Abstract
The SARS-coronavirus (SARS-CoV) is a newly emerged, highly pathogenic agent that caused over 8,000 human infections with nearly 800 deaths between November 2002 and September 2003. While direct person-to-person transmission via respiratory droplets accounted for most cases, other modes have not been ruled out. Faecal shedding is common and prolonged and has caused an outbreak in Hong Kong. We studied the stability of SARS-CoV under different conditions, both in suspension and dried on surfaces, in comparison with other human-pathogenic viruses, including human coronavirus HCoV-229E. In suspension, HCoV-229E gradually lost its infectivity completely while SARS-CoV retained its infectivity for up to 9 days; in the dried state, survival times were 24 h versus 6 days. Thermal inactivation at 56°C was highly effective in the absence of protein, reducing the virus titre to below detectability; however, the addition of 20% protein exerted a protective effect resulting in residual infectivity. If protein-containing solutions are to be inactivated, heat treatment at 60°C for at least 30 min must be used. Different fixation procedures, e.g. for the preparation of immunofluorescence slides, as well as chemical means of virus inactivation commonly used in hospital and laboratory settings were generally found to be effective. Our investigations confirm that it is possible to care for SARS patients and to conduct laboratory scientific studies on SARS-CoV safely. Nevertheless, the agent’s tenacity is considerably higher than that of HCoV-229E, and should SARS re-emerge, increased efforts need to be devoted to questions of environmental hygiene.
Keywords: SARS-associated coronavirus, Virus infectivity, Stability, Thermal inactivation, Disinfection
Introduction
The severe acute respiratory syndrome (SARS) is a novel infectious disease that first occurred in November 2002 in China. SARS is caused by a newly emerged virus belonging to the coronaviridae family, provisionally termed SARS-coronavirus (SARS-CoV) [6]. Available sequence data indicate that SARS-CoV is clearly different from all previously known coronaviruses [13]. A new, fourth genetic lineage has been proposed for SARS-CoV [9], although others have suggested it may be an early split-off from the group 2 lineage [16].
Although human-to-human transmission of SARS-CoV is less efficient than for example in most influenza A viruses, the recent SARS epidemic was characterised by several explosive outbreaks [1,17]. Most cases were the result of direct transmission via respiratory droplets during close personal contact, and adequate respiratory protective measures were shown to be effective [14]. However, there are a number of instances when transmission occurred through other means that are often still not well defined. In the “Hotel M” episode, cases occurred in individuals that had never met a SARS-infected individual face-to-face [11]. At the Amoy Gardens high-rise housing estate, transmission probably occurred through SARS-CoV shed in the faeces of a patient [7]. In mainland China, around 50% of probable SARS patients did not have an apparent history of close personal contact with another case [8]. At least a proportion of these cases might have arisen from modes of transmission other than droplets.
While the wearing of face masks is the single most important precaution against SARS in hospital settings [14], it is of practical interest to study the stability of the virus under different conditions encountered in various environments. In addition, the validation of chemical and physical means for rendering SARS-CoV non-infectious is important. Given their remote relatedness, SARS-CoV may well behave differently from other coronaviruses. We therefore studied the stability of SARS-CoV in suspension and dried on surfaces in comparison to HCoV-229E.
Materials and methods
Viruses and cells
SARS-CoV isolate FFM-1 [5] was obtained from the sputum of a patient hospitalised with a diagnosis of probable SARS in the Isolation Unit of Frankfurt University Hospital, Germany. Herpes simplex virus type 1 (HSV-1, strain McIntyre) and human adenovirus type 3 were obtained from American Type Culture Collection (ATCC, Manassas, Va., USA; ATCC nos. VR-539 and VR-847), and human coronavirus strain 229E was kindly provided by Dr. J. Ziebuhr, University of Würzburg, Germany. SARS-CoV, adenovirus and HSV-1 were grown in Vero cell cultures (African green monkey kidney, ATCC no. CCL-81) while human coronavirus 229E was propagated in human embryonic lung fibroblasts (ATCC no. CCL-137). The maintenance medium consisted of minimum essential medium (MEM) without fetal calf serum (FCS) and containing 100 IU/ml of penicillin and 100 μg/ml of streptomycin. Virus stocks were stored at −80°C. Infectious virus titres were determined as 50% tissue culture infective doses (TCID50) in confluent cells in 96-well microtitre plates [12]. In accordance with WHO recommendations, all work involving infectious SARS-CoV was performed under biosafety level (BSL)-3 conditions in a BSL-3 facility.
Viral stability
Virus infectivity after various pre-treatments was assessed by performing virus titrations. To assess virus stability in solution, cell culture supernatants containing known concentrations of infectious virus were kept under different conditions. To assess the efficacy of thermal inactivation procedures, solutions were incubated at 56°C or 60°C with or without 20% FCS as protein additive for 30 min. In addition, 500 μl of virus suspension were applied to a polystyrene Petri dish (diameter 9 cm) and left to dry at room temperature (RT; 21–25°C). Thereafter, the dishes were stored at RT for different periods of time, before the “dried virus” was resuspended in 500 μl MEM. Residual SARS-CoV infectivity was detected by recognition of cytopathic effect (CPE) on Vero cells and additionally by immunostaining of infected cells using convalescent serum from a SARS patient (Fig. 1) as described before [2,3].
Fig. 1A–C.
SARS-CoV replication in Vero cells determined by immune peroxidase staining using serum from the index patient. Infected cells were stained 24 h (A) and 48 h (B) post-infection. Mock-infected cells are also shown (C)
Effects of different commonly used fixation solutions on the infectivity of SARS-CoV
SARS-CoV-infected Vero cells were fixed onto microscope slides as for immunofluorescence assays. Cells were fixed for different periods of time using acetone alone, an acetone/methanol (40:60) mixture, 100% ethanol, 70% ethanol, and a 1:1 mixture of ethanol and phosphate buffered saline (PBS). After storage at −80°C for 24–72 h, the cells were scratched from the slide, resuspended in MEM and inoculated onto confluent Vero cell monolayers in 12.5 cm2 flasks and incubated at 37°C. At 7-day intervals, cells were passaged. After two passages, flasks were microscopically examined for virus-specific CPE, and immune peroxidase staining was performed as described previously [2,3].
Susceptibility of SARS-CoV to different chemical disinfectants
The following compounds were tested: 2-propanol (70 and 100%) (Roth, Karlsruhe, Germany), Desderman N (78% ethanol, 0.2% 2-biphenylol) (Schülke & Mayr, Norderstedt, Germany), Sterillium (45% 2-propanol, 30% 1-propanol) (Bode Chemie, Hamburg, Germany), formaldehyde (0.7 and 1%) and glutardialdehyde (0.5%) (Merck, Darmstadt, Germany), Incidin plus (2%; containing 26% glucoprotamin) (Henkel/Ecolab, Düsseldorf, Germany). In addition, wine vinegar (acid concentration 6%, sugar concentration 5% w/v; Doktorenhof, Venningen, Germany), which might be used as a (hand) disinfectant or as a spray for inhalation, was analysed.
For each of the experiments, eight parts of the compound (adapted to RT) were mixed with one part of the virus suspension and one part of FCS or MEM, respectively. Immediately after incubation for defined periods of time at RT, the mixture was put into an ice bath to avoid an extension of the effective incubation period. Then, serial 10-fold dilutions with ice-cold MEM were done to assess virus titres as described above. All tests were performed in triplicate, and for each experiment, a virus control containing MEM instead of disinfectant was included.
If the cytotoxic effect of a disinfectant was still present at a dilution of 1:1,000, the virus-disinfectant mixture was membrane-filtred after incubation using Amicon Ultra4 Filter units 100 kDa (Millipore, Schwalbach, Germany) in accordance with the manufacturer’s instructions. By increasing the virus concentration approximately 100-fold whilst retaining the concentration of the disinfectant, it became possible to assess a reduction in virus titre of >3log10 despite the agent’s cytotoxicity.
Cytotoxic effects
Cytotoxic effects caused by the compounds at various dilutions were assessed in confluent layers of Vero cells grown in 96-well plates using the MTT cell proliferative Kit I (Roche, Mannheim, Germany) as published previously [2,3].
Results
Figure 2 shows the decline of infectious virus titres of all four viruses tested over time, both in suspension (Fig. 2c,d) and dried (Fig. 2a, b), and in the presence of 10% FCS as a protein additive (Fig. 2, b, d) or with no protein added (Fig. 2, a, c). All experiments were kept at RT and residual infectious titres were tested at different timepoints (0 h, 24 h, 48 h, 72 h, day 6 and day 9) post-infection.
Fig. 2a–d.
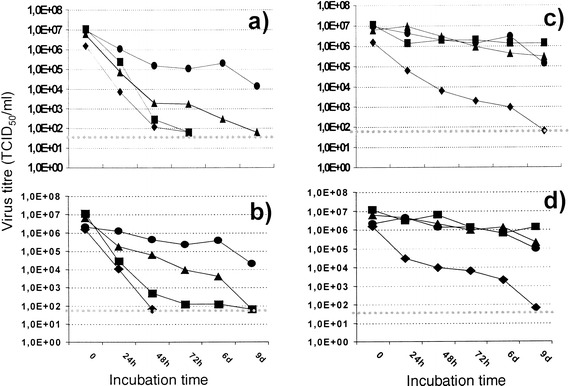
In vitro stability of SARS-CoV, HCoV-229E, HSV-1 and adenovirus type 3 either in suspension or dried. Infected cell culture supernatants were incubated at RT either in suspension (c, d) or dried on a plastic surface (a, b), in the presence (b, d) or absence (a, c) of 10% FCS. Values are means from three independent experiments. The SD did not exceed 20%. ▲ SARS-CoV (FFM1), ♦ h-CoV (E229), ■ HSV-1, ● adenovirus type 3, ⋅⋅⋅⋅⋅⋅ detection limit
In suspension, only HCoV-229E gradually lost its infectivity, while the other three viruses, including SARS-CoV, were stable for the entire duration of the experiment. The addition of FCS had no effect. In the dried state, a gradual loss of infectivity was observed for all four viruses tested. HCoV-229E and HSV-1 were most sensitive and completely lost their infectivity within 72 h, with or without added FCS. This is in agreement with previous studies [15]. In contrast, dried SARS-CoV retained its infectivity for as long as 6 days, with 10% FCS exerting a protective influence throughout. Only after 9 days in a dried state had SARS-CoV completely lost its infectivity. Adenovirus type 3 was the most stable amongst the viruses tested, retaining infectivity as late as day 9, when the experiment was terminated.
The effect of exposure to different temperatures and the influence of protein additive on the infectivity of SARS-CoV is presented in Table 1. Heat treatment at 56°C over 30 min reduced the virus titre below the detection limit; however, in the presence of 20% FCS the reduction factors were only 1.93log10 instead of >5.01log10. Incubation at 60°C for 30 min resulted in no infectious virus remaining, regardless of the presence of the protein additive. At 4°C (control), there was no loss of infectious titre.
Table 1.
Effect of different temperatures and a protein additive on the infectivity of SARS-CoV. The initial input virus titre was 7.18±0.37log10
| Temperature (°C) | Protein additive | Virus titre (TCID50/ml [log10]) after a contact time of 30 min | Minimal reduction factor (log10) |
|---|---|---|---|
|
4 (as control) |
no | 6.68±0.41 | 0 |
| 20% FCS | 6.43±0.45 | 0 | |
| 56 | no | ≤1.8±0 | ≥5.01 |
| 20% FCS | 4.55±0.33 | 1.93 | |
| 60 | no | ≤1.8±0 | ≥5.01 |
| 20% FCS | ≤1.8±0 | ≥5.01 |
The evaluation of the efficacy of different commonly used fixation procedures in eliminating the infectivity of SARS-CoV shows that with an initial virus titre of 6.55log10, no residual infectivity was detected after fixation with ice-cold acetone for 90 s, an ice-cold acetone-methanol mixture (40:60) for 10 min, 70% ethanol for 10 min or and 100% ethanol for 5 min. However, after fixation with a 1:1 mixture of PBS and ethanol (100%) for 5 min, low-level residual infectivity was observed but not quantified.
All four commonly used brands of hand disinfectants were able to render SARS-CoV non-infectious within 30 sec of contact. Table 2 presents the data on their SARS-Co-virucidal efficacy. Isopropanol 70% and 100% achieved a >3.31log10 reduction of virus infectivity after 30 s, while Desderman reduced the virus titre by >5.01log10 and Sterillium by >2.78log10. The actual reduction factors shown varied because of the effects of ultrafiltration necessitated by the different cytotoxicities of the compounds. In addition, three more disinfectants, formaldehyde, glutardialdehyde and Incidin plus, were assessed for their anti-SARS-CoV potency. All three rendered SARS-CoV non-infectious. The minimum reduction factor for formaldehyde (0.7 and 1%) was >3.01log10, for glutardialdehyde (0.5%) >4.01log10, and for Incidin plus >1.68log10, after 2 min of incubation. The reduction factor for wine vinegar was ≥3.0log10, achieved within 60 s.
Table 2.
Viricidal activity of different disinfectants against SARS-CoV
| Treatment | Virus titre (TCID50/ml [log10]) (after contact time of x s) | Minimal reduction factor (log10) |
|---|---|---|
| 2-Propanola (100%) | ≤1.8±0 (30 s) | ≥3.31 |
| 2-Propanola (70%) | ≤1.8±0 (30 s) | ≥3.31 |
| Desdermanb (78% ethanol) | ≤1.8±0 (30 s) | ≥5.01 |
| Sterilliumc (45% 2-propanol, 30% 1-propanol) | ≤3.8±0 (30 s) | ≥2.78 |
| Wine vinegard | ≤2.80 ± 0 (60 s) | ≥ 3.0 |
| Formaldehyde (0.7%) b | ≤3.8±0 (120 s) | ≥3.01 |
| Formaldehyde (1.0%) b | ≤3.8±0 (120 s) | ≥3.01 |
| Glutardialdehyde (0.5%) b | ≤2.8±0 (120 s) | ≥4.01 |
|
Incidin pluse (2%) (26% glucoprotamin) |
≤4.8±0 (120 s) | ≥1.68 |
aInput virus titre 5.55±0.44
bInput virus titre 7.18±0.37, tested by membrane filtration
cInput virus titre 6.95±0.37, tested by membrane filtration
dInput virus titre 5.93±0.13
eInput virus titre 6.48±0.37, tested by membrane filtration
Discussion
Although the SARS outbreak seems to have been halted for the time being, indicating that the stringent control measures taken to prevent person-to-person transmission were effective, it remains important to assess the risk for other modes of spread, for example via fomites or excretions. Reports from member laboratories of the WHO SARS network indicated that the virus is stable in faeces and urine at RT for at least 1–2 days and even more stable (up to 4 days) in stool from patients with diarrhoea (which has a higher pH than normal stool) [4,18]; however, neither the time required for complete inactivation nor quantitative data were reported. Our data allow a more meaningful interpretation, in that they compare the behaviour of SARS-CoV with that of other important human-pathogenic viruses.
In a considerable proportion of probable SARS cases in China, direct close contact with another SARS patient could not be elucidated [8]; thus at least some of these cases may have arisen from indirect transmission. It is therefore important to obtain information about the tenacity of SARS-CoV in the environment under different conditions. Furthermore, it is critical to assess how efficiently commonly used disinfection methods are able to reliably inactivate SARS-CoV.
Our experimental data show that SARS-CoV is considerably more stable than the previously identified human coronavirus HCoV-229E. In a dried state, SARS-CoV retained residual infectivity even after 6 days while HCoV-229E completely lost its infectivity within 24 h.
Thermal inactivation of SARS-CoV at 56° and 60°C is highly effective; however, in the presence of protein (20%), infectivity was only reduced by less than 2log10 at 56°C after 30 min (and also after 60 min; data not shown). This has implications for the handling of clinical specimens from SARS patients; for example, pre-treatment of sera at 56°C for 30 min might not be sufficient for full inactivation. On the other hand, as SARS-CoV viraemia does not seem to reach high titres [10], a moderate reduction of residual infectivity might be sufficient to enable the safe performance of serological laboratory assays.
Another question of practical importance concerns the absence of infectivity of chemically fixed SARS-CoV-infected cells. Indirect immunofluorescence assays (IFA) are widely utilised for the detection of SARS-CoV-specific antibodies in patient sera, and are normally carried out under limited safety conditions (BSL-2 instead of BSL-3). We demonstrated that with one exception (PBS/ethanol 100% (1:1) over 5 min), all fixatives were able to eliminate infectivity.
In summary, we have shown that despite its considerably higher environmental stability compared to the previously characterised human coronavirus HCoV-229E, SARS-CoV can easily be inactivated thermally and chemically. This should be borne in mind in case SARS re-emerges, for the often critical clinical state of SARS patients requires frequent determinations of standard haematological and clinical chemistry parameters. These may be done with confidence, provided standard infection safety precautions are adhered to. Our data presented here contribute to a better understanding of the stability of SARS-CoV in different environmental situations. We also demonstrate the efficacy of various means of SARS-CoV inactivation, as a contribution to improving laboratory safety.
References
- 1.Centers Morb Mortal Wkly Rep Surveill Summ. 2003;52:241. [Google Scholar]
- 2.Cinatl Lancet. 2003;361:2045. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cinatl Lancet. 2003;362:293. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Department of Communicable Disease Surveillance and Response (2003) Consensus document on the epidemiology of severe acute respiratory syndrome (SARS). WHO/CDS/CSR/GAR/2003.11. WHO, Geneva
- 5.Drosten N Engl J Med. 2003;348:1967. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 6.Drosten Trends Mol Med. 2003;9:325. doi: 10.1016/S1471-4914(03)00133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong Kong Department of Health (2003) Outbreak of severe acute respiratory syndrome (SARS) at Amoy Gardens, Kowloon Bay, Hong Kong.http://www.info.gov.hk/info/ap/pdf/amoy_e.pdf
- 8.Liang W, Zhu Z, Guo J, Liu Z, He X, Zhou W, Chin DP, Schuchat A (2003) Severe acute respiratory syndrome, Beijing, 2003.http://www.cdc.gov/ncidod/EID/vol10no03–0553.htm [DOI] [PMC free article] [PubMed]
- 9.Marra Science. 2003;300:1399. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 10.Peiris Lancet. 2003;361:1767. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poutanen N Engl J Med. 2003;348:1995. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 12.Reed Am J Hyg. 1938;27:493. [Google Scholar]
- 13.Rota Science. 2003;300:1394. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 14.Seto Lancet. 2003;361:1519. doi: 10.1016/S0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sizun J Hosp Infect. 2000;46:55. doi: 10.1053/jhin.2000.0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snijder J Mol Biol. 2003;331:991. doi: 10.1016/S0022-2836(03)00865-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Environmental Health Team (2003a) Report on Amoy Gardens. http://www.info.gov.hk/info/ap/who-amoye.pdf
- 18.World Health Organization (2003b) First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network.http://www.who.int/csr/sars/survival_2003_05_04/en/index.html


