Abstract
Patients with atrial fibrillation (AF) and acute coronary syndrome (ACS) are at high risk of stroke, recurrent coronary ischemic events, and cardiovascular mortality. The composition of antithrombotic therapy including an oral anticoagulant and antiplatelet drug(s) should be tailored according to the individual patient’s risk profile, to reduce the bleeding risk and maintain antithrombotic effect. There is no single antithrombotic treatment regimen that would fit to all patients with AF and ACS. However, available data promote the use of full‐dose direct oral anticoagulants (DOACs) (dabigatran 150 mg twice daily or apixaban 5 mg twice daily) or rivaroxaban 15 mg once daily in patients with AF and ACS or percutaneous coronary intervention (PCI). For many patients, a DOAC plus P2Y12 inhibitor early after ACS and/or PCI would be optimal, whereas a longer course of triple therapy should be used in patients at high thrombotic risk.
Keywords: acute coronary syndrome, antiplatelets, atrial fibrillation, oral anticoagulation, percutaneous coronary intervention, triple therapy
Essentials.
Atrial fibrillation (AF) is common among patients with vascular disease.
Studies on antithrombotic management in patients with AF and acute coronary syndrome (ACS) were assessed.
Balancing the risk of ischemia and stroke and bleeding in patients with AF and ACS remains challenging.
Direct oral anticoagulant–based management strategies are preferred.
1. INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia in adults, coexisting with vascular disease in about 30% of patients. Over 80% of patients with AF have ≥1 stroke risk factor(s), thus requiring stroke prevention therapy, most commonly using oral anticoagulants (OACs).1 Given that the estimated global prevalence of AF is 1% to 3% and around 20% of patients with AF would need a percutaneous coronary intervention (PCI), about 1 to 3 million Europeans with AF taking OACs may require PCI.2, 3, 4, 5
Patients with AF and acute coronary syndrome (ACS) (ie, unstable angina, non–ST‐segment elevation myocardial infarction [MI] or ST‐segment elevation MI) have particularly high risk of recurrent coronary events (ie, MI or stent thrombosis), stroke, and cardiovascular mortality.6
Preventing stroke, recurrent cardiac ischemia, and stent thrombosis using a combined antithrombotic therapy needs to be balanced against the risk of major bleeding (including intracranial hemorrhage ICH; Figure 1).1, 7 The use of dual antiplatelet therapy (DAPT) alone would not sufficiently protect patients against stroke, whereas OAC monotherapy, either a direct oral anticoagulant (DOAC) or vitamin K antagonist (VKA), would not protect patients against new coronary events.8, 9 Triple therapy (TT) using DAPT in combination with an OAC effectively prevents vascular ischemic events but is associated with considerably increased risk of bleeding.10
Figure 1.
Balancing the risks in the patients with atrial fibrillation who present with an acute coronary syndrome and/or undergo percutaneous coronary intervention/stenting
2. OVERVIEW OF PUBLISHED DATA
Various studies have addressed the challenging management of patients with AF and ACS. Observational studies have shown that in AF patients after MI/PCI, dual antithrombotic therapy (clopidogrel and OAC) was equal to or better than TT in terms of benefit (MI or coronary death, fatal or nonfatal ischemic stroke, and all‐cause mortality) and safety outcomes (fatal or nonfatal bleeding).11 In the Management of Patients With Atrial Fibrillation Undergoing Coronary Artery Stenting (AFCAS) registry,12 TT, DAPT, and dual antithrombotic therapy (VKA with clopidogrel) had similar 1‐year efficacy (stroke/transient ischemic events, peripheral embolism, MI, revascularization, definite/probable stent thrombosis) and safety (minor and major bleedings), but the study was limited by a low rate of adverse events and relatively small size of the group taking VKA with clopidogrel.
In the warfarin era, the What Is the Optimal Antiplatelet and Anticoagulant Therapy in Patients With Oral Anticoagulation and Coronary Stenting (WOEST) trial assessed the use of antiplatelet therapy in patients on a VKA.13 The use of dual antithrombotic therapy (clopidogrel and a VKA) was compared to triple therapy (VKA and clopidogrel plus aspirin). Dual antithrombotic therapy was associated with significantly lower risk of Thrombolysis in Myocardial Infarction (TIMI) minor and major bleeding in comparison to TT (of note, there was no significant difference in major bleeds). However, the trial was small; not all patients were taking OACs for AF‐related stroke prevention (69% of patients had AF) and 25% to 30% of participants had an ACS; radial access was chosen in only 25% to 27% of patients; and TT was continued for 12 months. Notably, the WOEST trial also showed that patients taking TT had a higher risk of mortality compared with those on dual antithrombotic therapy (ie, clopidogrel and a VKA).
In the contemporary era of DOACs, post hoc analyses of the landmark DOACs trials for stroke prevention in AF showed consistent efficacy and safety of the respective DOAC versus warfarin irrespective of the concomitant aspirin use or nonuse.14, 15, 16, 17 Although patients concomitantly using an antiplatelet drug (mostly aspirin) and OAC (either a DOAC or warfarin) were at higher risk of both ischemic and bleeding events compared with those on OAC monotherapy, the rates of hemorrhagic stroke or ICH were consistently lower with DOACs in comparison to warfarin.14, 15, 16, 17
Contemporary observational studies consistently reported findings similar to those substudies. The Danish nationwide registry–based study, for example, reported that among patients with AF and MI and/or PCI, those taking a DOAC plus DAPT had a significantly lower risk of bleeding than patients taking a VKA plus DAPT, with no significant differences in all‐cause mortality, ischemic stroke, or MI between the 2 treatment regimens.18 The study was limited by lack of significant parameters (International Normalized Ratio [INR], blood pressure, creatinine clearance, estimated glomerular filtration rate, and alanine amino transferase). There was also no possibility to compare safety and efficacy of particular DOACs because of the limited number of patients.
Four recent open‐label, randomized, controlled clinical trials investigated the use of DOACs in patients with AF with a recent ACS and/or undergoing a PCI19, 20, 21, 22 see Table 1. The PIONEER AF‐PCI (an open‐label, randomized, controlled, multicenter study exploring 2 treatment strategies of rivaroxaban and dose‐adjusted oral VKA treatment strategy in subjects with AF undergoing PCI)19 was an exploratory study comparing bleeding rates across 3 strategies (low‐dose rivaroxaban [2.5 mg twice daily] plus DAPT vs. rivaroxaban 15 mg once daily, or 10 mg once daily in patients with a creatinine clearance of <50 mL/min, plus clopidogrel [dual antithrombotic therapy with DOAC or TT with DOAC] vs. TT with VKA plus DAPT) in patients with AF after a PCI with stent placement. The primary safety end point was defined as the percentage of patients with either TIMI major bleeding, minor bleeding, or bleeding requiring medical attention events by the end of 12 months of randomized therapy. Secondary safety end points were defined as the incidence of each component of the bleeding composite (TIMI major bleeding, minor bleeding, and bleeding requiring medical attention), the composite of adverse cardiovascular events (cardiovascular death, stroke, and MI), as well as cardiovascular death, MI, stroke, and stent thrombosis. The treatment regimens with rivaroxaban 15 mg once daily plus clopidogrel for 12 months or rivaroxaban 2.5 mg twice daily plus DAPT for 1, 6, or 12 months were associated with lower rates of the composite end point of bleeding than TT with VKA plus DAPT for 1, 6, or 12 months.23 All 3 strategies were associated with similar rates of composite outcome of cardiovascular mortality, MI, or stroke; however, the trial was underpowered to establish superiority or noninferiority of evaluated strategies regarding these outcomes.
Table 1.
Randomized clinical trials of combined antithrombotic therapies in patients with AF with ACS or PCI/Stenting
| PIONEER‐AF PCI 23 | RE‐DUAL PCI 20 | ENTRUST‐AF PCI 22 | AUGUSTUS 24 | |
|---|---|---|---|---|
| Objective | Rivaroxaban + P2Y12 inhibitor or DAPT versus VKA + DAPT in patients with NVAF undergoing PCI | Dabigatran + P2Y12 inhibitor versus VKA + DAPT in patients with NVAF undergoing PCI | Edoxaban + P2Y12 inhibitor versus VKA + DAPT in patients with NVAF undergoing PCI | Apixaban + ASA/placebo versus VKA + ASA/placebo in patients with NVAF and ACS or PCI |
| Population size | 2124 | 2725 | 1506 | 4600 |
| Treatments |
Rivaroxaban 15 mg once daily + P2Y12 inhibitor, rivaroxaban 2.5 mg once daily + P2Y12 inhibitor + ASA, then rivaroxaban 15 mg once daily + ASA, VKA + P2Y12 inhibitor + ASA, then VKA + ASA |
Dabigatran 120 mg or 110 mg twice daily + P2Y12 inhibitor, VKA + P2Y12 inhibitor + ASA |
Edoxaban 60 mg once daily or 30 mg once daily + P2Y12 inhibitor, VKA + P2Y12 inhibitor + aspirin |
Apixaban 5 mg or 2.5 mg twice daily + ASA/placebo VKA + ASA/placebo |
| Duration | 12 mo | 6‐30 mo | 12 mo | 6 mo |
| Primary outcome | Clinically significant bleeding | Major or clinically relevant nonmajor bleeding event | Major or clinically relevant nonmajor bleeding | Major or clinically relevant nonmajor bleeding |
| Main secondary composite end point | The composite of death from cardiovascular causes, myocardial infarction, or stroke | The composite of thromboembolic events (myocardial infarction, stroke, or systemic embolism), death, or unplanned revascularization (PCI or coronary artery bypass grafting). | The composite of cardiovascular death, stroke, systemic embolic events, myocardial infarction, and definite stent thrombosis | The composite of death or hospitalization; the composite of death or ischemic events (stroke, myocardial infarction, stent thrombosis [definite or probable], or urgent revascularization) |
| Analysis period | Treatment‐emergent period | Time to first event | Day 1 to 12 mo | Time to first event |
Abbreviations: ACS, acute coronary syndrome; ASA, acetylsalicylic acid; DAPT, dual antiplatelet therapy; NVAF, nonvalvular atrial fibrillation; PCI, percutaneous coronary intervention; TTR, time in therapeutic range; VKA, vitamin K antagonists.
The aim of the Evaluation of Dual Therapy with Dabigatran Versus Triple Therapy With Warfarin in Patients With AF That Undergo a PCI With Stenting (REDUAL‐PCI)20 was to compare dual antithrombotic therapy with dabigatran (dabigatran 150 mg or 110 mg twice daily plus clopidogrel or ticagrelor) to TT (warfarin plus clopidogrel or ticagrelor plus aspirin) in patients with AF after PCI. The primary end point was defined as a major or clinically relevant nonmajor (CRNM) bleeding event. The secondary end points were defined as a composite efficacy end point of MI, stroke, or systemic embolism; death or unplanned revascularization; or a combined end point of thromboembolic events or death as well as the individual thromboembolic events and definite stent thrombosis. Compared with TT, both dual antithrombotic therapy regimens (ie, dabigatran 110 mg twice daily or 150 mg twice daily plus a P2Y12 inhibitor) were associated with significantly lower risk of major bleeding and CRNM bleeding. Dual antithrombotic therapy with dabigatran (both dabigatran doses taken together) was noninferior to TT regarding the rates of MI, stroke, or systemic embolism. The study was underpowered to evaluate efficacy according to dabigatran dose. Moreover, with respect to the results for the safety and efficacy end points, it is only possible to speculate on the relative contributions of the omission of aspirin and the type of OAC in the dual antithrombotic therapy groups and the triple‐therapy group.
Both the PIONEER AF‐PCI and RE‐DUAL PCI trial clearly showed that dual therapy including the respective DOAC plus a P2Y12 inhibitor (mostly clopidogrel) was associated with significantly less major bleeding in comparison to TT with VKA plus dual antiplatelet therapy. However, these trials could not distinguish whether the bleeding risk reduction was driven by the use of a DOAC or nonuse of aspirin, or both.
Apixaban was tested in an open‐label, 2 × 2 factorial, randomized controlled trial evaluating the safety of apixaban versus VKA and aspirin versus placebo in patients with AF and ACS and/or PCI (the AUGUSTUS trial).21 Patients with AF and an ACS and/or PCI within the preceding 14 days were randomized to apixaban 5 mg twice daily (or apixaban 2.5 mg twice daily in patients fulfilling dose reduction criteria) versus VKA and to aspirin versus aspirin‐placebo. The primary outcome was major bleeding defined by the ISTH or CRNM bleeding. Secondary outcomes included the composite end point of death, stroke, MI, stent thrombosis, or urgent hospitalization and the composite of death, hospitalization, and first hospitalization for any cause. Dual antithrombotic therapy (apixaban plus P2Y12 inhibitor without aspirin) was associated with significantly lower incidence of bleeding when compared to regimen with VKA, aspirin, or both. Apixaban without aspirin was also correlated with significantly lower incidence of hospitalizations than VKA, aspirin, or both. There were no significant differences in ischemic events, although the rates of stent thrombosis and cardiovascular events were numerically higher in the placebo‐treated patients compared to those on aspirin, while the rate of stroke was lower in patients on apixaban than in those on VKA.24 One of the major study limitations is the poor quality of anticoagulation with VKA (time in therapeutic range [TTR] in patients on VKA was lower than in previous randomized trials).
In the Safety and Efficacy of an Edoxaban‐Based Antithrombotic Regimen in Patients With AF Following Successful PCI With Stent Placement (the ENTRUST‐AF PCI) trial,22 AF patients undergoing PCI for stable coronary artery disease or ACS were randomized to dual antithrombotic therapy with a DOAC (edoxaban [60 mg once daily or 30 mg once daily where dose reduction criteria were fulfilled] with a P2Y12 inhibitor for 12 months) or TT with VKA plus P2Y12 inhibitor and aspirin (100 mg once daily for 1‐12 months). The primary end point was defined as the composite of major or CRNM bleeding defined by the ISTH. The composite of cardiovascular death, stroke, systemic embolic events, MI, and definite stent thrombosis was defined as the main efficacy outcome. Edoxaban 60 mg once daily plus a P2Y12 inhibitor was noninferior to TT with VKA regarding major or CRNM bleeding events at 12 months. The main efficacy outcome (composite of cardiovascular death, stroke, systemic embolic events, MI, or definite stent thrombosis) were similar in both treatment arms. The study was limited by its open‐label design. It was also underpowered and limited by a small proportion of patients medicated with a more potent P2Y12 inhibitor than clopidogrel.
In a network meta‐analysis that included the WOEST, PIONEER AF‐PCI, RE‐DUAL PCI, and AUGUSTUS trials and 4 different treatment strategies (ie, DOAC or VKA plus a P2Y12 inhibitor dual regimens and DOAC or VKA plus a P2Y12 and aspirin TT regimens),25 DOAC‐based dual treatments were associated with fewer TIMI major bleedings and less ICH compared with VKA with DAPT, and there was no statistically significant difference in the rates of major adverse cardiac events, all‐cause death, and cardiovascular mortality among the 4 treatment regimens. No statistically significant differences were revealed with respect to stroke, MI, and stent thrombosis among the treatment regimens (see Table 2).
Table 2.
Summarized outcomes of trials assessing treatment regimens in patients with AF with ACS or PCI/stenting 25
| Outcomes | VKA + DAPT (reference) | |||||
|---|---|---|---|---|---|---|
| VKA + P2Y12 inhibitor | DOAC + DAPT | DOAC + P2Y12 inhibitor | ||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| TIMI major bleeding | 0.58 | 0.31‐1.08 | 0.70 | 0.38‐1.23 | 0.49 | 0.30‐0.82 |
| TIMI major and minor bleeding | 0.49 | 0.26‐0.92 | 0.63 | 0.33‐1.17 | 0.43 | 0.25‐0.76 |
| Primary safety outcome (trial defined) | 0.45 | 0.21‐0.92 | 0.64 | 0.31‐1.31 | 0.47 | 0.25‐0.85 |
| ICH | 1.44 | 0.40‐5.22 | 0.54 | 0.15‐1.92 | 0.26 | 0.08‐0.79 |
| All‐cause death | 0.84 | 0.40‐1.56 | 1.04 | 0.54‐1.98 | 1.02 | 0.59‐1.74 |
| Cardiovascular death | 0.82 | 0.42‐1.49 | 0.94 | 0.53‐1.63 | 1.11 | 0.70‐1.75 |
| Primary MACE (trial defined) | 0.96 | 0.60‐1.46 | 0.94 | 0.60‐1.15 | 1.02 | 0.71‐1.97 |
| MI | 1.25 | 0.77‐1.99 | 1.13 | 0.72‐1.78 | 1.18 | 0.81‐1.72 |
| Stroke | 1.02 | 0.36‐2.65 | 0.91 | 0.35‐2.32 | 0.77 | 0.34‐1.67 |
| Stent thrombosis | 1.08 | 0.46‐2.31 | 0.93 | 0.40‐2.17 | 1.41 | 0.71‐2.76 |
| Hospitalization | 0.86 | 0.57‐1.23 | 0.80 | 0.55‐1.13 | 0.80 | 0.59‐1.08 |
Odds ratio < 1 favors nonreference strategy; odds ratio > 1 favors reference strategy.
Abbreviations: CI, confidence interval; DAPT, dual antiplatelet therapy; DOAC, direct oral anticoagulants; ICH, intracranial hemorrhage; MACE, major adverse cardiac event; MI, myocardial infarction; OR, odds ratio; TIMI, Thrombolysis in Myocardial Infarction; VKA, vitamin K antagonist.
In another meta‐analysis,26 data from the PIONEER AF‐PCI, RE‐DUAL PCI, and AUGUSTUS trials were evaluated. The use of DOACs was associated with significantly lower rates of bleeding and similar rates of ischemic events (stroke, MI, and stent thrombosis) and all‐cause death/cardiovascular mortality than VKA‐based treatments (Table 3). Dual antithrombotic therapy was associated with a significantly lower incidence of bleeding; similar rates of stroke, MI, and all‐cause/cardiovascular death; and a significantly greater risk of stent thrombosis when compared with TT (Table 3).
Table 3.
Summarized outcomes of PIONEER‐PCI, RE‐DUAL PCI, and AUGUSTUS trials26
| Outcomes | Dual antithrombotic therapy versus triple antithrombotic therapy | DOAC‐based antithrombotic treatment regimen versus VKA‐based antithrombotic treatment regimen | ||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| ISTH major bleeding | 0.60 | 0.49‐0.73 | 0.58 | 0.48‐0.70 |
| Stroke | 1.01 | 0.68‐1.51 | 0.84 | 0.56‐1.28 |
| Myocardial infarction | 1.21 | 0.96‐1.54 | 0.98 | 0.78‐1.25 |
| Stent thrombosis | 1.67 | 1.02‐2.73 | 1.10 | 0.68‐1.77 |
| All‐cause death | 1.05 | 0.83‐1.34 | 1.02 | 0.80‐1.30 |
| CV death | 1.13 | 0.81‐1.56 | 1.11 | 0.80‐1.55 |
Abbreviations: CI, confidence interval; CV; cardiovascular; DOAC, direct oral anticoagulant; OR, odds ratio; VKA, vitamin K antagonist.
Overall, available data support the use of full‐dose DOAC (dabigatran 150 mg twice daily or apixaban 5 mg twice daily) or rivaroxaban 15 mg once daily in patients with AF and ACS or PCI. An initial course of first months after ACS or PCI may be prudent, particularly in patients at high risk of recurrent ischemia.
3. TT IN PATIENTS WITH AF AND ACS FROM DISCHARGE TO 12 MONTHS AFTER ACS
Owing to increased stroke risk in patients with ACS and AF, such patients should be considered for concomitant OAC use.27 There is no single strategy of TT use that would be suitable for all patients with AF and ACS. The composition and duration of TT should be individualized based on the estimated bleeding and cardioembolic and atherothrombotic risks in each patient.28, 29, 30, 31, 32 Stroke risk should be evaluated using the CHA2DS2‐VASc (congestive heart failure, hypertension, age ≥75 years, diabetes, stroke/transient ischemic attack, vascular disease, age 65‐74 years, sex category) score, while the risk of ischemic events risk can be determined using the Global Registry of Acute Coronary Events (GRACE) score.29 The risk of bleeding risk is dynamic and should be regularly reassessed using the HAS‐BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR) score. Modifiable bleeding risk factors should be identified and actively managed.28, 33, 34 Of note, the use of a formal bleeding risk score has been shown to be superior to relying only on an approach based on modifiable bleeding risk factors.35
4. BLEEDING PREVENTION AND BLEEDING REDUCTION STRATEGIES
TT is associated with increased rates of major bleeding ranging from 5% to 15% at 1 year.36, 37, 38, 39, 40 Unfortunately, major bleeding may result in a 2‐ to 8‐fold increase in the risk of mortality and nonfatal cardiovascular events.41, 42, 43, 44, 45
The risk of bleeding varies with different antithrombotic treatment regimens. The risk of bleeding with aspirin together with clopidogrel is higher during the early months after initiation. Bleeding on warfarin is also highest in the early period after the drug initiation (initial 3 months).46 Clinical risk scores may facilitate decision making regarding the most suitable treatment strategy, but their predictive ability is generally modest, and interscore variability is present.47 Another hurdle is that bleeding risk is closely related to the stroke risk and some thromboembolic risk factors such as older age, uncontrolled hypertension, or history of stroke have also been identified as bleeding risk factors.48 Thus, patients with high bleeding risk also have a high risk of stroke.
To minimize bleeding risk, while maintaining antithrombotic effect,49 VKA therapy should be well managed, with a high TTR (>65%‐70%),50 and the lowest effective dose of aspirin should be chosen. Regarding DOACs, the doses approved for stroke prevention should be used. Data on concomitant use of prasugrel or ticagrelor in patients taking OACs are scarce, and the risk of bleeding with either of these drugs may be excessive in combination with aspirin in anticoagulated patients.31, 51, 52 Hence, clopidogrel is the preferred antiplatelet agent regarding PCI in patients with indications for OACs.9, 28, 53 Importantly, new‐generation drug‐eluting stents and radial approach should be used to minimize bleeding risk, while proton pump inhibitors should be considered in all patients receiving TT to minimize gastrointestinal bleeding.51 Concomitant use of nonsteroidal anti‐inflammatory drugs should be avoided in patients with AF and ACS (Figure 2).49
Figure 2.
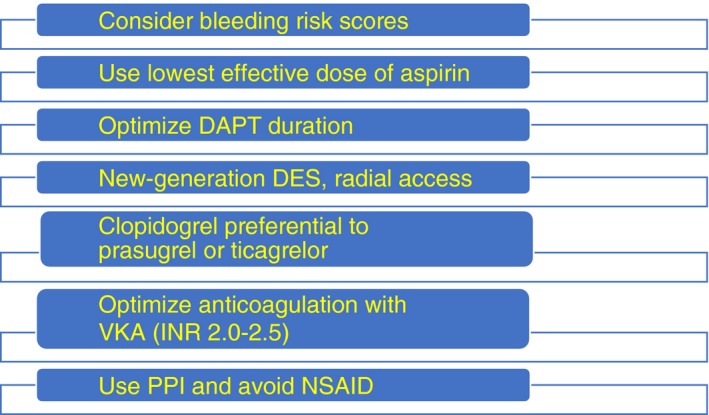
Strategies to reduce bleeding risk. DAPT, dual antiplatelet therapy; DES, drug‐eluting stent; INR, International Normalized ratio; NSAID, nonsteroidal anti‐inflammatory drug; PPI, proton pump inhibitor; VKA, vitamin K antagonist
5. CHALLENGES IN COMBINED ANTITHROMBOTIC AND ANTIPLATELET THERAPY
One of the most challenging patients among AF population are those with AF and end‐stage renal disease on hemodialysis. Severe chronic kidney disease (CKD) elevates the risk of major and intracranial bleeding, and this risk may be greater by the use of OAC or/and antiplatelet therapy. The evidence for antithrombotic therapy declines with the renal function.54 There are no randomized trials dedicated to OACs in patients undergoing hemodialysis. Of note, there are currently 3 ongoing randomized trials of stroke prevention in patients with AF on hemodialysis: the AXADIA trial,55 comparing apixaban 2.5 mg twice daily to phenprocoumon; the AVKDIAL trial (Oral Anticoagulation in Hemodialysis Patients), comparing VKA to no antithrombotic treatment; and the RENAL‐AF (Renal Hemodialysis Patients Allocated Apixaban Versus Warfarin in Atrial Fibrillation) trial, comparing apixaban 5 mg twice daily to warfarin. In one systematic review and meta‐analysis56 of 14 observational studies, the use of warfarin in patients with AF on hemodialysis was not associated with ischemic stroke or ICH. The use of warfarin in the above‐mentioned population of patients was not associated with a clear benefit or harm. In individuals with CKD who start an OAC, concomitant antiplatelet therapy including low‐dose aspirin may substantially make bleeding risk higher and should be used judiciously. In dialysis‐dependent patients, individualized decision making may be appropriate.
6. FUTURE DIRECTIONS
Many questions regarding optimal combination(s) of antiplatelet and antithrombotic therapy in patients with AF and ACS or elective PCI remain unanswered, including optimal timing of aspirin cessation and adequate P2Y12 inhibitor(s) to be used concomitantly with OACs.57 The role of aspirin instead of a P2Y12 inhibitor in dual therapy in different clinical settings also needs further research, as well as the time trends in recurrent coronary ischemic events with dual therapy versus TT and risk difference with dual therapy versus TT in different clinical settings (ie, ACS vs elective PCI, etc). New large‐scale randomized clinical trials investigating these issues are unlikely, but patient‐level meta‐analyses of available studies could provide additional insights.
Also, the search for efficacious anticoagulant(s) associated with minimal bleeding risk continues. Research on anticoagulant agents targeting factor XI or XII in the coagulation cascade are promising developments.57, 58, 59, 60 Evolutions in antithrombotic therapies for ACS are also apparent.7
7. CONCLUSIONS
Balancing the prevention of stroke and recurrent coronary ischemic events against the risk of bleeding in patients with AF and ACS or elective PCI may be challenging. To mitigate the risk of bleeding in these patients, DOAC‐based treatment strategies should be used in preference to VKA in DOAC‐eligible patients, and duration of TT should be minimized. For many patients, a DOAC plus P2Y12 inhibitor early after ACS and/or PCI would be optimal, whereas a longer course of TT should be used in patients at high thrombotic risk.
8. ISTH 2019 MELBOURNE REPORT
At the ISTH Congress in Melbourne, there was much focus on AF risk assessment and aspects of its management.
For example, Osman61 reported that machine learning algorithms had a good performance in predicting stroke outcomes (among others, type of stroke, 1‐year survival, and post‐stroke depression). Abdulrehman et al62 evaluated the real‐world use and appropriateness of idarucizumab, which appeared to be in concordance to institutional criteria, mostly for trauma‐related hemorrhages without knowledge of dabigatran concentration. Costa et al63 assessed the effect of a patient‐oriented educational strategy focused on low‐income patients with poor anticoagulation control. Patients with AF on warfarin for at least 6 months and with TTR <60% were enrolled and the intervention group participated in educational sessions based on a patient‐oriented care approach, which seem to improve anticoagulation management compared to the control group.
In the Effect of Bleeding Risk and Frailty Status on Anticoagulation Patterns in Octogenarians With Atrial Fibrillation (FRAIL‐AF) study, Joosten et al64 examined whether switching from INR‐guided VKA therapy to a DOAC‐based management strategy compared to continuing VKA therapy was safe in frail elderly patients with AF.
Foulon et al65 evaluated the link between plasma dabigatran concentrations and thrombinography/fibrinography parameters measured using the thrombodynamics system in an in vitro study and in 18 subjects aged >80 years receiving dabigatran for AF. They found that thrombodynamics enables a reliable analysis of the concentration‐dependent effect of dabigatran on thrombinography and some fibrinography parameters.
Douketis et al66 reported that in dabigatran and rivaroxaban‐medicated patients who interrupted therapy for an elective surgery/procedure, DOAC level cannot be reliably determined by a normal prothrombin time or an activated partial thromboplastin time. In apixaban‐medicated patients, an undetectable/minimal DOAC level might be reported based on a normal thrombin time.
Konigsbrugge et al67 evaluated the risk of major bleeding in hemodialyzed patients associated with antithrombotic treatment (the Vienna Investigation of Atrial fibrillation and Thromboembolism in Hemodialysis Patients [VIVALDI]). The incidence of major bleedings was greater in individuals on hemodialysis and the risk of major bleeding was equally elevated in patients on anticoagulation and antiplatelet drugs.
RELATIONSHIP DISCLOSURE
GYHL has been a consultant for Bayer/Janssen, BMS/Pfizer, Medtronic, Boehringer Ingelheim, Novartis, Verseon, and Daiichi‐Sankyo. He has been a speaker for Bayer, BMS/Pfizer, Medtronic, Boehringer Ingelheim, and Daiichi‐Sankyo. No fees are directly received personally. TSP has been a consultant for Bayer/Jansen and BMS/Pfizer (no fees). MK declared no conflict of interest.
AUTHOR CONTRIBUTIONS
MK reviewed the literature and drafted the manuscript. TSP and GYHL critically revised the manuscript.
Kozieł M, Potpara TS, Lip GYH. Triple therapy in patients with atrial fibrillation and acute coronary syndrome or percutaneous coronary intervention/stenting. Res Pract Thromb Haemost. 2020;4:357–365. 10.1002/rth2.12319
Handling Editor: Cihan Ay.
REFERENCES
- 1. Lip G, Freedman B, De Caterina R, Potpara TS. Stroke prevention in atrial fibrillation: Past, present and future. Comparing the guidelines and practical decision‐making. Thromb Haemost. 2017;117(7):1230–9. [DOI] [PubMed] [Google Scholar]
- 2. Bjorck S, Palaszewski B, Friberg L, Bergfeldt L. Atrial fibrillation, stroke risk, and warfarin therapy revisited. Stroke. 2013;44(11):3103–8. [DOI] [PubMed] [Google Scholar]
- 3. Lip GYH, Laroche C, Dan G‐A, Santini M, Kalarus Z, Rasmussen LH, et al. A prospective survey in European Society of Cardiology member countries of atrial fibrillation management: baseline results of EURObservational Research Programme Atrial Fibrillation (EORP‐AF) Pilot General Registry. Europace. 2013;16(3):308–19. [DOI] [PubMed] [Google Scholar]
- 4. Lane DA, Raichand S, Moore D, Connock M, Fry‐Smith A, Fitzmaurice DA. Combined anticoagulation and antiplatelet therapy for high‐risk patients with atrial fibrillation: a systematic review. Health Technol Assess. 2013;17(30):1–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kralev S, Schneider K, Lang S, Suselbeck T, Borggrefe M. Incidence and severity of coronary artery disease in patients with atrial fibrillation undergoing first‐time coronary angiography. PLoS ONE. 2011;6(9):e24964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bang CN, Gislason GH, Greve AM, Bang CA, Lilja A, Torp‐Pedersen C, et al. New‐onset atrial fibrillation is associated with cardiovascular events leading to death in a first time myocardial infarction population of 89,703 patients with long‐term follow‐up: a nationwide study. J Am Heart Assoc. 2014;3(1):e000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sibbing D, Angiolillo DJ, Huber K. Antithrombotic therapy for acute coronary syndrome: past, present and future. Thromb Haemost. 2017;117(7):1240–8. [DOI] [PubMed] [Google Scholar]
- 8. Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events (ACTIVE W): a randomised controlled trial. Lancet. 2006;367(9526):1903–12. [DOI] [PubMed] [Google Scholar]
- 9. Lip GYH, Collet JP, Haude M, Byrne R, Chung EH, Fauchier L, et al. 2018 Joint European consensus document on the management of antithrombotic therapy in atrial fibrillation patients presenting with acute coronary syndrome and/or undergoing percutaneous cardiovascular interventions: a joint consensus document of the European Heart Rhythm Association (EHRA), European Society of Cardiology Working Group on Thrombosis, European Association of Percutaneous Cardiovascular Interventions (EAPCI), and European Association of Acute Cardiac Care (ACCA) endorsed by the Heart Rhythm Society (HRS), Asia‐Pacific Heart Rhythm Society (APHRS), Latin America Heart Rhythm Society (LAHRS), and Cardiac Arrhythmia Society of Southern Africa (CASSA). Europace. 2019;21(2):192–3. [DOI] [PubMed] [Google Scholar]
- 10. van Rein N, Heide‐Jorgensen U, Lijfering WM, Dekkers OM, Sorensen HT, Cannegieter SC. Major bleeding rates in atrial fibrillation patients on single, dual, or triple antithrombotic therapy. Circulation. 2019;139(6):775–86. [DOI] [PubMed] [Google Scholar]
- 11. Lamberts M, Gislason GH, Olesen JB, Kristensen SL, Schjerning Olsen AM, Mikkelsen A, et al. Oral anticoagulation and antiplatelets in atrial fibrillation patients after myocardial infarction and coronary intervention. J Am Coll Cardiol. 2013;62(11):981–9. [DOI] [PubMed] [Google Scholar]
- 12. Rubboli A, Schlitt A, Kiviniemi T, Biancari F, Karjalainen PP, Valencia J, et al. One‐year outcome of patients with atrial fibrillation undergoing coronary artery stenting: an analysis of the AFCAS registry. Clin Cardiol. 2014;37(6):357–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open‐label, randomised, controlled trial. Lancet. 2013;381(9872):1107–15. [DOI] [PubMed] [Google Scholar]
- 14. Dans AL, Connolly SJ, Wallentin L, Yang S, Nakamya J, Brueckmann M, et al. Concomitant use of antiplatelet therapy with dabigatran or warfarin in the randomized evaluation of long‐term anticoagulation therapy (RE‐LY) trial. Circulation. 2013;127(5):634–40. [DOI] [PubMed] [Google Scholar]
- 15. Shah R, Hellkamp A, Lokhnygina Y, Becker RC, Berkowitz SD, Breithardt G, et al. Use of concomitant aspirin in patients with atrial fibrillation: Findings from the ROCKET AF trial. Am Heart J. 2016;179:77–86. [DOI] [PubMed] [Google Scholar]
- 16. Alexander JH, Lopes RD, Thomas L, Alings M, Atar D, Aylward P, et al. Apixaban vs. warfarin with concomitant aspirin in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2014;35(4):224–32. [DOI] [PubMed] [Google Scholar]
- 17. Xu H, Ruff CT, Giugliano RP, Murphy SA, Nordio F, Patel I, et al. Concomitant use of single antiplatelet therapy with edoxaban or warfarin in patients with atrial fibrillation: analysis from the ENGAGE AF‐TIMI48 trial. J Am Heart Assoc. 2016;5(2):e002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sindet‐Pedersen C, Lamberts M, Staerk L, Nissen Bonde A, Berger JS, Pallisgaard JL, et al. Combining oral anticoagulants with platelet inhibitors in patients with atrial fibrillation and coronary disease. J Am Coll Cardiol. 2018;72(15):1790–800. [DOI] [PubMed] [Google Scholar]
- 19. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt F, Wildgoose P, et al. An open‐label, randomized, controlled, multicenter study exploring two treatment strategies of rivaroxaban and a dose‐adjusted oral vitamin K antagonist treatment strategy in subjects with atrial fibrillation who undergo percutaneous coronary intervention (PIONEER AF‐PCI). Am Heart J. 2015;169(4):472–8.e5. [DOI] [PubMed] [Google Scholar]
- 20. Cannon CP, Bhatt DL, Oldgren J, Lip GYH, Ellis SG, Kimura T, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017;377(16):1513–24. [DOI] [PubMed] [Google Scholar]
- 21. Lopes RD, Vora AN, Liaw D, Granger CB, Darius H, Goodman SG, et al. An open‐Label, 2 x 2 factorial, randomized controlled trial to evaluate the safety of apixaban vs. vitamin K antagonist and aspirin vs. placebo in patients with atrial fibrillation and acute coronary syndrome and/or percutaneous coronary intervention: Rationale and design of the AUGUSTUS trial. Am Heart J. 2018;200:17–23. [DOI] [PubMed] [Google Scholar]
- 22. Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, et al. Edoxaban‐based versus vitamin K antagonist‐based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST‐AF PCI): a randomised, open‐label, phase 3b trial. Lancet. 2019;394(10206):1335–43. [DOI] [PubMed] [Google Scholar]
- 23. Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375(25):2423–34. [DOI] [PubMed] [Google Scholar]
- 24. Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380(16):1509–24. [DOI] [PubMed] [Google Scholar]
- 25. Lopes RD, Hong H, Harskamp RE, Bhatt DL, Mehran R, Cannon CP, et al. Safety and efficacy of antithrombotic strategies in patients with atrial fibrillation undergoing percutaneous coronary intervention: a network meta‐analysis of randomized controlled trials. JAMA Cardiol. 2019;4(8):747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Potpara TS, Mujovic N, Proietti M, Dagres N, Hindricks G, Collet JP, et al. Revisiting the effects of omitting aspirin in combined antithrombotic therapies for atrial fibrillation and acute coronary syndromes or percutaneous coronary interventions: meta‐analysis of pooled data from PIONEER‐AF PCI, RE‐DUAL PCI, and AUGUSTUS trials. Europace. 2019;22(1):33–46. [DOI] [PubMed] [Google Scholar]
- 27. Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14(11):627–8. [DOI] [PubMed] [Google Scholar]
- 28. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018;154(5):1121–201. [DOI] [PubMed] [Google Scholar]
- 29. Valgimigli M, Bueno H, Byrne RA, Collet J‐P, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2017;39(3):213–60. [DOI] [PubMed] [Google Scholar]
- 30. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli‐Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST‐segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39(2):119–77. [DOI] [PubMed] [Google Scholar]
- 31. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37(3):267–315. [DOI] [PubMed] [Google Scholar]
- 32. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–962. [DOI] [PubMed] [Google Scholar]
- 33. Lip GY, Lane DA. Bleeding risk assessment in atrial fibrillation: observations on the use and misuse of bleeding risk scores. J Thromb Haemost. 2016;14(9):1711–4. [DOI] [PubMed] [Google Scholar]
- 34. Borre ED, Goode A, Raitz G, Shah B, Lowenstern A, Chatterjee R, et al. Predicting thromboembolic and bleeding event risk in patients with non‐valvular atrial fibrillation: a systematic review. Thromb Haemost. 2018;118(12):2171–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chao TF, Lip GYH, Lin YJ, Chang SL, Lo LW, Hu YF, et al. Incident risk factors and major bleeding in patients with atrial fibrillation treated with oral anticoagulants: a comparison of baseline, follow‐up and delta HAS‐BLED scores with an approach focused on modifiable bleeding risk factors. Thromb Haemost. 2018;118(4):768–77. [DOI] [PubMed] [Google Scholar]
- 36. Holmes DR Jr, Kereiakes DJ, Kleiman NS, Moliterno DJ, Patti G, Grines CL. Combining antiplatelet and anticoagulant therapies. J Am College Cardiol. 2009;54(2):95–109. [DOI] [PubMed] [Google Scholar]
- 37. Paikin JS, Wright DS, Crowther MA, Mehta SR, Eikelboom JW. Triple antithrombotic therapy in patients with atrial fibrillation and coronary artery stents. Circulation. 2010;121(18):2067–70. [DOI] [PubMed] [Google Scholar]
- 38. Sorensen R, Hansen ML, Abildstrom SZ, Hvelplund A, Andersson C, Jorgensen C, et al. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet. 2009;374(9706):1967–74. [DOI] [PubMed] [Google Scholar]
- 39. Lamberts M, Olesen JB, Ruwald MH, Hansen CM, Karasoy D, Kristensen SL, et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation. 2012;126(10):1185–93. [DOI] [PubMed] [Google Scholar]
- 40. Zhao HJ, Zheng ZT, Wang ZH, Li SH, Zhang Y, Zhong M, et al. "Triple therapy" rather than "triple threat": a meta‐analysis of the two antithrombotic regimens after stent implantation in patients receiving long‐term oral anticoagulant treatment. Chest. 2011;139(2):260–70. [DOI] [PubMed] [Google Scholar]
- 41. Manoukian SV, Feit F, Mehran R, Voeltz MD, Ebrahimi R, Hamon M, et al. Impact of major bleeding on 30‐day mortality and clinical outcomes in patients with acute coronary syndromes: an analysis from the ACUITY Trial. J Am Coll Cardiol. 2007;49(12):1362–8. [DOI] [PubMed] [Google Scholar]
- 42. Kwok CS, Khan MA, Rao SV, Kinnaird T, Sperrin M, Buchan I, et al. Access and non‐access site bleeding after percutaneous coronary intervention and risk of subsequent mortality and major adverse cardiovascular events: systematic review and meta‐analysis. Circ Cardiovasc Interv. 2015;8(4):e001645. [DOI] [PubMed] [Google Scholar]
- 43. Ndrepepa G, Berger PB, Mehilli J, Seyfarth M, Neumann FJ, Schomig A, et al. Periprocedural bleeding and 1‐year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol. 2008;51(7):690–7. [DOI] [PubMed] [Google Scholar]
- 44. Verheugt FW, Steinhubl SR, Hamon M, Darius H, Steg PG, Valgimigli M, et al. Incidence, prognostic impact, and influence of antithrombotic therapy on access and nonaccess site bleeding in percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4(2):191–7. [DOI] [PubMed] [Google Scholar]
- 45. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the bleeding academic research consortium. Circulation. 2011;123(23):2736–47. [DOI] [PubMed] [Google Scholar]
- 46. Gomes T, Mamdani MM, Holbrook AM, Paterson JM, Hellings C, Juurlink DN. Rates of hemorrhage during warfarin therapy for atrial fibrillation. CMAJ. 2013;185(2):E121–E127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koziel M, Ding WY, Kalarus Z, Lip GYH. Considerations when restarting anticoagulants in patients with atrial fibrillation after bleeding. Exp Rev Hematol. 2019;12(10):845–55. [DOI] [PubMed] [Google Scholar]
- 48. Roldan V, Marin F, Manzano‐Fernandez S, Gallego P, Vilchez JA, Valdes M, et al. The HAS‐BLED score has better prediction accuracy for major bleeding than CHADS2 or CHA2DS2‐VASc scores in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. 2013;62(23):2199–204. [DOI] [PubMed] [Google Scholar]
- 49. Angiolillo DJ, Goodman SG, Bhatt DL, Eikelboom JW, Price MJ, Moliterno DJ, et al. Antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention. A North American perspective‐2016 update. Circ Cardiovasc Interv. 2016;9(11):e004395. [DOI] [PubMed] [Google Scholar]
- 50. Rossini R, Musumeci G, Lettieri C, Molfese M, Mihalcsik L, Mantovani P, et al. Long‐term outcomes in patients undergoing coronary stenting on dual oral antiplatelet treatment requiring oral anticoagulant therapy. Am J Cardiol. 2008;102(12):1618–23. [DOI] [PubMed] [Google Scholar]
- 51. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: executive summary. Europace. 2018;20(8):1231–42. [DOI] [PubMed] [Google Scholar]
- 52. Sarafoff N, Martischnig A, Wealer J, Mayer K, Mehilli J, Sibbing D, et al. Triple therapy with aspirin, prasugrel, and vitamin K antagonists in patients with drug‐eluting stent implantation and an indication for oral anticoagulation. J Am College Cardiol. 2013;61(20):2060–6. [DOI] [PubMed] [Google Scholar]
- 53. Capodanno D, Angiolillo DJ. Management of antiplatelet and anticoagulant therapy in patients with atrial fibrillation in the setting of acute coronary syndromes or percutaneous coronary interventions. Circ Cardiovasc Interv. 2014;7(1):113–24. [DOI] [PubMed] [Google Scholar]
- 54. Konigsbrugge O, Ay C. Atrial fibrillation in patients with end‐stage renal disease on hemodialysis: magnitude of the problem and new approach to oral anticoagulation. Res Pract Thromb Haemost. 2019;3(4):578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Reinecke H, Jurgensmeyer S, Engelbertz C, Gerss J, Kirchhof P, Breithardt G, et al. Design and rationale of a randomised controlled trial comparing apixaban to phenprocoumon in patients with atrial fibrillation on chronic haemodialysis: the AXADIA‐AFNET 8 study. BMJ Open. 2018;8(9):e022690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Harel Z, Chertow GM, Shah PS, Harel S, Dorian P, Yan AT, et al. Warfarin and the risk of stroke and bleeding in patients with atrial fibrillation receiving dialysis: a systematic review and meta‐analysis. Can J Cardiol. 2017;33(6):737–46. [DOI] [PubMed] [Google Scholar]
- 57. Weitz JI, Harenberg J. New developments in anticoagulants: past, present and future. Thromb Haemost. 2017;117(7):1283–8. [DOI] [PubMed] [Google Scholar]
- 58. Dimitropoulos G, Rahim SMZ, Moss AS, Lip GYH. New anticoagulants for venous thromboembolism and atrial fibrillation: what the future holds. Expert Opin Investig Drugs. 2018;27(1):71–86. [DOI] [PubMed] [Google Scholar]
- 59. Weitz JI, Fredenburgh JC. 2017 scientific sessions Sol Sherry distinguished lecture in thrombosis: factor XI as a target for new anticoagulants. Arterioscler Thromb Vasc Biol. 2018;38(2):304–10. [DOI] [PubMed] [Google Scholar]
- 60. Buller HR, Bethune C, Bhanot S, Gailani D, Monia BP, Raskob GE, et al. Factor XI antisense oligonucleotide for prevention of venous thrombosis. N Engl J Med. 2015;372(3):232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Osman M. Using machine learning for predicting length of hospital stay after stroke diagnosis, survival, stroke type and post‐stroke depression. Res Pract Thromb Haemost. 2019;30–1. [Google Scholar]
- 62. Abdulrehman J, Lindsay D, Elbaz C, Lin Y, Sholzberg M, Selby R. A retrospective analysis of the real‐world use of idarucizumab at two tertiary centres in Toronto, Canada. Res Pract Thromb Haemost. 2019;733.31624793 [Google Scholar]
- 63. Costa J, Marcolino M, Torres H, Rezende R, Souza R, Barbosa H, et al. The impact of an educational intervention in patients with atrial fibrillation treated with warfarin: protocol of a clinical trial. Res Pract Thromb Haemost. 2019;3(S1):4. [Google Scholar]
- 64. Joosten L, Rutten F, Hoes A, Investigators GGftF‐A . Design of the FRAIL‐AF trial: safety of switching from VKA to DOAC in frail elderly with atrial fibrillation. Res Pract Thromb Haemost. 2019;3(S1):16. [Google Scholar]
- 65. Foulon G, Jourdi G, Cavalie C, Gouin‐Thibault I, Pautas E, Gaussem P, et al. Effect of dabigatran in the very elderly on thrombinography and fibrinography parameters as assessed with the thrombodynamics System. Res Pract Thromb Haemost. 2019;3(S1):131. [Google Scholar]
- 66. Douketis J, Spyropoulos A, Duncan J, Li N, Arnold D, Moffat K, et al. Coagulation function tests to measure direct oral anticoagulant (DOAC) levels: comparison of routine and DOAC‐specific tests in the PAUSE study. Res Pract Thromb Haemost. 2019;3(S1):153–4. [Google Scholar]
- 67. Konigsbruegge OMH, Schmaldienst S, Auinger M, Lorenz M, Klauser‐Braun M, Kletzmayr J, et al. Incidence and risk of major bleeding in patients with end‐stage renal disease on hemodialysis with and without antithrombotic therapy: prospective results of the Vienna InVestigation of AtriaL fibrillation and thromboembolism in HemoDIalysis patients (VIVALDI). Res Pract Thromb Haemost. 2019;3(S1):15–6.30656271 [Google Scholar]


