Abstract
Background
Hepatocellular carcinoma (HCC) is a common malignancy with a steadily rising incidence and associated morbidity and mortality. Cirrhosis of the liver is presently the leading risk factor for developing HCC. Abdominal imaging, with or without alpha-fetoprotein (AFP) testing, every 6 months is the current surveillance strategy for patients at risk. The available biomarkers for detecting this cancer at an early stage have inadequate sensitivity and specificity.
Methods
The Hepatocellular carcinoma Early Detection Strategy (HEDS) study, a multi-center initiative of the National Cancer Institutes’ (NCI) Early Detection Research Network (EDRN), launched an effort to establish what has become the nation’s largest comprehensive biorepository and database on patients at high risk of developing HCC. The cohort has been developed in six clinical centers across the country. Subjects are enrolled for a five-year period involving data and specimen collection every six months in accordance with standard surveillance for HCC. Extensive clinical data are collected and specimens are stored at a central repository.
Results
The database and biorepository contain longitudinally collected clinical data and serum and plasma samples from 1482 participants with cirrhosis and without evidence of HCC at baseline. Fifty-six percent are male, 85% Caucasian, 30% have a history of chronic HCV and 71% have compensated cirrhosis.
Conclusions
The HEDS cohort provides opportunities for the continued study of the incidence and course of HCC in a comprehensively followed population of patients at high risk for this malignancy. Further, the EDRN biorepository provides a distinct opportunity for the development of novel biomarkers.
Keywords: research design, hepatocellular carcinoma, cancer surveillance, biomarker, real-world
Introduction
Hepatocellular carcinoma (HCC) is a growing cause of cancer-related death in the United States.1 Among all malignancies, HCC presented the largest increase in incidence over the past decade.1–3 The National Cancer Institute Surveillance Epidemiology and End Results database, U.S. Vital Statistics, and the Department of Veteran Administration showed the increasing incidence of HCC can be attributed primarily to the high prevalence of hepatitis C virus (HCV) and the progressive nature of liver disease to cirrhosis.4–6 Chronic HCV, hepatitis B (HBV), alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD), and autoimmune diseases can all progress to cirrhosis. As such, patients with these conditions are at high risk of developing HCC, with annual incidence rates up to 2% per year.5, 6 Despite the highly effective oral direct-acting antiviral (DAA) regimens for HCV, a significant percentage of these patients have cirrhosis and remain at risk for HCC.7 While convincing evidence suggests achieving sustained viral response (SVR) decreases the risk of developing initial HCC in patients with HCV cirrhosis, there remain conflicting data on the effect of HCV treatment on the recurrence of HCC in patients with cirrhosis.8–10 Though HBV treatment with oral nucleos(t)ides is known to decrease HCC risk, treated patients especially with cirrhosis remain at significant risk for HCC.11–14 NAFLD must be seriously considered in the context of HCC surveillance, as it has become the leading cause of chronic liver disease in our country.15, 16 Patients with NAFLD are at an increased risk of developing HCC, even if they do not have cirrhosis.16, 17
Surveillance guidelines for HCC include twice-yearly ultrasound (US) imaging with or without assessment of the biomarker alpha-fetoprotein (AFP).18, 19 However, there have been limitations with regard to the sensitivity and specificity of AFP and US.20, 21 Preliminary data suggest that a combination of AFP >10 ng ml−1 or a composite AFP index achieves adequate sensitivity for early detection of HCC.22 Additional biomarkers that have been evaluated and used in clinical practice include AFP-L3 and des-carboxy prothrombin (DCP), but these have not been demonstrated superiority over AFP as single markers.20, 23 The GALAD (Gender, Age, AFP-L3, AFP, DCP) model combined simple demographic data with these biomarkers in an attempt to improve upon the AFP sensitivity and specificity.24, 25 This model was recently validated in a large cohort for the diagnosis of HCC, distinguished from non-HCC malignancy, and warrants prospective clinical studies for its role in HCC surveillance.25 Investigators have also evaluated other biomarkers including GP73, kininogen, osteopontin, and certain glycoproteins, but none of these have been validated in clinical practice for HCC surveillance.26, 27
Given the continued rise in incidence and mortality of HCC, along with the limitations of the current surveillance strategy, the development of improved methods for early detection are of utmost importance. To that end, the NIH-sponsored Early Detection Research Network (EDRN) continues to support multi-center studies focused on developing novel biomarkers and further examining the risk factors, incidence, and course of HCC in the US population.
Methodology
The EDRN’s multidisciplinary approach to liver cancer screening and surveillance research
Des-Carboxy Prothrombin Validation (DCP-V) case/control study
In the year 2000, the EDRN collaborative group was established to assess the role of then available markers for the detection of early stage HCC. An EDRN Phase II case/control study on the validation of DCP as a biomarker for detecting incipient HCC (the “DCP-V” study) was initiated and comprised of two well defined groups of patients with cirrhosis. The first group included patients with cirrhosis and HCC (cases); the second included patients with cirrhosis without detected HCC (controls). The study involved collection of serum and plasma samples for all patients at their routine clinic visits. The purpose of the study was to assess the accuracy of DCP and AFP-L3 versus AFP alone in detecting early HCC.20
Hepatocellular carcinoma Early Detection Strategy (HEDS)
In sequence to the DCP-V study and under the continued administration of the EDRN, the HEDS study was designed and implemented. Centers participating in HEDS include: Stanford University, Saint Louis University, Mayo Clinic, Mount Sinai University Hospital, University of Michigan, and the University of Pennsylvania; the Fred Hutchinson Cancer Research Center in Seattle, WA, serves as the Data Management Coordinating Center.
This study has been conducted in accordance with the elements of Good Clinical Practice, as defined by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Prior to the initiation of study-specific procedures, study candidates at each center voluntarily signed informed consent forms consistent with the ethical guidelines of the Declaration of Helsinki and approved by each centers’ Institutional Review Board.
The HEDS study was designed to address the following aims:
Aim 1: to determine the incidence rate of HCC and the performance of US, AFP, AFP-L3%, and DCP in the detection of preclinical HCC; Aim 2: to determine the cost-effectiveness of surveillance strategies for HCC; Aim 3: to determine the performance of biomarkers in the prognosis of patients with HCC; and Aim 4: to establish a biorepository of longitudinally collected biospecimens from patients with cirrhosis to be used as a reference set for future EDRN research.
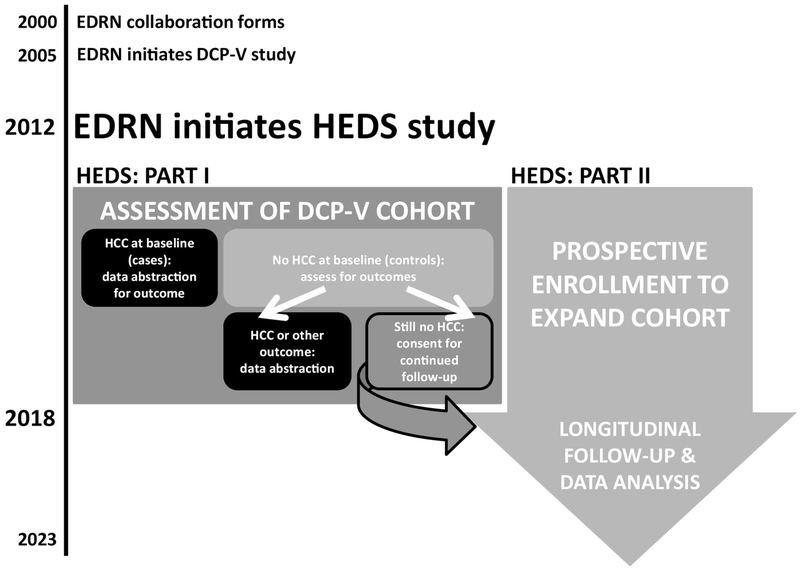
To accomplish these aims, the HEDS study is being conducted in two parts, as described in Figure 1. Part I involved the follow-up of patients enrolled in the DCP-V study, and Part II involves the prospective enrollment of patients with cirrhosis. Clinical, laboratory, and imaging data, as well as serum and plasma biospecimens are collected longitudinally from participants for up to five years; data are collected only from participants who maintain the absence of evidence of HCC on imaging.
Figure 1. Schematic timeline of the EDRN’s liver cancer research efforts, with concentration on the HEDS project.
Part I of the HEDS study involved the evaluation of all subjects enrolled in the DCP-V study. DCP-V controls who remained cancer-free at the time of assessment were “rolled over” to the HEDS study for continued follow-up (Part II). Part II also involved the enrollment of new controls to expand the cancer-free cirrhotic cohort to 1550 subjects. All subjects are expected to adhere to the biannual follow-up visit schedule, per standard of care for this population.
Gathering a distinguished cohort for long-term monitoring
In the DCP-V study, information on the treatment, response, and survival of 372 HCC cases was collected to assess the factors (including biomarkers) that determined prognosis and best staging system for these patients. In Part I of the HEDS study, their medical records were reviewed to determine if they achieved outcomes including: liver transplant, death, or lost to follow-up. Outcomes were recorded and no further follow-up is being conducted. Those who were enrolled in DCP-V as cirrhotic controls and were found to have achieved an outcome of: diagnosis of HCC, death, liver transplant, or lost to follow up, were not approached to consent for continued monitoring in HEDS.
Instead, the outcomes were recorded as one-time abstractions and no further follow-up is being conducted. Specifically, for those that developed HCC, abstracted data included: treatment(s) performed, response to treatment(s), disease progression, tumor recurrence(s) after response and date, de novo HCC, death and dates, or the last dates before lost to follow-up. Eligibility criteria for all subjects enrolled in DCP-V is described in Table 1.
Table 1.
Descriptions and key eligibility criteria of cohorts enrolled in DCP-V and HEDS studies.
| DCP-V | HEDS n = 1482 | |
|---|---|---|
| CASES (HCC) n = 372 | CONTROLS (NO HCC) n = 374 | |
| - AFP labs within 180 days - All other labs within past 90 days - Diagnosis of HCC based on: histology*, two imaging tests** - MELD < 15 OR INR < 1.5, t.bili < 1.7 and history of intrinsic renal disease - No prior or current treatment of HCC - No cancer history within 5 years*** - No Participation in a trial for HCC treatment - No undiagnosed liver mass |
- Albumin, Bilirubin, Creatinine and INR labs within past 90 days - Imaging**** showing no HCC within 180 days - Diagnosis of cirrhosis based on: histology, image showing cirrhotic liver with splenomegaly and platelets < 120 mm3, Esophageal or gastric varices on endoscopy AND presence of chronic liver disease - MELD < 15 -OR- INR < 1.5, t.bili < 1.7 and history of intrinsic renal disease - No significant hepatic decompensation****** - No hepatorenal syndrome - AFP labs within 180 days, AFP < 20 ng/ml***** - No cancer history within 5 years*** - No prior solid organ transplant |
- Albumin, Bilirubin, Creatinine and
INR labs within 180 days****
- Imaging**** showing no HCC within 180 days - Diagnosis of cirrhosis based on: histology, imaging showing cirrhotic liver with splenomegaly AND platelets < 120 mm-3, Elastography indicating cirrhosis, FibroTest result of F4, Varices AND chronic liver disease - MELD < 15 -OR- INR < 1.5, T.Bili < 1.7 and history of intrinsic renal disease - No significant hepatic decompensation****** - No hepatorenal syndrome - Not listed for liver transplantation and noted as “exception” - No known AIDS related diseases - No significant co-morbid conditions with life expectancy < 1 year - No cancer history within 5 years*** - No prior solid organ transplant |
DCP-V controls who remained HCC-free at time of abstraction were screened to meet eligibility criteria for HEDS enrollment prior to being approached for consent to continue follow-up in HEDS study.
required if only one lesion < 2 cm is present) – Must have CT/MRI to calculate stage
with at least one (CT/MRI/Angiography) within the past 3 months or up to 2 weeks after consent showing evidence of arterial hypervascularization, and the other within the past 6 months (US, CT, MRI/Angiography) indicating a mass in the liver.
(excluding non-melanoma skin cancer)
within 6 months prior to enrollment OR up to 2 weeks after consent
without proof of a dynamic CT scan or triple phase MRI within 3 months prior to consent OR up to 2 weeks after consent indicating no mass
Grade 3–4 encephalopathy, refractory ascites, CTP class C
Subjects who were enrolled in DCP-V as cirrhotic controls and remained undiagnosed of HCC at the time of assessment were approached to provide consent to continued monitoring in Part II of the HEDS study. These subjects will be followed for up to five years; data and biospecimen collection will occur every 6 months.
An additional 1550 patients with cirrhosis and no history or evidence of HCC are being enrolled across all seven participating institutes for five-year longitudinal specimens and data collection. The updated key eligibility criteria for HEDS enrollment are described in Table 1. Data collected every 6 months include: ultrasound, AFP, liver function tests, complete blood counts, MELD scores and any changes in medical history, personal cancer history and family cancer history. Extending the follow up of this cohort has provided the opportunity for a more thorough examination of potential independent factors that lead to the development of HCC.
The HEDS study allows us to evaluate the performance of biomarkers, surveillance imaging studies, and other assessments conducted at the hepatologists’ discretion for the early detection of HCC. Most importantly, this cohort has formed a large, uniquely heterogeneous repository of serum and plasma samples from high risk patients prior to any clinical presentation of HCC. This biorepository provides the EDRN with a robust, third phase sample set for the testing and validation of highly sensitive and specific biomarkers. The unique cohort enrolled in the HEDS study will have a high impact in the field of HCC by providing temporally relevant data in conjunction with blood samples at matched time points.
Longitudinal data & biospecimen collection
Several parameters are established for each patient at baseline. These include clinical data from medical history, co-morbidities, and laboratory data (e.g. hematology, chemistry panels). Patient-reported data, including social history, substance abuse, coffee and tea consumption (quantitatively), and physical performance status are also obtained. These are further described in Table 2. Additionally, serum and plasma specimens are collected. Plasma samples for DNA analysis are collected and stored at baseline.
Table 2.
Covariates collected at baseline and throughout follow-up.
| Variable | Categorization of covariate | Baselin e | Time-Varying |
|---|---|---|---|
| Clinical & Participant Data | |||
| Etiology of liver disease | HCV, HBV, EtOH, NASH, PBC, PSC, AIH, hemochromatosis, Wilson’s, other | X | |
| Family history of liver disease | HCV, HBV, EtOH, NASH, PBC, PSC, AIH, hemochromatosis, Wilson’s, other | X | |
| Race | White, Black, Asian, other | X | |
| Ethnicity | Hispanic/Latino yes/no | X | |
| Sex | Male, Female | X | |
| Age | Continuous | X | X |
| Body mass index | Continuous | X | X |
| Physical performance status | Fully active, ambulatory but unable to work, restricted to chair/bed | X | X |
| Ascites | Binary yes/no | X | X |
| Hepatic encephalopathy | Binary yes/no | X | X |
| Varices | Binary yes/no | X | X |
| Hepatorenal syndrome | Binary yes/no | X | X |
| HCV status | No HCV, HCV with viremia, HCV with SVR | X | X |
| HCV antiviral use | (LIST) | X | X |
| Statin use (current, previous) | Atorvastatin, fluvastatin, lovastatin, pravastatin, rosuvastatin, simvastatin | X | X |
| Metformin use (current, previous) | Binary yes/no | X | X |
| # Children, # Siblings | Continuous | X | X |
| Smoking (current, previous) | Continuous | X | X |
| Alcohol (current, previous) | Continuous | X | X |
| Coffee and tea consumption | Amount per day/week/month | X | X |
| Personal and immediate family cancer history & diagnosis | Bladder, brain, cervix, esophagus, kidney, leukemia, lymphoma, pancreas, rectum, stomach, bone, breast, colon, head/neck, liver, lung, ovary, prostate, melanoma, thyroid | X | X |
| Laboratory Data | |||
|
Chemistry: ALT, AST, ALP,
albumin, bilirubin, creatinine, sodium |
Continuous | X | X |
|
Hematology: Hct, Hgb, WBC,
Plts |
Continuous | X | X |
| INR | Continuous | X | X |
| AFP | Continuous | X | X |
Abbreviations: HCV=hepatitis C virus; NASH=non-alcoholic steatohepatitis; HBV=hepatitis B virus; PBC=primary biliary cirrhosis; PSC=primary sclerosing cholangitis; AIH=autoimmune hepatitis; AST=aspartate aminotransferase; ALT=alanine aminotransferase; ALP=alkaline phosphatase; Hct=hematocrit; Hbg=hemoglobin; WBC=white blood cells; Plts=platelets; INR=international normalized ratio
As part of the continued monitoring, clinical and laboratory data are collected every six months and include: US/MRI/CT imaging results, AFP, liver function tests, complete blood counts, MELD scores and any changes in medical history, personal cancer history and family cancer history. Participants are also asked about changes to their tobacco/alcohol use and coffee and tea consumption. Serum and plasma specimens are collected longitudinally for novel biomarker analysis.
Sample Size and Statistical Power
Statistical considerations are for final data collected from both Parts I and II of HEDS. An interim analysis was conducted in February 2016 to check the accrual assumptions. It was observed that the DCP-V cohort eligible for continued observation in HEDS was very small (n = 23). The study power was mainly determined by the number of incidence HCC cases. To ensure the study would achieve the target number of incidence HCC cases (n = 80), the final sample size requirement was increased to 1,550. The updated study protocol also requires every recruited subject be followed for at least 60 months, thus the participants’ follow up will continue until the last recruited subject has been followed up for 60 months. This updated sample size and the follow up length is expected to yield about 105 HCC cases.
To date, there are 1482 participants enrolled in the HEDS study. The cohort is comprised of participants of a wide age range (from 18 to 87 years, median age 60), 56% of which are male, and 84% of which are Caucasian, as depicted in Table 3. All participants have been diagnosed with cirrhosis, as confirmed by biopsy and/or other clinical assessments. At present, 71% have compensated cirrhosis, as defined by Child-Turcotte-Pugh class. Reported etiologies of liver disease include HCV, HBV, NASH, PBC, PSC, AIH, alcoholic hepatitis, hemochromatosis, Wilson’s disease, Alpha-1 antitrypsin deficiency, and cryptogenic cirrhosis. HCV and HBV underlie cirrhosis within approximately 30% and 2% of the cohort, respectively. Non-viral hepatitis, including fatty liver disease (19%), alcoholic hepatitis (21%), autoimmune hepatitis (5%), and cholestatic disease (8%), account for the other largely observed causes of liver disease. Approximately half of all participants enrolled have a family history of cancer.
Table 3.
Demographics and clinical characteristics of HEDS cohort at baseline.
| HEDS (n = 1482) | |
|---|---|
| Male, n (%) | 808 (54.5%) |
| BMI (kg/m2) | |
| Median (25th percentile, 75th percentile) | 30.7 (26.9, 36.0) |
| Min, max | 7.6, 87.1 |
| Race, n (%) | |
| White or Caucasian | 1250 (84.3%) |
| Black or African-American | 98 (6.6%) |
| Asian | 32 (2.2%) |
| Other | 102 (6.9%) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 159 (10.7%) |
| Age (y) | |
| Median (25th percentile, 75th percentile) | 60 (54, 65) |
| Min, max | 18, 87 |
| Etiology of liver disease | |
| History of HCV infection, n (%) | 439 (29.6%) |
| SVR achieved | 97 (22.1%) |
| HBV infection, n (%) | 27 (1.8%) |
| NASH | 279 (18.8%) |
| Alcoholic hepatitis | 313 (21.1%) |
| Autoimmune hepatitis | 80 (5.4%) |
| Cholestatic liver disease (PBC, PSC) | 112 (7.6%) |
| Other | 109 (7.4%) |
| Child-Turcotte-Pugh Class A, n (%) | 1048 (70.7%) |
| Alcohol use, n current (%) | 941 (63.5%) |
| Tobacco use, n (%) | |
| Current | 217 (14.6%) |
| Former | 834 (56.3%) |
| Fully active performance status, n (%) | 979 (66.1%) |
| Medications of interest, n ever taken (%) | |
| DAA therapy for HCV | 134 (9.0%) |
| Statins | 344 (23.2%) |
| Metformin | 112 (7.6%) |
| Interferon therapy | 28 (1.9%) |
| Non-DAA antiviral agents | 292 (19.7) |
| Coffee and tea consumption, n (%) | 854 (57.6%) |
| Obesity, n (%) | 804 (54.2%) |
| Cancer history (excl. liver), n (%) | 98 (6.6%) |
| Family cancer history, n (%) | |
| Liver cancer | 65 (4.3%) |
| Other cancer | 706 (47.6%) |
| Family liver disease history, n (%) | 291 (19.6%) |
Cohort data are inclusive of DCP-V subjects who have continued in follow-up through the HEDS study.
Discussion
It has been widely acknowledged that the presence of cirrhosis, regardless of etiology, is the leading risk factor for the development of HCC.4–6 Evidence supporting the presently published incidence rates in the US, as well as the current screening and surveillance recommendations, were compiled from patient populations with cirrhosis primarily caused by untreated HCV.5–7 Further, most studies describing the incidence of HCC after successful HCV therapy were retrospective in nature and analyzed select cohorts such as the Veterans Affairs population; this predominantly male population may not accurately reflect the incidence of HCC in the wider spectrum patient population. One strength of the HEDS cohort is that approximately half of those currently enrolled are female. Emerging evidence suggests that sex hormones could influence the development of HCC; the HEDS cohort may help to further examine this question.28
With the recent emergence of highly effective combined DAA regimens for treating HCV, along with the therapies effective in suppressing HBV infection, it is critical to prospectively re-evaluate HCC risk and incidence in patients with HCV/HBV-cirrhosis. Approximately 22% of patients with HCV-cirrhosis in the HEDS cohort have achieved SVR. Many of these patients were treated with DAA regimens. Continued follow-up will shed valuable insight on the contentious question of whether or not these regimens affect the development of HCC.10 Many studies have demonstrated that DAA treatment is not presently associated with an increased risk of developing HCC; further, achieving SVR is associated with a decreased risk of HCC and improved HCC-free survival.7, 8, 29 Previously reported events on the development of HCC subsequent to DAA therapy appear to be explained by pre-existing risk factors for HCC such as HCV genotype 3 infection, male gender, and alcohol abuse.30 HCC recurrence following DAA therapy has been a contentious observation in patients with liver cancer history, and even with an aggressive course despite HCV cure.10, 31 The potential to assess risk and progression of HCC among patients who have achieved SVR, utilizing prospectively gathered time-varying clinical and laboratory data, is a noteworthy characteristic that distinguishes the HEDS cohort from previously studied patient populations with HCV. The likelihood that some individuals in this group have failed therapy also provides the opportunity to compare HCC incidence between patients who have been successfully treated and those remain uncured.
Alcoholic liver disease is another commonly reported etiology of cirrhosis among previously published cohorts.4, 32 To distinguish the effects of alcoholism from moderate alcohol intake across patients with cirrhosis, the types and amounts of alcohol consumed by subjects are key parameters for analysis in HEDS. Information collected on alcohol consumption is broken down into patient-reported estimations of weekly consumptions of beer/wine/liquor per decade of life. For example, a patient who has confirmed that she has consumed at least one alcoholic beverage per month during a twelve-month period is further asked to approximate the number of drinks consumed per week during her twenties, thirties, etc. Additional patient-reported, lifestyle-related data collected in the HEDS study include tobacco use, coffee and tea consumption, and performance status, factors which may influence HCC development.33–35
Non-alcoholic fatty liver disease (NAFLD) is a condition of paramount interest in the field of Hepatology. NAFLD has grown in prevalence alongside other conditions contributing to metabolic syndrome, and has become the leading cause of chronic liver disease in our country.15, 16 Currently in the U.S., between 30–40% of adults have NAFLD. It is estimated that 20% of these individuals will develop steatohepatitis.36 This progression is marked by the histological presence of inflammation in addition to the adipose deposition and fibrosis observed in NAFLD. Patients with NAFLD are at an increased risk of developing HCC.16 There is evidence of development of HCC even in patients with NAFLD who do not have cirrhosis. In a recent experience, 20% of NAFLD patients with HCC showed no evidence of cirrhosis.17 Prospective studies on large cohorts of patients with NAFLD at various stages of liver disease will be valuable for the continued examination of the influence of hepatic steatosis on the development of HCC. The HEDS cohort will shed further insight on those with cirrhosis. Non-alcoholic steatohepatitis (NASH) is the underlying cause of cirrhosis in approximately 20% of the HEDS cohort. Additionally, the HEDS cohort is representative of groups at increased risk of developing fatty liver, including populations that are Hispanic/Latino (11%), obese (54%), and have type 2 diabetes mellitus (T2DM) (8%). Though NAFLD can affect people of all ages, races, and ethnicities, it is reportedly most common among Hispanic adults and non-Hispanic white adults.37–39 Individuals with NAFLD are also more likely to progress to NASH if they have other characteristics of metabolic syndrome such as hypertension and dyslipidemia. For patients affected by the latter, there exist preliminary data showing a potential HCC-protective mechanism of statin therapy.40 The HEDS population presents an opportunity for continued study of this phenomenon, as 23% of enrolled patients have been treated with statin regimens. Metformin is another medication of interest in populations at increased risk of developing cancer, considering evidence suggesting its potential protective mechanism against pancreatic cancer.41 Metformin warrants further exploration for its implications beyond treating T2DM in humans, based on cardioprotective and neuroprotective effects demonstrated in animal studies.42, 43 Of the HEDS cohort, approximately 8% are currently being treated with metformin.
Performance status is a notable variable within the context of this population, considering its influence on treatment algorithms and determining outcomes in patients with HCC.44 Further, it can help to assess liver disease-related morbidity and mortality, as has been shown by its impact in predicting 3-month post discharge mortality in patients with cirrhosis.45 Through the HEDS study, information on patients’ performance status is obtained longitudinally; this will enable the continued exploration of this lifestyle factor’s impact on outcomes related to HCC. Performance status as it relates to physical activity is another valuable area of study, especially pertinent in the realm of chronic disease prevention and treatment. Though the HEDS study exclusively follows a population affected by chronic liver disease, differences in performance status among patients within this cohort may be worth examining. Performance status has been shown to be an important prognostic factor in HCC.46 Therefore, the prognosis of patients with HCC can be comprehensively determined by hepatic function, performance status, and tumor burden in this study and can help in developing HCC risk-prediction models as had been done by others. 47, 48
In conclusion, through its vast and unique and national dataset and biorepository, the EDRN has established a heterogeneous and well-defined cohort of patients with cirrhosis, welcoming opportunities for assessing the performance of novel biomarkers and prospectively studying myriad variables associated with the development of HCC in the United States. This sample population will provide updated data for determining the present incidence rate, the categorization of high risk subgroups, and the characteristics and prognosis of patients with HCC. The HEDS study will provide valuable feedback on the performance of imaging, AFP, AFP-L3%, and DCP in the detection of preclinical HCC, and the cost-effectiveness of these surveillance strategies. Additionally, the HEDS cohort will provide a unique opportunity to prospectively validate the GALAD model in the early detection of HCC, and potentially improve upon it with the addition of novel biomarkers.
Acknowledgements
We appreciate the generous support from the National Cancer Institute. This publication was supported under Award Number U24CA086368. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Funding
This work was supported by the National Cancer Institute of the National Institutes of Health under Award Number U24CA086368.
Abbreviations
- AFP
Alpha-fetoprotein
- CT
Computed tomography imaging
- DAA
Direct-Acting Antiviral
- DCP
Des-carboxy Prothrombin
- DCP-V
Des-carboxy Prothrombin Validation (Vanguard) study
- EDRN
Early Detection Research Network
- HBV
Hepatitis B Virus
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C Virus
- HEDS
Hepatocellular carcinoma Early Detection Strategy
- MELD
Model for End- Stage Liver Disease
- MRI
Magnetic Resonance Imaging
- NAFLD
Non-alcoholic fatty liver disease
- NASH
Non-alcoholic steatohepatitis
- NCI
National Cancer Institute
- NIH
National Institutes of Health
- T2DM
Type 2 Diabetes Mellitus
- US
Ultrasound imaging
Footnotes
Declaration of Conflicting Interests
Neehar D. Parikh serves on the advisory board for Bayer and Eisai Co, Ltd., and as a consultant to Bristol-Myers Squibb and Exelixis, Inc. The other authors have no disclosures.
Trial registry URL: https://edrn.nci.nih.gov/protocols/316-hepatocellular-carcinoma-early-detection-strategy
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016; 122: 1312–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massarweh NN and El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer control : journal of the Moffitt Cancer Center. 2017; 24: 1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singal AG and El-Serag HB. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015; 13: 2140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Serag HB and Everhart JE. Improved survival after variceal hemorrhage over an 11-year period in the Department of Veterans Affairs. The American journal of gastroenterology. 2000; 95: 3566–73. [DOI] [PubMed] [Google Scholar]
- 5.Davila JA, Morgan RO, Shaib Y, McGlynn KA and El-Serag HB. Hepatitis C infection and the increasing incidence of hepatocellular carcinoma: a population-based study. Gastroenterology. 2004; 127: 1372–80. [DOI] [PubMed] [Google Scholar]
- 6.Howe HL, Wu X, Ries LA, et al. Annual report to the nation on the status of cancer, 1975–2003, featuring cancer among U.S. Hispanic/Latino populations. Cancer. 2006; 107: 1711–42. [DOI] [PubMed] [Google Scholar]
- 7.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y and El-Serag HB. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology. 2017; 153: 996–1005 e1. [DOI] [PubMed] [Google Scholar]
- 8.Backus LI, Belperio PS, Shahoumian TA and Mole LA. Impact of Sustained Virologic Response with Direct-Acting Antiviral Treatment on Mortality in Patients with Advanced Liver Disease. Hepatology. 2017. [DOI] [PubMed] [Google Scholar]
- 9.Reig M, Marino Z, Perello C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. Journal of hepatology. 2016; 65: 719–26. [DOI] [PubMed] [Google Scholar]
- 10.Kutala B, Valla D and Marcellin P. Things fall apart with hepatocellular carcinoma and direct-acting antivirals. Expert opinion on drug safety. 2018; 17: 107–9. [DOI] [PubMed] [Google Scholar]
- 11.Ahn J, Lee HM, Lim JK, et al. Entecavir safety and effectiveness in a national cohort of treatment-naive chronic hepatitis B patients in the US - the ENUMERATE study. Alimentary pharmacology & therapeutics. 2016; 43: 134–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin D, Yang HI, Nguyen N, et al. Reduction of chronic hepatitis B-related hepatocellular carcinoma with anti-viral therapy, including low risk patients. Alimentary pharmacology & therapeutics. 2016; 44: 846–55. [DOI] [PubMed] [Google Scholar]
- 13.Lok AS, McMahon BJ, Brown RS, Jr., et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology. 2016; 63: 284–306. [DOI] [PubMed] [Google Scholar]
- 14.Hoang JK, Yang HI, Le A, et al. Lower liver cancer risk with antiviral therapy in chronic hepatitis B patients with normal to minimally elevated ALT and no cirrhosis. Medicine. 2016; 95: e4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH and Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2015; 13: 643–54 e1–9; quiz e39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012; 55: 2005–23. [DOI] [PubMed] [Google Scholar]
- 17.Kanwal F, Kramer JR, Mapakshi S, et al. Risk of Hepatocellular Cancer in Patients with Non-alcoholic Fatty Liver Disease. Gastroenterology. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang BH, Yang BH and Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. Journal of cancer research and clinical oncology. 2004; 130: 417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67: 358–80. [DOI] [PubMed] [Google Scholar]
- 20.Marrero JA, Feng Z, Wang Y, et al. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009; 137: 110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S, Bent S and Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Annals of internal medicine. 2003; 139: 46–50. [DOI] [PubMed] [Google Scholar]
- 22.Biselli M, Conti F, Gramenzi A, et al. A new approach to the use of alpha-fetoprotein as surveillance test for hepatocellular carcinoma in patients with cirrhosis. British journal of cancer. 2015; 112: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lok AS, Sterling RK, Everhart JE, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010; 138: 493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson PJ, Pirrie SJ, Cox TF, et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014; 23: 144–53. [DOI] [PubMed] [Google Scholar]
- 25.Berhane S, Toyoda H, Tada T, et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016; 14: 875–86 e6. [DOI] [PubMed] [Google Scholar]
- 26.Wang M, Long RE, Comunale MA, et al. Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009; 18: 1914–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marrero JA, Romano PR, Nikolaeva O, et al. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. Journal of hepatology. 2005; 43: 1007–12. [DOI] [PubMed] [Google Scholar]
- 28.Yeh SH and Chen PJ. Gender disparity of hepatocellular carcinoma: the roles of sex hormones. Oncology. 2010; 78 Suppl 1: 172–9. [DOI] [PubMed] [Google Scholar]
- 29.Ioannou GN, Green PK and Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li DK, Ren Y, Saikh O, et al. The Incidence of Hepatocellular Carcinoma Is not Increased in Individuals with Chronic Hepatitis C After Treatment with Interferon-free Regimens: an ERCHIVES Study Open forum infectious diseases. Oxford University Press, 2017, p. S41. [Google Scholar]
- 31.Cariani E, Pilli M, Barili V, et al. Natural killer cells phenotypic characterization as an outcome predictor of HCV-linked HCC after curative treatments. Oncoimmunology. 2016; 5: e1154249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.El-Serag HB and Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014; 60: 1767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelucchi C, Gallus S, Garavello W, Bosetti C and La Vecchia C. Cancer risk associated with alcohol and tobacco use: focus on upper aero-digestive tract and liver. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2006; 29: 193–8. [PMC free article] [PubMed] [Google Scholar]
- 34.Shih WL, Chang HC, Liaw YF, et al. Influences of tobacco and alcohol use on hepatocellular carcinoma survival. International journal of cancer. 2012; 131: 2612–21. [DOI] [PubMed] [Google Scholar]
- 35.Koh WP, Robien K, Wang R, Govindarajan S, Yuan JM and Yu MC. Smoking as an independent risk factor for hepatocellular carcinoma: the Singapore Chinese Health Study. British journal of cancer. 2011; 105: 1430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spengler EK and Loomba R. Recommendations for Diagnosis, Referral for Liver Biopsy, and Treatment of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Mayo Clinic proceedings. 2015; 90: 1233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres DM, Williams CD and Harrison SA. Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2012; 10: 837–58. [DOI] [PubMed] [Google Scholar]
- 38.Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011; 140: 124–31. [DOI] [PubMed] [Google Scholar]
- 39.Brunt EM, Wong VW, Nobili V, et al. Nonalcoholic fatty liver disease. Nature reviews Disease primers. 2015; 1: 15080. [DOI] [PubMed] [Google Scholar]
- 40.Yi C, Song Z, Wan M, Chen Y and Cheng X. Statins intake and risk of liver cancer: A dose-response meta analysis of prospective cohort studies. Medicine. 2017; 96: e7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li D, Yeung SC, Hassan MM, Konopleva M and Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009; 137: 482–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Messaoudi S, Rongen GA, de Boer RA and Riksen NP. The cardioprotective effects of metformin. Current opinion in lipidology. 2011; 22: 445–53. [DOI] [PubMed] [Google Scholar]
- 43.Patil SP, Jain PD, Ghumatkar PJ, Tambe R and Sathaye S. Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neuroscience. 2014; 277: 747–54. [DOI] [PubMed] [Google Scholar]
- 44.Nishikawa H, Kita R, Kimura T, et al. Clinical implication of performance status in patients with hepatocellular carcinoma complicating with cirrhosis. Journal of Cancer. 2015; 6: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tandon P, Reddy KR, O’Leary JG, et al. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology. 2017; 65: 217–24. [DOI] [PubMed] [Google Scholar]
- 46.Hsu CY, Lee YH, Hsia CY, et al. Performance status in patients with hepatocellular carcinoma: determinants, prognostic impact, and ability to improve the Barcelona Clinic Liver Cancer system. Hepatology. 2013; 57: 112–9. [DOI] [PubMed] [Google Scholar]
- 47.Orman ES, Ghabril M and Chalasani N. Poor Performance Status Is Associated With Increased Mortality in Patients With Cirrhosis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016; 14: 1189–95 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tapper EB and Su GL. Does Karnofsky Performance Status of Patients With Cirrhosis on the Transplant Waitlist Meet the Eyeball Test? Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016; 14: 1196–8. [DOI] [PubMed] [Google Scholar]