Abstract
In this study, two human bocaviruses (HBoV), HBoV2 and HBoV3, that were detected previously in enteric samples were characterized genetically. Nearly complete genome sequences of three HBoV2 variants and one HBoV3 variant originating from Thailand and the UK were compared to published HBoV sequences. HBoV2 showed divergence from HBoV1 throughout the genome, while the HBoV3 sequence grouped phylogenetically with HBoV1 in the non-structural region and with HBoV2 sequences in the structural gene, consistent with its proposed recombinant origin. Compared to HBoV1 and HBoV3, HBoV2 shows substantially greater intra-species diversity, consistent with a longer period of human circulation.
Keywords: Human Bocavirus, HBoV2, HBoV3, Complete coding sequence
Infections of the respiratory tract by respiratory syncytial virus (RSV), influenza virus, parainfluenzavirus (PIV) and human rhinovirus (HRV) are a major cause of morbidity and mortality worldwide, especially in children. Despite recent advances in molecular-based diagnostics for respiratory virus detection, a proportion of apparently viral respiratory illness remains undiagnosable, consistent with the circulation of additional viral respiratory pathogens. In the past few years, a variety of molecular-based virus discovery methods have been successfully applied to identify new respiratory pathogens. These include human metapneumovirus (hMPV) [1], human coronavirus (HCoV)-NL63 [2], HCoV-HKU1 [3] and human bocavirus (HBoV) [4]. These methods have also revealed the presence of other viruses in the respiratory tract, such as WU [5] and KI polyomavirus [6], which are not associated with respiratory disease [7].
For HBoV, the evidence of a significant aetiological role in respiratory disease is more convincing [4, 8, 9]. HBoV was originally detected in respiratory samples by application of a random PCR/cloning technique with pooled respiratory samples, followed by bioinformatic analysis of sequences of the resulting clones [4]. The genome of the virus was most similar to those of bovine parvovirus and canine minute virus, both members of the genus Bocavirus, family Parvoviridae, to which HBoV has now been added. The genome contains open reading frames (ORFs) encoding the nonstructural protein (NS1) and at least two capsid proteins (VP1 and VP2). Moreover, as found in the animal bocaviruses, HBoV contains a third middle ORF encoding a second non-structural protein of unknown function (NP1) [4]. Following its discovery, the global prevalence of HBoV had been reported to range from 1.5 to 19% [4, 10–21]. Not only can HBoV be detected in respiratory samples, but it can also be detected in stool samples at frequencies ranging from 0.8 to 9.1% [22–29].
More recently, application of virus discovery methods directly to faecal samples revealed the presence of two new types of HBoV. A highly divergent variant of HBoV, described as HBoV type 2, was identified from stool samples by using random PCR, cloning, library sequencing and bioinformatics analysis [30]. HBoV2 showed amino acid similarities of 78, 67 and 80% in the NS1, NP1 and VP1/VP2 genes, respectively, to the prototype HBoV, described as HBoV1 in the remainder of the paper [30]. Following this, a third genetically divergent variant of HBoV described as HBoV3 was identified from stool samples from Australian children [31]. This virus showed amino acid similarities to HBoV1 of 87.3, 86.8, 76.7 and 75.4% for NS1, NP1, VP1 and VP2, respectively. In previous studies, detection frequencies of between 0.6 and 17.2% were reported for HBoV2 in faecal samples [30–35], 2.7% for HBoV3 in faecal samples [31], and 2.3 to 4.3% for HBoV2 in respiratory samples [36, 37]. In the current study, we have determined the complete genome sequences of members of both species of HBoV to further investigate their diversity and phylogenetic relationships across the genome.
A total of 6,500 nasopharyngeal (NP) aspirates and 3,000 faecal samples from Edinburgh, UK, and Bangkok, Thailand, were screened for HBoV2 [35]. HBoV2/HBoV3 variants were detected in 14 and 2 faecal samples from the UK and Thailand, respectively, but not in any respiratory samples [35].
Two samples from Thailand (CU47TH and CU54TH) and two samples from the UK (CU1557UK and CU2139UK) were selected, and their complete coding sequences were analyzed. The semi-nested PCR used primers conserved between HBoV1 and the prototype HBoV2 sequence, NC_012042 (Table 1). The amplification mixture contained 4 μl of 5× GoTaq Buffer (Promega, WI), 0.3 mM dNTP (Promaga, WI), 0.5 μM forward primer, 0.5 μM reverse primer, 0.2 U/μl GoTaq DNA polymerase (Promaga, WI), and 2 μl of DNA template in first-round PCR. One μl from the first PCR product was used as a template in the second-round PCR and added to nuclease-free water to a final volume of 20 μl. PCR cycles of both rounds included denaturation at 94°C for 3 min, followed by 35 cycles of denaturation at 94°C for 18 s, 50°C for 21 s, and 72°C for 1.30 min. Amplification products from the second round of PCR were sequenced directly in both the forward and reverse direction using BigDye 3.0 (ABI, CA) and sequenced by GenePool, University of Edinburgh. Sequences were aligned and assembled using Simmonics v1.7 (http://www2.warwick.ac.uk/fac/sci/bio/research/devans/bioinformatics/simmonics/) and SeqMan™ II software from DNASTAR, Inc. Complete coding sequences of CU47TH, CU54TH, CU1557UK and CU2139UK have been submitted to GenBank and have been assigned the accession numbers GU048662-65. Sequences were aligned with reference and other published sequences of HBoV1-HBoV3 using ClustalX v1.8 [38]. Similarity/divergence was determined using MegAlign software from DNASTAR, Inc.
Table 1.
Primers for amplification of the complete HBoV coding sequence
| Primer name | Direction | Primer sequences (5–3′) | Position* |
|---|---|---|---|
| HBoV-1S | Sense | GCCGGCAGACATATTGGATTCCAA | 1–24 |
| HBoV_280AS | For sequence only | ACATAAGTRAAAGCAGGTTGAGAAAAA | 308–282 |
| HBoV_1306IAS | Inner anti-sense | RTGCATGCCVARSACYTGTTC | 1362–1306 |
| HBoV_1515OAS | Outer anti-sense | GTTTTRCCTGTTGARGCAGGVCCRTAA | 1541–1515 |
| HBoV_1098S | Sense | GGAACAWCTKCCTGAGGTAG | 1101–1120 |
| HBoV_2729IAS | Inner anti-sense | GARTGCCAGTARAACCCACACC | 2750–2729 |
| HBoV_2762OAS | Outer anti-sense | CATTAAAGATWSAATTAGTVCCATCTCTAG | 2791–2762 |
| HBoV_2621S | Sense | ACCAAGYGAYGAAGACGARGG | 2621–2641 |
| HBoV_3770IAS | Inner anti-sense | TTGTDARRYGCTGCCARTC | 3788–3770 |
| HBoV_3801OAS | Outer anti-sense | GCATTKYTYKAGGYYTRAAGC | 3821–3801 |
| HBoV_3685S | Sense | CSMARGWGGAAAATYMCAGCG | 3685–3705 |
| HBoV_4826IAS | Inner anti-sense | GTAKATGTTTAGRTATGAGTCTGCRTT | 4852–4826 |
| HBoV_4871OAS | Outer anti-sense | TCWACYTCCCAKACAATYTCRCA | 4893–4871 |
| HBoV_4726S | Sense | AMACACAATMATKGATCCWTTYGATG | 4726–4751 |
| HBoV_5188IAS | Inner anti-sense | CTAGGTTCGAGACGGYAACACC | 5209–5188 |
| HBoV_5221OAS | Outer anti-sense | CAGCTCCYCCCACAATGYACA | 5241–5221 |
* Reference position from GenBank database accession number NC_00745
The three HBoV2 sequences obtained in this study yielded nucleotide similarity values of 92.0–92.3% when compared to the prototype HBoV2 sequence NC_012042, and 94.7–95.2% when compared to the PK-2255 variant [30]. The HBoV3 sequence CU2139 showed 99.5% similarity to the prototype NC_012564 sequences from Australia [31].
Sliding window analysis and phylogenetic analysis were used to determine sequence relationships across the genome. For sliding window analysis, a window size of 300 nucleotides, incrementing by 30 nucleotides (Fig. 2), was used in the program Sequence Distance within Simmonic 2005 v1.7 sequence editor. For phylogenetic analysis, trees were constructed by neighbor-joining (NJ), implemented in MEGA 4.0 [39], using 1,000 bootstrap-replicated datasets to determine the robustness of the tree topology. Separate trees were generated using nucleotide and inferred amino acid sequences (Fig. 1).
Fig. 2.
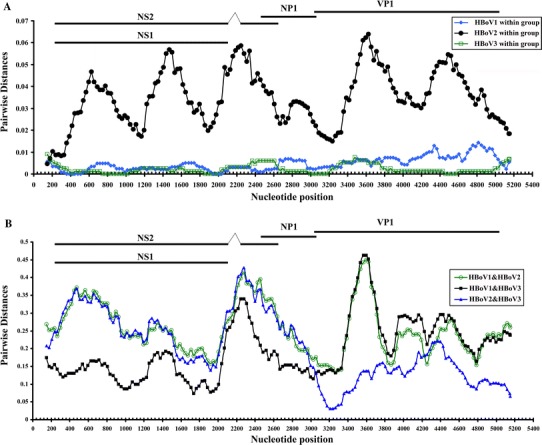
Sliding windows analysis of intra- and inter-species diversity of HBoV. The data show mean pairwise nucleotide sequence divergence within HBoV species (a) and between species (b)
Fig. 1.
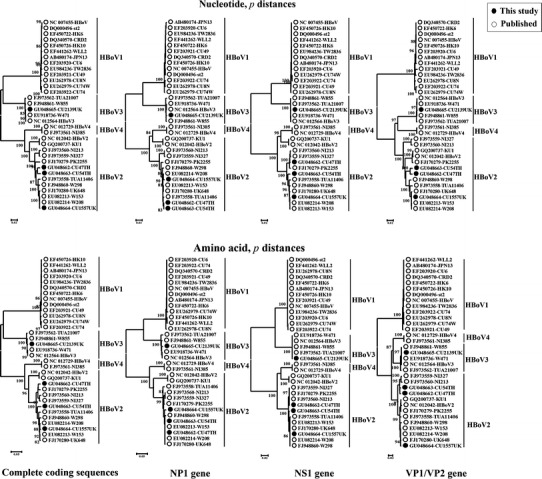
Phylogenic analysis of human bocaviruses (HBoVs) using complete coding sequences and each of the three open reading frames. Bootstrap values ≥80% are shown at each branch. This analysis incorporates currently published sequences of HBoV4 (NC_012279 and FJ973561) [42]
Of the three HBoV types, complete genome sequences of HBoV1 and HBoV3 from a wide range of geographical locations showed consistently low levels of intra-type variability (Table 2; >99.5% nucleotide sequence identity in all three genes for HBoV3, 99.0–100.0% for HBoV1; Fig. 2, blue and green plots). This contrasts with a mean identity of only 94.1% between HBoV2 variants collected over a geographical range similar to that of HBoV1 and HBoV3. By phylogenetic analysis, CU47TH and CU54TH from Thailand group most closely with PK2255 (FJ170279) from Pakistan, while CU1557UK from the UK was most similar to the other UK strain UK648 (FJ170280) (Fig. 1). Within-species variability was unevenly distributed across the genome. As described previously, variability within HBoV1 was greatest in VP1 [40] and lowest in NS1 and NP1, while both structural a non-strucural genes of the 5 HBoV3 sequences varied minimally throughout. Further sequences from HBoV3 are required to further substantiate this observation.
Table 2.
Nucleotide and amino acid sequences comparison of members of different HBoV species
| Comparison, gene | Comparison no. | Mean identity (range) | |
|---|---|---|---|
| Nucleotide | Amino acid | ||
| Within HBoV1 | |||
| Nearly complete genome | 15 | 99.5% (99.0–100.0%) | ND |
| NS1 | 15 | 99.6% (99.5–100.0%) | 99.9% (99.8–100.0%) |
| NP1 | 15 | 99.6% (99.1–100.0%) | 99.6% (99.1–100.0%) |
| VP1/VP2 | 15 | 99.3% (98.5–100.0%) | 99.6% (99.1–100.0%) |
| Within HBoV2 | |||
| Nearly complete genome | 13 | 94.1% (88.3–99.9%) | ND |
| NS1 | 13 | 96.2% (92.5–99.9%) | 96.9% (93.8–100.0%) |
| NP1 | 13 | 95.5% (91.1–100.0%) | 94.1% (88.1–100.0%) |
| VP1/VP2 | 13 | 97.1% (94.1–100.0%) | 98.2% (96.4–100.0%) |
| Within HBoV3 | |||
| Nearly complete genome | 5 | 99.6% (99.2–100.0%) | ND |
| NS1 | 5 | 99.9% (99.8–100.0%) | 100.0% (100.0–100.0%) |
| NP1 | 5 | 99.8% (99.6–100.0%) | 99.3% (98.6–100.0%) |
| VP1/VP2 | 5 | 99.8% (99.5–100.0%) | 99.7% (99.3–100.0%) |
| Between HBoV1 and HBoV2 | |||
| Nearly complete genome | 28 | 74.1% (71.9–76.2%) | ND |
| NS1 | 28 | 74.2% (73.8–74.6%) | 77.7% (77.2–78.1%) |
| NP1 | 28 | 75.4% (75.0–75.7%) | 67.6% (66.7–68.5%) |
| VP1/VP2 | 28 | 78.1% (77.7–78.5%) | 79.8% (79.2–80.3%) |
| Between HBoV1 and HBoV3 | |||
| Complete coding sequences | 20 | 79.9% (78.1–81.6%) | ND |
| NS1 | 20 | 87.9% (86.9–88.9%) | 91.15% (91.1–91.2%) |
| NP1 | 20 | 85.9% (85.6–86.1%) | 82.9% (82.2–83.6%) |
| VP1/VP2 | 20 | 77.8% (76.9–78.7%) | 80.0% (79.8–80.1%) |
| Between HBoV2 and HBoV3 | |||
| Nearly complete genome | 18 | 78.8% (76.9–80.6%) | ND |
| NS1 | 18 | 74.3% (74.0–74.6%) | 76.6% (76.2–77.0%) |
| NP1 | 18 | 75.7% (74.9–76.4%) | 68.2% (67.6–68.5%) |
| VP1/VP2 | 18 | 87.9% (86.9–88.8%) | 91.0% (90.8–91.2%) |
Translated NS1 and NP1 sequences of HBoV2 and HBoV3 from this study showed some amino acid differences when compared to the sequence of the prototype of each species. Because the functions of the HBoV proteins have not been studied, the effect of these amino acid substitutions is unknown. However, critical sites in the VP1-encoded phospholipase A2 (PLA2), which hydrolyzes phospholipids into free fatty acids and lysophospholipids [41] and varies between HBoV types, have been identified in pavoviruses and also studied in HBoV. All HBoV2 and HBoV3 sequences show conservation of four critical sites (P21, H41, D42 and E63) in the VP1 gene.
Sliding window analysis of members of the three species of HBoV showed that NS1 and NP1 genes of HBoV3 were more closely related to those of HBoV than to those of HBoV2, whereas they were more similar to HBoV2 in the VP1/VP2 gene (Fig. 2b). These findings matched those of phylogenetic analysis, where the clustering of HBoV3 with HBoV1 in the non-structural gene was replaced by clustering with HBoV2 sequences in the S region. These findings are consistent with the previous hypothesis of a recombination event in the evolutionary histoy of HBoV3 [31]. In their and our own analysis, the recombination break point was located precisely at the beginning of the VP1 gene [31], but we found no evidence for the second proposed recombination breakpoint at the start of NP1.
In conclusion, combining the three complete HBoV2 and one HBoV3 coding sequences generated in the current study with the other published sequences has revealed marked differences in intra-type diversity and provides further evidence for a recombinant origin of HBoV3. Further work is required to develop effective screening and type identification methods for the HBoV variants and to compare their prevalences and potential disease associations. In particular, whether HBoV3 shows an exclusively or predominantly enteric site of replication similar to that of HBoV2 [35] is important to resolve, as is determining the genome regions responsible for these observed differences in tropism and, potentially, for their different pathogenicities.
Acknowledgments
We would like to express our gratitude for the supported from Postdoctoral Fellowship of Ratchadaphiseksomphot Endowment Fund, Graduate School, Chulalongkorn University; Royal Golden Jubilee PhD program, the Thailand Research Fund; Chulalongkorn University Centenary Academic Development Project; the higher commission on Higher Education, Ministry of Education; Center of Excellence in Clinical Virology, Chulalongkorn University; and King Chulalongkorn Memorial Hospital, Thailand. We also would like to thank Gillian Fewster and the staff at the Microbiology Laboratory, Western General Hospital, Edinburgh, for providing faecal surveillance samples in Edinburgh, UK.
References
- 1.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Hoek L, Pyrc K, Jebbink MF, Vermeulen-Oost W, Berkhout RJ, Wolthers KC, Wertheim-van Dillen PM, Kaandorp J, Spaargaren J, Berkhout B. Identification of a new human coronavirus. Nat Med. 2004;10:368–373. doi: 10.1038/nm1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lau SK, Woo PC, Yip CC, Tse H, Tsoi HW, Cheng VC, Lee P, Tang BS, Cheung CH, Lee RA, So LY, Lau YL, Chan KH, Yuen KY. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol. 2006;44:2063–2071. doi: 10.1128/JCM.02614-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allander T, Andreasson K, Gupta S, Bjerkner A, Bogdanovic G, Persson MA, Dalianis T, Ramqvist T, Andersson B. Identification of a third human polyomavirus. J Virol. 2007;81:4130–4136. doi: 10.1128/JVI.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaynor AM, Nissen MD, Whiley DM, Mackay IM, Lambert SB, Wu G, Brennan DC, Storch GA, Sloots TP, Wang D. Identification of a novel polyomavirus from patients with acute respiratory tract infections. PLoS Pathog. 2007;4:e64. doi: 10.1371/journal.ppat.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalianis T, Ramqvist T, Andreasson K, Kean JM, Garcea RL. KI, WU and Merkel cell polyomaviruses: a new era for human polyomavirus research. Semin Cancer Biol. 2009;19:270–275. doi: 10.1016/j.semcancer.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Schildgen O, Müller A, Allander T, Mackay IM, Völz S, Kupfer B, Simon A. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin Microbiol Rev. 2008;21:291–304. doi: 10.1128/CMR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schildgen O. Human bocavirus: increasing evidence for virulence. Pediatr Pulmonol. 2010;45:118–119. doi: 10.1002/ppul.21159. [DOI] [PubMed] [Google Scholar]
- 10.Bastien N, Brand K, Dust K, Ward D, Li Y. Human Bocavirus infection, Canada. Emerg Infect Dis. 2006;12:848–850. doi: 10.3201/eid1205.051424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi EH, Lee HJ, Kim SJ, Eun BW, Kim NH, Lee JA, Lee JH, Song EK, Kim SH, Park JY, Sung JY. The association of newly identified respiratory viruses with lower respiratory tract infections in Korean children, 2000–2005. Clin Infect Dis. 2006;43:585–592. doi: 10.1086/506350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manning A, Russell V, Eastick K, Leadbetter GH, Hallam N, Templeton K, Simmonds P. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J Infect Dis. 2006;194:1283–1290. doi: 10.1086/508219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplan NM, Dove W, Abu-Zeid AF, Shamoon HE, Abd-Eldayem SA, Hart CA. Human bocavirus infection among children, Jordan. Emerg Infect Dis. 2006;12:1418–1420. doi: 10.3201/eid1209.060417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weissbrich B, Neske F, Schubert J, Tollmann F, Blath K, Blessing K, Kreth HW. Frequent detection of bocavirus DNA in German children with respiratory tract infections. BMC Infect Dis. 2006;6:109. doi: 10.1186/1471-2334-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allander T, Jartti T, Gupta S, Niesters HG, Lehtinen P, Osterback R, Vuorinen T, Waris M, Bjerkner A, Tiveljung-Lindell A, van den Hoogen BG, Hyypiä T, Ruuskanen O. Human bocavirus and acute wheezing in children. Clin Infect Dis. 2007;44:904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung JY, Han TH, Kim SW, Kim CK, Hwang ES. Detection of viruses identified recently in children with acute wheezing. J Med Virol. 2007;79:1238–1243. doi: 10.1002/jmv.20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fry AM, Lu X, Chittaganpitch M, Peret T, Fischer J, Dowell SF, Anderson LJ, Erdman D, Olsen SJ. Human bocavirus: a novel parvovirus epidemiologically associated with pneumonia requiring hospitalization in Thailand. J Infect Dis. 2007;195:1038–1045. doi: 10.1086/512163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau SK, Yip CC, Que TL, Lee RA, Au-Yeung RK, Zhou B, So LY, Lau YL, Chan KH, Woo PC, Yuen KY. Clinical and molecular epidemiology of human bocavirus in respiratory and fecal samples from children in Hong Kong. J Infect Dis. 2007;196:986–993. doi: 10.1086/521310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chieochansin T, Samransamruajkit R, Chutinimitkul S, Payungporn S, Hiranras T, Theamboonlers A, Poovorawan Y. Human bocavirus (HBoV) in Thailand: clinical manifestations in a hospitalized pediatric patient and molecular virus characterization. J Infect. 2008;56:137–142. doi: 10.1016/j.jinf.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacques J, Moret H, Renois F, Lévêque N, Motte J, Andréoletti L. Human Bocavirus quantitative DNA detection in French children hospitalized for acute bronchiolitis. J Clin Virol. 2008;43:142–147. doi: 10.1016/j.jcv.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villa L, Melón S, Suárez S, Alvarez-Argüelles ME, Gónzalez D, Morilla A, Boga JA, Rodríguez J, de Oña M. Detection of human bocavirus in Asturias, Northern Spain. Eur J Clin Microbiol Infect Dis. 2008;27:237–239. doi: 10.1007/s10096-007-0419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albuquerque MC, Rocha LN, Benati FJ, Soares CC, Maranhão AG, Ramírez ML, Erdman D, Santos N. Human bocavirus infection in children with gastroenteritis, Brazil. Emerg Infect Dis. 2007;13:1756–1758. doi: 10.3201/eid1311060671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JI, Chung JY, Han TH, Song MO, Hwang ES. Detection of human bocavirus in children hospitalized because of acute gastroenteritis. Infect Dis. 2007;196:994–997. doi: 10.1086/521366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vicente D, Cilla G, Montes M, Pérez-Yarza EG, Pérez-Trallero E. Human bocavirus, a respiratory and enteric virus. Emerg Infect Dis. 2007;13:636–637. doi: 10.3201/eid1304.061501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campe H, Hartberger C, Sing A. Role of Human Bocavirus infections in outbreaks of gastroenteritis. J Clin Virol. 2008;43:340–342. doi: 10.1016/j.jcv.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Cheng WX, Jin Y, Duan ZJ, Xu ZQ, Qi HM, Zhang Q, Yu JM, Zhu L, Jin M, Liu N, Cui SX, Li HY, Fang ZY. Human bocavirus in children hospitalized for acute gastroenteritis: a case-control study. Clin Infect Dis. 2008;47:161–167. doi: 10.1086/589244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chieochansin T, Thongmee C, Vimolket L, Theamboonlers A, Poovorawan Y. Human bocavirus infection in children with acute gastroenteritis and healthy controls. Jpn J Infect Dis. 2008;61:479–481. [PubMed] [Google Scholar]
- 28.Yu JM, Li DD, Xu ZQ, Cheng WX, Zhang Q, Li HY, Cui SX, Miao-Jin, Yang SH, Fang ZY, Duan ZJ (2008) Human bocavirus infection in children hospitalized with acute gastroenteritis in China. J Clin Virol 42:280–285 [DOI] [PubMed]
- 29.Szomor KN, Kapusinszky B, Rigó Z, Kis Z, Rózsa M, Farkas A, Szilágyi A, Berencsi G, Takács M. Detection of human bocavirus from fecal samples of Hungarian children with acute gastroenteritis. Intervirology. 2009;52:17–21. doi: 10.1159/000210834. [DOI] [PubMed] [Google Scholar]
- 30.Kapoor A, Slikas E, Simmonds P, Chieochansin T, Naeem A, Shaukat S, Alam MM, Sharif S, Angez M, Zaidi S, Delwart E. A newly identified bocavirus species in human stool. J Infect Dis. 2009;199:196–200. doi: 10.1086/595831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arthur JL, Higgins GD, Davidson GP, Givney RC, Ratcliff RM. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog. 2009;5:e1000391. doi: 10.1371/journal.ppat.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chow BD, Esper FP. The human bocaviruses: a review and discussion of their role in infection. Clin Lab Med. 2009;29:695–713. doi: 10.1016/j.cll.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han TH, Kim CH, Park SH, Kim EJ, Chung JY, Hwang ES. Detection of human bocavirus 2 in children with acute gastroenteritis in South Korea. Arch Virol. 2009;154:1923–1927. doi: 10.1007/s00705-009-0533-3. [DOI] [PubMed] [Google Scholar]
- 34.Shan TL, Zhang W, Guo W, Cui L, Yuan CL, Dai XQ, Shen Q, Yang ZB, Zhu JG, Hua XG. The first detection of human bocavirus 2 infections in China. J Clin Virol. 2009;46:196–197. doi: 10.1016/j.jcv.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Chieochansin T, Kapoor A, Delwart E, Poovorawan Y, Simmonds P. Absence of detectable replication of human bocavirus species 2 in respiratory tract. Emerg Infect Dis. 2009;15:1503–1505. doi: 10.3201/eid1509.090394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han TH, Chung JY, Hwang ES. Human bocavirus 2 in children, South Korea. Emerg Infect Dis. 2009;15:1698–1700. doi: 10.3201/eid1510.090337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song JR, Jin Y, Xie ZP, Gao HC, Xiao NG, Chen WX, Xu ZQ, Yan KL, Zhao Y, Hou YD, Duan ZJ. Novel human bocavirus in children with acute respiratory tract infection. Emerg Infect Dis. 2010;16:324–327. doi: 10.3201/eid1602.090553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 40.Chieochansin T, Chutinimitkul S, Payungporn S, Hiranras T, Samransamruajkit R, Theamboolers A, Poovorawan Y. Complete coding sequences and phylogenetic analysis of Human Bocavirus (HBoV) Virus Res. 2007;129:54–57. doi: 10.1016/j.virusres.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Qu XW, Liu WP, Qi ZY, Duan ZJ, Zheng LS, Kuang ZZ, Zhang WJ, Hou YD. Phospholipase A2-like activity of human bocavirus VP1 unique region. Biochem Biophys Res Commun. 2008;365:158–163. doi: 10.1016/j.bbrc.2007.10.164. [DOI] [PubMed] [Google Scholar]
- 42.Kapoor A, Simmonds P, Slikas B, Li L, Bodhidatta L, Sethabutr O, Triki H, Bahri O, Oderinde B, Baba M, Bukbuk D, Besser J, Bartkus J, Delwart E. Bocaviruses are highly diverse, recombination prone, dispersed, and prevalent human enteric infections. J Infect Dis. 2010;201:1633–1643. doi: 10.1086/652416. [DOI] [PMC free article] [PubMed] [Google Scholar]


