Abstract
Background:
This study aimed to determine the prevalence of respiratory pathogens among newborns admitted to a neonatal medium care unit (NMCU) and to identify clinical predictors.
Methods:
A 1-y observational study was performed of neonates admitted to an NMCU in Amsterdam, The Netherlands. Nasopharyngeal samples were collected for the detection of respiratory viruses and bacteria by real-time PCR (RT-PCR). Cycle threshold (Ct) values were provided to estimate viral load. Predictors for the presence of study pathogens were identified.
Results:
From October 2010 through September 2011, 334 neonates (median age 1.3 d, 53.6% male) were included. Overall, 37 respiratory pathogens were detected in 34 children (10.2%): parainfluenza-1 (n = 9), human rhinovirus (n = 7), parainfluenza-3 (n = 6), respiratory syncytial virus (RSV, n = 6), Streptococcus pneumoniae (n = 3), adenovirus (n = 2), human coronavirus (n = 2), influenza A (n = 1), and bocavirus (n = 1). Neonates with higher viral loads (Ct <35; n = 11) were more often clinically ill than those with lower viral loads (Ct ≥35; n = 23). Two variables significantly contributed to the detection of study pathogens: age (odds ratio (OR) 1.21 for each day older; 95% confidence interval 1.12–1.30) and rhinorrhea (OR 6.71; 95% confidence interval 1.54–29.21).
Conclusion:
Respiratory pathogens seem to play a role in neonates admitted to an NMCU. The influence of respiratory pathogen detection on clinical management remains to be determined.
Subject terms: Respiratory tract diseases, Pathology, Paediatrics, Epidemiology
Main
Infections in neonates (newborn children aged until 28 d postpartum) have been known to cause significant mortality and long-term morbidity (1). One of the most important infectious disease syndromes in newborns is neonatal sepsis, with an estimated incidence of 1.5% during the first 72 h of life (2). Most infants with sepsis present with nonspecific signs and symptoms. The most common of these vague signs are temperature instability, lethargy, apnea, and poor feeding (3,4). Neonates with respiratory tract infection can present with a clinical picture that is consistent with sepsis, in addition to more classical symptoms such as tachypnea and hypoxemia (5).
The outcome of neonatal infections may be improved if illness is recognized early and appropriate antimicrobial agents are administered promptly (6). If sepsis cannot be reasonably excluded on clinical grounds, blood cultures should be obtained and empiric antibiotics should be administered. Unfortunately, laboratory investigations are not always helpful, and cultures of blood or other tissues are often negative or not possible to perform. The early detection of a (viral) respiratory tract infection could be useful because it might reduce the use of antibiotics (7).
Common pathogens causing respiratory illness in newborns are respiratory syncytial virus (RSV), influenza virus, adenovirus, rhinovirus, and parainfluenza viruses (2,3). Less common pathogens, including the more recently identified bocavirus, have also been detected in infants with acute respiratory infection (7,8). However, the prevalence of infection with respiratory pathogens in neonates, and therefore the extent of the clinical problem in this fragile pediatric population, remains largely unknown. Therefore, we aimed to determine the prevalence of respiratory pathogens, both viruses and some bacteria, among a population of neonates admitted to a neonatal medium care unit (NMCU) during a 1-y period. Secondary objectives were to compare the clinical course of neonates with and without respiratory pathogens and to identify risk factors and predictors for the presence of those pathogens.
Results
Patient Population and Characteristics
From 1 October 2010 until 30 September 2011, a total of 334 neonates were admitted to the NMCU of our hospital and screened by nasopharyngeal aspirate for the presence of respiratory pathogens. Median age at hospitalization was 1.3 d, 53.6% were male, and the median duration of admission was 4 d. Eighty-eight children (26.3%) were delivered preterm (i.e., <37 wk) and 99 (29.6%) were born after prolonged rupture of membranes (i.e., >24 h). Three hundred and nine neonates (92.5%) were admitted to the NMCU within 72 h from birth, and they were almost exclusively admitted from hospital labor and delivery; the remaining 25 (7.5%) were mostly transferred from home, with some from other hospitals. Undifferentiated perinatal infection (i.e., perinatal infection of unknown origin) was diagnosed in 79 newborns (23.7%), whereas a diagnosis of perinatal sepsis was established in 5 (1.5%); antibiotics were given to 108 (32.3%). These and other relevant characteristics are summarized in Table 1. Of note, a specific diagnosis (i.e., infection or other diagnosis) was made for 227 neonates during admission; the remaining 107 neonates were merely clinically observed because of premature age (n = 21) or delivery complications (n = 86) such as prolonged rupture of membranes, cesarean section, assisted vaginal delivery, or maternal fever during delivery. As displayed in Table 1, 8 of 138 performed blood cultures (5.8%) were positive for bacterial pathogens. The following bacteria were cultured: Staphylococcus aureus (n = 2), Staphylococcus epidermidis (n = 2), Micrococcus lylae (n = 1), Escherichia coli (n = 1), Streptococcus sanguis (n = 1), and Staphylococcus capitis (n = 1).
Table 1.
Characteristics of neonates (n = 334) admitted to a medium care unit (October 2010–September 2011)
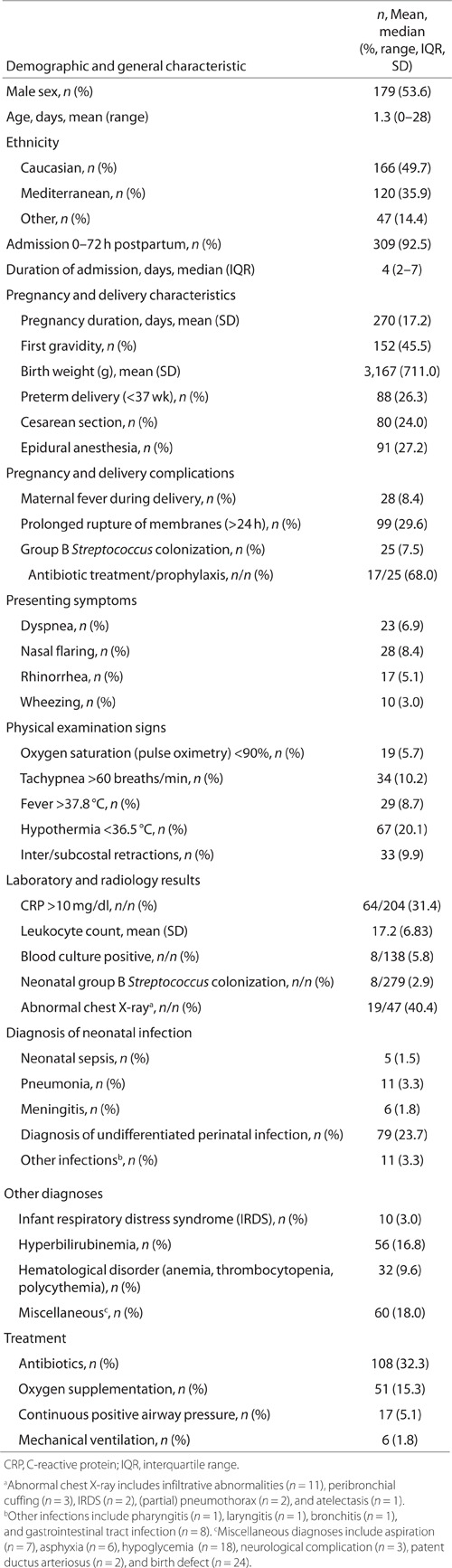
The details regarding antibiotic duration and type have not been shown. An extended course of antibiotics (i.e., >3 d) was administered to 77 of 108 neonates (71.3%) who received antibiotic treatment. Antibiotic treatment was guided by local protocols. Initial antibiotic treatment in the case of presumed sepsis or undifferentiated perinatal infection consisted of amoxicillin combined with gentamicin, followed by a switch from gentamicin to cefotaxim if antibiotic treatment had to be continued for more than 3 d. In the case of a diagnosis of meningitis being considered, flucloxacillin was added to the above-mentioned regimen. The antibiotic used in the case of community-acquired pneumonia was amoxicillin, and cefotaxim was used if the pneumonia was hospital acquired. The choice of antibiotics to be used was guided by the results of available positive microbiology culture reports.
Respiratory Pathogens Studied
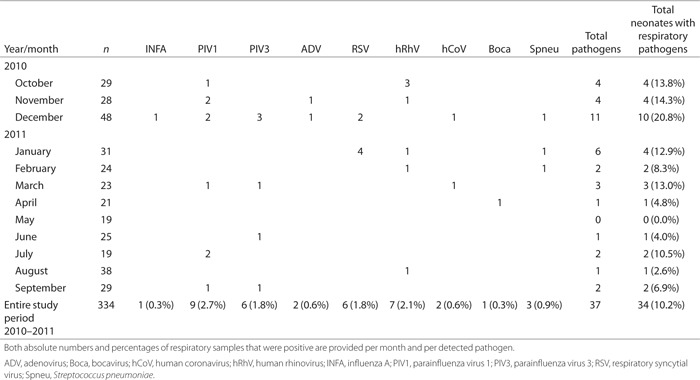
Table 2 demonstrates the study pathogens that were identified via real-time PCR (RT-PCR) on nasopharyngeal aspirates in all newborn children who were admitted during the 1-y study period to the NMCU department of our hospital. Overall, 37 pathogens were detected in 34 children, who comprise 10.2% of the total population. Mean cycle threshold (Ct) value of positive samples was 34.59 (SD 8.48). Twenty-one percent of study pathogens were identified in the month of December, with parainfluenza viruses being detected in 5 of 10 positive samples. Regarding the entire study period, parainfluenza-1 (n = 9), human rhinovirus (n = 7), parainfluenza-3 (n = 6), and RSV (n = 6) were most frequently detected. Human rhinovirus was the most frequently detected pathogen in October (three of four instances); parainfluenza viruses comprised almost half of the identified pathogens in November and December (7 of 15); and RSV showed its peak activity in January (four of six). Although Streptococcus pneumoniae (n = 3) had been detected as a bacterial pathogen by RT-PCR, blood cultures remained negative. Influenza B, parainfluenza-2, parainfluenza-4, human metapneumovirus, Chlamydia pneumoniae, Mycoplasma pneumoniae, and Legionella species were not detected at all. The two coronaviruses that were detected were human coronavirus OC43 and NL63. The simultaneous presence of two pathogens was observed in three children: all were positive for RSV combined with either human rhinovirus, human coronavirus OC43, or S. pneumoniae.
Table 2.
Respiratory pathogens identified in neonates admitted to a medium care unit
Analysis of Variables Associated With Detection of Study Pathogens
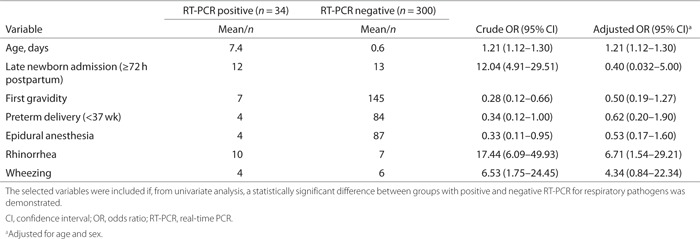
Demographic and clinical characteristics from Table 1 were compared between neonates with RT-PCR positive for any study pathogen (n = 34) and those with a negative RT-PCR result (n = 300). From this univariate analysis, we identified seven statistically significant (P < 0.05) predictor variables: age, late newborn admission (≥72 h postpartum), first gravidity, preterm delivery (<37 wk), epidural anesthesia, rhinorrhea, and wheezing; these were subsequently added to a logistic regression model (see Table 3 for statistics). In addition to these significant contributing variables, some nonsignificant differences (comparing the RT-PCR-positive and RT-PCR-negative groups) were observed with regard to other variables: male sex (67.6 vs. 52.0%; P = 0.083), median duration of admission (3 d vs. 4 d; P = 0.089), mean birth weight (3,366 grams vs. 3,144 grams; P = 0.089), and mean leukocyte count (14.7 × 109/l vs. 17.5 109/l; P = 0.066). The remaining characteristics from Table 1 were comparable between the two groups, including diagnosis of undifferentiated perinatal infection (23.5% vs. 23.7%) and pneumonia (2.9% vs. 3.3%). The positive blood culture results (n = 8) were restricted to the group of neonates for whom nasopharyngeal samples were RT-PCR-negative for study pathogens.
Table 3.
Risk of RT-PCR positive for respiratory pathogens per selected variable
To explore a possible association between the amount of virus detected in a positive sample and certain clinical characteristics, an arbitrary cutoff of the Ct value was used to compare neonates with higher viral loads (Ct <35; n = 11) with those with lower viral loads (Ct ≥35; n = 23). Neonates with positive samples that contained higher virus concentrations were older (mean age 19 vs. 2 d; P < 0.001), suffered more frequently from rhinorrhea (54.5% vs. 17.4%; P = 0.026), had a higher body temperature measured at admission (mean 37.4 °C vs. 36.9 °C; P = 0.018), had more abnormal sounds on lung auscultation (45.5% vs. 8.7%; P = 0.013), were more frequently diagnosed with bronchiolitis (36.4% vs. 4.3%; P = 0.014), and required more oxygen supplementation (36.4% vs. 8.7%; P = 0.048).
Because RSV infections can take a serious course in neonates, we specifically compared the clinical course of neonates with RT-PCR-confirmed RSV infection (n = 7) with those with positive results for other pathogens (n = 27); we found that duration of admission was significantly longer among those with RSV infection (median duration 7.5 vs. 3 d; P = 0.046). Other variables relating to clinical course, such as antibiotic therapy, oxygen supplementation, and ventilatory support, did not differ between the groups. Likewise, we compared the clinical course between neonates with RT-PCR-confirmed dual infection (n = 3) and mono infection (n = 31): although dual-infected children had significantly more nasal flaring (66.7% vs. 9.7%; P = 0.008), inter/subcostal retractions (66.7% vs. 12.9%; P = 0.020), and abnormal lung auscultation sounds (66.7% vs. 16.1%; P = 0.039), other relevant variables relating to clinical outcome (e.g., duration of admission), and illness severity (e.g., vital signs) were comparable between groups. Furthermore, none of the dual-infected neonates needed oxygen supplementation or (invasive) respiratory assistance.
In addition to comparing variables between neonates with and without a positive RT-PCR for study pathogens, another comparison was made based on presenting signs of respiratory infection (dyspnea, nasal flaring, rhinorrhea, and wheezing). Of 39 neonates with respiratory symptoms, 12 (30.8%) had a positive RT-PCR, whereas of 295 neonates without respiratory symptoms, 22 (7.5%) had a positive RT-PCR. The difference in proportion was statistically significant (P < 0.001). Comparing neonates for whom a specific diagnosis was made during their hospital stay (n = 223) with those who were merely observed clinically (n = 107), the difference in RT-PCR positivity rate was not statistically significant (12.3% vs. 5.6%; P = 0.058). Of those neonates who required continuous positive airway pressure (n = 17), two (11.8%) were positive for any study pathogen; none who required mechanical ventilation (n = 6) had a positive RT-PCR.
Table 3 summarizes the results of the logistic regression analysis. Odds ratios (ORs) were calculated and adjusted for age and sex to estimate the risk of a positive RT-PCR for study pathogens per included variable. After adjustment, two variables were identified as significant contributors to the model: age (OR 1.21 for each day older; 95% confidence interval 1.12–1.30) and symptoms of rhinorrhea (OR 6.71; 95% confidence interval 1.54–29.21). For rhinorrhea, we calculated the predictive characteristics of this symptom within our population: we determined a positive predictive value of 58.8% and negative predictive value of 92.4%.
Discussion
This prospective observational study describes a population of 334 neonates who have been admitted to the NMCU of our hospital in the period from 1 October 2010 until 30 September 2011. A respiratory pathogen was detected by RT-PCR on nasopharyngeal aspirates in a total of 34 newborn children (10.2%). Parainfluenza-1 (n = 9), human rhinovirus (n = 7), parainfluenza-3 (n = 6), and RSV (n = 6) were most frequently detected.
To our knowledge, this is the first study that determined the prevalence of respiratory pathogens among neonates admitted to a medium care unit. Other studies of neonates have been performed in neonatal intensive care units (8,9,10), and are therefore not entirely comparable to our medium care unit population, which is generally considered to be a milder neonatal patient category. Moreover, these studies solely used immunofluorescence and/or culture, and the range of studied respiratory pathogens was different from and less extensive than ours. Other studies are even less comparable to our study because they have focused on selected pediatric populations of older children (up until 5 y of age) with an acute respiratory infection (11,12,13). In our study population, parainfluenza virus was the most identified pathogen (15 of 37 detected pathogens), whereas most of the other studies have in common that RSV was the most frequently detected virus (RSV accounted for 6 of 37 detected pathogens in our study) (8,9,10,12,13). Besides the study populations not being entirely comparable, another explanation for this discrepancy might be the fact that we had noticed a milder than usual RSV epidemic in the winter of 2010–2011 among children in our hospital in general.
RT-PCR has been used as the diagnostic method in our study, as opposed to other neonatal studies (8,9,10). In a study comparing multiplex PCR assays and conventional techniques for diagnosing respiratory virus infections in children admitted to the hospital with an acute respiratory illness, PCR was more sensitive and had the advantages of a shorter delay in specific diagnosis and a lower cost than immunofluorescence or culture (14). Another study concluded that RT-PCR for respiratory viruses was found to be a sensitive and reliable method in pediatric intensive care unit patients with lower respiratory tract infection, with a twofold increase in the diagnostic yield as compared with conventional methods (15). However, a limitation of the use of PCR assays has been acknowledged by studies showing that asymptomatic carriage of a respiratory virus occurs frequently in young children (16) and the presence of viral nucleic acids may not always reflect an association with infectious virus production (17). This limitation most likely holds true for newborns as well, as is reflected by our findings showing that respiratory pathogens have been detected in 7.5% of neonates without respiratory symptoms. In addition, blood cultures remained negative in three children of whom nasopharyngeal aspirates tested positive for S. pneumoniae by RT-PCR in our study. Asymptomatic colonization with this specific bacterial pathogen has been demonstrated by Bisgaard et al. in 9% of neonates born from mothers with asthma (18). In general, the Ct value may be of help in differentiating symptomatic from asymptomatic infections. Our data show that neonates in whom respiratory samples tested positive and contained lower Ct levels (i.e., higher viral loads) indeed were more often clinically ill than neonates with low viral loads. Regardless, our study design does not really allow us to draw conclusions regarding asymptomatic carriership because all the neonates from our population were hospitalized, and therefore we did not have a true “asymptomatic” comparison group.
We compared demographic and clinical characteristics between neonates with a positive RT-PCR for any study pathogen with those with a negative RT-PCR result, and found that seven variables showed statistically significant differences and therefore were potentially associated with the risk of a positive RT-PCR result for respiratory pathogens: age, late newborn admission, first gravidity, preterm delivery, epidural anesthesia, rhinorrhea, and wheezing. After adjustment for confounding, only age (OR 1.21 for each day older; 95% confidence interval 1.12–1.30) and rhinorrhea (OR 6.71; 95% confidence interval 1.54–29.21) remained significant contributors to an increased risk of RT-PCR positivity for study pathogens. The risk factors for infection have been most thoroughly characterized for RSV. Prematurity and young age have been shown to be independent risk factors for RSV-associated hospitalizations (19). Birth at the onset of the RSV season and other environmental factors, such as the presence of (school-aged) siblings and day care attendance, have also been designated as significant risk factors for severe RSV infection (20,21). Similar to the results from our unadjusted univariate analysis, a study in which newborns infected with a range of respiratory viruses were compared with newborns without infection showed that wheezing was one of the symptoms that was significantly more frequently noticed in the presence of respiratory virus infection (9).
For neonates with a nonspecific syndrome of infection of unknown origin, there are currently no guidelines for the use of respiratory pathogen diagnostics. In clinical practice, these cases are usually regarded as possible sepsis and, after the collection of blood samples for bacterial culture, empirical antibiotics are then initiated to conform with guidelines (22). However, antibiotics in neonatal infections, especially the frequent use of aminoglycosides, can lead to serious toxicity (23). In addition, from a cost–effectiveness study, it has been shown that treatment of children aged 0–36 mo with lower respiratory tract infection causes a considerable economic burden, which is for the most part caused by (duration of) hospitalization (24). Studies on the value of RT-PCR diagnostics in children have shown conflicting results as to whether the introduction of this method as a diagnostic tool for neonatal infection might be of benefit (25,26). The results of our study, in which all pediatricians were blinded to the RT-PCR results, show that median duration of clinical admission was 1 d shorter among those neonates with a positive result (nonsignificant difference; P = 0.089). Other variables, relating to clinical course and outcome, were comparable between the two groups. Therefore, for neonates hospitalized with nonspecific signs and symptoms, the possible advantage of performing RT-PCR assays for the detection of respiratory pathogens remains to be elucidated. Of note here, we did show that positive blood cultures, which are considered to be the ultimate proof of bacterial infection, were not seen at all among neonates with a nasopharyngeal aspirate positive for respiratory pathogens as detected by RT-PCR. This might be a cautious argument in support of withholding antibiotics or decreasing their use when respiratory viruses as the etiological agents are detected using RT-PCR assays on respiratory samples.
There are some limitations of our study that need to be discussed. First, although we are the first to describe a neonatal population in a medium care setting, we have restricted ourselves to milder cases as compared with neonates admitted to an intensive care setting. It would be interesting and useful to repeat our research among unselected neonates who are hospitalized, irrespective of the intensity of the provided care. Another limitation is that we have identified only 34 neonates with a respiratory pathogen. Even though this comprises 10.2% of the total population, the absolute numbers that were used for the analyses are relatively small, especially when considering the detected pathogens separately. To increase the power to detect any relevant difference between groups, a longer follow-up period would be of added value. A last limitation is that our study design does not yet allow us to draw solid conclusions on the usefulness of RT-PCR, and therefore its role in clinical practice. Only a randomized controlled trial would give us definite answers.
In conclusion, this study has demonstrated that respiratory pathogens were present in 1 of 10 unselected neonates admitted to an NMCU. Parainfluenza viruses, rhinovirus, and RSV were most important with respect to frequency of detection. Increasing age and symptoms of rhinorrhea significantly contributed to an increased risk of detection of any respiratory pathogen. Therefore, practitioners might be advised to consider ordering respiratory viral/bacterial testing of nasopharyngeal specimens in neonates with rhinorrhea, especially when they are older. There was no significant difference regarding use of antibiotics or duration of hospital stay; however, pediatricians were blinded to RT-PCR results. The question of whether clinical management will be influenced by the knowledge of respiratory viruses being present in respiratory samples of newborn children remains to be answered.
Methods
Study Design and Population
The design of the study was observational; data were prospectively collected from 1 October 2010 until 30 September 2011. During the study period, all neonates (i.e., both early and late newborn children aged until 28 days postpartum) who were admitted to the NMCU of the Slotervaart Hospital were consecutively included. Slotervaart Hospital is a 410-bed teaching hospital in Amsterdam, The Netherlands, serving a low- to middle-income urban population of about 140,000 inhabitants, consisting of 18% children and 49% ethnic minorities, most of them of Moroccan or Turkish origin. The study has been approved by the institutional medical ethics committee (METC Slotervaartziekenhuis en Reade); the committee judged that it was not necessary to ask the parents/caregivers for informed consent.
Data Collection
Epidemiological and clinical data were collected by using an extensive standardized form. Electronic patient files were used to provide the required information. Data included demographic and general characteristics, pregnancy and delivery characteristics and complications, presenting symptoms, physical examination signs, diagnoses, and treatment. Additional laboratory and radiology investigations, including microbiology blood cultures, were performed at the full discretion of the responsible pediatrician, and the results of those investigations, if applicable, were also included in the database.
Respiratory Pathogens Studied
From all neonates, a diagnostic nasopharyngeal aspirate was collected as soon as possible after NMCU admission. Total nucleic acids were extracted from the clinical samples using the automated MagnaPureLC Isolation platform (Roche Applied Science, Penzberg, Germany). RT-PCR was performed on extracted samples for detection of 18 study pathogens, both viruses and some bacteria. Multiplex RT-PCRs were performed for parainfluenza 1–4, human coronavirus OC43/229E/NL63, influenza A and B virus, adenovirus, human rhinovirus, Chlamydia pneumoniae, and Mycoplasma pneumoniae. Monoplex RT-PCRs were performed for RSV, human metapneumovirus, Legionella species, bocavirus, and S. pneumoniae. Phocine herpesvirus and equine arteritis virus were spiked to the clinical samples and used as internal controls to monitor nucleic acid isolation and inhibition of the RT-PCR (27). Ct values were provided for each positive result in order to estimate viral load, with lower Ct values corresponding to higher viral loads. All pediatricians and pediatric residents that were involved with delivered patient care were blinded to the RT-PCR results.
Statistical Analysis
Statistical analysis was performed using the SPSS software package (version 18.0; SPSS, Chicago, IL). Continuous variables were summarized as means or medians, and for categorical variables, percentages were calculated. To determine which independent variables significantly contributed to the prediction of the outcome of RT-PCR positive for study pathogens, a univariate analysis was performed: demographic and clinical characteristics were compared by using a Student’s t-test or nonparametric test for continuous variables and χ2 or Fisher’s exact test for categorical variables, as appropriate. If a significant contribution was found for any of the independent factors in the univariate analysis, they were added as variables to a logistic regression model. Crude ORs were adjusted for age and sex. In general, a P value of <0.05 was considered statistically significant.
Statement of Financial Support
No financial assistance was received to support this study.
References
- 1.Jason JM. Infectious disease-related deaths of low birth weight infants, United States, 1968 to 1982. Pediatrics. 1989;84:296–303. [PubMed] [Google Scholar]
- 2.Stoll BJ, Hansen N, Fanaroff AA. Changes in pathogens causing early-onset sepsis in very-low-birth-weight infants. N Engl J Med. 2002;347:240–7. doi: 10.1056/NEJMoa012657. [DOI] [PubMed] [Google Scholar]
- 3.Klein JO, Marcy SM . Bacterial sepsis and meningitis. In: Remington JS, Klein JO, eds. Infectious Diseases of the Fetus and Newborn Infant. Philadelphia, Pennsylvania: WB Saunders, 1995:835–90.
- 4.Voora S, Srinivasan G, Lilien LD, Yeh TF, Pildes RS. Fever in full-term newborns in the first four days of life. Pediatrics. 1982;69:40–4. [PubMed] [Google Scholar]
- 5.Korpela J, Campbell J, Singh N . Healthcare-associated infections. In: MacDonald M, Seshia M, Mullett M, eds. Avery’s Neonatology. Philadelphia, Pennsylvania: Wolters Kluwer/Lippincott Williams & Wilkins, 2005:1366–7.
- 6.Schelonka R, Freij B, McCracken G Jr . Bacterial and fungal infections. In: MacDonald M, Seshia M, Mullett M, eds. Avery’s Neonatology. Philadelphia, Pennsylvania: Wolters Kluwer/Lippincott Williams & Wilkins, 2005:1235–8.
- 7.Tregoning JS, Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verboon-Maciolek MA, Krediet TG, Gerards LJ, Fleer A, van Loon TM. Clinical and epidemiologic characteristics of viral infections in a neonatal intensive care unit during a 12-year period. Pediatr Infect Dis J. 2005;24:901–4. doi: 10.1097/01.inf.0000180471.03702.7f. [DOI] [PubMed] [Google Scholar]
- 9.Vieira RA, Diniz EM, Vaz FA. Clinical and laboratory study of newborns with lower respiratory tract infection due to respiratory viruses. J Matern Fetal Neonatal Med. 2003;13:341–50. doi: 10.1080/jmf.13.5.341.350. [DOI] [PubMed] [Google Scholar]
- 10.Rudd PT, Carrington D. A prospective study of chlamydial, mycoplasmal, and viral infections in a neonatal intensive care unit. Arch Dis Child. 1984;59:120–5. doi: 10.1136/adc.59.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–6. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 12.Bezerra PG, Britto MC, Correia JB. Viral and atypical bacterial detection in acute respiratory infection in children under five years. PLoS ONE. 2011;6:e18928. doi: 10.1371/journal.pone.0018928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vos N, Vankeerberghen A, Vaeyens F, Van Vaerenbergh K, Boel A, De Beenhouwer H. Simultaneous detection of human bocavirus and adenovirus by multiplex real-time PCR in a Belgian paediatric population. Eur J Clin Microbiol Infect Dis. 2009;28:1305–10. doi: 10.1007/s10096-009-0780-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freymuth F, Vabret A, Cuvillon-Nimal D. Comparison of multiplex PCR assays and conventional techniques for the diagnostic of respiratory virus infections in children admitted to hospital with an acute respiratory illness. J Med Virol. 2006;78:1498–504. doi: 10.1002/jmv.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Pol AC, Wolfs TF, Jansen NJ, van Loon AM,, Rossen JW. Diagnostic value of real-time polymerase chain reaction to detect viruses in young children admitted to the paediatric intensive care unit with lower respiratory tract infection. Crit Care. 2006;10:R61. doi: 10.1186/cc4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen RR, Wieringa J, Koekkoek SM. Frequent detection of respiratory viruses without symptoms: toward defining clinically relevant cutoff values. J Clin Microbiol. 2011;49:2631–6. doi: 10.1128/JCM.02094-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright PF, Deatly AM, Karron RA. Comparison of results of detection of rhinovirus by PCR and viral culture in human nasal wash specimens from subjects with and without clinical symptoms of respiratory illness. J Clin Microbiol. 2007;45:2126–9. doi: 10.1128/JCM.02553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bisgaard H, Hermansen MN, Buchvald F. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–95. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- 19.Hall CB, Weinberg GA, Iwane MK. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Figueras-Aloy J, Carbonell-Estrany X, Quero-Jiménez J. FLIP-2 Study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr Infect Dis J. 2008;27:788–93. doi: 10.1097/INF.0b013e3181710990. [DOI] [PubMed] [Google Scholar]
- 21.Simoes EA. Environmental and demographic risk factors for respiratory syncytial virus lower respiratory tract disease. J Pediatr. 2003;143(Suppl 5):S118–26. doi: 10.1067/S0022-3476(03)00511-0. [DOI] [PubMed] [Google Scholar]
- 22.Muller-Pebody B, Johnson AP, Heath PT, Gilbert RE, Henderson KL, Sharland M. Empirical treatment of neonatal sepsis: are the current guidelines adequate. Arch Dis Child Fetal Neonatal Ed. 2011;96:F4–8. doi: 10.1136/adc.2009.178483. [DOI] [PubMed] [Google Scholar]
- 23.Fanos V, Dall’Agnola A. Antibiotics in neonatal infections: a review. Drugs. 1999;58:405–27. doi: 10.2165/00003495-199958030-00003. [DOI] [PubMed] [Google Scholar]
- 24.Ehlken B, Ihorst G, Lippert B. Economic impact of community-acquired and nosocomial lower respiratory tract infections in young children in Germany. Eur J Pediatr. 2005;164:607–15. doi: 10.1007/s00431-005-1705-0. [DOI] [PubMed] [Google Scholar]
- 25.Brozanski BS, Jones JG, Krohn MJ, Jordan JA. Use of polymerase chain reaction as a diagnostic tool for neonatal sepsis can result in a decrease in use of antibiotics and total neonatal intensive care unit length of stay. J Perinatol. 2006;26:688–92. doi: 10.1038/sj.jp.7211597. [DOI] [PubMed] [Google Scholar]
- 26.Wishaupt JO, Russcher A, Smeets LC, Versteegh FG, Hartwig NG. Clinical impact of RT-PCR for pediatric acute respiratory infections: a controlled clinical trial. Pediatrics. 2011;128:e1113–20. doi: 10.1542/peds.2010-2779. [DOI] [PubMed] [Google Scholar]
- 27.Templeton KE, Scheltinga SA, Beersma MF, Kroes AC, Claas EC. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza a and influenza B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol. 2004;42:1564–9. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


