Abstract
Three 4-month-old kittens from the same litter were presented, two of which were exhibiting cerebellar signs. Euthanasia was requested. No cerebellum atrophy was disclosed on necropsy. General cerebellar anatomy was normal, including the thickness of the cortical layers, myelination, and neurons of the deep cerebellar nuclei. In the ataxic cat vermis, Purkinje cells were lacking along broad parasagittal bands symmetrically disposed relative to the midline. Many Purkinje cells were also lacking in the hemispheres. The nodulus and the flocculus were normal. Surviving Purkinje cells had frequent main dendrite swellings visible with anti-calbindin and anti-microtubule associated protein. In affected regions, calbindin and phosphorylated neurofilaments immunesera stained numerous axonal torpedoes located in the granular layer and the folial white matter. They were also present in processes of the deep cerebellar nuclei and lateral vestibular nucleus. Loss of synaptic endings onto the neurons of these nuclei was evident. Hypertrophied Purkinje cell recurrent axons and enhanced retrograde synaptic endings were present in the granular layer. Bergmann glia was strongly labeled by anti-GFAP, but no abnormal supplementary fibers were seen. None of these alterations were present in the normal sister. However, abnormal vacuolation of the Purkinje cell main dendrites was evident in all three cats, but not in six unrelated control cats that were 3–6 months old. The inferior olive and pontine nuclei were also normal. The two ataxic cats had a primary Purkinje cell degeneration that shared many common features with the abnormal Purkinje cells of the nervous mutant mouse.
Keywords: Cat, Purkinje cells, Abiotrophy, Immunohistochemistry, Nervous mouse
Introduction
Neonatal and infantile ataxia have long been described in kittens [11], dogs [21], and mice [5, 12, 13,17]. These entities share the common feature of becoming obvious within the first weeks or months of life. These animal models may help to better understand similar diseases in humans ([14] for review).
Congenital ataxia in cats, characterized by early and stable cerebellar symptoms, has been related to fetal or neonatal infection by the feline panleukopenia virus [4,7]. Recently, two early-onset cortical cerebellar degenerations (CCD) have been described in cats; they were characterized by prominent Purkinje cell degeneration, moderate granule cell number decrease, moderate inferior olive degeneration, and normal pontine nuclei. One of these entities was autosomic recessive [1,6], and the other [22] was of unknown origin. Prominent Purkinje cell decay was reported in three kittens from the United Kingdom [24]. In the latter cases, changes in the medullary nuclei were also noted. A neuroaxonal dystrophy that involved lateral cuneate and olivary nuclei, as well as the dorsal tegmentum beside the cerebellar cortex, has also been reported in kittens [3,25].
This paper reports on pure cortical cerebellar degeneration involving the Purkinje cells only, occurring in two of three kittens from the same litter. Ataxia was visible as soon as they began to walk. The third cat of the litter, albeit clinically normal, displayed mild Purkinje cell modifications as compared with controls. The pathological features in these cats were original as they involved the Purkinje cell only. They share many similarities with the pathological features of the nervous mouse.
Case report
Three 4-month-old kittens of the same litter, all female, were presented because two of them exhibited a progressively worsening gait disturbance. According to the owners, the litter resulted from the mating of the queen by one of her sons. General examination was unremarkable, and blood tests for feline coronavirus antibodies and feline leukemia virus antigen were negative. Cat 1 had a normal gait, whereas cats 2 and 3 exhibited mild cerebellar signs characterized by intention tremors and limb hypermetria. Signs were more obvious in cat 3. Cat 3 underwent head MRI imaging that was normal. Notably, the size of the cerebellum was similar to that of a healthy, age-matched control. At the owners’ request, the three kittens were euthanized, and partial necropsy was allowed. Cat 1 was used as a control, as well as six other kittens, 3–6 months of age, which were euthanized for untreatable neurological signs. These controls had meningomyeloencephalitis lesions but no cerebellar degeneration.
Materials and methods
Brains and cranial cervical cord were immediately removed after death and fixed by immersion in 4% paraformaldehyde, in 0.1 M phosphate buffer, pH 7.4. Fixation was made for 4 days at 4ºC, followed by 24-h rinsing in 0.1 M phosphate buffer and embedding in paraffin. Specimens were cut semi-serially at 5 µm. Every 20th section was stained with Luxol fast blue and cresyl violet [9], and some of them with hematoxylin and eosin.
For immunohistochemistry, the peroxidase-anti-peroxidase (PAP) technique was used as described earlier [15]. Nine primary antiserum were used: polyclonal rabbit serum against calbindin (courtesy of Dr. E. Lawson, 1:5000), calretinin (SWannt, 1:1000), microtubule associated protein (MAP2) (courtesy of Dr. J. P. Brion, 1:1500), tau (courtesy of Dr. J. P. Brion, 1:1000), glial fibrillary acidic protein (GFAP) (Dako, 1:10000), S100 (Dako, 1:5000), monoclonal mouse antibodies against beta III tubulin subunit (clone 5G8, Promega, 1:500), phosphorylated neurofilaments (clone RT97, Boehringer, 1:200), and synaptophysin (Sigma, 1:200).
Results
Macroscopically, all parts of the brain and the spinal cord, including the cerebellum, appeared normal in the three cats. The immunesera that were used in this study had the same cell specificity as already described in mice, rats, and, in our previous report, in cats [15].
The whole neuron population of the cerebellar cortex was examined with Klüver-Barrera staining [9] and MAP2 immunolabeling. No cell category was missing in the ataxic cats, and thickness of the different layers was as in controls. An external granular layer was no longer present. All the neurons of the granular and molecular layers were present and normal. Only the Purkinje cells were abnormal, with a moderate loss of about 15%.
Purkinje cells were immunolabeled by four different immunesera. As in normals, calbindin was present in the whole cell up to the synaptic endings. MAP2 and beta III tubulin were expressed in the cell bodies and main dendrites only, while RT97 was expressed in the axons. Abnormal features were identical in cats 2 and 3.
In the vermis cortex, densely packed Purkinje cells alternated with areas devoid of these cells. It was particularly evident with calbindin immunostaining (Fig. 1a); missing Purkinje cells appeared as areas of the molecular layer lacking immunostaining, contrasting with areas that contained dense calbindin-positive Purkinje cell dendrites. Staining with MAP2 immunesera (Fig. 1b) showed normal basket and stellate cells in these regions of the molecular layer. The calbindin-negative bands were arranged symmetrically relative to the midline (Fig. 1c). The pattern was identical in the two affected cats. Lobule X and ventral lobule IX retained their Purkinje cells in both cats (Fig. 1c).
Fig. 1a–e.
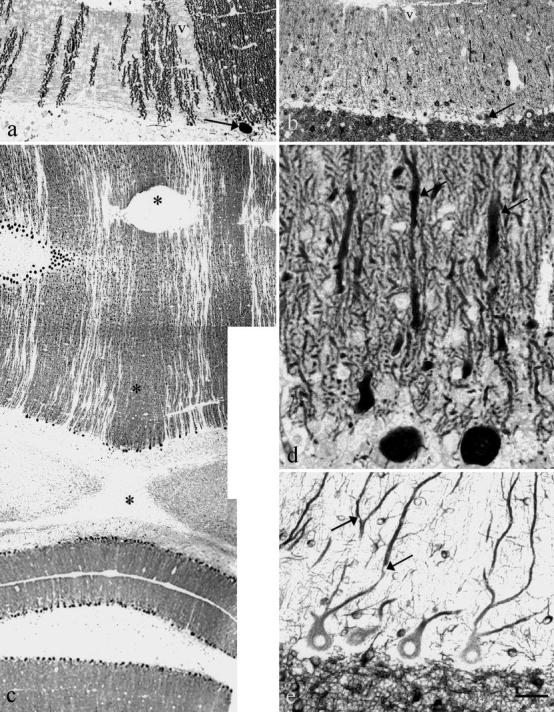
Purkinje cell dendrites in cat 2 ( a, b, d) and cat 3 ( c, e). Anti-calbindin ( a, c, d) and anti-MAP2 ( b, e). a, b Corresponding area from two adjacent transversal sections showing gaps in calbindin-positive dendrites in the molecular layer ( a) with normal numbers of stellate and basket cells ( b). One Purkinje cell body is visualized by both immunesera (arrows in a and b ). A blood vessel (v) is also visible in both sections. c Symmetrical parasagittal gaps without calbindin immunoreactivity in the vermis. The nodulus (bottom of the figure) is spared. The stars indicate the midline. d Dendritic swellings (arrows) and abnormal branching. e Empty vacuoles (arrows). Bar = a, b:160 µm; c:320 µm; d:40 µm; e:60 µm
The second zone of Purkinje cell loss was located in the hemispheres, which were more depopulated than the vermis. Many patches devoid of Purkinje cells alternated with regions where their number was almost normal. The flocculus was entirely spared.
The surviving Purkinje cells located in the affected part of the cerebellum had mild modifications of their dendritic tree, such as swellings of the main dendrites (Fig. 1d), abnormal shape and arborization of the secondary dendrites, and small round empty vacuoles (Fig. 1e). The vacuoles were also observed in the normal cat of the litter but never in the six controls. Anti-calbindin and anti-MAP2 were used to visualize them.
In all parts of the cerebellum but lobes IX and X and the flocculus, many axonal torpedoes were present in the granular layer and the folial white axes. They were heavily contrasted by anti-calbindin (Fig. 2a), and some of them were also labeled with anti-phosphorylated neurofilaments (Fig. 2b). Many spheroids were also present in the deep cerebellar nuclei and in the lateral vestibular nucleus.
Fig. 2a–f.
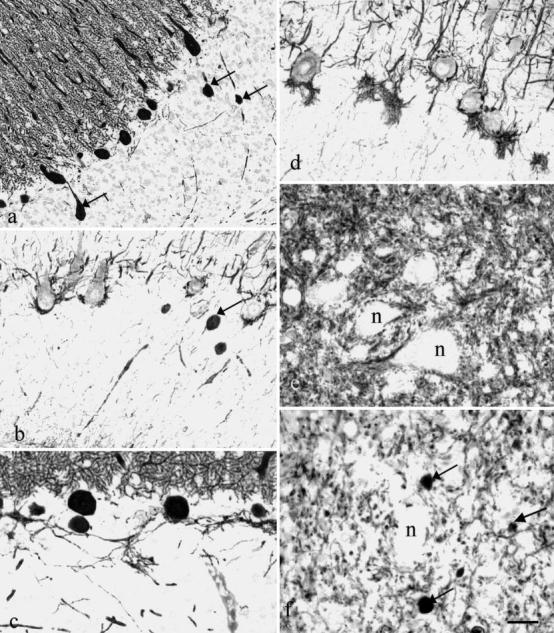
In the granular layer ( a – d) of cats 2 ( a) and 3 ( b, c, d), numerous spheroids are present on the Purkinje cell axons, stained by anti-calbindin (arrows in a ) and by clone RT97 (arrow in b ). Moderately increased calbindin-positive recurrent synapses are visible in c. RT97-positive baskets are either normal ( b) or hypertrophied ( d). Neurons (n) in the normal deep cerebellar nuclei of cat 1 ( e) are covered by calbindin-positive synapses, which are almost absent in cat 3 ( f). The arrows in f point out spheroids. Bar = a:83 µm; b:30 µm; c, d:35 µm; e, f:22 µm
A low number of Purkinje cell axons gave rise to enlarged recurrent collaterals. Abnormally numerous, calbindin-positive, recurrent synapses were observed in the immediate vicinity of Purkinje cells (Fig. 2c) and in the superficial quarter of the granular layer.
Basket cell axons were identified using immuneserum RT97, raised against phosphorylated neurofilaments. Most of them were normal (Fig. 2b). Shrunken empty baskets were rarely encountered, while hypertrophied baskets were often seen in the same region as hypertrophied RT97-positive terminals (Fig. 2d).
The deep cerebellar nuclei and the lateral vestibular nuclei had normal-shaped neurons and a highly abnormal Purkinje terminal field. In normal cats, innumerable calbindin-positive axons, coming from the Purkinje cells, run between these neurons and end as large synapses that cover almost 30% of their linear pericaryal surface (Fig. 2e). In the two mutants, the number of calbindin-positive fibers running between the neurons in these nuclei was extremely decreased, and many spheroids were visualized by calbindin (Fig. 2f) and RT97 immunesera. Almost no calbindin-positive synapses were visible on the neuron surface (Fig. 2f). The drop in this synaptic covering was much more dramatic than expected from the Purkinje cell loss.
Glial cells appeared moderately increased in number, but their processes were abnormally strongly stained. Anti-GFAP stained an enhanced number of processes, both in the granular layer and the white axes. In addition, the processes of Bergmann glia were also vigorously stained, but there was no additional positive fiber in the molecular layer. Labeling with anti-S100 was unremarkable.
Other encephalic and spinal structures were normal, including the basal ganglia, inferior olive, pontine nuclei, red nucleus, substantia nigra, nucleus gracilis, cuneatus, and cuneatus lateralis. The posterior column of the cervical spinal cord and the spinocerebellar tracts were normal.
Discussion
Cases reported here have similarities with the mouse mutant group, characterized by a primary Purkinje cell abiotrophy beginning after a normal differentiation [10] at the end of weaning. Purkinje cells of these mutants acquire normal synapses with afferent and efferent neurons but fail to survive, leaving empty baskets as a track of their previous presence [20]. Among these mice, the “nervous” mutant [17] shares many features with our cats from a neuropathological point of view. Indeed, in the young nervous mice, preferential death of vermis Purkinje cells occurs along longitudinal strips, symmetrical relative to the midline [23]. Degeneration is more prominent in the hemispheres [17], and affected Purkinje cells have axonal torpedoes [16,20]. Regions of preferentially surviving Purkinje cells include the nodulus and the flocculus [23]. Dendritic modifications are also mild, with swellings and slight abnormalities at the branching points [2,16]. Recurrent collaterals are only occasionally encountered in nervous mice [16], and increased density and staining of basket cells terminals are present [2]. In cats as in mice, symmetrical parasagittal stripes of Purkinje cells are characterized by differential protein expressions [18]. A resulting difference of sensitivity to abiotrophic processes has been proposed in mice [23] and could similarly exist in cats.
In the present cases, the pathological changes of the Purkinje axons were prominent in their terminal domain; i.e., the deep cerebellar nucleus and the lateral vestibular nucleus. Indeed, the loss of terminal arborizations and synaptic endings was extremely high, contrasting with the rather high number of surviving Purkinje cells. This is pathognomonic of the so-called “dying-back” process degenerations [19], as are also the axonal spheroids [8,19], arciform collaterals [19], and increased recurrent plexuses in the granular layer [19]. This process causes an early subtotal elimination of Purkinje cell input in the deep cerebellar and lateral vestibular nuclei and explains the cerebellar signs observed in the two ataxic cats, in spite of the relatively high number of surviving Purkinje cells.
The cats of this report have some features in common with the ataxic cat of Taniyama et al. [22] but also many differences. Prominent hemispheric degeneration and axonal torpedoes are similar, but in the Japanese case, these torpedoes were eosinophilic; the number of granule cells and the cortical thickness were reduced while the flocculus and nodulus were also degenerated. As this cat was 3 months old, the length of evolution cannot be responsible for the dissimilarities. Comparison was more difficult with the cats described by Inada et al. [6] that were euthanized at the age of 12 months. However, torpedoes were not observed onto the residual Purkinje cell axons.
Many breed-related cerebellar degenerations (abiotrophies) have been described in the dog species, with variable onset ages [21]. Most were purely cortical, but the three cerebellar cortical layers were usually involved. Purkinje cell axonal swellings were described in several reports. In some entities, a gradient of increasing degenerative changes has been reported, with either the vermis, the hemispheres, or the paravermian area most affected. Degeneration organized in parasagittal strips was not observed in these cases.
Acknowledgment
We are grateful to Dr J. P. Brion, from the Faculty of Medicine of the Free University of Brussels, who gave us several of the immunesera used in this report. This investigation was supported by a grant from the Fonds de la Recherche Scientifique Medicale Belge (1.5025.02 to LP).
References
- 1.Aye Acta Neuropathol. 1998;96:379. doi: 10.1007/s004010050908. [DOI] [PubMed] [Google Scholar]
- 2.Brion Dev Brain Res. 1988;44:221. doi: 10.1016/0165-3806(88)90220-9. [DOI] [PubMed] [Google Scholar]
- 3.Carmichael J Vet Diagn Invest. 1993;5:585. doi: 10.1177/104063879300500414. [DOI] [PubMed] [Google Scholar]
- 4.Csiza Infect Immun. 1971;3:838. doi: 10.1128/iai.3.6.838-846.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guenet J Hered. 1983;74:105. doi: 10.1093/oxfordjournals.jhered.a109729. [DOI] [PubMed] [Google Scholar]
- 6.Inada Am J Vet Res. 1996;57:296. [PubMed] [Google Scholar]
- 7.Johnson Nature. 1967;214:175. doi: 10.1038/214175a0. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi Acta Neuropathol. 1990;80:145. doi: 10.1007/BF00308917. [DOI] [PubMed] [Google Scholar]
- 9.Klüver J Neuropathol Exp Neurol. 1953;12:400. doi: 10.1097/00005072-195312040-00008. [DOI] [PubMed] [Google Scholar]
- 10.Landis J Cell Biol. 1973;57:782. doi: 10.1083/jcb.57.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockard J Comp Neurol. 1956;104:403. doi: 10.1002/cne.901040305. [DOI] [PubMed] [Google Scholar]
- 12.Mullen Proc Natl Acad Sci USA. 1976;73:208. [Google Scholar]
- 13.Phillips J Genet. 1960;57:35. [Google Scholar]
- 14.Ramaekers Brain. 1997;120:1739. doi: 10.1093/brain/120.10.1739. [DOI] [PubMed] [Google Scholar]
- 15.Résibois Vet Pathol. 2004;41:20. doi: 10.1354/vp.41-1-20. [DOI] [PubMed] [Google Scholar]
- 16.Rossi J Neurosci. 1995;15:2040. doi: 10.1523/JNEUROSCI.15-03-02040.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sidman RL, Green MC (1970) Nervous, a new mutant mouse with cerebellar disease. In: Sabourdy M (ed) Les mutants pathologiques chez l’animal, leur intéret dans la recherche biomédicale. CNRS, Paris, pp 69–79
- 18.Sillitoe Brain Res. 2003;997:1. doi: 10.1016/S0006-8993(03)02569-1. [DOI] [Google Scholar]
- 19.Sotelo J Neurocytol. 1990;19:737. doi: 10.1007/BF01188042. [DOI] [PubMed] [Google Scholar]
- 20.Sotelo Brain Res. 1979;175:11. doi: 10.1016/0006-8993(79)90511-0. [DOI] [PubMed] [Google Scholar]
- 21.Summers BA, Cummings JF, deLahunta A (1995) Hereditary, familial, and idiopathic degenerative diseases. In: Summers BA, Cummings JF, deLahunta A (eds) Veterinary neuropathology. Mosby, St Louis, pp 208–350.
- 22.Taniyama Vet Pathol. 1994;31:710. doi: 10.1177/030098589403100614. [DOI] [PubMed] [Google Scholar]
- 23.Wassef Dev Biol. 1987;124:379. doi: 10.1016/0012-1606(87)90490-8. [DOI] [PubMed] [Google Scholar]
- 24.Willoughby Vet Rec. 2002;151:295. doi: 10.1136/vr.151.10.295. [DOI] [PubMed] [Google Scholar]
- 25.Woodward Am J Pathol. 1974;74:551. [PMC free article] [PubMed] [Google Scholar]


