Abstract
Formins are one of the central players in the assembly of most actin networks in cells. The sensitivity of these processive molecular machines to mechanical tension is now well established. However, how the activity of formins is affected by geometrical constraints related to network architecture, such as filament cross-linking and formin spatial confinement, remains largely unknown. Combining microfluidics and micropatterning, we reconstituted in vitro mDia1 formin-elongated filament bundles induced by fascin, with different geometrical constraints on the formins, and measured the impact of these constraints on formin elongation rate and processivity. When filaments are not bundled, the anchoring details of formins have only a mild impact on their processivity and do not affect their elongation rate. When formins are unanchored, we show that filament bundling by fascin reduces both their elongation rate and their processivity. Strikingly, when filaments elongated by surface-anchored formins are cross-linked together, formin elongation rate immediately decreases and processivity is reduced up to 24-fold depending on the cumulative impact of formin rotational and translational freedom. Our results reveal an unexpected crosstalk between the constraints at the filament and the formin levels. We anticipate that in cells the molecular details of formin anchoring to the plasma membrane strongly modulate formin activity at actin filament barbed ends.
Keywords: Formin, actin, bundle, cytoskeleton, microfluidics, fluorescence polarization
To perform complex cellular functions and mechanotransduction at the micron scale, actin filaments assemble to create networks that vary in size, structure, and dynamics. Actin filaments are continuously generated, polymerized, cross-linked to each other, or attached to membranous cellular compartments. The intertwining of actin filament assembly and cross-linking is tightly regulated in space and time to shape the various cytoskeletal structures, such as the cell cortex, stress fibers, or transverse arcs.1
Formins, together with Ena/VASPs, are actin binding proteins that have the unique ability to processively track actin filament barbed ends and increase their elongation rates.2−5 Members of the formin family are head-to-tail homodimers with two functional formin homology domains, FH1 and FH2 (recently reviewed in (6 and 7)). The FH2 homodimer interacts with the last subunits of the actin filament barbed end, adopts different conformations in rapid equilibrium, and gates actin monomer addition or removal from the barbed end.8 The FH1 domains are disordered domains harboring multiple polyproline tracks to which profilin and profilin-actin complexes bind. These two functional domains work together to speed up actin filament barbed end elongation.9,10
The mechanosensitivity of formins is now well established. Applying a pulling force on a formin bound to an actin filament led to an increase in barbed end elongation rate for mDia1,11−14 mDia2,15,16 and Bni1p,17 whereas the opposite effect was observed for Cdc1216 and for Bni1p in the absence of profilin.17 The processivity of mDia1 and mDia2 formins was shown to depend on the efficiency of FH1 domains to bind, with the help of profilin, to the actin filament barbed end and to thereby increase the lifetime of the FH2 interaction with the barbed end.15 Most importantly, formin mDia1 and mDia2 processivity was shown to be severely reduced when a pulling force was applied on those formins with a 3 pN pulling force increasing the formin dissociation rate more than 10-fold.15 Lastly, observations using magnetic tweezers to probe the increase in elongation rate with force revealed that mDia1 formins were also sensitive to torque.13
These observations have provided novel insights into the molecular details of formin function and crucial evidence for the high sensitivity of formins to biochemical and mechanical conditions to which they are exposed. However, these insights were obtained for individual formins elongating single isolated actin filaments. This situation is rarely, if ever, encountered in cells where formins elongate filaments which are cross-linked together into various networks, such as the cell cortex, filopodia, and stress fibers.18 Furthermore, in cells formins are specifically localized to membranes thanks mostly to interactions with other proteins like GTPases, IQGAP1, or IRSp53 (reviewed in refs (19 and 20)). Formin localization is mediated by domains at the amino-terminus of the FH1 domain or by FH1 itself in the case of its interaction with IRSp53. It is thus necessary to study formins in a more physiological context, where formins are anchored and filaments are cross-linked, to better understand the impact of these constraints on their activity.
Here, using in vitro approaches, we investigated the activity of mDia1(FH1FH2DAD) formins (hereafter referred to as formins) in various geometrical configurations with the fascin-induced bundle geometry as a case study. As readouts of formin activity, we monitored the elongation rate of filament barbed ends and the formin detachment rate. We first examined, separately, the consequences of geometrical constraints on formins (anchoring) and on filaments (bundling). Different anchoring conditions were tested, binding formins from either end (FH1 or FH2 side) on a solid or a fluid surface. None of these conditions had a significant impact on formin activity at the barbed ends of independent, individual filaments (Figure 1). When we bundled filaments elongating with free, unanchored formins, we found that bundling on its own slowed down elongation and enhanced formin detachment (Figure 2). We next combined filament bundling and formin anchoring and found that formin activity was affected further, in ways that depended on the anchoring features (Figures 3–5). These results show that details of formin anchoring, which appear inconsequential when elongating single filaments, become crucial when filaments are bundled together. We determined that in order to function efficiently when filaments are bundled, anchored formins must have both rotational freedom (Figure 4) and translational freedom (Figure 5).
Figure 1.
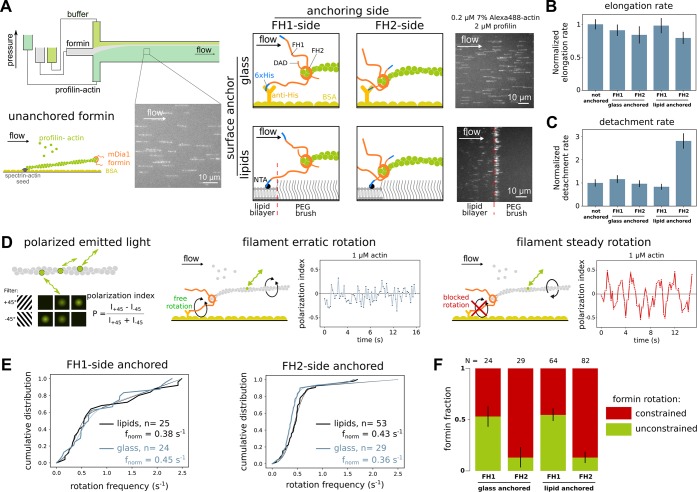
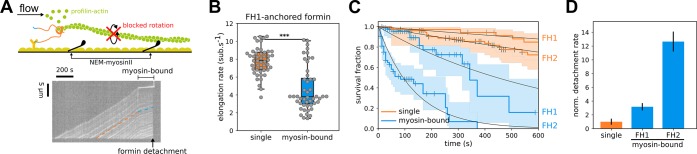
Formin anchoring may impact its rotational freedom but does not affect single actin filament elongation rates. (A) In a microfluidics chamber, shown in upper left, formin-induced actin filament elongation is monitored for unanchored formins (bottom left) or for formins specifically anchored to either glass or a lipid bilayer (right) by a 6× Histidine tag located either at their N- or C-terminus. Typical fields of view showing alexa488-labeled actin filaments aligned by the microfluidics flow. (B) Formin-induced actin filament mean elongation rate in the presence of 0.2 μM 7% alexa488-labeled actin and 2 μM profilin, as a function of formin anchoring (error bar is sample standard deviation) (see Supporting Information Figure 1A). (C) Formin detachment rate as a function of formin anchoring in the presence of 0.2 μM 7% alexa488-labeled actin and 2 μM profilin (error bar is standard error, see Supporting Information Methods and Supporting Information Figure 1A). (D) The polarization of the emitted light indicates the orientation of a single fluorescently labeled actin subunit. The polarization index P = (I+ 45 – I–45)/(I+45 + I–45) is determined by measuring the emitted intensity through two orthogonal polarization filters (I+ 45 and I–45). Depending on the rotation constraints at the anchoring point, an actin filament elongated by an anchored formin will exhibit either an erratic variation of polarization (left) or a steady oscillation of the polarization signal (right). For each sketched situation, a typical experimental data curve is shown as an example. (E) Cumulative distributions of the main frequency of the polarization signal for filaments elongated by (left) FH1-anchored formins or (right) FH2-anchored formins, anchored either on glass or lipids, in the presence of 1 μM unlabeled actin. Experimental data are fitted by the weighted sum of the cumulative distribution functions of a normal distribution (the mean frequency, fnorm, is indicated for each case) and a random distribution. (F) The fraction of formins identified as rotationally constrained or rotationally unconstrained was determined by fitting the cumulative distributions, as shown in panel E.
Figure 2.
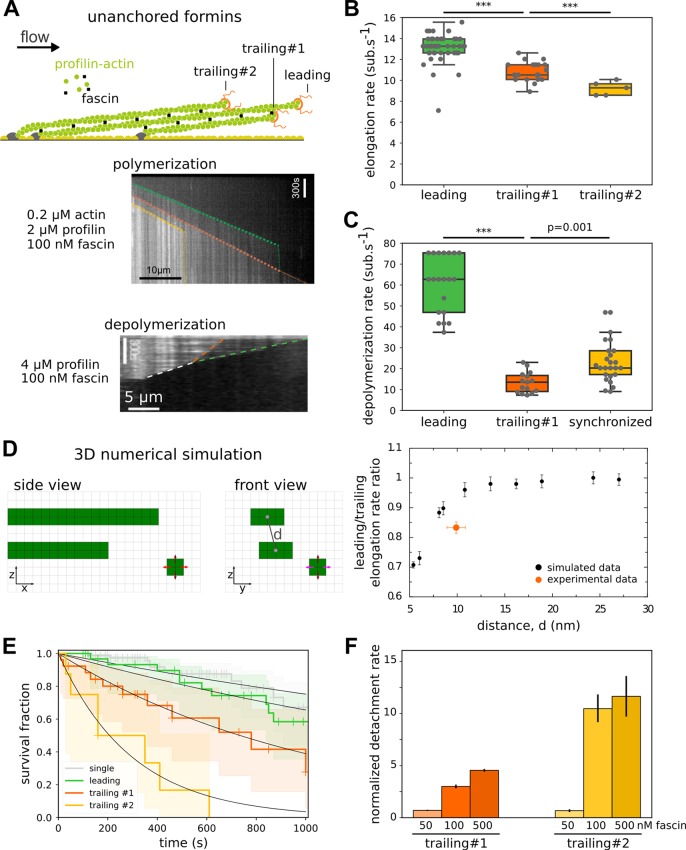
Fascin-induced bundling slows down trailing actin filament barbed end elongation and increases trailing formin detachment rate. (A) Sketch depicting a fascin-induced bundle where barbed ends are elongated by unanchored formins, identified as “leading”, “trailing #1”, and “trailing #2” depending on the relative barbed end positions The kymographs show either the elongation of bundled filaments by formins, in the presence of 0.2 μM actin, 2 μM profilin, and 100 nM fascin, for leading (green dashed line), trailing #1 (orange dashed line), and trailing #2 (yellow dashed line) formins or the depolymerization of bundled filaments by formins in the presence of 4 μM profilin and 100 nM fascin, for leading formin (green dashed line), trailing #1 formin (orange dashed line), and “synchronized” leading and trailing formins (white dashed line). Abrupt drops in the filament elongation rate were interpreted as formin-barbed end detachment events. (B) Mean filament elongation rate for leading (n = 52), trailing #1 (n = 21), or trailing #2 (n = 5) unanchored formins in conditions shown in panel A middle. Error bar is sample standard deviation. (C) Mean filament depolymerization rate for leading (n = 20), trailing (n = 15), and synchronized (n = 25) unanchored formins in conditions shown in panel A bottom. Error bar is sample standard deviation. (D) (Left) sketch representing a three-dimensional numerical simulation of actin monomer diffusion and a leading and a trailing filament separated by a distance, d, between their main axis. simulation mesh size = 2.7 nm (see Supporting Information Methods). (right) Comparison between simulated and experimental measurements of the leading/trailing elongation rate ratio, as a function of the distance d. (E) Survival fraction of formin-bound barbed ends as a function of time for single filaments (gray, n = 80) or bundled filaments whose barbed end is leading (green, n = 34), trailing #1 (orange, n = 28), and trailing #2 (yellow, n = 8), fitted by a monoexponential decay function. (F) Normalized detachment rate of trailing formins, relative to leading formin detachment rate, as a function of fascin concentration. (error bar is standard error of the fit).
Figure 3.
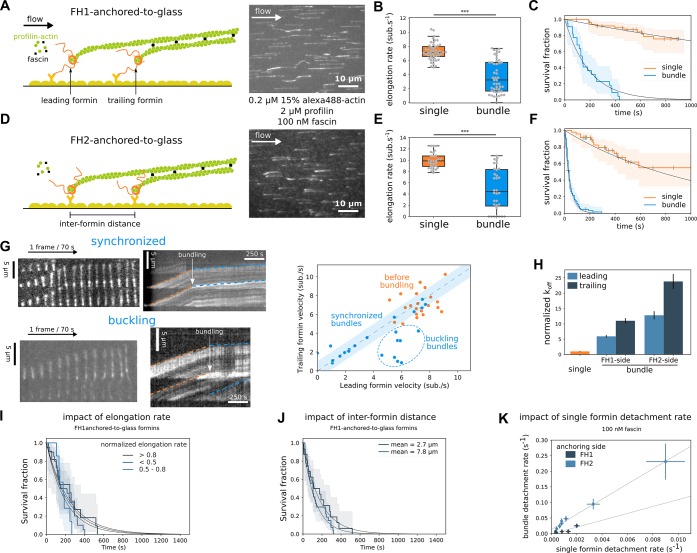
Fascin-induced bundling affects glass-anchored formin elongation and detachment rates. (A,D) Sketches of a 2-filament bundle elongated by (A) FH1-anchored-to-glass or (D) FH2-anchored-to-glass formins, and respective typical fields of view showing alexa488-labeled actin filaments bundling together. Filament unbinding from the formin-decorated surface is interpreted as a formin-barbed end detachment event and used to quantify formin off rate koff. (B) Barbed end elongation rate distribution for single (n = 48) or bundled (n = 48) filaments elongated by FH1-anchored-to-glass formins in the presence of 0.2 μM actin, 2 μM profilin, and 100 nM fascin. (C) Survival fractions of FH1-anchored-to-glass single (n = 51) or bundled filaments (n = 22), in the presence of 0.2 μM actin, 2 μM profilin, and 100 nM fascin. (E) Barbed end elongation rate distribution of single (n = 29) or bundled (n = 32) filaments elongated by FH2-anchored-to-glass formins in the presence of 0.2 μM actin, 2 μM profilin, and 100 nM fascin. (F) Survival fractions of FH2-anchored-to-glass single (n = 46) or bundled filaments ( n = 85), in the presence of 0.2 μM actin, 2 μM profilin, and 100 nM fascin. (G) Typical images and corresponding kymographs of 2-filament bundles where bundling leads to either (top) “synchronized” elongation of the two filaments, or (bottom) “buckling” of the leading filament. Pairwise elongation rates for trailing and leading filaments in 2-filament bundles for FH1-anchored-to-glass formins, before (orange) or during (blue) bundle elongation, in the presence of 0.2 μM actin, 2 μM profilin, and 100 nM fascin. (H) Normalized detachment rate of the leading and trailing FH1-anchored-to-glass or FH2-anchored-to-glass formins, relative to the detachment rate of single formins (error bars are standard error of the fit). (I) Survival fractions of FH1-anchored-to-glass formin-bound bundled filaments for three populations where the normalized elongation rate, relative to the elongation rate before bundling, is higher than 0.8, lower than 0.5 or in between (n = 11, 20, and 7 bundles respectively; log-rank test p-value = 0.31, 0.28, 0.34 between subset pairs). (J) Survival fractions of FH1-anchored-to-glass formin-bound bundled filaments for two populations where the formin-formin distance is 2.7 ± 0.8 and 7.8 ± 3.3 μm (average ± standard deviation, n = 16 and 27 bundles, respectively; log-rank test p-value = 0.193). (K) Bundle detachment rate as a function of single formin detachment rate at 100 nM fascin obtained by varying actin and profilin concentrations, for FH1-anchored-to-glass or FH2-anchored-to-glass formins (error bars are standard errors).
Figure 5.
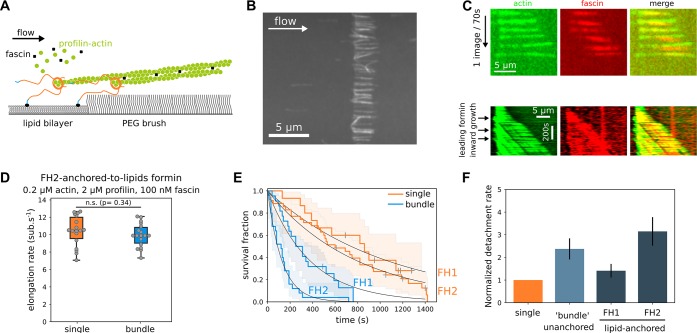
Fascin-induced bundling moderately affects lipid-anchored formin detachment rate but not elongation rate. (A) Sketch of a fascin-induced 2-filament bundle elongated by lipid-anchored formins in a microfluidics flow. (B) Typical field of view of filament bundles elongated by FH1-anchored-to-lipids formins at the edge of a lipid square pattern, in the presence of 0.2 μM 15% alexa488-labeled actin, 2 μM profilin, and 100 nM fascin, in a microfluidics flow. (C) (Top) Raw data of a 2-filament bundle imaged with both 15% alexa488-labeled actin and 100% alexa568-labeled fascin; (bottom) Kymograph of a 2-filament bundle where a leading filament moves upstream as its elongation rate is higher than the elongation rate of the trailing filament. Filament unbinding from the formin-decorated surface is interpreted as a formin-barbed end detachment event and used to quantify formin off rates, koff. (D) Elongation rate distributions of single filaments (n = 18) or 2-filament bundles (n = 18) elongated by FH2-anchored-to-lipids formins (Student’s t test p-value = 0.34). (E) Survival fractions of lipid-anchored formin-bound single filaments or 2-filament bundles for FH1-anchored-to-lipids (n = 15 and 22 filaments, for single filaments and 2-filament bundles respectively) or FH2-anchored-to-lipids formins (n = 35 and 25 filaments, for single filaments and 2-filament bundles respectively) in the presence of 0.2 μM actin, 2 μM profilin, and 100 nM fascin. (F) Normalized individual formin detachment rates for either FH1-anchored-to-lipids or FH2-anchored-to-lipids individual formins elongating 2-filament bundles, relative to the detachment rate of formins elongating single, unbundled filaments (orange bar). For comparison, the average detachment rate of both leading and trailing unanchored formins is shown (light blue bar). (Error bars are standard errors.)
Figure 4.
Single surface attachment of the filaments reduces single formin elongation and processivity. (A) (top) Sketch of a glass-anchored formin elongating a single filament whose rotation is blocked by surface attachment upon binding of a NEM–myosin to the filament side. (bottom) Kymograph showing an actin filament elongated by a FH1-anchored-to-glass formin, whose elongation is slowed down upon filament binding to a surface-bound NEM–myosin, until the filament unbinds from the surface-anchored formin (arrow). Filament unbinding from the formin-decorated surface is interpreted as a formin-barbed end detachment event and used to quantify formin off rates koff. (B) Distribution of elongation rates of individual actin filaments, elongated by FH1-anchored-to-glass formins, bound or not to NEM-myosins attached to the glass surface (n = 48 for both conditions; Student’s t test p-value <0.001). (C) Survival fractions of formin-bound single filaments attached to the surface via a NEM–myosin or not, for FH1-anchored-to-glass (n = 78 for NEM–myosin-bound and 35 for single filaments) or FH2-anchored-to-glass formins (n = 53 for NEM–myosin-bound and 76 for single filaments) in the presence of 0.2 μM actin and 2 μM profilin. (D) Normalized formin detachment rates for either FH1-anchored-to-glass or FH2-anchored-to-glass individual formins, whose elongating filaments are rotationally blocked by a surface-attached NEM-myosin, relative to glass-anchored formins elongating rotationally unconstrained single filaments.
Anchoring a Single Formin mDia1 Weakly Affects Its Activity
In cells, activated formins are bound to membranes compartments, via direct binding to lipids or through protein complexes.21,22 To study the impact of formin anchoring, we used an in vitro microfluidics approach where we tested four different anchoring schemes and compared them to situations where the formins were not anchored; formins were anchored by either their N-terminus or their C-terminus and either to proteins adsorbed on glass (referred to as “FH1-anchored-to-glass” and “FH2-anchored-to-glass” formins) or to a freely diffusing lipid bilayer bordered by a PEG brush (referred to as “FH1-anchored-to-lipids” and “FH2-anchored-to-lipids” formins) (see Figure 1A and Supporting Information Methods).
In all these configurations, individual anchored formins were probed in terms of elongation rate and processivity (Figure 1B,C, Supporting Information Figure 1A) and directly compared to unanchored formins within the same microfluidics chamber therefore exposed to the same biochemical conditions.
All anchored formins displayed slightly reduced elongation rates compared to unanchored formins, that did not exceed 20% in reduction. All anchored formins were as processive as unanchored formins except FH2-anchored-to-lipids formins, which were ∼3-fold less processive (Figure 1C). The fact that among all the anchoring configurations that we tested, FH2-anchored-to-lipids corresponds to the shortest distance between the FH2 dimer and the underlying surface (Figure 1A, no antibody being used with lipids) already suggests that anchoring details may have important consequences.
To further characterize our different anchoring schemes, we looked at how anchoring formins either via their FH1 or via their FH2 side could affect their ability to freely rotate around their surface attachment point. This aspect is important because due to the helical structure of the actin filament, it appears that the formin needs to be able to rotate around the filament axis as it tracks the growing barbed end.3,8,23 It has recently been reported that, for different surface-anchoring schemes, putting different constraints on formin rotation could affect their ability to elongate filaments.13 Here, for FH1-side anchored formins, the FH1 domain can be considered as a flexible linker with a contour length of ∼40 nm, connecting the formin to the surface.24 In contrast, when formins are anchored by their FH2 side, the DAD domain, which lies C-terminus of FH2 and is composed of a short α-helix followed by an unstructured region, can be considered as a linker of ∼10 nm in contour length.20 FH2-anchoring thus constitutes a shorter tether than FH1-anchoring, and we anticipated that it would affect the formin’s ability to rotate around its anchoring point as the tether length is similar to the FH2-bound actin filament barbed end diameter (∼8–10 nm).8
In order to assess filament rotation around its main axis, we measured the polarization of the light emitted by a single fluorescently labeled actin subunit incorporated within the filament (Figure 1D).23,25 This allowed us to determine the polarization index of this subunit, which varies over time as the filament rotates around its axis (which itself points in the fixed direction of the flow). If the filament cannot rotate around its axis, the polarization index remains constant (Supporting Information Figure 1). If the filament is anchored to the surface via a formin, we can expect two main outcomes depending on the formin’s ability to rotate: (i) if the formin can rotate freely around its anchoring point, thermal fluctuations should cause the filament to twirl around its axis, and the polarization index is expected to vary rapidly and erratically over time; (ii) if the formin cannot rotate, then the filament should rotate steadily around its axis as it elongates, and the polarization index is expected to oscillate at a rate matching the elongation rate divided by the filament helicity. Individual single molecule fluorescence polarization traces lasted a few seconds before the fluorophore photobleached or the filament detached from anchored formins. Their visual inspection did not reveal any obvious switching between the erratic and steady rotation behaviors, thus indicating stable subpopulations at the time scale of a few seconds. The polarization traces were processed by fast Fourier transform in order to determine the main frequency of the signal. Filaments exhibiting an erratic rotation (i) were expected to have randomly distributed main frequencies, whereas filaments exhibiting a steady rotation (ii) were expected to have a main frequency imposed by the filament’s helicity and elongation rate, thus following a normal distribution. Consistently, for each type of formin anchoring, the cumulative distributions of the main frequencies could be well fitted by the weighted sum of a random distribution and a normal distribution centered on a frequency which was compatible with the filament elongation rate (Figure 1E, rotation at 0.4 Hz would correspond to an elongation rate of ∼11 s–1, consistent with our conditions). The relative weight of these two distributions thus allowed us to quantify the fraction of filaments in each category. Surprisingly, regardless of the solid (glass) or fluid (lipids) nature of the surface, we found that approximately 54% of filaments elongated by FH1-anchored formins were in situation (i) whereas this proportion was only 13% for FH2-anchored formin elongated filaments (Figure 1F). Therefore, as could be anticipated from the aforementioned structural details of the physical link connecting formins to the surface, most FH1-anchored formins were able to rotate around their anchoring point, whereas the rotation of FH2-anchored formins was mostly blocked.
Our results thus far show that the anchored formin’s ability to diffuse on the surface (lipid- versus glass-anchored) and its ability to rotate around its anchoring point (FH1- versus FH2-anchoring) only mildly affect its ability to rapidly elongate individual actin filaments and only increased its detachment rate when the formin-to-surface distance is of the order of 10 nm (FH2-anchored-to-lipids).
In a Fascin-Induced Bundle, Unanchored Trailing Formins Are Slower and Less Processive
We next sought to investigate how filament cross-linking, on its own, could affect their elongation by unanchored formins. We used fascin as a way to cross-link filaments into parallel bundles (Figure 2A). First, we characterized the bundling activity of fascin, using fluorescently labeled fascin. We observed that fascin binds cooperatively to zipper two parallel actin filaments with an effective affinity constant of Kd ∼ 75 nM (Supporting Information Figure 2A) which is in agreement with previous reports.26,27 After two filaments were bundled together by exposure to 100 nM fascin, the bundle could be maintained by fascin concentrations as low as 10 nM, for durations exceeding tens of seconds, in a fascin concentration-dependent manner (Supporting Information Figure 2B,C). This result indicates that bundle maintenance is easier to achieve than its formation.
On single filaments (not bundled), the presence of fascin in solution had no detectable impact on formin elongation rate or processivity, for fascin concentrations as high as 1 μM (Supporting Information Figure 2D).
We then tested the impact of filament bundling on formin-induced polymerization and depolymerization of filament barbed ends (Figure 2A). When filaments elongated by unanchored formins were bundled together by 100 nM fascin, we observed that the barbed end elongation of the leading filament was unaffected, i.e., consistent with single filament observations. In contrast, the elongation rates of the first and second trailing filaments were reduced by 18% and 30%, respectively (Figure 2B, Supporting Information Figure 2E). This decrease in elongation rate upon fascin-induced bundling is not specific to formins, because we also observed a similar reduction for trailing free barbed ends in bundles formed in the absence of formins (Supporting Information Figure 2F), as previously reported.27 This effect could originate from monomer diffusion being partially hindered by the presence of neighboring filaments within the bundle, 8–12 nm away.28−30 We tested this hypothesis by simulating monomer diffusion and barbed end binding on a 3D lattice (see Methods, Supporting Information Figure 2J–L). We found that in the presence of a neighboring filament 8 nm away from the barbed end, the polymerization rate dropped by less than 10% (Figure 2D). Thus, hindrance of monomer diffusion alone seems insufficient to explain the observed decrease in the elongation rate of trailing filament barbed ends both in the presence or absence of formins. One additional contribution could originate from the change in filament conformation induced by fascin bundling,31 which could alter the barbed ends of trailing filaments and decrease their affinity for monomeric actin.
In the presence of 4 μM profilin and 100 nM fascin, the depolymerization of the leading barbed end was similar to that of single filaments. In contrast, the depolymerization of the trailing barbed end was reduced by 78% (Figure 2C), which is a much stronger effect than in the polymerization regime (Figure 2B). Remarkably, when the leading formin caught up with the trailing formin, both filament barbed ends depolymerized synchronously at an intermediate rate and remained in close proximity (Figure 2A, bottom). The observed slower trailing formin depolymerization may originate from the combined effect of barbed end conformational change already effective in the polymerization regime, and the stabilization of terminal actin subunits by fascin connections to neighboring filaments. A similar decrease in depolymerization rate and the ensuing barbed end synchronization were observed in the absence of formins (Supporting Information Figure 2G).
We next quantified the impact of bundling on the processivity of unanchored formins. In the presence of 100 nM fascin and various concentrations of profilin-actin, the detachment rate of the first and second trailing formins were increased ∼3- and 12-fold, respectively, relative to the leading formin, which is unaffected by fascin-induced bundling (Figure 2E, Supporting Information Figure 2H). To further quantify the impact of bundling on formin activity, we varied the fascin concentration between 50 and 500 nM. At 50 nM fascin, although the filaments visually appeared to be bundled together and trailing formin elongation rates were reduced compared to those of the leading one (Supporting Information Figure 2I), we found that, surprisingly, the detachment rate of the leading, first, and second trailing formins were all identical (Figure 2F). In the presence of 500 nM fascin, the detachment rates of trailing formins were increased to a similar extent as what is observed with 100 nM fascin (Figure 2F). Therefore, it appears that low fascin concentrations affect the elongation rate but not the processivity of the formins in the bundle.
Overall, these results indicate that formin elongation rate is affected by fascin-induced bundling, potentially originating from filament under-twisting31 and monomer diffusion hindrance, which happens even at low fascin concentration, whereas formin processivity appears to be more sensitive to the efficient barbed end zippering occurring at higher fascin concentrations.
Anchoring Formins to a Solid Surface Further Hinders Their Activity in Filament Bundles
We next sought to investigate how formin anchoring may affect their activity when filaments’ rotation is impaired by cross-linking. In the presence of profilin-actin and 100 nM fascin, as filaments elongate from glass-anchored formins, pairwise bundles form with random distances between the two formins, typically 1–20 μm (Figure 3A, Supporting Information Movie 1 and Movie 2). As a standard condition, we used low actin and profilin concentrations to elongate filaments from formins at a moderate rate (∼8 subunits/s) with the intent to keep filaments reasonably short (∼10–30 μm), in order to form mainly 2-filament bundles, but similar results were obtained for higher actin and profilin concentrations (see below, Figure 3K).
When bundling occurred, we observed that the elongation rates of both leading and trailing formins abruptly changed, dropping on average to 50% of the elongation rate before bundling. In bundles, both the FH1-anchored-to-glass and the FH2-anchored-to-glass formin elongation rates were distributed quite uniformly between almost zero and the elongation rate observed before bundling (Figure 3B,E). As filament elongation proceeded, two typical behaviors were observed (Figure 3G). Either the leading filament elongated faster than the trailing filament and buckled (35%, n = 8 out of 23) or the two filaments elongated synchronously (65%, n = 15 out of 23). In cases where the leading filament buckled between the leading and trailing formin attachment points, the buckling force was estimated to be lower than 0.15 pN (see Supporting Information Methods). For trailing formins, this force adds up to the minimal viscous pulling force applied to the trailing filament and is expected to accelerate elongation not slow it down.11−13 We therefore concluded that the applied forces likely play no role in the slowing down of formin elongation rates.
In these bundles, we also quantified the rate at which filaments detached from the glass-anchored formins. We found that fascin-induced bundling strongly increased the detachment of formins (Figure 3C,F). The detachment rate in bundles was significantly higher for FH2-anchored-to-glass formins than for FH1-anchored-to-glass formins. Notably, we observed that the processivity of the leading formin was affected by fascin-induced bundling (Figure 3H). Leading formins dissociated from barbed ends before trailing formins in 35% of the cases, therefore more frequently than what we observed for unanchored formins (25%). This frequency was independent of the formin anchoring side (Figure 3H), of fascin concentration (Supporting Information Figure 3A,B), and of whether leading filaments buckled or not (Supporting Information Figure 3C).
Bundled formin detachment rate was independent of formin elongation rate reduction induced by bundling (Figure 3I) and of the distance between the leading and trailing formins (when comparing two subpopulations of bundles with average distances of 2.7 ± 0.8 μm or 7.8 ± 3.3 μm between bundled formins) (Figure 3J). When varying profilin–actin concentrations, we observed that bundle formin detachment rates scaled with the single formin detachment rates (Figure 3K). We also checked that applying a significant pulling force (by working with stronger microfluidics flow rates) increased formin bundle detachment rates but did not affect the amplitude of the increase due to bundling (Supporting Information Figure 3D).
Formin detachment rate increase caused by fascin-induced bundling was significantly different depending on the anchoring side, thus on the anchoring details. To further investigate this aspect, we performed measurements with heterodimeric formin constructs, comprising the FH2 dimer, one FH1 domain, and only one anchoring point (located either on the FH1-side or the FH2-side, see Methods and Supporting Information Figure 3G). Each of these formin heterodimers could thus bind to the glass surface via a single anchoring point. Heterodimeric formin processivity was reduced to the same extent as for homodimeric formins (Supporting Information Figure 3E,F), suggesting that the observed processivity reduction for homodimeric formins cannot be attributed to a double anchoring of the formins on the surface, which would impede their rotation as filaments elongate.
Overall, compared to single formins, the detachment rate of FH1-anchored-to-glass formins was increased 6- and 11-fold for leading and trailing formins, respectively, whereas for FH2-anchored-to-glass formins this reduction was more pronounced, respectively by 13- and 24-fold (Figure 3H). Taken together, those results indicate that in the situation of bundles where formins are statically anchored to a substrate, the anchoring of formins amplifies the effect of fascin-induced bundling to reduce formin elongation rate and processivity.
Preventing the Rotation of a Single Filament Has the Same the Impact as Bundling on Anchored Formin Activity
To test if the strong reduction in anchored formin activity requires the bundling of filaments by fascin, or if it can occur by only blocking filament rotation, we performed experiments where filaments elongating from glass-anchored formins were bound to inactivated glass-anchored NEM-myosins to prevent filament rotation (Figure 4A and Supporting Information Movie 3). Single formin elongation rates dropped and spread similarly to what we observed for glass-anchored formin elongating filaments bundled by fascin (Figure 4B). This observation is reminiscent of what has been observed by Mizuno and colleagues with formin mDia1 elongated filaments occasionally attaching to the surface through biotin–avidin interaction.32 Formin detachment rate increased upon NEM–myosin binding, similarly to what was observed for leading glass-anchored formins in fascin bundles, with FH2-anchored-to-glass formins detaching faster than FH1-anchored-to-glass formins (Figure 4C,D). Using this assay, we thus showed that a single attachment point along the side of the filament is enough to account for most of the change in formin activity observed in fascin bundle experiments. This suggests that the dominant effect of fascin-induced bundling, regarding the activity of glass-anchored formin, is to block filament rotation.
Lipid-Anchored Formins Efficiently Elongate Actin Filament Bundles
In cells, formins are anchored to lipid membranes and can potentially move independently from each other.33 We thus investigated the impact of filament bundling in the more physiological situation where formins are anchored to a lipid bilayer.
Filaments gathered at the edge of the lipid bilayer (Figure 5A and Supporting Information Movie 4) were bundled together by flowing in fascin in addition to profilin–actin (Figure 5B). Starting with a low density of single filaments allowed us to form mostly 2-filament bundles, whose elongation was then monitored. Here, the distance between bundled formins was much smaller than in the case of glass-anchored formins and below the optical resolution of our TIRF microscope (∼200 nm). Therefore, we used fluorescently labeled fascin to monitor when the bundled region moved away from the lipid edge (Figure 5C), which we identified as formin dissociation events. In contrast to glass-anchored formins, lipid-anchored 2-filament bundles grew steadily, and the elongation rate was not reduced compared to single filaments (Figure 5D). For some bundles, we could observe leading formins moving upstream as they elongated filament barbed ends away from the lipid pattern edge and the trailing formin (Figure 5C, bottom), possibly indicating a slower elongation of the trailing formin, reminiscent of what we observed for unanchored trailing formins (Figure 2B). Interestingly, the detachment rate of lipid-anchored formins appeared similar to that of unanchored formins (Figure 5F). The ∼2-fold difference between FH1-anchored-to-glass and FH2-anchored-to-glass formins detachment rate (Figure 3H,K) was still present for lipid-anchored formins, indicating that the tether length still plays an important role even for lipid-anchored formins in a bundle.
Overall, the results obtained from lipid-anchored formins show that (1) the translational freedom of formins thanks to the fluidity of the lipid bilayer seems to allow a smoother and more processive filament elongation by formins when filament rotation is blocked by fascin-induced bundling, whereas (2) this process is still dependent on the tether length connecting the formins to the surface.
Discussion
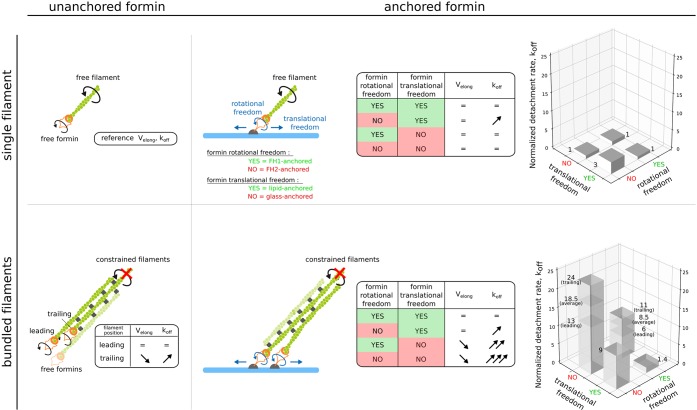
In this work, we investigated the impact of geometrical constraints on the activity of formin mDia1, quantified by the rate at which it elongates barbed ends and by the rate at which it detaches from them, which we summarized in Figure 6. First, we showed that having a barbed end within a fascin-induced bundle slowed down its elongation and increased formin detachment rate (Figure 2). As revealed by numerical simulations, these effects, in part, can be attributed to the presence of the neighboring filament which acts as an obstacle hindering monomer diffusion. Formin steric hindrance as well as a barbed end conformational change may also contribute to these effects. We also observed that FH2-anchored-to-lipids formins exhibit an increase of their detachment rate. This indicates that being very close to the surface can hinder formin activity. This observation is reminiscent of what was observed in a previous study, where Bni1p formin activity was affected by the height of the chromium barrier it was pushed against by a microfluidics flow.17 These observations, together with the impact of fascin-induced bundling, indicate that formins are very sensitive to the proximity of other components (e.g., membrane-bound proteins).
Figure 6.
Schematic summary of filament bundling and formin anchoring impact on formin activity.Depending on filaments being bundled by fascin or not, and whether formin translational or rotational freedom are blocked or not, formin-induced actin filament elongation and formin detachment rate are differently affected. Top, left: a single filament elongated by an unanchored formin is chosen as the reference for the comparison of elongation rates (Velong) and formin detachment rates (koff). Bottom, left: when fascin bundles filaments, the activity of the formin elongating the leading filament is unaffected, whereas the elongation rate is reduced and the detachment rate is increased for formins elongating trailing filaments. Top, right: the activity of surface-anchored formins elongating individual filaments is not affected by anchoring, except in the case of a short formin-to-surface distance. Bottom, right: Simultaneously blocking filament rotation by fascin-induced bundling and formin rotational or translational freedom, strongly impacts both formin elongation rate and detachment rate, for both leading and trailing filaments.
More strikingly, we found that the application of constraints to both actin filaments (bundling) and formins (anchoring) had a significant impact on formin activity (Figures 3 and 5). This unexpected cross-talk between constraints located micrometers apart is illustrated by our observation that formin anchoring details, which may play no role when elongating individual filaments (Figure 1), matter significantly as soon as filaments are bundled. In particular, constraints relative to formin’s ability to rotate around its anchoring point appear to have a strong impact on formin activity when filaments are bundled. Remarkably, formins anchored to a lipid bilayer via their N-terminus, hence with minimal constraints, are able to elongate bundled filaments as efficiently as if they were not anchored (Figure 5). Notably, in the case of lipid surfaces where lipid diffusion may favor the double anchoring of formin dimers, such a situation still appears to allow formins to reorient and to function with minimal hindrance.
Interestingly, the issue of formin rotational freedom upon anchoring has recently been investigated in the context of pulling forces applied to single filaments by Yu and colleagues.13 In this study, the authors used magnetic beads to pull on filaments and found that piconewton pulling forces applied to formins that were free to rotate allowed them to elongate filaments extremely rapidly, close to the theoretical diffusion limit in the presence of profilin–actin.14 In contrast, when applying significant forces to our freely rotating formins, the elongation rates remained comparable to what we have reported earlier using microfluidics11,15 and to what others have reported using optical traps12 or myosins16 to apply pulling forces. Control experiments in the latter study indicate that the effect they observe (on mDia2 and Cdc12 formins) can indeed be attributed to tension. Nonetheless, the contribution of active myosins to torsional constraints is not fully understood34 and may be worth investigating further, following our results.
Thanks to these earlier reports that used different means to apply mechanical tension to actin filaments, the fact that formins are sensitive to external forces is now well established.7 Here, we show that formins are also sensitive to the geometrical organization of the filaments they elongate. When filaments are cross-linked and cannot rotate around their main axis, hindering the rotation of the anchored formin results in the generation of a mechanical torque as the filaments elongate, and, as a consequence, the formin’s elongation rate is reduced and its off-rate is increased. This sensitivity to geometry, where mechanical stress is not applied from the outside but rather generated by the protein’s activity in a specific context, is reminiscent of our recent work showing that cofilin binding applies a mechanical torque to cross-linked filaments, and thereby greatly enhances their severing.25
Here, we focused on fascin-induced bundling as a means to cross-link filaments, a situation typically encountered in filopodia35 and invadopodia.36 More generally, most actin cross-linkers and network geometries are likely to also block filament rotation and to have a similar impact on formin activity, as exemplified by our experiments with NEM-myosins (Figure 4). As we show, the details of formin anchoring are of great importance in this context, and these can certainly take different forms in cells. The binding of Diaphanous-related formins directly to membranes or to membrane-bound proteins and activators via their N-terminal regions37−39 likely allows them to maintain some rotational freedom while being anchored. This freedom may be tuned by a number of other factors, such as the membrane lipid composition or the presence of other proteins. In particular, in the context of filopodia, the presence of Ena/VASP and its potential interaction with formins40,41 may alter formin’s rotational freedom and thereby its ability to efficiently elongate barbed ends for significant durations. Future studies will certainly shed light on how the rotational freedom of membrane-anchored formins can be modulated and regulate the assembly dynamics of actin filaments in cells.
Acknowledgments
We thank all members of the G.R.-L./A.J. laboratory for their help, especially Hugo Wioland for help in the fluorescence polarization analysis and Sandy Jouet for protein purifications. We acknowledge funding from the French ANR (Grants “MuscActin” and “Conformin” to G.R.-L.) and the European Research Council (Grant StG-679116 to A.J.).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.nanolett.9b02241.
Methods; three figures showing the supplementary data of formin activity in bundles; one table summarizing the population sample sizes and statistical significance tests for the data presented in the main figures (PDF)
Supplementary movie #1: Randomly spaced FH1-anchored-to-glass formins elongate filaments in the presence of 0.2 μM 15% alexa488-labeled actin, 2 μM profilin and 100 nM fascin. As the filaments elongate they encounter and bundle with other filaments due to fascin crosslinking. Scale bar, 10 μm. Flow direction: right (AVI)
Supplementary movie #2: Annotated sub-region of supplementary movie #1. Numbers (either ‘2’ or ‘3’) indicate the size of a bundle; ‘up. detach.’ (respectively ‘down. detach.’) indicates that the upstream (resp. downstream) filament detaches from the ‘leading’ (resp. ‘trailing’) formin of a 2-filament bundle (AVI)
Supplementary movie #3: Randomly spaced FH1-anchored-to-glass formins elongate filaments in the presence of 0.2 μM 15% alexa488-labeled actin and 2 μM profilin. Filaments may attach to randomly spaced NEM-myosins non-specifically adsorbed to the glass surface. Scale bar, 10 μm. Flow direction: right (AVI)
Supplementary movie #4: FH1-anchored-to-lipid formins that have collected at the lipid/PEG brush boundary elongate filaments in the presence of 0.5 μM actin, 2 μM profilin and 100 nM fascin. Green: alexa488-labeled actin; Red: alexa568-labeled fascin. Scale bar, 10 μm. Flow direction: right (AVI)
The authors declare no competing financial interest.
Supplementary Material
References
- Blanchoin L.; Boujemaa-Paterski R.; Sykes C.; Plastino J. Actin Dynamics, Architecture, and Mechanics in Cell Motility. Physiol. Rev. 2014, 94, 235–263. 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- Hansen S. D.; Mullins R. D. VASP is a processive actin polymerase that requires monomeric actin for barbed end association. J. Cell Biol. 2010, 191, 571–584. 10.1083/jcb.201003014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar D. R.; Pollard T. D. Insertional assembly of actin filament barbed ends in association with formins produces piconewton forces. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 14725–14730. 10.1073/pnas.0405902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero S.; et al. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 2004, 119, 419–429. 10.1016/j.cell.2004.09.039. [DOI] [PubMed] [Google Scholar]
- Zigmond S. H.; et al. Formin leaky cap allows elongation in the presence of tight capping proteins. Curr. Biol. 2003, 13, 1820–1823. 10.1016/j.cub.2003.09.057. [DOI] [PubMed] [Google Scholar]
- Courtemanche N. Mechanisms of formin-mediated actin assembly and dynamics. Biophys. Rev. 2018, 10, 1553–1569. 10.1007/s12551-018-0468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann D.; Kovar D. R. Feeling the force: formin’s role in mechanotransduction. Curr. Opin. Cell Biol. 2019, 56, 130–140. 10.1016/j.ceb.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Otomo T.; et al. Structural basis of actin filament nucleation and processive capping by a formin homology 2 domain. Nature 2005, 433, 488–494. 10.1038/nature03251. [DOI] [PubMed] [Google Scholar]
- Paul A.; Pollard T. D. The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation. Curr. Biol. 2008, 18, 9–19. 10.1016/j.cub.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vavylonis D.; Kovar D. R.; O’Shaughnessy B.; Pollard T. D. Model of formin-associated actin filament elongation. Mol. Cell 2006, 21, 455–466. 10.1016/j.molcel.2006.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jégou A.; Carlier M.-F.; Romet-Lemonne G. Formin mDia1 senses and generates mechanical forces on actin filaments. Nat. Commun. 2013, 4, 1883. 10.1038/ncomms2888. [DOI] [PubMed] [Google Scholar]
- Kubota H.; et al. Biphasic Effect of Profilin Impacts the Formin mDia1 Force-Sensing Mechanism in Actin Polymerization. Biophys. J. 2017, 113, 461–471. 10.1016/j.bpj.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.; et al. mDia1 senses both force and torque during F-actin filament polymerization. Nat. Commun. 2017, 8, 1650. 10.1038/s41467-017-01745-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M.; et al. Effects of Mechanical Stimuli on Profilin- and Formin-Mediated Actin Polymerization. Nano Lett. 2018, 18, 5239. 10.1021/acs.nanolett.8b02211. [DOI] [PubMed] [Google Scholar]
- Cao L.; et al. Modulation of formin processivity by profilin and mechanical tension. eLife 2018, 7, e34176 10.7554/eLife.34176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann D.; et al. Mechanoregulated inhibition of formin facilitates contractile actomyosin ring assembly. Nat. Commun. 2017, 8, 703. 10.1038/s41467-017-00445-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtemanche N.; Lee J. Y.; Pollard T. D.; Greene E. C. Tension modulates actin filament polymerization mediated by formin and profilin. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 9752–9757. 10.1073/pnas.1308257110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D.; Goode B. L. Formins at a glance. J. Cell Sci. 2013, 126, 1–7. 10.1242/jcs.107250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönichen A.; Geyer M. Fifteen formins for an actin filament: a molecular view on the regulation of human formins. Biochim. Biophys. Acta, Mol. Cell Res. 2010, 1803, 152–163. 10.1016/j.bbamcr.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Kühn S.; Geyer M. Formins as effector proteins of Rho GTPases. Small GTPases 2014, 5, e983876 10.4161/sgtp.29513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X.; Kawauchi K.; Shivashankar G. V.; Bershadsky A. D. Novel localization of formin mDia2: importin β-mediated delivery to and retention at the cytoplasmic side of the nuclear envelope. Biol. Open 2015, 4, 1569–1575. 10.1242/bio.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti R.; et al. INF2-mediated actin polymerization at the ER stimulates mitochondrial calcium uptake, inner membrane constriction, and division. J. Cell Biol. 2018, 217, 251–268. 10.1083/jcb.201709111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno H.; et al. Rotational movement of the formin mDia1 along the double helical strand of an actin filament. Science 2011, 331, 80–83. 10.1126/science.1197692. [DOI] [PubMed] [Google Scholar]
- Horan B. G.; Zerze G. H.; Kim Y. C.; Vavylonis D.; Mittal J. Computational modeling highlights the role of the disordered Formin Homology 1 domain in profilin-actin transfer. FEBS Lett. 2018, 592, 1804–1816. 10.1002/1873-3468.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wioland H.; Jegou A.; Romet-Lemonne G. Torsional stress generated by ADF/cofilin on cross-linked actin filaments boosts their severing. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 2595–2602. 10.1073/pnas.1812053116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D.; et al. Cofilin cooperates with fascin to disassemble filopodial actin filaments. J. Cell Sci. 2011, 124, 3305–3318. 10.1242/jcs.086934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman J. D.; Bilancia C. G.; Peifer M.; Kovar D. R. Ena/VASP Enabled is a highly processive actin polymerase tailored to self-assemble parallel-bundled F-actin networks with Fascin. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 4121–4126. 10.1073/pnas.1322093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S.; et al. Mechanism of actin filament bundling by fascin. J. Biol. Chem. 2011, 286, 30087–30096. 10.1074/jbc.M111.251439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.; et al. Molecular mechanism of fascin function in filopodial formation. J. Biol. Chem. 2013, 288, 274–284. 10.1074/jbc.M112.427971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramaki S.; Mayanagi K.; Jin M.; Aoyama K.; Yasunaga T. Filopodia Formation by Cross-linking of F-actin with Fascin in Two Different Binding Manners. Cytoskeleton 2016, 73, 365. 10.1002/cm.21309. [DOI] [PubMed] [Google Scholar]
- Shin H.; Purdy Drew K. R.; Bartles J. R.; Wong G. C. L.; Grason G. M. Cooperativity and frustration in protein-mediated parallel actin bundles. Phys. Rev. Lett. 2009, 103, 238102. 10.1103/PhysRevLett.103.238102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno H.; Tanaka K.; Yamashiro S.; Narita A.; Watanabe N. Helical rotation of the diaphanous-related formin mDia1 generates actin filaments resistant to cofilin. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, E5000. 10.1073/pnas.1803415115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashida C.; et al. Actin polymerization-driven molecular movement of mDia1 in living cells. Science 2004, 303, 2007–2010. 10.1126/science.1093923. [DOI] [PubMed] [Google Scholar]
- Leijnse N.; Oddershede L. B.; Bendix P. M. An updated look at actin dynamics in filopodia. Cytoskeleton 2015, 72, 71. 10.1002/cm.21216. [DOI] [PubMed] [Google Scholar]
- Vignjevic D.; et al. Role of fascin in filopodial protrusion. J. Cell Biol. 2006, 174, 863–875. 10.1083/jcb.200603013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A.; et al. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr. Biol. 2010, 20, 339–345. 10.1016/j.cub.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aspenström P. Formin-binding proteins: Modulators of formin-dependent actin polymerization. Biochim. Biophys. Acta, Mol. Cell Res. 2010, 1803, 174–182. 10.1016/j.bbamcr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Ramalingam N.; et al. Phospholipids regulate localization and activity of mDia1 formin. Eur. J. Cell Biol. 2010, 89, 723–732. 10.1016/j.ejcb.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Rousso T.; Shewan A. M.; Mostov K. E.; Schejter E. D.; Shilo B.-Z. Apical targeting of the formin Diaphanous in Drosophila tubular epithelia. eLife 2013, 2, e00666 10.7554/eLife.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzik M.; McClain L. M.; Gupton S. L.; Gertler F. B. Ena/VASP regulates mDia2-initiated filopodial length, dynamics, and function. Mol. Biol. Cell 2014, 25, 2604–2619. 10.1091/mbc.e14-02-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilancia C. G.; et al. Enabled negatively regulates diaphanous-driven actin dynamics in vitro and in vivo. Dev. Cell 2014, 28, 394–408. 10.1016/j.devcel.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.