐blind placebo controlled RCT, randomisation into two treatment groups by block randomisation using random numbers Duration of study: 12/52; assessment at 4/52, 8/52, 12/52 | |
| Participants | 29 post‐menopausal women Inclusion criteria: stress urinary incontinence documented by pad‐weighing test, >1year post‐menopausal including iatrogenic menopause, no oestrogen treatment < 2/12 prior to study,patients expected to comply with protocol including treatment on outpatient basis, stable cystometry, no evidence of obstruction Exclusion criteria: neurological disease and senility, diabetes mellitus, liver dysfunction, previous cancer of breast or uterus, hypertension (diastolic > 100 mmHg), concomitant treatment with drugs affecting the lower urinary tract | |
| Interventions | Group A (n=15): placebo for 4/52 (period 1), then phenylpropanolamine (PPA) 50mg twice daily + placebo for 4/52 (period 2), then PPA 50mg twice daily + oestriol 4mg daily for 4/52 (period 3) Group B (n=14): placebo for 4/52 (period 1), then oestriol 4mg daily + placebo for 4/52 (period 2), then PPA 50mg twice daily + oestriol 4mg daily for 4/52 (period 3) | |
| Outcomes | Subjective drug preference, 3‐day urinary diary, incontinence, median voiding frequency, mean number of leakage episodes, pad test, vaginal cytology, urine cultures, side effects, heart rate, BP | |
| Notes | Adverse events: 5 in placebo period, 6 in PPA period, 5 in oestriol period, 7 in PPA + oestriol period Losses to follow up: 1 during PPA period, 1 after period 2 of study (not specified which group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation into two treatment groups by block randomisation using random numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation into two treatment groups by block randomisation using random numbers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Women were blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | no description |
Wilson 1987 S.
| Methods | RCT using statistical tables for random allocation of 2 drugs Duration of study: 12/52, assessment at 6/52 + 11/52 | |
| Participants | 36 post‐menopausal women
Inclusion criteria: serum gonadotrophin in post‐menopausal range, GSI and stable detrusor function on cystourethrography, no HRT in previous 3/12, no contraindications to oestrogen therapy
Exclusion criteria: outflow obstruction 6 had hysterectomy |
|
| Interventions | Group A (n=18): piperazine oestrone sulphate 3mg at night for 3/52 followed by one treatment‐free week Group B (n=18): placebo for 3/52, followed by one treatment‐free week | |
| Outcomes | 7 day bladder diary, 2 hour pad test (22 women only), number of micturitions, pad changes/24 hours, urethral pressure profile, vaginal cytology, oestrone, oestradiol, FSH, LH levels, subjective assessment: patients were asked if they were much improved, improved or no better Adverse events: A: 2, 1 palpitations and trembling after 5/7, 1 subendocardial infarct after 5/52 , other adverse events: leg pain, breast discomfort, chest pain, nausea | |
| Notes | Losses to follow up: group A: 2 (same women as in adverse events) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Standard statistical tables for random allocation of two drugs |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 18 oestrogen group two failed to complete (not included in analysis) 18 placebo group |
Zullo 2005 L.
| Methods | RCT to determine if topical oestrogen therapy can help prevent overactive bladder symptoms after TVT Duration of study 6 months Follow up review 1,3 and 6 months after surgery | |
| Participants | 56 post‐menopausal women with SUI having a TVT + 3 who were not randomised Inclusion criteria: no periods for last 12 months, serum oestradiol level less than 150pmol/l in patients who have undergone hysterectomy, diagnosis of USI (urodynamic diagnosis), no contraindications to local estrogen therapy, no contraindications to vaginal surgery and informed consent Exclusion criteria: urogenital prolapse above grade 1, detrusor overactivity, intrinsic urethral sphincter deficiency, previous urogynaecology surgery, endometrial thickness more than 4mm, previous estrogen therapy within past 6 months, unexplained uterine bleeding, or history of diabetes mellitus or insipidus, congestive cardiac failure, diuretic therapy or neoplasms Age (years), mean (SD): A, 56.4 (4.9); B, 55.9 (6.4) Parity: A, 1.8 (0.8); B, 1.6 (0.7) BMI: A, 26.4 (2.4), B: 25.5 (2.5) Groups comparable on these baseline characteristics Pre‐operative assessment included TV scan, standardised urogynaecology history clinical examination, urodynamic evaluation. 10 grade VAS, degree of vaginal defects, cough stress test, cotton swab test, pressure flow study, electromyography | |
| Interventions | Group A (n=28) intravaginal oestriol ovules (1mg) daily for one month, then 2mg once weekly for 5 months as maintenance therapy Group B (n=28) no oestriol | |
| Outcomes | Successful treatment (cure) of SUI was defined as no leakage of urine during the cough stress test and urodynamic test, and no leakage episodes reported in a 7 day voiding diary Cure of UI: 53/56 (95%) of women cured at 6 months but no data available for each group UTI in first month after surgery: 4/56 (7%) but no data available for each group Frequency at 6 months: group A: 2/28, group B: 5/28 Urgency: group A: 1/28,group B: 8/28 No significant increase in endometrial thickness from baseline value in group A, no other adverse effects reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | no description |
| Allocation concealment (selection bias) | Unclear risk | no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors blind to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 59 had TVT, three women who were eligible after TVT refused to take part. 28 oestrogen group, 28 placebo |
BMI = body mass index BP = blood pressure DO = detrusor overactivity (previously known as DI, detrusor instability) FSH = follicle stimulating hormone HRT = hormone replacement therapy ITT = intention to treat IU/l = international units per litre LH = luteinising hormone MSU = midstream specimen of urine pH = measure of acidity/alkalinity PPA = phenylpropanolamine RCT = randomised controlled trial SUI = stress urinary incontinence (symptom diagnosis)
UI = urinary incontinence USI = urodynamic stress incontinence (previously known as GSI, genuine stress incontinence) UTI = urinary tract infection UUI = urgency urinary incontinence
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brandstettter 1961 | Unclear if RCT, published in 1961 and in German |
| Brown 2001 | Not all women were incontinent at baseline. Outcomes looking at oestrogen's effects on urinary tract infections as opposed to urinary incontinence |
| Brys 2009 | Outcomes not relevant to this review |
| Capobianco 2012 L | Not all women incontinent at baseline |
| Cardozo 1990 | Only a paragraph in a review, author contacted for more data, data have never been published, were collected from 1983‐1985, no data available any more |
| Castillo 1999 | Looking at vascular resistance index of periurethral vessels in patients with stress urinary incontinence treated with local oestrogens. No data though 'clinical improvement was found in 15 patients, most of whom received oestrogens' |
| Chompootaweep 1998 SL | No mention of incontinence at baseline. The focus of the study was to investigate urogenital symptoms (vaginal dryness, dyspareunia, urinary frequency and urinary urgency) |
| Ciaccia 2002 | Not all women were incontinent at baseline |
| Ciaccia 2002a | Cannot use data, does not state numbers randomised |
| Delmas PD | Study did not look at oestrogen but raloxifene instead |
| Donaldson 2006 | Not all women post‐menopausal at baseline |
| Ettinger 1999 | No incontinence outcomes in this study |
| Ewies 2010 | Not an RCT |
| Foidart 1991 | Looking at urogenital symptoms and systemic post‐menopausal complaints, incontinence only mentioned as part of "Urogenital Index" |
| Foster 12017 | RCT using topical oestrogen cream and biofeedback combinations in four arms. Not all women incontinent at baseline |
| Gokdeniz 1999 | Women not incontinent at baseline |
| Goldstein 2011 | Lasofoxifene given as intervention but no oestrogen |
| Hirai 2009 | Not an RCT |
| Holtedahl 2000 | Urodynamic examination, no oestrogen therapy given |
| Howie 1997 | Short abstract only, no results given |
| Johnston 2000 | Raloxifene given as intervention but no oestrogen |
| Kagan 2010 | RCT looking at oestrogen as treatment for vulvar/vaginal atrophy. No mention of incontinence |
| Karp 2010 | Women not incontinent at baseline |
| Kok 1999 | All women treated with 2 mg oestradiol, dose finding study of dydrogesterone |
| Koski 2011 | Not an RCT |
| Long 2006 | RCT but not all women incontinent at baseline |
| Mikkelsen 1995 | Looking at long‐term effect of treatment with oestradiol 3/52 before vaginal repair, only 7 patients with incontinence which is not further specified |
| Molander 1990 | No mention of incontinence, brief description of frequency and urgency only |
| Nelken 2011 L | Women were not incontinent in trial. OAB only |
| Nilsson 1994 | RCT of transdermal oestrogen versus placebo but for severe urogenital estrogen deficiency (not urinary incontinence) |
| Notelovitz 1995 | 67 women with symptoms of urinary urgency, but no comment on cure of urinary symptoms, only pH results given |
| Palomba 2001 | RCT of oestriol cream versus no cream prior to surgery, no outcomes on incontinence. Italian publication |
| Poreba 1981 | RCT with four arms, unclear allocation concealment, not enough usable information given, paper written in Polish |
| Raz 1993 | No outcomes looking at incontinence. Trial focused on recurrent urinary tract infections |
| Reid 2004 | Not all women were incontinent at baseline. Same study reported in 2005 by Goldstein et al |
| Rud 1980 | RCT but included 6/38 continent women and 9/38 premenopausal women. Outcomes not reported separately |
| Schmidbauer 1992 | Not RCT |
| Serati 2009 | Not all women incontinent at baseline |
| Sherman 2003 | Women not incontinent at baseline |
| Speroff 2003 | Women not incontinent at baseline |
| Steinauer 2005 | Excluded as trial looking at effect of treatment on continent women |
| Stovall 2008 | Not an RCT |
| Tammela 1988 | Effect of prostaglandin vs placebo, not oestrogen |
| Vaccaro 2008 | Inclusion criteria did not involve women diagnosed with incontinence |
| Valente 2000 | Effect of HRT on calcium and collagen, no mention of urinary symptoms |
| Vardy 2003 | RCT but not all patients were incontinent at baseline |
| Vestergaard 2003 | RCT with four arms, two randomised tow patient choice. Excluded as only 70% of women incontinent at baseline |
| von Holst 2000 | Not all participants were incontinent at baseline |
| Waetjen 2004 | Raloxifene not an oestrogen |
| Waetjen 2011 | No intervention given to the cohort of women. The study was looking at serum oestradiol levels in the blood and its association with urinary incontinence |
| Weisberg 2005 | Women not incontinent at baseline |
| Wozniakowska 2008 | No outcomes of interest |
| Yang 2011 | Not an RCT, text in Chinese |
Characteristics of studies awaiting assessment [ordered by study ID]
Bergman 1985.
| Methods | 17 women were 'subdivided' into 2 groups ‐ unclear how this was achieved |
| Participants | 17 post‐menopausal women with genuine stress incontinence |
| Interventions | 11 women in vaginal oestrogen treatment group and 6 in no treatment group |
| Outcomes | Urodynamics |
| Notes | Original review authors wrote to authors ‐ no reply? |
Characteristics of ongoing studies [ordered by study ID]
Sant 2002.
| Trial name or title | A comparative study between oestrogen replacement therapy, anticholinergic treatment and a combination of both in the management of detrusor instability in post‐menopausal women |
| Methods | |
| Participants | 80 participants, 4 random groups post‐menopausal women, symptoms of detrusor instability, positive cystometry, no contraindications to treatment |
| Interventions | Group A: tolterodine 2mg twice daily Group B: oestrogen 2mg+norethisterone 1mg daily in one tablet Group C: both drugs Group D: placebo |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Tried to contact, no success |
Contributions of authors
Birgit Moehrer, Andrew Hextall and Simon Jackson conducted the first version of this review. Birgit Moehrer wrote most of the review with help from Andrew Hextall. Andrew Hextall and Simon Jackson wrote the initial protocol for the review.
June Cody, Karen Richardson and Cathryn Glazener updated the review in 2009; they selected trials, carried out data abstraction and analysis and redrafted the text and conclusions. Birgit Moehrer and Andrew Hextall provided clinical support for the update of the review and approved the final text.
June Cody and Madeleine Jacobs updated the review in 2012; they selected trials, redrafted the text and conclusions.
Declarations of interest
Simon Jackson was an author on one of the included studies and Andrew Hextall on another. However, data extraction was done independently.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ahlstrom 1990 S {published data only}
- Ahlström K, Sandahl B, Sjöberg B, Ulmsten U, Stormby N, Lindskog M. Effect of combined treatment with phenylpropanolamine and estriol, compared with estriol treatment alone, in postmenopausal women with stress urinary incontinence. Gynecologic and Obstetric Investigation 1990;30(1):37‐43. [MEDLINE: ; 338] [Google Scholar]
Assassa 2003 L {published data only}
- Assassa RP, Jagger C, Williams K, McGrother C, Mayne C. Randomised double‐blind placebo controlled trial of topical oestrogen in the treatment of female bladder symptoms (Abstract number 140 (205)). International Urogynecology Journal and Pelvic Floor Dysfunction 2003;14 Suppl 1:43. [Google Scholar]
Beisland 1984 L {published data only}
- Beisland HO, Fossberg E, Moer A, Sander S. Urethral sphincteric insufficiency in postmenopausal females: treatment with phenylpropanolamine and estriol separately and in combination. Urologia Internationalis 1984;39:211‐6. [MEDLINE: ; 690] [Google Scholar]
Blom 1995 S {published data only}
- Blom M, Sommers B. The effects of an estradiol transdermal therapuetic system, alone and in combination with naproxen, on urge incontinence in elderly women: a pilot study. Current Therapeutic Research, Clinical and Experimental 1995;56(10):1100‐4. [6083] [Google Scholar]
Cardozo 1993 S {published data only}
- Cardozo L, Rekers H, Tapp A, Barnick C, Shepherd A, Schussler B, et al. Oestriol in the treatment of postmenopausal urgency: a multicentre study. Maturitas 1993;18(1):47‐53. [MEDLINE: ; 1223] [Google Scholar]
Cardozo 2001 L {published and unpublished data}
- Benness C. Vaginal oestradiol for postmenopausal urinary symptoms ‐ a double‐blind placebo‐controlled study [MD Thesis]. University of Sydney, 1992. [16396] [Google Scholar]
- Benness C. Vaginal oestradiol for postmenopausal urinary symptoms ‐ a double‐blind placebo‐controlled study [abstract]. Proceedings of the FIGO meeting, Stockholm. 1992. [16397]
- Benness CJ, Wise BG, Cutner A, Cardozo L. Does low dose vaginal estradiol improve frequency and urgency in postmenopausal women?. American Urogynecology Society 13th annual meeting, Sept 27‐30 1992. Cambridge, Massachussetts, USA, 1992:281. [14578]
- Cardozo LD, Wise BG, Benness CJ. Vaginal oestradiol for the treatment of lower urinary tract symptoms in postmenopausal women ‐ A double‐blind placebo‐controlled study. Journal of Obstetrics and Gynaecology 2001;21(4):383‐5. [15720] [Google Scholar]
Dessole 2004 L {published data only}
- Dessole S, Rubattu G, Ambrosini G, Gallo O, Capobianco G, Cherchi PL, et al. Efficacy of low‐dose intravaginal estriol on urogenital aging in postmenopausal women. Menopause 2004;11(1):49‐56. [16649] [Google Scholar]
Ek 1980 S {published data only}
- Ek A, Anderson KE, Gullberg B, Ulmsten U. Effect of oestradiol and combined norephedrin and oestradiol treatment on female stress incontinence. Zentralbibliothek Gynaekologie 1980;102(15):839‐44. [MEDLINE: ; 777] [Google Scholar]
Enzelsberger 1990 L {published data only}
- Enzelsberger H, Schatten C, Kurz C, Zorzi P. Treatment of female urge incontinence by local oestrogen therapy [Zur Behandlung der weiblichen Urge‐Inkontinenz durch lokale Oestrogentherapie]. Gynaekologische Rundschau 1990;30 Suppl 1:235‐6. [MEDLINE: ; 305] [Google Scholar]
Enzelsberger 1991a L {published data only}
- Enzelsberger H, Kurz C, Schatten C, Huber J. Effects of intravaginal application of oestriol tablets in women with urge incontinence [Zur Wirksamkeit einer intravaginalen Oestrioltablettenapplikation bei Frauen mit Urge‐Inkontinenz]. Geburtshilfe und Frauenheilkunde 1991;51(10):834‐8. [MEDLINE: ; 238] [Google Scholar]
Enzelsberger 1991b L {published data only}
- Enzelsberger H, Kurz C, Schatten C, Huber J. Effects of intravaginal application of oestriol tablets in women with urge incontinence [Zur Wirksamkeit einer intravaginalen Oestrioltablettenapplikation bei Frauen mit Urge‐Inkontinenz]. Geburtshilfe und Frauenheilkunde 1991;51(10):834‐8. [MEDLINE: ; 238] [Google Scholar]
Fantl 1996 S {published data only}
- Fantl JA, Richard C, Bump C, Robinson D, McClish DK, Wyman JF, and the Continence Program for Women Research Group. Efficacy of estrogen supplementation in the treatment of urinary incontinence. Obstetrics and Gynecology 1996;88(5):745‐9. [4860] [Google Scholar]
Grady 2001 S {published data only}
- Grady D, Applegate W, Bush T, Furberg C, Riggs B, Hulley SB. Heart and Estrogen/Progestin Replacement Study (HERS): Design, methods and baseline characteristics. Controlled Clinical Trials 1998;19:314‐35. [15451] [Google Scholar]
- Grady D, Brown JS, Vittinghoff E, Applegate W, Warner E, Snyder T. Postmenopausal hormones and incontinence: the Heart and Estrogen/Progestin Replacement Study. Obstetrics and Gynecology 2001;97(1):116‐20. [11845] [Google Scholar]
- Sherman AM, Shumaker SA, Kancler C, Zheng B, Reboussin DM, Legault C, et al. Baseline health‐related quality of life in postmenopausal women with coronary heart disease: the Estrogen Replacement and Atherosclerosis (ERA) trial. Journal of Women's Health 2003;12(4):351‐62. [Google Scholar]
Henalla 1989 L {published data only}
- Henalla SM, Hutchins CJ, Castleden CM. Conservative management of urethral sphincter incompetence [Abstract]. Neurourology and Urodynamics 1987;6(3):191‐2. [2689] [Google Scholar]
- Henalla SM, Hutchins CJ, Robinson P, MacVicar J. Non‐operative methods in the treatment of female genuine stress incontinence of urine. Journal of Obstetrics and Gynaecology 1989;9:222‐5. [2637] [Google Scholar]
Henalla 1990 L {published data only}
- Henalla SM, Millar DR, Wallace KJ. Surgical versus conservative management for post‐menopausal genuine stress incontinence of urine [Abstract]. Neurourology and Urodynamics 1990;9(4):436‐7. [5117] [Google Scholar]
Hendrix (hysterectomy) S {published data only}
- Hendrix SL, Cochrane BB, Nygaard IE, Handa VL, Barnabei VM, Iglesia C, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA 2005;293(8):935‐48. [20122] [Google Scholar]
Hendrix (no hysterect) S {published data only}
- Hendrix SL, Cochrane BB, Nygaard IE, Handa VL, Barnabei VM, Iglesia C, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA 2005;293(8):935‐48. [20122] [Google Scholar]
Hendrix 2005 S {published data only}
- Hendrix SL, Cochrane BB, Nygaard IE, Handa VL, Barnabei VM, Iglesia C, et al. Effects of estrogen with and without progestin on urinary incontinence. JAMA 2005;293(8):935‐48. [20122] [Google Scholar]
- Pal L, Hailpern SM, Santoro NF, Freeman R, Barad D, Kipersztok S, et al. Association of pelvic organ prolapse and fractures in postmenopausal women: analysis of baseline data from the Women's Health Initiative Estrogen Plus Progestin trial.[see comment]. Menopause 2008;15(1):59‐66. [Google Scholar]
Hilton 1990 SL {published data only}
- Hilton P, Tweddell AL, Mayne C. Oral and intravaginal estrogens alone and in combination with alpha‐adrenergic stimulation in genuine stress incontinence. International Urogynecology Journal 1990;1:80‐6. [2675] [Google Scholar]
Ishiko 2001 S {published data only}
- Ishiko O, Hirai K, Sumi T, Tatsuta I, Ogita S. Hormone replacement therapy plus pelvic floor muscle exercise for postmenopausal stress incontinence. A randomized, controlled trial. Journal of Reproductive Medicine 2001;46(3):213‐20. [12340] [Google Scholar]
Jackson 1999 S {published data only}
- Jackson S, Shepherd A, Abrams P. Does oestrogen supplementation improve the symptoms of postmenopausal urinary stress incontinence? A double blind placebo controlled trial. Neurourology and Urodynamics 1997;16 (5):350‐1. [5837] [Google Scholar]
- Jackson S, Shepherd A, Abrams P. Subjective benefit from HRT in postmenopausal urinary stress incontinence? A double blind placebo controlled trial. International Urogynaecology Journal and Pelvic Floor Dysfunction 1997;8(1 Suppl):51. [5170] [Google Scholar]
- Jackson S, Shepherd A, Abrams P. The effect of oestradiol on objective urinary leakage in postmenopausal stress incontinence; a double blind placebo controlled trial [Abstract]. Neurourology and Urodynamics 1996;15 (4):322‐3. [4616] [Google Scholar]
- Jackson S, Shepherd A, Brookes S, Abrahams P. The effect of oestrogen supplementation on post‐menopausal urinary stress incontinence: a double‐blind placebo‐controlled trial. British Journal of Obstetrics and Gynaecology 1999;106(7):711‐8. [MEDLINE: ; 8840] [Google Scholar]
Judge 1969 S {published data only}
- Judge TG. The use of quinestradol in elderly incontinent women, a preliminary report. Gerontologia Clinica 1969;11(3):159‐64. [MEDLINE: ; 885] [Google Scholar]
Kinn 1988 S {published data only}
- Kinn AC, Lindskog M. Estrogens and phenylpropanolamine in combination for stress urinary incontinence in postmenopausal women. Urology 1988;32(3):273‐80. [MEDLINE: ; 476] [Google Scholar]
Kurz 1993 L {published data only}
- Kurz C, Nagele F, Sevelda P, Enzelsberger H. Intravesical application of estriol in sensory urge incontinence ‐ a prospective study [Intravesikal appliziertes Oestriol bei sensorischer Urgeinkontinenz ‐ eine prospektive Studie]. Geburtshilfe und Frauenheilkunde 1993;53(8):535‐8. [MEDLINE: ; 99] [Google Scholar]
Liapis 2010 L {published data only}
- Liapis A, Bakas P, Georgantopoulou C, Creatsas G. The use of oestradiol therapy in postmenopausal women after TVT‐O anti‐incontinence surgery. Maturitas 2010;66(1):101‐6. [Google Scholar]
Lose 2000 L {published data only}
- Lose G, Englev E. A randomised study to compare the efficacy of a vaginal ring (estradiol) with a vaginal pessary (estriol) in postmenopausal women (PMW) with urinary symptoms (US) [Abstract]. Proceedings of the 8th International Congress on Menopause; 1996 Nov 3‐7; Sydney. 1996:87. [11866]
- Lose G, Englev E. Oestradiol‐releasing vaginal ring versus oestriol vaginal pessaries in the treatment of bothersome lower urinary tract symptoms. British Journal of Obstetrics and Gynaecology 2000;107(8):1029‐34. [MEDLINE: ; 9816] [Google Scholar]
Melis 1997 L {published data only}
- Melis GB, Paoletti AM, Murgia C, Piras B, Pilia I, Romagnino S, et al. Vaginal oestriol and benzidamine in the treatment of urogenital disturbances of the climacteric [Estriolo vaginale e benzidamina nel trattamento dei disturbi uroginecologici del climaterio]. Giornale Italiano Di Ostetricia e Ginecologica 1997;19(5):303‐12. [5929] [Google Scholar]
Ouslander 2001 S {published data only}
- Ouslander J, Greendale G, Uman G, Schnelle J. Effects of oral oestrogen/progestin among incontinent female nursing home residents [Abstract]. Neurourology and Urodynamics 1998;17(4):165‐6. [5693] [Google Scholar]
- Ouslander JG, Greendale GA, Uman G, Lee C, Paul W, Schnelle J. Effects of oral estrogen and progestin on the lower urinary tract among female nursing home residents. Journal of the American Geriatrics Society 2001;49:803‐7. [MEDLINE: ; 15403] [Google Scholar]
Rufford 2003 S {published and unpublished data}
- Rufford J, Hextall A, Cardozo L, Khullar V. A double blind placebo controlled trial on the effects of 25mg oestradiol implants on the urge syndrome in postmenopausal women. Personal communication. [16452]
Sacco 1990 L {published data only}
- Sacco F, Rigon G, Carbone A, Sacchini D. Transvaginal estrogen therapy for stress incontinence [Terapia estrogenica transvaginale dell'incontinenza urinaria da sforzo]. Minerva Ginecologica 1990;42(12):539‐44. [MEDLINE: ; 10496] [Google Scholar]
Samsioe 1985 S {published data only}
- Samsioe G, Jansson I, Mellstroem D, Svanborg A. Occurrence, nature and treatment of urinary incontinence in a 70‐year‐old female population. Maturitas 1985;7(4):335‐42. [MEDLINE: ; 609] [Google Scholar]
Tinelli 2007 L {published data only}
- Tinelli A, Malvasi A, D'Anna L, Tinelli R, Perrone A, Tinelli FG. Presurgical promestriene therapy in postmenopausal women with stress urinary incontinence. Gynecological Endocrinology 2007;23(8):445‐50. [Google Scholar]
Tseng 2007 L {published data only}
- Tseng LH, Liang CC, Chang YL, Lin YH, Soong YK, Wang A. A randomized comparative study of vaginal estrogen cream in postmenopausal women with overactive bladder syndrome (Abstract number 27). International Urogynecology Journal and Pelvic Floor Dysfunction 2007;18 Suppl 1:15‐6. [Google Scholar]
Walter 1978 S {published data only}
- Walter S, Wolf H, Barlebo H, Jensen HK. Urinary incontinence in postmenopausal women treated with oestrogens. A double‐blind clinical trial. Urologia Internationalis 1978;33:135‐43. [2659] [Google Scholar]
Walter 1990 S {published data only}
- Walter S, Kjærgaard B, Heisterberg L, Jakobsen H, Klarskov P, Lose G, et al. Stress urinary incontinence in postmenopausal women treated with phenylpropanolamine and estrogen: a randomised blind placebo‐controlled study. Proceedings of the 18th Annual Meeting of the International Continence Society, Oslo, Norway, 1‐3 September. 1988:282‐3. [12052]
- Walter S, Kjærgaard B, Lose G, Andersen JT, Heisterberg L, Jakobsen H, et al. Stress urinary incontinence in postmenopausal women treated with oral estrogen (estriol) and an alpha‐adrenoceptor‐stimulating agent (phenylpropanolamine): a randomized double‐blind placebo‐controlled study. International Urogynaecology Journal 1990;1:74‐9. [2657] [Google Scholar]
Wilson 1987 S {published data only}
- Wilson PD. Female genuine stress incontinence. An objective study of aspects of its aetiology, investigation and conservative treatment [MD Thesis]. Glasgow: Glasgow University, 1981:218‐24. [27332] [Google Scholar]
- Wilson PD, Faragher B, Butler B, Bu'lock D, Robinson EL, Brown ADG. Treatment with oral piperazine oestrone sulphate for genuine stress incontinence in postmenopausal women. British Journal of Obstetrics and Gynaecology 1987;94(6):568‐74. [MEDLINE: ; 513] [Google Scholar]
Zullo 2005 L {published data only}
- Zullo MA, Plotti F, Calcagno M, Palaia I, Muzii L, Manci N, et al. Vaginal estrogen therapy and overactive bladder symptoms in postmenopausal patients after a tension‐free vaginal tape procedure: a randomised clinical trial. Menopause 2005;12(4):421‐7. [20805] [Google Scholar]
References to studies excluded from this review
Brandstettter 1961 {published data only}
- Brandstetter F. [Results of the medical therapy of functional urine incontinence]. Wiener Klinische Wochenschrift 1961;73(33/34):556‐7. [21999] [Google Scholar]
Brown 2001 {published data only}
- Brown JS, Vittinghof E, Kanaya AM, Agarwal SK, Hulley S, Foxman B, et al. Urinary tract infections in postmenopausal women: Effect of hormone therapy and risk factors. Obstetrics and Gynecology 2001;98(6):1045‐52. [Google Scholar]
Brys 2009 {published data only}
- Brys M, Szyllo K, Romanowicz‐Makowska H, Dobrowolski Z, Maslowska I, Krajewska W. Expression of estrogen and progesterone receptor genes in endometrium, myometrium and vagina of postmenopausal women treated with estriol. Sao Paulo Medical Journal = Revista Paulista de Medicina 2009;127(3):128‐33. [Google Scholar]
Capobianco 2012 L {published data only}
- Capobianco G, Donolo E, Borghero G, Dessole F, Cherchi PL, Dessole S. Effects of intravaginal estriol and pelvic floor rehabilitation on urogenital aging in postmenopausal women. Archives of Gynecology and Obstetrics 2012;285(2):397‐403. [Google Scholar]
Cardozo 1990 {published data only}
- Cardozo L. Role of estrogens in the treatment of female urinary incontinence. Journal of the American Geriatics Society 1990;38(3):326‐8. [MEDLINE: ; 366] [Google Scholar]
Castillo 1999 {published data only}
- Castillo E, Saibene H, Varela R, Souza M. Periurethral vascular resistance index and local estrogen therapy in patients with stress urinary incontinence. Proceedings of the 29th Annual Meeting of the International Continence Society, Denver, Colorado, 23‐26 August 1999. 1999:147. [9916]
Chompootaweep 1998 SL {published data only}
- Chompootaweep S, Nunthapisud P, Trivijitsilp P, Sentrakul P, Dusitsin N. The use of two estrogen preparations (a combined contraceptive pill versus conjugated estrogen cream) intravaginally to treat urogenital symptoms in postmenopausal Thai women: a comparative study. Clinical Pharmacology and Therapeutics 1998;64(2):204‐10. [MEDLINE: ; 12894] [Google Scholar]
- Chompootaweep S, Nunthapisud P, Trivijitsilp P, Sentrakul P, Dusitsin N. The use of two estrogen preparations (a combined contraceptive pill versus conjugated estrogen cream) intravaginally to treat urogenital symptoms in postmenopausal Thai women: a comparative study [Abstract]. Proceedings of the 8th International Congress on the Menopause; Sydney; Nov 3‐7, 1996. 1996:87‐8. [5184]
Ciaccia 2002 {published data only}
- Ciaccia A, Goldstein S, Johnson S, Watts S, Plouffe L Jr. Incidence and severity of urinary incontinence in postmenopausal women participating in a placebo controlled clinical trial of raloxifene and oestrogen. The 10th World Congress on the Menopause 2002. [17186]
Ciaccia 2002a {published data only}
- Ciaccia A, Goldstein S, Johnson S, Watts S, Draper M, Plouffe L. Incidence and severity of urinary incontinence in postmenopausal women participating in a placebo‐controlled trial of raloxifene and estrogen (Abstract number P378). Journal of the American Geriatrics Society 2002; Vol. 50, issue 4:S134.
Delmas PD {published data only}
- Delmas PD, Bjarnason NH, Mitlak BH, Ravoux AC, Shah AS, Huster WJ, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations and uterine endometrium in postmenopausal women. New England Journal of Medicine 1997 Dec 4;337(23):1641‐7. [Google Scholar]
Donaldson 2006 {published data only}
- Donaldson MM, Thompson JR, Matthews RJ, Dallosso HM, McGrother CW, Leicestershire MRC Incontinence Study Group. The natural history of overactive bladder and stress urinary incontinence in older women in the community: a 3‐year prospective cohort study. Neurourology and Urodynamics 2006; Vol. 25, issue 7:709‐16.
Ettinger 1999 {published data only}
- Ettinger B, Black DM, Mitlak BH, Knickerbocker RK, Nickelsen T, Genant HK, et al. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3 year randomised clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) investigators. JAMA 1999 Aug 18;282(7):637‐45. [Google Scholar]
Ewies 2010 {published data only}
- Ewies AA, Alfhaily F. Topical vaginal estrogen therapy in managing postmenopausal urinary symptoms: a reality or a gimmick?. Climacteric 2010;13(5):405‐18. [Google Scholar]
Foidart 1991 {published data only}
- Foidart JM, Vervliet J, Buytaert P. Efficacy of sustained‐release vaginal oestriol in alleviating urogenital and systemic climacteric complaints. Maturitas 1991;13(2):99‐107. [MEDLINE: ; 12895] [Google Scholar]
Foster 12017 {published data only}
- Foster DC, Palmer M, Marks J. Effects of vulvovaginal estrogen on sensorimotor response of the lower genital tract: A randomised Controlled Trial. Obstetrics and Gynaecology 1999;94(2):232‐7. [12017] [Google Scholar]
Gokdeniz 1999 {published data only}
- Gokdeniz R, Taskin O, Atmaca R, Burak F, Wheeler JM. The impact of Hormone Replacement Therapy (HRT) and tibolone (OD) on urodynamic function in postmenopausal women: a randomized controlled study [abstract number P‐113]. Fertility and Sterility 1999;72 Suppl 1:127. [Google Scholar]
Goldstein 2011 {published data only}
- Goldstein SR, Neven P, Cummings S, Colgan T, Runowicz CD, Krpan D, et al. Postmenopausal Evaluation and Risk Reduction With Lasofoxifene (PEARL) trial: 5‐year gynecological outcomes. Menopause 2011;18(1):17‐22. [Google Scholar]
Hirai 2009 {published data only}
- Hirai K, Tsuda H. Estrogen and urinary incontinence. International Journal of Urology 2009;16(1):45‐8. [Google Scholar]
Holtedahl 2000 {published data only}
- Holtedahl K, Verelst M, Schiefloe A, Hunskaar S. Usefulness of urodynamic examination in female urinary incontinence‐‐lessons from a population‐based, randomized, controlled study of conservative treatment. Scandinavian Journal of Urology and Nephrology 2000;34(3):169‐74. [Google Scholar]
Howie 1997 {published data only}
- Howie H, Heimer G, Larson G, Feldt G, Sundstroem G, Risberg B. The effect of estrogen on stress urinary incontinence in postmenopausal women, preliminary results [Abstract]. Proceedings of the International Continence Society, 27th Annual Meeting; Yokohama, Japan; 1997 Sep 23‐26;. 1997:297. [5848]
Johnston 2000 {published data only}
- Johnston CC Jr, Bjarnason NH, Cohen FJ, Shah A, Lindsay R, Mitlak BH, et al. Long‐term effects of raloxifene on bone mineral density, bone turnover, and serum lipid levels in early postmenopausal women: three‐year data from 2 double‐blind, randomized, placebo‐controlled trials. Archives of Internal Medicine 2000;160(22):3444‐50. [39832] [Google Scholar]
Kagan 2010 {published data only}
- Kagan R, Williams RS, Pan K, Mirkin S, Pickar JH. A randomized, placebo‐ and active‐controlled trial of bazedoxifene/conjugated estrogens for treatment of moderate to severe vulvar/vaginal atrophy in postmenopausal women. Menopause 2010;17(2):281‐9. [Google Scholar]
Karp 2010 {published data only}
- Karp D, Jean‐Michel M, Peterson T, Johnston Y, Suciu G, Aguilar V, et al. Optimizing post‐operative healing following vaginal reconstructive surgery: a triple arm randomized clinical trial of an estradiol‐releasing vaginal ring (Abstract number 55). Neurourology and Urodynamics 2010;29(6):887‐8. [Google Scholar]
Kok 1999 {published data only}
- Kok AL, Burger CW, Weijer PH, Voetberg GA, Peters‐Muller ER, Kenemans P. Micturition complaints in postmenopausal women treated with continuously combined hormone replacement therapy: a prospective study. Maturitas 1999;31(2):143‐9. [MEDLINE: ; 11868] [Google Scholar]
- Kok ALM, Burger CW, Weijer PHM, Voetberg GA, Peters‐Muller ERA, Kenemans P. The effect of the dydrogesterone dose in continuously combined HRT regimens on micturition complaints. Proceedings of the 8th International Congress on Menopause, Sydney, Australia, 3‐7 November. 1996:88. [11867]
Koski 2011 {published data only}
- Koski ME, Chermansky CJ. Does estrogen have any real effect on voiding dysfunction in women?. Current Urology Reports 2011;12(5):345‐50. [Google Scholar]
Long 2006 {published data only}
- Long C, Lui C, Hsu S, Chen Y, Wu C, Tsai E. A randomized comparative study of the effects of oral and topical estrogen therapy on the lower urinary tract of hysterectomised postmenopausal women. American Society for Reproductive Medicine 2006;85(1):155‐60. [22078] [Google Scholar]
Mikkelsen 1995 {published data only}
- Mikkelsen AL, Felding C, Clausen HV. Clinical effects of preoperative oestradiol treatment before vaginal repair operation. A double‐blind randomized trial. Gynecologic and Obstetric Investigation 1995;40(2):125‐8. [MEDLINE: ; 2851] [Google Scholar]
Molander 1990 {published data only}
- Molander U, Milsom I, Ekelund P, Mellstrom D, Eriksson O. Effect of oral oestriol on vaginal flora and cytology and urogenital symptoms in the post‐menopause. Maturitas 1990;12(2):113‐20. [MEDLINE: ; 11269] [Google Scholar]
Nelken 2011 L {published data only}
- Nelken RS, Ozel BZ, Leegant AR, Felix JC, Mishell DR Jr. Randomized trial of estradiol vaginal ring versus oral oxybutynin for the treatment of overactive bladder. Menopause 2011;18(9):962‐6. [Google Scholar]
Nilsson 1994 {published data only}
- Nilsson K, Heimer GM. Ultra‐low‐dose transdermal estrogen therapy in postmenopausal urogenital estrogen deficience ‐ a placebo‐controlled study. Menopause 1994;1(4):191‐7. [26559] [Google Scholar]
Notelovitz 1995 {published data only}
- Notelovitz M. Estrogen therapy in the management of problems associated with urogenital ageing: a simple diagnostic test and the effect of the route of hormone administration. Maturitas 1995;22 Suppl:31‐3. [MEDLINE: ; 2712] [Google Scholar]
Palomba 2001 {published data only}
- Palomba S, Napolotano V, Sammartino A, Spiezio SA, Vassallo M, Mandato V, et al. Effect of estriol treatment per vaginum before Burch culposuspension. Minerva Ginecologica 2000;53(2):141‐5. [12281] [Google Scholar]
Poreba 1981 {published data only}
- Poreba R, Dudkiewicz J. Evaluation of selected pharmacological preparations in the treatment of stress urinary incontinence in women [Ocena wybranych preparatow farmacologicznych w leczeniu wysilkowego nietrzymania moczu u kobiet]. Ginekologia Polska. 1981;52(2):193‐9. [MEDLINE: ; 745] [Google Scholar]
Raz 1993 {published data only}
- Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections [see comments]. New England Journal of Medicine 1993;329(11):753‐6. [Google Scholar]
Reid 2004 {published data only}
- Goldstein SR, Johnson S, Watts NB, Ciaccia AV, Elmerick D, Muram D. Incidence of urinary incontinence in postmenopausal women treated with raloxifene or estrogen. Menopause 2005;12(2):160‐4. [19160] [Google Scholar]
- Reid IR, Eastell R, Fogelman I, Adachi JD, Rosen A, Netelenbos C, et al. A comparison of the effects of raloxifene and conjugated equine estrogen on bone and lipids in healthy postmenopausal women. Archives of Internal Medicine 2004;164(8):871‐9. [19160] [Google Scholar]
Rud 1980 {published data only}
- Rud T. The effects of estrogens and gestagens on the urethral pressure profile in urinary continent and stress incontinent women. Acta Obstetricia Gynecologica Scandinavica 1980;59(3):265‐70. [MEDLINE: ; 788] [Google Scholar]
Schmidbauer 1992 {published data only}
- Schmidbauer CP. Vaginal estriol administration in treatment of postmenopausal urinary incontinence [Vaginale Oestriolapplikation zur Behandlung der postmenopausalen Harninkontinenz]. Urologe 1992;31(6):384‐9. [MEDLINE: ; 168] [Google Scholar]
Serati 2009 {published data only}
- Serati M, Salvatore S, Uccella S, Cardozo L, Bolis P. Is there a synergistic effect of topical oestrogens when administered with antimuscarinics in the treatment of symptomatic detrusor overactivity?. European Urology 2009;55(3):713‐9. [Google Scholar]
Sherman 2003 {published data only}
- Sherman AM, Shumaker SA, Sharp P, Reboussin DM, Kancler C, Walkup M, et al. No effect of HRT on health‐related quality of life in postmenopausal women with heart disease. Minerva Ginecologica 2003;55(6):511‐7. [Google Scholar]
Speroff 2003 {published data only}
- Speroff L, for the United States VR Investigator Group. Efficacy and tolerability of a novel estradiol vaginal ring for relief of menopausal symptoms. Obstetrics and Gynecology 2003;102(4):823‐34. [Google Scholar]
Steinauer 2005 {published data only}
- Steinauer JE, Subak LL, Grady D, Vittinghoff E, Brown J. Hormonal therapy for prevention or urinary Incontinence: The HERS study. Obstetrics and Gynecology 2003;101(4 Suppl):10. [17197] [Google Scholar]
- Steinauer JE, Waetjen E, Vittinghoff E, Subak LL, Hulley SB, Grady D, et al. Postmenopausal hormone therapy: does it cause incontinence?. American College of Obstetricians and Gynaecologists 2005;106(5):940‐5. [21291] [Google Scholar]
Stovall 2008 {published data only}
- Stovall DW, Pinkerton JV. Estrogen agonists/antagonists in combination with estrogen for prevention and treatment of menopause‐associated signs and symptoms. Women's Health 2008;4(3):257‐68. [Google Scholar]
Tammela 1988 {published data only}
- Tammela T, Kontturi, M, Kaar, K. [Prostaglandin management of urine retention after surgery for incontinence]. Duodecim 1988;104(20):1621‐4. [Google Scholar]
Vaccaro 2008 {published data only}
- Vaccaro C, Fellner A. The effects of local vaginal estrogen in postmenopausal women with pelvic organ prolapse: A randomized control trial. ClinicalTrials.gov (www.clinicaltrials.gov) (accessed 12 April 2010) 2008.
Valente 2000 {published data only}
- Valente M, Dettori C, Feraudo E, Re ME. Pelvic‐floor: new therapeutic approach (Abstract). International Urogynecology Journal 2000;11 Suppl 1:97. [Google Scholar]
Vardy 2003 {published data only}
- Vardy M, Lindsay R, Scotti RJ, Mikhail M, Richart RM, Neives J, et al. Short‐term urogenital effects of raloxifene, tamoxifen and estrogen. American Journal of Obstetrics and Gynaecology 2003;189(1):81‐8. [16541] [Google Scholar]
Vestergaard 2003 {published data only}
- Vestergaard P, Hermann AP, Stilgren L, Tofteng CL, Sorensen OH, Eiken P, et al. Effects of 5 years of hormonal replacement on menopausal symptoms and blood pressure ‐ a randomised controlled study. Maturitas 2003;46(2):123‐32. [16679] [Google Scholar]
von Holst 2000 {published data only}
- Holst T, Salbach B. Efficacy and tolerability of a new 7‐day transdermal estradiol patch versus placebo in hysterectomized women with postmenopausal complaints. Maturitas 2000;34(2):143‐53. [Google Scholar]
Waetjen 2004 {published data only}
- Waetjen LE, Brown JS, Modelska K, Blackwell T, Vittinghoff E, Cummings SR, et al. Effect of raloxifene on urinary incontinence: a randomized controlled trial.[see comment]. Obstetrics and Gynecology 2004;103(2):261‐6. [Google Scholar]
Waetjen 2011 {published data only}
- Waetjen LE, Johnson WO, Xing G, Feng WY, Greendale GA, Gold EB, et al. Serum estradiol levels are not associated with urinary incontinence in midlife women transitioning through menopause. Menopause 2011;18(12):1283‐90. [Google Scholar]
Weisberg 2005 {published data only}
- Weisberg E, Ayton R, Darling G, Farrell E, Murkies A, O'Neill S, et al. Endometrial and vaginal effects of low‐dose estradiol delivered by vaginal ring or vaginal tablet. Climacteric 2005;8(1):83‐92. [Google Scholar]
Wozniakowska 2008 {published data only}
- Wozniakowska E, Milart P, Paszkowski T, Szkodziak P. Comparison of oestradiol and oestriol influence on pulsatility indices in periurethral vessels in women suffering from urinary stress incontinence (Abstract number 245). Proceedings of the 38th Annual Meeting of the International Continence Society (ICS), 2008 Oct 20‐24, Cairo, Egypt 2008.
Yang 2011 {published data only}
- Yang X, Yu Q. Hormone replacement therapy and urinary incontinence in post‐menopausal women. Chinese Journal of Obstetrics and Gynaecology 2011 Apr;46(4):314‐6. [Google Scholar]
References to studies awaiting assessment
Bergman 1985 {published data only}
- Bergman A, Bhatia NN. Estrogen induced cytological and functional urethral changes in postmenopausal women with genuine stress incontinence. Proceedings of the International Continence Society (ICS), 15th Annual Meeting, London, UK, 3‐6 September, 1985:304‐5. [10927]
References to ongoing studies
Sant 2002 {published data only}
- Sant M, Galea P, Brincat MP. A comparative study between oestrogen replacement therapy, anticholinergic treatment and a combination of both in the management of detrusor instability in postmenopausal women. The 10th World Congress on the Menopause 2002. [17185]
Additional references
Alhasso 2005
- Alhasso AA, Glazener CMA, Pickard R, N'Dow JMO. Adrenergic drugs for urinary incontinence in adults. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD001842.pub2; CD001842] [DOI] [Google Scholar]
Barlow 1997
- Barlow DH, Cardozo LD, Francis RM, Griffin M, Hart DM, Stephens E, et al. Urogenital ageing and its effect on sexual health in older British women. British Journal of Obstetrics and Gynecology 1997;104(1):87‐91. [MEDLINE: ; 26] [Google Scholar]
Benness 1991
- Benness C, Gangar K, Cardozo LD, Cutner A, Whitehead M. Do progestogens exacerbate incontinence in women on HRT?. Neurourology and Urodynamics 1991;10:316‐8. [Google Scholar]
Blakeman 1996
- Blakeman PJ, Hilton P, Bulmer JN. Mapping oestrogen and progesterone receptors throughout the female lower urinary tract. Neurourology and Urodynamics 1996;15(4):324‐5. [Google Scholar]
BNF 2002
- British Medical Association and the Royal Pharmaceutical Society of Great Britain. British National Formulary. Oxford: Pharmaceutical Press, 2002. [ISSN: 0260‐535X] [Google Scholar]
Boyle 2012
- Boyle R, Hay‐Smith EJC, Cody JD, Mørkved S. Pelvic floor muscle training for prevention and treatment of urinary and faecal incontinence in antenatal and postnatal women. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD007471.pub2; CD007471] [DOI] [Google Scholar]
Crown 1979
- Crown S, Crisp AH. Crown‐Crisp Experimental Index. Kent, Hodder and Stoughton Educational, 1979. [8059] [Google Scholar]
Cutner 1992
- Cutner A, Carey A, Cardozo LD. Lower urinary tract symptoms in early pregnancy. Journal of Obstetrics and Gynecology 1992;12:75‐8. [Google Scholar]
Dean 2006
- Dean N, Ellis G, Herbison GP, Wilson D. Laparoscopic colposuspension for urinary incontinence in women. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD002239.pub2; CD002239] [DOI] [Google Scholar]
Dumoulin 2010
- Dumoulin C, Hay‐Smith J. Pelvic floor muscle training versus no treatment, or inactive control treatments, for urinary incontinence in women. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD005654.pub2; CD005654] [DOI] [Google Scholar]
Glazener 2001
- Glazener CMA, Cooper K. Anterior vaginal repair for urinary incontinence in women. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001755; CD001755] [DOI] [Google Scholar]
Glazener 2004
- Glazener CMA, Cooper K. Bladder neck needle suspension for urinary incontinence in women. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003636.pub2; CD003636] [DOI] [Google Scholar]
Glazener 2012
- Glazener CMA, Lapitan MCM. Urodynamic studies for management of urinary incontinence in children and adults. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD003195.pub2; CD003195] [DOI] [Google Scholar]
Hannestad 2000
- Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community‐based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord‐Trondelag. Journal of Clinical Epidemiology 2000; Vol. 53, issue 11:1150‐7.
Haylen 2010
- Haylen BT, Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourology and Urodynamics 2010;29(1):4‐20. [Google Scholar]
Herbison 2002
- Herbison GP, Dean N. Weighted vaginal cones for urinary incontinence. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD002114; CD002114] [DOI] [Google Scholar]
Higgins 2003
- Higgins JPT, Thomspon SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org. 2011.
Iosif 1981
- Iosif CS, Batra S, Ek A, Astedt B. Estrogen receptors in the human female lower urinary tract. American Journal of Obstetrics and Gynecology 1981;141(7):817‐20. [MEDLINE: ; 34] [Google Scholar]
Iosif 1984
- Iosif CS, Bekassy Z. Prevalence of genito‐urinary symptoms in the late menopause. Acta Obstetricia Gynecologica Scandinavica 1984;63(3):257‐60. [MEDLINE: ; 704] [Google Scholar]
Jackson 1996
- Jackson S, Donovan J, Brookes S, Eckford S, Swithinbank L, Abrams P. The Bristol Female Lower Urinary Tract Symptoms questionnaire: development and psychometric testing. British Journal of Urology 1996;77(6):805‐12. [MEDLINE: ; 8058] [Google Scholar]
Jolleys 1988
- Jolleys JV. Reported prevalence of urinary incontinence in women in a general practice. BMJ (Clinical Research Edition) 1988;296(6632):1300‐2. [MEDLINE: ; 12044] [Google Scholar]
Kelleher 1997
- Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. British Journal of Obstetrics and Gynaecology 1997;104(12):1374‐9. [MEDLINE: ; 5489] [Google Scholar]
Kernan 2000
- Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of haemorrhagic stroke. New England Journal of Medicine 2000;343(25):1826‐32. [Google Scholar]
Kirchin 2012
- Kirchin V, Page T, Keegan PE, Atiemo K, Cody JD, McClinton S. Urethral injection therapy for urinary incontinence in women. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD003881.pub3; CD003881] [DOI] [Google Scholar]
Kondo 1990
- Kondo A, Kato K, Saito M, Otani T. Prevalence of handwashing incontinence in females in comparison with stress and urge incontinence. Neurourology and Urodynamics 1990;9:330‐1. [Google Scholar]
Lapitan 2012
- Lapitan MCM, Cody JD. Open retropubic colposuspension for urinary incontinence in women. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD002912.pub5; CD002912] [DOI] [Google Scholar]
Lipp 2011
- Lipp A, Shaw C, Glavind K. Mechanical devices for urinary incontinence in women. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD001756.pub5; CD001756] [DOI] [Google Scholar]
Madhuvrata 2012
- Madhuvrata P, Cody JD, Ellis G, Herbison GP, Hay‐Smith EJC. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD005429.pub2; CD005429] [DOI] [Google Scholar]
Marjoribanks 2012
- Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD004143.pub4] [DOI] [Google Scholar]
Nabi 2006
- Nabi G, Cody JD, Ellis G, Hay‐Smith J, Herbison GP. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003781.pub2; CD003781] [DOI] [Google Scholar]
Ogah 2009
- Ogah J, Cody JD, Rogerson L. Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006375.pub2; CD006375] [DOI] [Google Scholar]
Rehman 2011
- Rehman H, Bezerra CCB, Bruschini H, Cody JD. Traditional suburethral sling operations for urinary incontinence in women. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD001754.pub3; CD001754] [DOI] [Google Scholar]
Rossouw 2007
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, et al. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 2007;297(13):1465‐77. [Google Scholar]
Roxburgh 2007
- Roxburgh C, Cook J, Dublin N. Anticholinergic drugs versus other medications for overactive bladder syndrome in adults. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2007, issue 4. [DOI: 10.1002/14651858.CD003190.pub4; CD003190] [DOI]
Sandvik 1993
- Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. Journal of Epidemiology and Community Health 1993;47(6):497‐9. [MEDLINE: ; 4595] [Google Scholar]
Shumaker 1994
- Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fantl JA. Health‐related quality of life measures for women with urinary incontinence: the Incontinence Impact Questionnaire and Urogenital Distress Inventory Continence Program in Women (CPW) Research Group. Quality of Life Research 1994;3(5):291‐306. [MEDLINE: ; 22] [Google Scholar]
Smith 1993
- Smith P. Estrogens and the urogenital tract. Studies on steroid hormone receptors and a clinical study on a new estradiol‐releasing vaginal ring. Acta Obstetricia Gynecologica Scandinavica Supplement 1993;157:1‐26. [MEDLINE: ; 15434] [Google Scholar]
Thomas 1980
- Thomas TM, Plymat KR, Blannin J, Meade TW. Prevalence of urinary incontinence. British Medical Journal 1980;281(6250):1243‐5. [MEDLINE: ; 6676] [Google Scholar]
Van Geelen 1981
- Geelen JM, Doesburg WH, Thomas CM, Martin CB Jr. Urodynamic studies in the normal menstrual cycle: the relationship between hormonal changes during the menstrual cycle and the urethral pressure profile. American Journal of Obstetrics and Gynecology 1981;141(4):384‐92. [MEDLINE: ; 1] [Google Scholar]
Ware 1993
- Ware JE. Measuring patients' views: the optimum outcome measure. BMJ 1993;306(6890):1429‐30. [MEDLINE: ; 1] [Google Scholar]
References to other published versions of this review
Moehrer 2003
- Moehrer B, Hextall A, Jackson S. Oestrogens for urinary incontinence in women. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD001405] [DOI] [Google Scholar]


