Abstract
Purpose
The increased risk of morbidity and mortality from certain microbial infections and the demonstrated improvements in the clinical course of some autoimmune diseases support the existence of pregnancy-related alterations in immune status. Elucidating the changes in innate and adaptive immunity during gestation may improve pregnancy outcomes and facilitate the development of targeted therapies for autoimmune diseases.
Method
The Viral Immunity and Pregnancy (VIP) study evaluated over 50 subjects longitudinally at three time points during pregnancy and at two time points post-delivery. Leukocyte enumeration was performed; functional responses of NK cells and CD4 T cells were analyzed, and soluble factors such as cytokines, defensins, and steroid hormones were measured in maternal blood.
Results
In comparison to the post-partum period, the latter part of pregnancy was characterized by significant increases in blood phagocytes and pDCs and decreases in the number and activity of NK and T cells. Alterations were found in antimicrobial proteins and serum cytokines.
Conclusions
These data show that pregnancy is not a period of immunosuppression but an alteration in immune priorities characterized by a strengthening of innate immune barriers and a concomitant reduction in adaptive/inflammatory immunity in the later stages of pregnancy.
Keywords: Pregnancy, immunity, influenza, cytokines, gestation, Th2
Introduction
Pregnant women are at increased risk for both morbidity and mortality from an array of pathogens. Pregnancy-related increases in disease severity have been reported for CMV [1], SARS [2], Varicella Zoster [3], Listeria monocytogenes [4], malaria [5], as well as influenza virus. Numerous reports suggest that influenza infection results in increased morbidity and mortality during pregnancy with the greatest risk in the second and third trimesters [6–11]. In all recorded influenza pandemics including 1918, 1957, and 2009, pregnant women exposed to the pandemic strain experienced significantly increased death rates [6, 7, 10]. Moreover, women that survive influenza infection faced increased risks of adverse pregnancy outcomes, including preterm birth and fetal death [10–15].
Many hypotheses have been advanced to explain these epidemiological data using Sir Peter Medawar's posit as a springboard [16]. Medawar hypothesized that some type of immune suppression must occur to allow a semi-allogeneic fetus to escape attack. Subsequent research at the maternal–fetal interface and maternal peripheral circulation resulted in sometimes conflicting hypotheses, including that pregnancy involves a shift toward Th2 immunity [17] or variants thereof [18], a controlled state of inflammation [19], or a combination of both depending on the stage of pregnancy [20]. Evidence for and against each of these hypotheses can be found in the literature. In no small measure, the lack of consensus on the exact nature of phenotypic changes to immunity during pregnancy can be explained by limitations in previous investigations, including differences in study design, the use of animal models, or a focus on one narrow aspect of immunity. Additionally, the anatomical site of analysis (peripheral blood/spleen versus uterine environment) may have a profound impact on the conclusions drawn. Study design is critical. Most published studies have been cross-sectional, but substantial interpersonal variability in immunologic parameters poses a significant challenge to data interpretation.
The present study enrolled pregnant women early in pregnancy and followed them to at least 6 months postpartum in order to evaluate selective components of the maternal systemic immune response that might explain the increased severity and complications of influenza infection during the latter stages of pregnancy. This approach differed from other investigations which focused primarily on the maternal–fetal interface, an approach that was used as the basis for the Th2 hypothesis [17]. That report found no bias toward Th2 cytokine production from unstimulated or ConA stimulated splenocytes during pregnancy [21]. Other groups that suggested an inflammatory view of pregnancy either focused on the maternal–fetal interface or on particular cell types, such as monocytes, that they reported to have increased activity [22]. In our longitudinal study, blood was collected from women three times during pregnancy and twice in the post partum period in an effort to monitor some elements of the immune system known to be important for influenza virus control. Cellular and soluble components of blood representing innate and adaptive immune elements were analyzed. In order to remove the influence of interpersonal variability in immune response, each woman's pregnant immune profile was compared to her own 6-month measurements. In this paper, we present the alterations and compensatory changes we observed in maternal blood cell counts, NK, and T cell function and serum defensins as pregnancy progressed.
Methods
Study Design
The Viral Immunity in Pregnancy (VIP) project was funded by a NIH-NIAID contract (Immune Responses to Virus Infections During Pregnancy; Contact No. HHSN266200500028C) and enrolled pregnant women into two different prospective, observational, longitudinal cohorts: a vaccine cohort, which assessed immunological responses during pregnancy to inactivated influenza vaccine (Sperling et al. manuscript in press); and also, an immune response cohort. The aim of the immune-response cohort, the subject of this current paper, was to examine whether the different trimesters of pregnancy, characterized by unique hormonal environments, are associated with identifiable and discrete changes in maternal innate and adaptive immunity. Both cohorts were approved by the Mount Sinai School of Medicine Program for the Protection of Human Subjects/Institutional Review Board. Study recruitment started in 2006 and continued until 2010.
Subjects for the immune response cohort were enrolled from Mount Sinai Hospital's Obstetrics and Gynecology Diagnostic and Treatment Center (D&TC) practice. Pregnant women were eligible for study participation if they were at least 18 years of age, up to 20 weeks gestation, with a documented viable pregnancy. The study was designed to survey healthy women without significant co-morbid medical conditions or concomitant medications that would substantially impact immunologic function. Therefore, exclusion criteria for study participation were: (1) individuals with known or suspected HIV infection; (2) individuals with a history of splenectomy; (3) individuals with known auto-immune disorders (e.g., systemic lupus erythematosus or rheumatoid arthritis); (4) individuals who required chronic systemic immunosuppressive therapy (women who use topical steroids (inhaled or nasal) or intermittent oral steroids for the treatment of asthma remained eligible; (5) individuals who had received any immune globulin (including Rhogam or blood derived products within 3 months of study enrollment; (6) individuals who had anemia, defined by hemoglobin (Hgb) < 10 g/dL at the first prenatal visit; (7) individuals unable to give informed consent; and (8) individuals who did not plan to remain in the geographic area after delivery. The target enrollment size was 50 women, each donating five blood samples (250 visits).
Blood and serum samples were obtained at three time-points during pregnancy: during the first 20 weeks of gestation, at 26–28 weeks of gestation, at 34–36 weeks, and twice post partum, at 5–6 weeks, and 6 months post-delivery. The specimen biorepository was linked to comprehensive maternal clinical data (age, weight, co-morbid medical conditions, concomitant medications/vaccinations, prior obstetrical history, allergies, asthma/atopy, depression/stress assessments, prior influenza vaccination history, alcohol/drug use, and smoking/s-hand smoke exposures), and pregnancy outcome data (birth weight, route of delivery, apgar score).
Blood Cell Enumeration
Fifty milliliters of peripheral blood was drawn into EDTA purple top tubes (BD). An aliquot of fresh whole blood was used for cell enumeration using Miltenyi Blood Dendritic Cell Enumeration Kit (Miltenyi Biotec, Bergisch Gladbach, Germany) or Beckman Coulter Flow-Count Fluorospheres and cell enumeration system (Beckman Coulter, Brea, CA). Antibody sets and staining strategies to identify cells include CD3+/CD8+, CD3+/CD4+ (T cells), CD19+ (B cells), CD14+ (monocytes), CD56+ (NK cells), CD19-/CD103+ (plasmacytoid DCs), and CD19-/CD11c (monocyte-derived DCs). Enumeration of granulocytes was calculated by exclusion from these subsets.
Cell Isolation
The remaining blood was loaded onto a Ficoll density gradient centrifugation (Histopaque; Sigma-Aldrich, St Louis, MO) to remove red blood cells. DCs/monocytes were first removed by positive selection. The flow-through fraction was then divided into two. Naïve CD4 T cells were isolated using Miltenyi negative selection beads. NK cells were isolated using negative selection beads.
T Cell Assay
Naïve CD4 cells were isolated and cultured at 106 cells/ml in RPMI supplemented with 1 mM sodium pyruvate, 2% human serum (Cambrex/Lonza, East Rutherford, NJ), and 1% penicillin/streptomycin (Invitrogen). Cells were cultured for 48 h either without stimulation or with toxic shock syndrome toxin-1 (TSST) at 50 ng/ml before cells were pelleted and supernatants stored at −80°C. Cytokines were analyzed using a Luminex 200 (Luminex, Austin, TX) and Millipore xMap cytokine/chemokine kits (Millipore, Billerica, MA). Supernatant from cell cultures were used according the manufacturer's protocol. To limit interassay variability, all samples for a patient were run on the same plate.
NK Cell Assay
NK cells were isolated to >85% purity by negative selection using Miltenyi beads. Cells were cultured in RPMI supplemented with 1 mM sodium pyruvate, 4% human serum (Cambrex/Lonza, East Rutherford, NJ), and 1% penicillin/streptomycin for 20 h either unstimulated or with IL-12 (4 ng/ml)/IL15 (60 pg/ml) stimulation. Secreted cytokines in the supernatant were analyzed using Luminex technology and Millipore xMap cytokine/chemokine kits and analyzed using Millipore Analyst software.
Defensin ELISA
Coagulated blood was isolated from each patient visit. Patient serum was stored at −80°C until the end of the study and assayed together. Capture enzyme-linked immunosorbent assays (ELISAs) for HNP1-3 (Hycult Biotechnology, Uden, Netherlands) was used according to the manufacturers' instructions to quantify the α-defensins 1–3 in the serum. Plates were read in an ELISA reader (Biotek Instruments, Winooski, VT) following the manufacturer's protocol, and data were analyzed using software from Applied Cytometry Systems.
Statistical Analysis
All analyses were conducted using SAS, Version 9.2 (SAS Inc., Cary, NC, USA). Data were transformed with natural logarithms (NK and T cell cytokines and defensins) or square root (cell fractions). Percentage of data below the limit of detection (LOD) was calculated for each cytokine and experimental stimulation. Changes in cytokine expression levels over the course of pregnancy in comparison to the 6-month postpartum visit were calculated using multivariable mixed models, accounting for repeated measures taken from the same woman over time.
If all of the measured cytokine values were detectable at all visits, linear mixed models with a random intercept were used to examine changes in expression level over time, using PROC MIXED. This procedure was also used to examine changes in concentrations in defensins and cell fractions. If no more than 50% of measured values were below the LOD, tobit models with a random intercept were used to examine changes in expression level over time, using PROC NLMIXED. If 50–75% of measured values were below the LOD, the quantitative expression data were converted to a 3-level categorical variable: (0) below the LOD; (1) above the LOD but below the third quartile of those with quantifiable cytokine level; and (2) at or above the third quartile. Changes in expression according to this three-level variable over time were analyzed using generalized linear mixed models, using PROC GLIMMIX, with a random intercept and a cumulative logit link function. We did not examine changes in expression level over time for cytokines in which fewer than 25% of the population had values above the LOD. In all cases, contrasts were done to test for significant differences in expression level between the second trimester and 6-month postpartum and third trimester and 6-month postpartum times. A Bonferroni correction was applied and significant two-tailed p values were considered to be 0.025 (0.05/2). Subjects were excluded from analyses if they missed more than one study-mandated blood draw or did not complete a final (~6 months post-delivery) visit and blood draw (the reference period).
Results
Characteristics of the Subjects
At study completion, 56 subjects had enrolled; 50 subjects completed all visits/blood-draws, 5 subjects were lost to follow-up, and 1 subject withdrew after signing consent. The enrolled population reflects the characteristics of the community surrounding Mount Sinai Hospital; 95% of the women reported Black or Hispanic race/ethnicity. The majority of our subjects had at least a high school education and were either married or living with a partner. Pre-pregnancy obesity was extremely prevalent in our population, with 63% of the population meeting the Institute of Medicine criteria for obesity (body mass index ≥30). Depression statistics were obtained by the Beck Depression Inventory and constitute generally mild depression. The rate of common pregnancy complications in this population was consistent with typically observed rates (Table I).
Table I.
Patient characteristics of the VIP study, Mount Sinai School of Medicine New York, New York 2005–2009 (n = 56)
| Characteristics | Frequency (%) |
|---|---|
| Race | |
| White | 1 (2%) |
| Black | 25 (45%) |
| Hispanic | 28 (50%) |
| American Indian | 2 (4%) |
| Education | |
| <High School | 9 (16%) |
| Finished High School | 17 (30%) |
| >High School | 30 (54%) |
| Marital status | |
| Married/living with partner | 39 (70%) |
| Divorced/widowed/separated/single and never married | 17 (30%) |
| Parity | |
| 0 | 20 (36%) |
| 1 | 16 (29%) |
| 2+ | 20 (36%) |
| Spontaneous abortions | |
| 0 | 37 (66%) |
| 1 | 12 (21%) |
| 2+ | 7 (13%) |
| Induced abortions | |
| 0 | 28 (50%) |
| 1 | 14 (25%) |
| 2+ | 14 (25%) |
| Obesity | 35 (63%) |
| Pregnancy induced | |
| Hypertension | 3 (5.4%) |
| Gestational diabetes | 1 (1.8%) |
| Preeclampsia | 5 (8.9%) |
| Preterm delivery | 5 (8.9%) |
| Depression | 21 (38%) |
| Mean (SD) | |
| Maternal age start of | |
| Pregnancy (years) | 26.5 (5.2) |
| Birthweight (grams) | 3,128 (596) |
| Gestational age at delivery (completed weeks) | 38.2 (2.4) |
| Most recent IPI (days) | 1,171 (1,647) |
| Most recent IPI from live birth (days) | 1,734 (1,315) |
Changes Occur in the Serum Components during Pregnancy
In a previous publication, we reported that many serum cytokines were reduced drastically during pregnancy including VEGF, GM-CSF, MCP-1, and IFNγ, while G-CSF and TNFα rose significantly [23]. We continued our analysis of soluble blood components by measuring serum α-defensins 1–3. α-defensins 1–3 are a family of cationic antimicrobial peptides that demonstrate a broad spectrum of microbicidal activity against bacterial, fungal, and viral pathogens [24]. α-Defensins 1–3 are secreted by many cell types including neutrophils, monocytes, mDCs [24–26]. These peptides have antimicrobial activity and are considered to be an early barrier to infection from bacteria and fungi as well as enveloped virus such as influenza, herpes virus, cytomegalovirus, and HIV [24]. We found that α-defensins 1–3 are significantly increased in the blood during pregnancy (Fig. 1). This increase is seen in the first trimester and continues to at least 35 weeks.
Fig. 1.
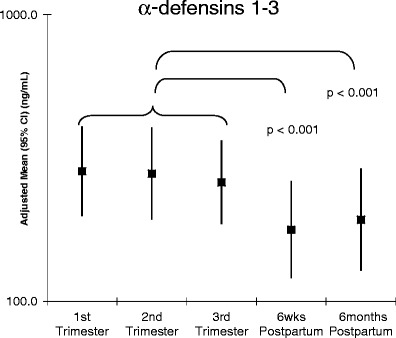
Alpha-defensins 1–3 are increased in peripheral blood during pregnancy. Serum was stored at −80°C until end of study and diluted to 1:100 in PBS before ELISA. All samples from each patient were analyzed on the same plate
Blood Phagocytes Rise during Pregnancy
Monocytes and neutrophils are the primary phagocytic cells in the body. Individuals with reduced levels of phagocytes are extremely susceptible to and often struggle to recover from infection. During pregnancy, a significant increase in the absolute numbers of both populations of phagocytes was observed (Fig. 2). The increase in each was highly significant before week 20, and this change was still observed at the 35–37-week sample. Baseline, defined by the 6-month post-partum bleed, was achieved by 6 weeks post-partum. These results suggest that a strengthening of the phagocytic barrier to infection occurs early in pregnancy, remains high throughout the pregnancy period, and reverts quickly to baseline post-delivery.
Fig. 2.
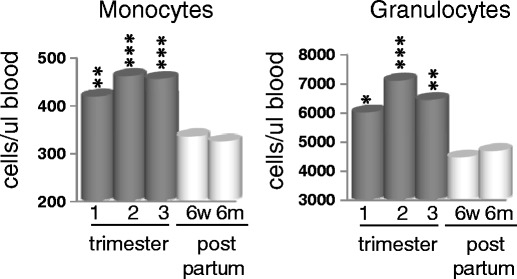
Monocytes and granulocytes increase in peripheral blood during pregnancy. Blood was drawn at each visit and incubated with the appropriate antibodies before being assessed by flow cytometry using Miltenyi cell enumeration beads. Monocytes are defined as CD14+ cells. Granulocytes were calculated based on the subtraction of T, B, NK, monocytes, and DCs from total leukocytes. Data is the mean of all patients and represented as cells per microliter blood. All p values are compared to 6 months post partum. *p < 0.05, **p < 0.005, ***p < 0.001
Blood Dendritic Cells are Elevated during Pregnancy
Using flow cytometry, we measured the numbers of plasmacytoid DCs (pDCs) and conventional DCs (mDCs) in blood during pregnancy and in the post partum period (Fig. 3). While both populations were elevated during pregnancy, we observed almost a twofold increase in pDCs. These cells are the primary type I IFN-producing cell in the body, and their elevation suggests that pregnant women may be capable of producing higher levels of type I IFN in response to virus. The function of blood mDCs has not been clearly defined.
Fig. 3.

Increase in pDCs and mDCs in peripheral blood during pregnancy. Plasmacytoid dendritic cells were defined as CD19−/CD103+ cells, while conventional DCs (mDCs) are defined as CD19−/CD11c+ cells. Data is the mean of all patients and represented as cells per microliter blood. *p < 0.05, ***p < 0.001
CD56dim NK Cells Decrease in the Last Two Trimesters of Pregnancy
NK cells function as effectors against virus-infected cells. While usually considered elements of the innate immune response, they are a major producer of IFNγ and a number of other cytokines that work to promote and support the adaptive response. To measure changes in NK cell numbers in blood, CD3 negative lymphocytes were gated on CD56 bright and dim (Fig. 4a). CD56 bright are overexpressed in the decidua and are immunoregulatory but non-cytolytic, while CD56 dim cells are both cytotoxic and secrete immunoregulatory cytokines. In the 6-month post-partum samples, approximately 85–90% of the peripheral blood NK cells were CD56 dim. CD56 dim cells were decreased as much as 38% during the second and third trimester of pregnancy (Fig. 4b), whereas the absolute number of CD56 bright cells did not change significantly at any time during pregnancy.
Fig. 4.
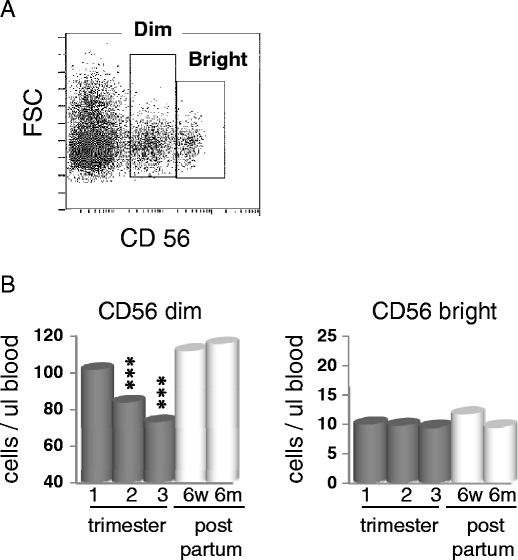
Decrease in CD56 dim cells during pregnancy. a RBC depleaded PBMCs were gated on the CD3− lymphocyte population prior to CD56 analysis. Dot blot shows a representation of analysis of CD56 dim vs. bright staining. b CD56 bright and CD56 dim cells were enumerated using Beckman Coulter Flow-Count fluorospheres. Data is the mean of all patients and represented as cells per microliter blood. ***p < 0.001
Function Changes in NK Cells during Pregnancy
An important goal of this study was to investigate cells in the steady state as well as following activation. Therefore we measured cytokine secretion from NK cells collected from patient samples and left in culture overnight. We found a significant decrease in spontaneous cytokine secretion in freshly cultured NK cells during pregnancy, especially during the second and third trimesters (Fig. 5a). IL-6, MIP-1α, MIP-1β, IFNγ, TNFα and GM-CSF secretion was significantly reduced in the second and third trimesters compared to the 6-month post-partum bleed. Levels of IL-6 and MIP-1β were reduced more that 65% in the third trimester, and the reduction was highly significant.
Fig. 5.
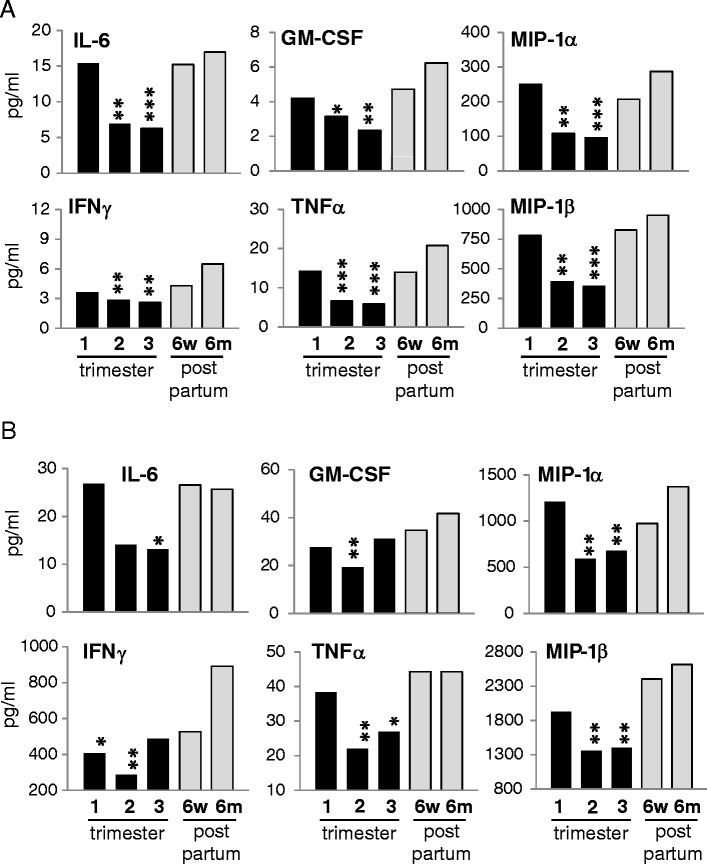
NK cell cytokine secretion during pregnancy is repressed. NK cells were freshly isolated by negative selection and cultured without stimulation (a) or with IL-12/IL-15 stimulation for 20 h (b). Supernatants were assayed by multiplex ELISA. Data is represented as the mean of all patients. All p values are compared to 6 months post partum. *p < 0.05, **p < 0.005, ***p < 0.001
NK cells are activated via cytokine released from infected cells including dendritic cells. To measure the response of NK cells following activation, we cultured NK cells overnight with IL-12/IL-15 (Fig. 5b). The secretion of IFNγ, TNFα, MIP-1α, MIP-1β, and IL-6 was increased over the steady state by stimulation, but the reduction in cytokine production during pregnancy remained significant, and for many cytokines was over a 50% reduction. In total, these data confirm a repression of NK cell number and function in the periphery in the second and third trimesters.
T Cells
T lymphocytes in blood were also decreased during pregnancy (Fig. 6). Both CD4 and CD8 T cells levels are significantly lower by 20 weeks of gestation. The percentage of CD3 cells in the total blood decrease from ~25% to 15% by week 26, although the ratio of CD4 to CD8 remains constant through gestation, suggesting that a specific T cell subtype is not being affected.
Fig. 6.
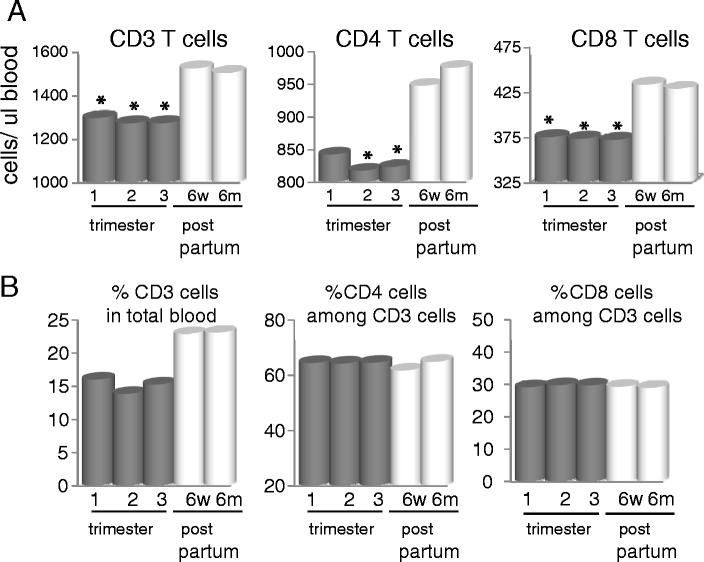
Decrease in CD3 T cells in blood during pregnancy. Blood was drawn at each visit and incubated with the appropriate antibodies before being assessed by flow cytometry using Miltenyi cell enumeration beads. Data is the mean of all patients and represented as cells per microliter blood (a) or as percentage of CD3 in total blood and percentage of CD4 or CD8 of total CD3+ cells (b). All p values are compared to 6 months post partum. *p < 0.05
It has been postulated that during normal pregnancy, there is a shift in the balance to a Th2 T cell phenotype. Since memory T cells are already polarized, we focused our analysis on the naïve CD4 T cell population. Freshly isolated CD4+/CD45RO- (naïve CD4) cells, upon stimulation with the superantigen TSST for 48 h, demonstrate a broad but selective suppression of cytokine secretion in the second and third trimesters (Fig. 7). Both Th1 (IFNγ, TNFα) and Th2 cytokines (IL-10, IL-13, IL-6) were downregulated. No significant difference was seen with IL-4 although the expression was quite low. The secretion of IL-2 appeared to be enhanced in the first trimester but otherwise not significantly regulated during pregnancy. In contrast to the other cytokines, IL-8 secretion was enhanced from T cells during pregnancy. These findings suggest a selective suppression of CD4 T cell cytokines during the second and third trimesters, although not along the conventional Th1/Th2 dichotomy. The cells were not functionally inert, as secretion of IL-8, IL-4, and IL-2 was the same or enhanced compared to the post partum period.
Fig. 7.
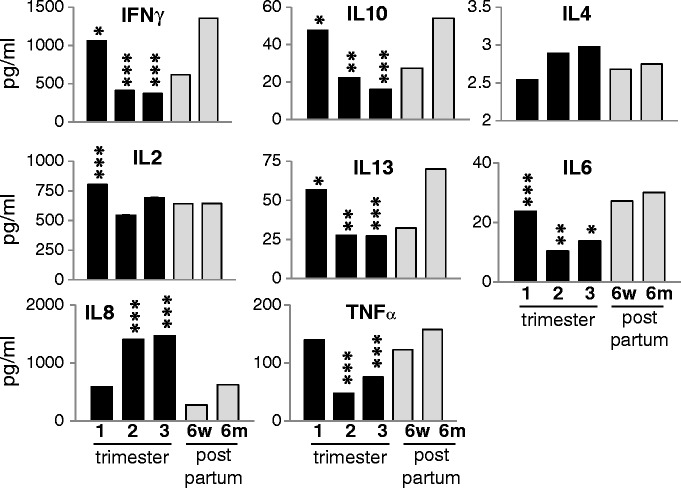
Naïve CD4 T cells have selective cytokine suppression during pregnancy. Naïve CD4 T cells were isolated by negative magnetic bead selection and cultured with TSST for 48 h. Culture supernatants were stored at −80°C until all samples of the patient were taken and assessed by multiplex ELISA. All p values are compared to 6 months post partum. *p < 0.05, **p < 0.005, ***p < 0.001
B Cells
Cell enumeration of CD19+ cells showed that B cells decrease in circulation by the third trimester (Fig. 8). This delayed reduction of B cells in blood might reflect the reports of Medina et al. that showed repression of B cell lymphopoiesis in the bone marrow during pregnancy in mice and humans [27, 28]. No reports of loss of antibody response to bacterial or viral infections during pregnancy have been published, and results from our VIP vaccine cohort suggest no loss of antibody response to inactivated influenza vaccine (Sperling et al., manuscript in press).
Fig. 8.

B cells are decreased in blood late in pregnancy. Blood was drawn at each visit and incubated with anti-CD19 antibody before being assessed by flow cytometry using Beckman Coulter cell enumeration kit. Data is represented as cells per microliter blood. *p < 0.05
Discussion
This study was performed to analyze the alterations in the systemic immune system that might contribute to the increased morbidity and mortality associated with influenza infection during the latter part of pregnancy. We observed that compensatory changes occur that appear to strengthen the barriers that prevent the establishment of infection as pregnancy progresses. These changes include a substantial increase both in the number of phagocytes and in the level of serum α-defensins 1–3 as well as alterations in the level of certain serum cytokines. Moreover, there is a striking rise in the type I IFN producing pDC population. However, NK and T cell number and function decrease, especially during the second and third trimesters, indicating a shift away from inflammatory Th1 responses and the production of IFNγ. Moreover, both NK and naïve T cells showed constitutive changes in a number of gene products in the steady state (data not shown). We were unable to identify any rise in the level of Th2 cytokines, either in circulating proteins or in supernatants collected from resting or stimulated NK or T cells. While peripheral B cell numbers decreased in the third trimester, no loss of B cell response to the influenza trivalent vaccine or alteration in immunoglobulin subtype was found (Sperling, et al., manuscript in press). The overall picture is one of increased defensive immunity with a concomitant decrease in NK and CD4 T cell inflammatory activity.
Many previous reports have shown immunological differences between pregnant and non-pregnant women. Some of these results appear to be contradictory, resulting at least in part to inherent interpersonal variability that affects cross-sectional studies. Furthermore, few, if any, investigations have considered multiple immune components simultaneously, with some groups focusing exclusively on NK cell activity while others focus on T cells, B cells, or monocytes. Additionally, many studies of the immunology of the human maternal–fetal interface compare unremarkable pregnancies to those complicated by adverse pregnancy outcomes. In these cases, it is impossible to disentangle the consequences of the disease from those inherent to immunological adaptations of pregnancy. Our longitudinal study reduces interpersonal variability by comparing each woman's immune function during pregnancy to her own response 6 months post-partum, thus eliminating the negative influence of interpersonal variability in immune profile. We also took a comprehensive approach to evaluating immunological adaptations in pregnancy, examining activity of cells in both innate and adaptive immunity, along with serum hormone levels, α-defensins 1–3, and peripheral blood cytokines. A complementary vaccine study during gestation was performed with a second cohort to analyze B cell responses. The results from both studies together suggest an alteration in systemic immunity consistent with a decrease in Th1 activity, an increase in phagocytic numbers and function, and no detectable elevation in Th2 activity.
Previous studies have reported changes in cellular blood components during pregnancy. Most of these studies compared immune responses between women at different stages of pregnancy to unrelated non-pregnant women, some with as little as five subjects per group [29–33]. Given the variability in immune profiles between individuals [23], it is not surprising that these reports are not in agreement with each other. In the cell enumeration studies presented here, we show an increase of phagocytic cells (both monocytes and granulocytes) and mDCs and pDC subsets in the blood of women during pregnancy compared to their own post-partum period. This increase is offset by a decrease in CD4 and CD8 cells, B cells, and CD56 dim NK cells.
M-CSF is increased in the blood during pregnancy [34], which could explain the increase in monocytes that we observe. We had previously reported an increase in serum G-CSF expression during pregnancy that could in part explain the increase in granulocytes [23]. In turn, this increase in phagocytes, or their increased activation state [22, 25, 35], could explain the rise in α-defensins 1–3, as neutrophils and other phagocytes are major producers of these peptides. These cascading events could be crucial to the success of a woman's defense against microbial infection in the pregnant state. Subsequent studies will evaluate phagocyte functional activation more comprehensively.
We found no significant decline of NK cells in the blood until after 20 weeks gestation relative to the 6-month post partum measures. By week 35, the absolute number of CD56dim cells declined by over 60%. The cells that remained had reduced spontaneous cytokine secretion as well as reduced secretion of cytokines when stimulated with either IL-12/IL-15 or with IL-2 (data not shown). NK cells play an important role in pregnancy. Uterine NK cells serve critical functions in trophoblast invasion as well as spiral artery remodeling [36, 37]. Loss of NK cells early in gestation may therefore be deleterious to the pregnancy, possibly resulting in poor placental invasion and perfusion. Placentation is completed by 20 weeks' gestation; thus, the declines in NK cell count and activity that we observed in late pregnancy may be consistent with their physiological functions. [38].
The theory that pregnancy requires a shift to a Th2 bias has been widely touted as well as widely criticized. Our data does not suggest any increase in Th2 cytokines in the serum [23] or from stimulated blood CD4 cells; in fact, we show that IL-10, IL-6, and IL-13 are downregulated along with Th1 cytokines throughout pregnancy in stimulated naïve CD4 T cells. The suppression of T cell activity is selective however. We found no decrease of IL-8, IL-2, and IL-4 secretion from naïve CD4 T cells during pregnancy.
The effect of pregnancy on humoral immunity is complex and somewhat ambiguous. B cell lymphopoiesis is suppressed early in pregnancy [27]. Populations of B cell precursors are reduced in the bone marrow. However, estrogen appears to increase survival and expansion of B cell subsets. Estrogen administration in vitro increases IgM and IgG production from PBMCs [39], and estrogen administration in vivo can lead to the increase of autoantibody production in SLE mouse models [40]. Together, these data suggest that pregnancy or associated hormones might negatively regulate new B cell development while enhancing antibody production in mature B cells. In our cohort, B cells were reduced in the periphery during the third trimester relative to the postpartum measures. However, from our influenza vaccine cohort (Sperling et al., manuscript in press), we found no loss of antibody response to the inactive flu vaccine during pregnancy. It can be argued that due to past exposure of antigenically similar virus strains, the influenza vaccine response is mostly reliant on a memory response [41], while estrogen reportedly affects naïve precursor B cells.
In a previous report analyzing the expression of serum cytokines from our cohort, we noted that serum cytokines are uniquely regulated during pregnancy [23]. Proinflammatory cytokines IFNγ and TNFα have divergent regulation, with IFNγ decreasing during pregnancy while the expression of TNFα increases. Although it is unclear what the overall consequence of this regulation is in vivo, we showed that circulating NK cells secrete more IFNγ in patients with high serum concentrations of IL-12, suggesting that these serum cytokines are functionally active in vivo. We further showed that peripheral blood mononuclear cells that were treated with mid-pregnancy serum were less able to inhibit viral replication than cells treated with post-pregnancy serum, suggesting that other cytokines, i.e., type I and II IFN, are active against virus infection in concentrations found in serum.
Whether pregnant women are more susceptible to virus infection or are just more prone to profound disease once infected, is controversial. We report an increase of phagocytic cells and serum α-defensins 1–3 that appears to persist throughout pregnancy. The abundance of pDCs may enhance the levels of type I IFN during a viral infection. As these are known to be vital and effective barriers to infection, their elevation might suggest no increase, and possibly even a decrease, in susceptibility during pregnancy. However, once an infection is established, the decreased efficacy of the important anti-viral effector cell populations (NK and T cells) during the late second and third trimesters, may compromise viral clearance, and these changes may underlie the reported increase in disease severity that have been reported in other studies [10–12]. Further studies are needed to determine whether these changes are dependent on one another or how they correlate with the increase in estrogen and progesterone expression.
Why systemic immunity changes in response to pregnancy remains unclear. Is there a need to protect the fetus because of its antigenic content, or are strong pro-inflammatory systemic responses harmful to the developing fetus? In the first scenario, the local immune regulation critical for blocking rejection of the fetus affects systemic responses non-specifically. For example, Tregs that might be generated against paternal or male antigens can produce cytokines that will act to suppress responses to other stimuli. If this is the case, then the altered immune response to influenza and other pathogens is merely a side effect of suppression of the alloresponse and any therapeutic efforts to bolster antiviral immunity might not be detrimental to mother or fetus. However, it is also possible that strong pro-inflammatory systemic immune responses to any target might be detrimental to the developing fetus and must be regulated. In this scenario, efforts aimed at increasing the immune response to an acute infection acquired in the perinatal period may have adverse effects on fetal survival. Studies are currently underway to address this question.
Conclusion
In summary, data from the current study as well as our other analyses from the VIP cohorts, strongly support a picture of suppression of selective elements of maternal immunity that are accompanied by compensatory changes to strengthen the barriers against the establishment of infection. Among subjects in our study, a substantial increase occurred in the level of phagocytes, serum cytokine levels change drastically, and serum defensins are elevated. Moreover, there was a striking rise in the type 1 IFN producing plasmacytoid DC population. However, in concert with these alterations, NK and T cell number and function decreased markedly, especially during the late second and third trimesters, indicating a shift away from inflammatory Th1 responses and the production of IFNγ. Moreover, both NK and naïve T cells showed constitutive changes in a number of gene products in the steady state. In contrast to the prevailing hypothesis, we found no indication of a rise of Th2 cytokines. While peripheral B cell numbers were observed to decrease in the third trimester, no loss of B cell response to the influenza trivalent vaccine was found in our other cohort (Sperling et al., manuscript in press). Overall, these changes can be characterized as a shift in the priorities of the systemic immune system with a selective repression of maternal immune responses and a shift to defensive immunity.
Acknowledgements
The study team would like to acknowledge the contributions of Bhakti Rawal, Melissa Larrier, and Sharon Czelusniak for technical support in the lab and Jordan Bragg and Joyce Preisinger for administrative support.
Funding
This work was supported by the National Institute of Allergies and Infectious Diseases—Division of Allergy, Immunology and Transplantation of the National Institutes of Health [grant number N01-AI-50028]. The funding source had no role in study design, collection, or interpretation of data.
Footnotes
Thomas A. Kraus and Stephanie M. Engel contributed equally to this work.
Contributor Information
Thomas A. Kraus, Email: Thomas.Kraus@mssm.edu
Stephanie M. Engel, Email: Stephanie.Engel@unc.edu
Rhoda S. Sperling, Email: Rhoda.Sperling@mssm.edu
Yungtai Lo, Email: yungtai.lo@einstein.yu.edu.
Sylvan Wallenstein, Email: Silvan.Wallenstein@mssm.edu.
Maria M. Escribese, Email: mescribese@cib.csic.es
Tricia Singh, Email: Tricia.Singh@mssm.edu.
Thomas M. Moran, Phone: +1-212-2416927, FAX: +1-212-9873653, Email: Thomas.Moran@mssm.edu
References
- 1.Lederman MM. Cell-mediated immunity and pregnancy. Chest. 1984;86(3 Suppl):6S–9S. doi: 10.1378/chest.86.3_Supplement.6S. [DOI] [PubMed] [Google Scholar]
- 2.Stockman LJ, et al. SARS during pregnancy, United States. Emerg Infect Dis. 2004;10(9):1689–1690. doi: 10.3201/eid1009.040244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harger JH, et al. Risk factors and outcome of varicella-zoster virus pneumonia in pregnant women. J Infect Dis. 2002;185(4):422–427. doi: 10.1086/338832. [DOI] [PubMed] [Google Scholar]
- 4.Braden CR. Listeriosis. Pediatr Infect Dis J. 2003;22(8):745–746. doi: 10.1097/01.inf.0000079439.30496.57. [DOI] [PubMed] [Google Scholar]
- 5.Shulman CE, Dorman EK. Importance and prevention of malaria in pregnancy. Trans R Soc Trop Med Hyg. 2003;97(1):30–35. doi: 10.1016/S0035-9203(03)90012-5. [DOI] [PubMed] [Google Scholar]
- 6.Harris J. Influenza occurring in pregnant women. Journal of the American Medical Association. 1919;72:978–980. [Google Scholar]
- 7.Greenberg M, et al. Maternal mortality in the epidemic of Asian influenza, New York City, 1957. Am J Obstet Gynecol. 1958;76(4):897–902. doi: 10.1016/0002-9378(58)90027-9. [DOI] [PubMed] [Google Scholar]
- 8.Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol. 1959;78:1172–1175. doi: 10.1016/0002-9378(59)90570-8. [DOI] [PubMed] [Google Scholar]
- 9.Neuzil KM, et al. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol. 1998;148(11):1094–1102. doi: 10.1093/oxfordjournals.aje.a009587. [DOI] [PubMed] [Google Scholar]
- 10.Klein SL, et al. The impact of sex, gender and pregnancy on 2009 H1N1 disease. Biol Sex Differ. 2010;1(1):5. doi: 10.1186/2042-6410-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14(1):95–100. doi: 10.3201/eid1401.070667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siston AM, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303(15):1517–1525. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid A. The effects of the 1918–1919 influenza pandemic on infant and child health in Derbyshire. Med Hist. 2005;49(1):29–54. doi: 10.1017/s0025727300008279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beigi RH. Pandemic influenza and pregnancy: a call for preparedness planning. Obstet Gynecol. 2007;109(5):1193–1196. doi: 10.1097/01.AOG.0000262051.71925.ac. [DOI] [PubMed] [Google Scholar]
- 15.Irving WL, et al. Influenza virus infection in the second and third trimesters of pregnancy: a clinical and seroepidemiological study. BJOG. 2000;107(10):1282–1289. doi: 10.1111/j.1471-0528.2000.tb11621.x. [DOI] [PubMed] [Google Scholar]
- 16.Billington WD. The immunological problem of pregnancy: 50 years with the hope of progress. A tribute to Peter Medawar. J Reprod Immunol. 2003;60(1):1–11. doi: 10.1016/S0165-0378(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 17.Wegmann TG, et al. Bidirectional cytokine interactions in the maternal–fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. 1993;14(7):353–356. doi: 10.1016/0167-5699(93)90235-D. [DOI] [PubMed] [Google Scholar]
- 18.Chaouat G. The Th1/Th2 paradigm: still important in pregnancy? Semin Immunopathol. 2007;29(2):95–113. doi: 10.1007/s00281-007-0069-0. [DOI] [PubMed] [Google Scholar]
- 19.Sargent IL, Borzychowski AM, Redman CW. NK cells and human pregnancy—an inflammatory view. Trends Immunol. 2006;27(9):399–404. doi: 10.1016/j.it.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63(6):425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin H, et al. Synthesis of T helper 2-type cytokines at the maternal–fetal interface. J Immunol. 1993;151(9):4562–4573. [PubMed] [Google Scholar]
- 22.Sacks GP, Redman CW, Sargent IL. Monocytes are primed to produce the Th1 type cytokine IL-12 in normal human pregnancy: an intracellular flow cytometric analysis of peripheral blood mononuclear cells. Clin Exp Immunol. 2003;131(3):490–497. doi: 10.1046/j.1365-2249.2003.02082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraus TA, et al. Peripheral blood cytokine profiling during pregnancy and post-partum periods. Am J Reprod Immunol. 2010;64(6):411–426. doi: 10.1111/j.1600-0897.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- 24.Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6(6):447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Garcia M, et al. Human immature monocyte-derived dendritic cells produce and secrete alpha-defensins 1-3. J Leukoc Biol. 2007;82(5):1143–1146. doi: 10.1189/jlb.0507295. [DOI] [PubMed] [Google Scholar]
- 26.Escribese, M., et al., Alpha-defensins 1-3 release by dendritic cells is reduced by estrogen Reproductive Biology and Endocrinology, 2011 (in press) [DOI] [PMC free article] [PubMed]
- 27.Medina KL, Smithson G, Kincade PW. Suppression of B lymphopoiesis during normal pregnancy. J Exp Med. 1993;178(5):1507–1515. doi: 10.1084/jem.178.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kincade PW, et al. Early B-lymphocyte precursors and their regulation by sex steroids. Immunol Rev. 2000;175:128–137. doi: 10.1111/j.1600-065X.2000.imr017502.x. [DOI] [PubMed] [Google Scholar]
- 29.Matthiesen L, et al. Lymphocyte subsets and autoantibodies in pregnancies complicated by placental disorders. Am J Reprod Immunol. 1995;33(1):31–39. doi: 10.1111/j.1600-0897.1995.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 30.Fiddes TM, et al. Phenotypic and functional evaluation of suppressor cells in normal pregnancy and in chronic aborters. Cell Immunol. 1986;97(2):407–418. doi: 10.1016/0008-8749(86)90410-7. [DOI] [PubMed] [Google Scholar]
- 31.Luppi P, et al. Normal pregnancy is associated with peripheral leukocyte activation. Am J Reprod Immunol. 2002;47(2):72–81. doi: 10.1034/j.1600-0897.2002.1o041.x. [DOI] [PubMed] [Google Scholar]
- 32.Kuhnert M, et al. Changes in lymphocyte subsets during normal pregnancy. Eur J Obstet Gynecol Reprod Biol. 1998;76(2):147–151. doi: 10.1016/S0301-2115(97)00180-2. [DOI] [PubMed] [Google Scholar]
- 33.Sabahi F, et al. Qualitative and quantitative analysis of T lymphocytes during normal human pregnancy. Am J Reprod Immunol. 1995;33(5):381–393. doi: 10.1111/j.1600-0897.1995.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 34.Praloran V, et al. Elevation of serum M-CSF concentrations during pregnancy and ovarian hyperstimulation. Br J Haematol. 1994;86(3):675–677. doi: 10.1111/j.1365-2141.1994.tb04809.x. [DOI] [PubMed] [Google Scholar]
- 35.Shibuya T, et al. Study on nonspecific immunity in pregnant women: increased chemiluminescence response of peripheral blood phagocytes. Am J Reprod Immunol Microbiol. 1987;15(1):19–23. doi: 10.1111/j.1600-0897.1987.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 36.Hanna J, et al. Decidual NK cells regulate key developmental processes at the human fetal–maternal interface. Nat Med. 2006;12(9):1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- 37.Burke SD, et al. Uterine NK cells, spiral artery modification and the regulation of blood pressure during mouse pregnancy. Am J Reprod Immunol. 2010;63(6):472–481. doi: 10.1111/j.1600-0897.2010.00818.x. [DOI] [PubMed] [Google Scholar]
- 38.Delgado SR, et al. Accounting for the peripartum loss of granulated metrial gland cells, a natural killer cell population, from the pregnant mouse uterus. J Leukoc Biol. 1996;59(2):262–269. [PubMed] [Google Scholar]
- 39.Kanda N, Tamaki K. Estrogen enhances immunoglobulin production by human PBMCs. J Allergy Clin Immunol. 1999;103(2 Pt 1):282–288. doi: 10.1016/S0091-6749(99)70503-8. [DOI] [PubMed] [Google Scholar]
- 40.Grimaldi CM, Michael DJ, Diamond B. Cutting edge: expansion and activation of a population of autoreactive marginal zone B cells in a model of estrogen-induced lupus. J Immunol. 2001;167(4):1886–1890. doi: 10.4049/jimmunol.167.4.1886. [DOI] [PubMed] [Google Scholar]
- 41.Wrammert J, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208(1):181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]


