Abstract
Emerging viruses represent a continuous threat to human health and to farmed animals, as evidenced on multiple occasions by outbreaks of influenza, henipavirus and SARS. Knowledge about the diversity of viromes present in reservoir species can lead to a better understanding of the origin of emerging pathogens. In this study, we extend the knowledge of astrovirus diversity in pigs by reporting the genetic characterization of an unknown astrovirus lineage. Phylogenetic analyses provided evidence that this porcine astrovirus lineage is unique and does not appear to share a recent common ancestor with any known mamastrovirus. The data reported in this study extend the number of porcine astrovirus lineages to a total of five, all of which most likely represent distinct species of different origins.
Keywords: ORF2 Gene, Complete Capsid, Mini Spin Column, Diarrheic Piglet, Capsid Code Region
The family Astroviridae consists of small (28–30 nm), non-enveloped, single-stranded positive-sense RNA viruses of approximately 7 kb in length. These viruses generally exhibit a distinctive five- or six-pointed star-shape appearance when viewed using electron microscopy. The AstV genome is arranged into three open reading frames (ORFs) designated ORF1a, ORF1b and ORF2. ORF1a and ORF1b are situated at the 5′ end of the genome and encode non-structural polyproteins, including a protease and an RNA-dependent RNA polymerase (RdRp). ORF2, situated at the 3′ end of the genome, encodes the structural capsid protein and is transcribed as a subgenomic mRNA [18, 19].
The family Astroviridae is separated into two genera; viruses of the genus Mamastrovirus infect mammals, and viruses of the genus Avastrovirus are found in avian hosts [19]. Mamastroviruses appear to have a broad host range, since they have been isolated from numerous host species, including humans, mink, sheep, pigs, rats, marine mammals, dogs, cheetahs, roe deer, cattle and bats [2, 4, 14, 19, 21, 24–26, 28]. The list of susceptible species is likely to continue growing as more species are investigated.
Astroviruses are generally associated with either mild or severe enteric disease symptoms such as diarrhea and vomiting in a number of mammalian species [18]. Human astrovirus (HuAstV) serotypes 1–8, which are the most extensively studied astroviruses, are known to be a common cause of diarrhea in young children, the elderly and the immunocompromised [1, 6, 11, 12]. In addition, a number of genetically distinct strains of HuAstV have recently been identified, characterized and proposed to represent novel AstV species based on genetic distance criteria [8, 15].
Porcine AstVs (PoAstV) were first detected by EM in the feces of a diarrheic piglet [3] and later isolated in culture [23]. Molecular characterization of the ORF2 gene from this isolate followed some years later [14]. In the last five years or so, different research groups have successfully used PCR approaches to investigate the presence and diversity of porcine astroviruses [13, 17, 20]. Collectively, these studies have unveiled an impressive genetic diversity among PoAstV strains. This diversity suggests different origins for the various PoAstV lineages and, presumably, a number of transpecies transmission events between susceptible mammalian hosts [14, 17]. In the present study, we report the identification and characterization of unforeseen and divergent PoAstV strains. Phylogenetic analysis of the 3′ end of this strain suggests that a novel and possibly fifth lineage of AstV exists in swine and heightens concerns about the role of pigs as a potential source of emerging astrovirus infections.
A total of 48 fecal samples originating from the individual cecal content of slaughtered adult pigs in Canada were collected in 2008–2009. Viral RNA was extracted as described previously and stored at −70°C until needed [17]. The general strategy employed for obtaining PoAstV sequences is summarized in Fig. 1. Briefly, primers panAV-F11 (forward), panAV-F12 (forward) and panAV-R1 (reverse) were originally designed by Chu and colleagues based on a conserved region situated in the RNA-dependent RNA polymerase (RdRp) gene of AstVs [5]. PCR conditions using these primers were as described by Luo and colleagues [17]. PCR products were analyzed on a Qiaxcel instrument (QIAGEN, Mississauga, Ontario, Canada). Amplicons of approximately 400 bp were then cloned using a TOPO 2.1 (T/A) cloning kit (Invitrogen, Mississauga, Ontario, Canada) and sequenced in both directions using the big dye v3.1 chemistry on a 3730xl instrument from Applied Biosystems (Foster City, CA). Based on the nucleotide sequences of the RdRp fragments from the four PoAstV 5 strains, specific forward primers were designed for 3′ RACE–PCR. Primers CC12FE (5′-ATCGCTATGTCCTGTTGCCTTCAG-3′) and CC12F1 (5′-TCTTAATGGTCCAGATGGCTGGGA-3′) were used in combination with primers (QT), (QO) and (QI) with PoAstV strain CC12 as described previously [17, 22]. Despite numerous attempts, we were unable to amplify the 3′end from the genomes of strains A5, D4 and D10, and hence, the 3′ RACE-PCR primer sequences are not shown. RACE-PCR products from strain CC12 were deposited on 1% agarose gels and stained with SYBR safe (Invitrogen), and fragments of approximately 3 kb were purified on mini spin columns (QIAGEN). Gel-purified amplicons were cloned using a PCR TOPO 2.1 (T/A) Cloning Kit (Invitrogen), and a minimum of three clones were selected and amplified in liquid broth, followed by plasmid DNA extraction using an alkaline lysis method (QIAprep Spin Miniprep, QIAGEN). Each clone was then completely sequenced in both directions using a primer walking strategy. Sequence editing, assembly and analysis were performed using BioEdit version 7.0.9.0 (http://www.mbio.ncsu.edu/bioedit/bioedit.html). Multiple sequence alignments were constructed with CLUSTAL W (version 1.6). Phylogenetic analysis was conducted using the neighbor-joining (NJ) method with p-distances for nucleotides and a Poisson distance correction calculation for amino acids using the Molecular Evolutionary Genetic analysis (MEGA 4.0) software with default settings, except that all missing data or gaps were completely ignored. Confidence values at the nodes were obtained by performing 1,000 bootstrap analyses.
Fig. 1.
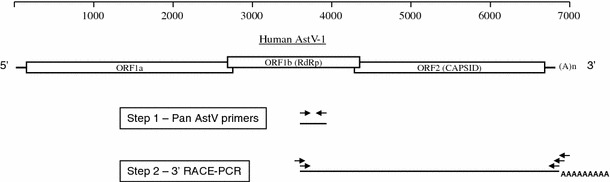
Schematic representation of the strategy used to PCR amplify PoAstV sequences. The upper panel represents the complete human AstV-1 genome. The 3 ORFs encoding the AstV proteins are boxed; the 5′ UTR, 3′ UTR and polyA tail are also shown. The two sequential steps used in the detection and amplification of the AstV sequence are shown in the lower part of the figure. The position and orientation of the primers used are indicated by arrows
We have previously reported the detection and characterization in Canadian pigs of novel and previously unknown AstVs belongs to the genus Mamastrovirus using a “broad-range” PCR strategy [17]. We hence applied the same strategy to screen a collection of 48 fecal samples from pigs slaughtered in 2008–2009. A total of 39 samples generated amplicons of the expected size, which were cloned and sequenced. Putative amino acid sequences from all strains revealed the presence of the characteristic “YGDD” motif located near the C-terminal region of the RdRp (not shown). This motif has been reported for all astrovirus polymerases characterized thus far. BLAST analysis revealed that most sequences were, in fact, closely related (>85% nucleotide identity) to known porcine AstV sequences previously characterized by Luo and colleagues [17]. However, a group of four related sequences revealed only approximately 70% nucleotide identity to bat and human AstV sequences in the database, suggesting that these sequences were divergent from known PoAstV strains and possibly novel. Pairwise identity comparisons of the partial RdRp nucleotide sequences revealed that the four strains were 80–90% identical to each other. Phylogenetic analysis of these sequences, in addition to prototypical animal AstV strains, confirmed their relatedness and grouped them in a unique lineage on a divergent branch distantly related to other known mamastroviruses (PoAstV 5 in Fig. 2a).
Fig. 2.
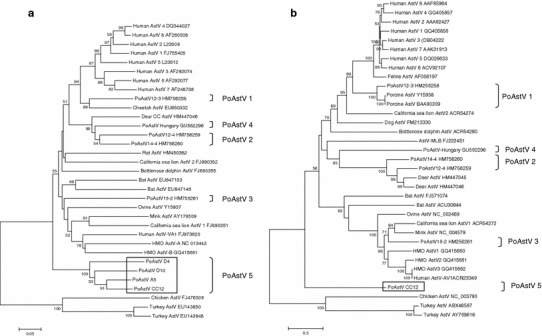
Phylogenetic analysis of astroviruses. a Phylogenetic tree based on a partial RdRp coding region (about 300 nt) using the neighbor–joining method with p-distances and 1000 bootstrap replicates; values >50% are shown. b Tree based on the complete ORF2 coding region of strain CC12 (about 800 aa). Selected representative astroviruses from the genera Mamastrovirus and Avastrovirus are depicted with their corresponding GenBank accession numbers. Novel PoAstV 5 strains characterized in the present study are boxed
To permit more in-depth genomic investigation and strengthen their taxonomic grouping, we performed 3′ RACE-PCR on these four PoAstV 5 samples. We were able to amplify and characterize a 3029 nt-long sequence from strain PoAstV CC12 only. It is unclear why 3′ RACE-PCR failed with the other samples. The amplified region from strain PoAstV CC12 included the 3′ end of the RdRp gene, the complete capsid gene, and the 3′ UTR (Fig. 1). The conserved motif situated at the junction between ORF1b and ORF2, which is thought to represent a regulatory element serving as a promoter for subgenomic RNA transcription, was present in strain PoAstV CC12: TTTGGGGGGGAGGACCAAAAAGAGACG ATG GC (following the convention of HuAstV, which places the initiation ATG codon, underlined, for ORF2 immediately upstream of the ORF1b stop codon) [18]. The ORF2 start codon of strain CC12, underlined, appeared in an optimal Kozak context for translation initiation (RNNAUGG, where R = A/G and N = A/T/G/C) [16]. The ORF2 gene is predicted to encode a capsid protein 735 amino acids long, which is comparable to most AstVs. The length of the 3′UTR is 101 nucleotides. The highly conserved stem-loop-II-like motif (s2 m) present in the 3′UTRs of most mamastroviruses is also present in strain PoAstV CC12 (not shown). This motif has been suggested to have an important function in viral RNA replication [14].
Phylogenetic analysis of the complete capsid coding region of PoAstV CC12 confirms that this strain forms a distinct lineage in the family Mamastroviridae, including previously identified porcine astroviruses from different continents (Fig. 2b). In addition, the tree topology revealed by our analysis is largely in agreement with previous studies [7–10, 15, 17, 21]. Pairwise amino acid comparisons reveal that strain PoAstV CC12 shares only between 25% and 30% aa identity in the complete ORF2 with prototypical AstV strains. Since phylogenetic analysis placed the CC12 strain in a unique lineage, we named this strain PoAstV 5, as there now appear to be five distinct lineages of AstVs in swine [13, 14, 17, 20], and this name maintains the continuity in the nomenclature of PoAstV strains. The genetic distance between PoAstV CC12 and the other strains is comparable to distances between members of established AstV species, suggesting that CC12 possibly represents a new, fifth PoAstV species.
Until recently, a relatively small number of astroviruses from very few hosts were known [18]. However, in the last few years, thanks in part to metagenomic analyses and broad-range PCR, a number of studies have revealed a wide array of divergent AstV strains in a growing number of mammalian species [4, 14, 15, 21, 24, 26–28]. The present work extends current knowledge about these agents and underscores the vast diversity and divergence of astrovirus strains harbored by swine [13, 17, 20]. PoAstVs now appear in five distinct lineages of the AstV evolutionary tree, which suggests different ancestral origins and numerous interspecies transmissions involving many mammalian species, including humans. As additional mammalian species are screened for the presence of AstVs, a clearer picture of the historical transmission path used by these viruses between different hosts might emerge and shed new light on the zoonotic potential of these viruses.
Emerging pathogens represent a constant threat to human health. Since the majority of these pathogens arise from animal reservoirs, a more thorough knowledge about the presence and diversity of viruses in animal species such as pigs could lead to a better characterization of the risk posed by such agents. In addition, a better understanding and appreciation of the virome present in wild and domestic animals could lead to early identification of the source of an emerging outbreak and therefore to faster and more targeted interventions to control and limit the spread of a disease. The discovery of novel PoAstV strains described here provides an example of how diverse these viruses are in this domestic reservoir species.
Acknowledgment
This work was supported by the Science Division of the Canadian Food Inspection Agency (CFIA).
Footnotes
The GenBank accession numbers for the sequences described in this study are JN088534–JN088537.
References
- 1.Akihara S, Phan TG, Nguyen TA, Hansman G, Okitsu S, Ushijima H. Existence of multiple outbreaks of viral gastroenteritis among infants in a day care center in Japan. Arch Virol. 2005;150:2061–2075. doi: 10.1007/s00705-005-0540-y. [DOI] [PubMed] [Google Scholar]
- 2.Atkins A, Wellehan JF, Jr, Childress AL, Archer LL, Fraser WA, Citino SB. Characterization of an outbreak of astroviral diarrhea in a group of cheetahs (Acinonyx jubatus) Vet Microbiol. 2009;136:160–165. doi: 10.1016/j.vetmic.2008.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridger JC. Detection by electron microscopy of caliciviruses, astroviruses and rotavirus-like particles in the faeces of piglets with diarrhoea. Vet Rec. 1980;107:532–533. [PubMed] [Google Scholar]
- 4.Chu DK, Chin AW, Smith GJ, Chan KH, Guan Y, Peiris JS, Poon LL. Detection of novel astroviruses in urban brown rats and previously known astroviruses in humans. J Gen Virol. 2010;91:2457–2462. doi: 10.1099/vir.0.022764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu DK, Poon LL, Guan Y, Peiris JS. Novel astroviruses in insectivorous bats. J Virol. 2008;82:9107–9114. doi: 10.1128/JVI.00857-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dennehy PH, Nelson SM, Spangenberger S, Noel JS, Monroe SS, Glass RI. A prospective case–control study of the role of astrovirus in acute diarrhea among hospitalized young children. J Infect Dis. 2001;184:10–15. doi: 10.1086/321007. [DOI] [PubMed] [Google Scholar]
- 7.Finkbeiner SR, Holtz LR, Jiang Y, Rajendran P, Franz CJ, Zhao G, Kang G, Wang D. Human stool contains a previously unrecognized diversity of novel astroviruses. Virol J. 2009;6:161. doi: 10.1186/1743-422X-6-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkbeiner SR, Kirkwood CD, Wang D. Complete genome sequence of a highly divergent astrovirus isolated from a child with acute diarrhea. Virol J. 2008;5:117. doi: 10.1186/1743-422X-5-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finkbeiner SR, Le BM, Holtz LR, Storch GA, Wang D. Detection of newly described astrovirus MLB1 in stool samples from children. Emerg Infect Dis. 2009;15:441–444. doi: 10.3201/eid1503.081213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkbeiner SR, Li Y, Ruone S, Conrardy C, Gregoricus N, Toney D, Virgin HW, Anderson LJ, Vinje J, Wang D, Tong S. Identification of a novel astrovirus (astrovirus VA1) associated with an outbreak of acute gastroenteritis. J Virol. 2009;83:10836–10839. doi: 10.1128/JVI.00998-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallimore CI, Taylor C, Gennery AR, Cant AJ, Galloway A, Iturriza–Gomara M, Gray JJ. Environmental monitoring for gastroenteric viruses in a pediatric primary immunodeficiency unit. J Clin Microbiol. 2006;44:395–399. doi: 10.1128/JCM.44.2.395-399.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray JJ, Wreghitt TG, Cubitt WD, Elliot PR. An outbreak of gastroenteritis in a home for the elderly associated with astrovirus type 1 and human calicivirus. J Med Virol. 1987;23:377–381. doi: 10.1002/jmv.1890230410. [DOI] [PubMed] [Google Scholar]
- 13.Indik S, Valicek L, Smid B, Dvorakova H, Rodak L. Isolation and partial characterization of a novel porcine astrovirus. Vet Microbiol. 2006;117:276–283. doi: 10.1016/j.vetmic.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Jonassen CM, Jonassen TO, Saif YM, Snodgrass DR, Ushijima H, Shimizu M, Grinde B. Comparison of capsid sequences from human and animal astroviruses. J Gen Virol. 2001;82:1061–1067. doi: 10.1099/0022-1317-82-5-1061. [DOI] [PubMed] [Google Scholar]
- 15.Kapoor A, Li L, Victoria J, Oderinde B, Mason C, Pandey P, Zaidi SZ, Delwart E. Multiple novel astrovirus species in human stool. J Gen Virol. 2009;90:2965–2972. doi: 10.1099/vir.0.014449-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991;266:19867–19870. [PubMed] [Google Scholar]
- 17.Luo Z, Roi S, Dastor M, Gallice E, Laurin MA, L’homme Y. Multiple novel and prevalent astroviruses in pigs. Vet Microbiol. 2011;149:316–323. doi: 10.1016/j.vetmic.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendez E, Arias CF. Astroviruses. In: Knipe DM, Howley PM, editors. Fields virology. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 981–1000. [Google Scholar]
- 19.Monroe SS (2005) Astroviridae. In: Carter MJ, Herrmann J, Mitchel JK Sanchez-Fauquier A (eds) Virus taxonomy. Eighth report of the International Committee on Taxonomy of Viruses.Elsevier, Amsterdam, pp 859–864
- 20.Reuter G, Pankovics P, Boros A. Identification of a novel astrovirus in a domestic pig in Hungary. Arch Virol. 2011;156:125–128. doi: 10.1007/s00705-010-0827-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera R, Nollens HH, Venn-Watson S, Gulland FM, Wellehan JF., Jr Characterization of phylogenetically diverse astroviruses of marine mammals. J Gen Virol. 2010;91:166–173. doi: 10.1099/vir.0.015222-0. [DOI] [PubMed] [Google Scholar]
- 22.Scotto–Lavino E, Du G, Frohman MA. 3′ end cDNA amplification using classic RACE. Nat Protoc. 2006;1:2742–2745. doi: 10.1038/nprot.2006.481. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu M, Shirai J, Narita M, Yamane T. Cytopathic astrovirus isolated from porcine acute gastroenteritis in an established cell line derived from porcine embryonic kidney. J Clin Microbiol. 1990;28:201–206. doi: 10.1128/jcm.28.2.201-206.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smits SL, Van LM, Kuiken T, Hammer AS, Simon JH, Osterhaus AD. Identification and characterization of deer astroviruses. J Gen Virol. 2010;91:2719–2722. doi: 10.1099/vir.0.024067-0. [DOI] [PubMed] [Google Scholar]
- 25.Toffan A, Jonassen CM, De BC, Schiavon E, Kofstad T, Capua I, Cattoli G. Genetic characterization of a new astrovirus detected in dogs suffering from diarrhoea. Vet Microbiol. 2009;139:147–152. doi: 10.1016/j.vetmic.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tse H, Chan WM, Tsoi HW, Fan RY, Lau CC, Lau SK, Woo PC, Yuen KY. Re-discovery and genomic characterization of bovine astroviruses. J Gen Virol. 2011;92(Pt 8):1888–1898. doi: 10.1099/vir.0.030817-0. [DOI] [PubMed] [Google Scholar]
- 27.Zhu AL, Zhao W, Yin H, Shan TL, Zhu CX, Yang X, Hua XG, Cui L. Isolation and characterization of canine astrovirus in China. Arch Virol. 2011;156(9):1671–1675. doi: 10.1007/s00705-011-1022-z. [DOI] [PubMed] [Google Scholar]
- 28.Zhu HC, Chu DK, Liu W, Dong BQ, Zhang SY, Zhang JX, Li LF, Vijaykrishna D, Smith GJ, Chen HL, Poon LL, Peiris JS, Guan Y. Detection of diverse astroviruses from bats in China. J Gen Virol. 2009;90:883–887. doi: 10.1099/vir.0.007732-0. [DOI] [PubMed] [Google Scholar]


