Abstract
Acute respiratory tract infections (ARTIs) due to various viruses are not only the most common causes of upper and lower respiratory infection but are also major causes of morbidity and mortality in children. In this study, we investigated the prevalence and clinical characteristics of children with virus-related ARTIs and determined the spectrum of respiratory viruses and their correlation with meteorological variables in Jiading District, Shanghai, China. Nasopharyngeal swabs from 2819 children with ARTIs were collected from August 2011 to December 2014, and used for detection of respiratory viruses by multiplex RT-PCR. Seventeen respiratory viruses were detected among 691 (24.5 %) of 2819 patients. The highest prevalence of respiratory viruses was detected in the age group of less than 1 year (29.0 %), and the prevalence decreased with age. This suggests that children less than one year old are the most susceptible to infection. Influenza virus (IFV) was the most frequently detected virus (5.8 %), followed by parainfluenza virus (PIV) (5.7 %), enterovirus (EV) (4.3 %), and respiratory syncytial virus (RSV) (3.6 %). Statistical analysis showed that epidemics of IFV, PIV and EV had distinct seasonal variations. Mean monthly temperature appeared to be the only meteorological factor associated with IFV and PIV infection. These findings will provide valuable information for decision-making, prevention and treatment of ARTIs in children.
Electronic supplementary material
The online version of this article (doi:10.1007/s00705-016-2866-z) contains supplementary material, which is available to authorized users.
Keywords: Respiratory Syncytial Virus, Meteorological Variable, Monthly Temperature, Acute Respiratory Tract Infection, Respiratory Virus
Introduction
Acute respiratory tract infections (ARTIs) are major risk factors associated with morbidity and mortality of infants and children worldwide and are caused by respiratory viruses and/or bacteria [1, 2]. The majority of ARTIs are ascribed to respiratory viruses; however, antibiotics were often used in the clinical treatment of ARTIs in some developing countries (e.g., China). Investigations of the prevalence of ARTIs and its correlation with viral pathogens are critical for improving the prevention and treatment of ARTIs.
There are more than 200 respiratory viruses that can cause ARTIs. Respiratory syncytial virus (RSV), human rhinovirus (HRV), human metapneumovirus (HMPV), human parainfluenza virus (PIV), human enterovirus (EV), influenza virus (IFV), human coronavirus (CoV), adenovirus (ADV), and human bocavirus (BoV) are the most common viral agents associated with ARTIs, accounting for around 70 % of ARTIs [3, 4]. As children with ARTIs often have similar clinical presentations, it is difficult for doctors to make decisions and prescriptions simply based on the physical symptoms. Therefore, investigating the clinical characteristics of children with virus-related ARTIs and the spectrum of respiratory viruses will facilitate the development of precise treatments for ARTIs.
The prevalence of respiratory viruses among children with ARTIs differs in different regions, and varies over time [5–12]. It has been relatively difficult to provide real-time surveillance of ARTIs in China because of its large population, vast territory and multivariate climatic factors. In several previous studies, the prevalence of respiratory viruses with ARTIs in Shanghai [6, 7], Wuhan [5], Harbin [8], Lanzhou [9, 10], Nanjing [13], Zhuhai [12] and Shantou [11] were investigated. The positive rates of respiratory viruses among patients with ARTIs in these cities were from 37.6 % to 78.7 %. RSV, HRV, and IFV-A were the most common respiratory viruses.
In this study, we report the clinical characteristics of respiratory viral infections in children in Shanghai, China, from August 2011 to December 2014, and investigated the etiologic agents. We found that the respiratory virus positive rate was the highest in children less than 1 year old, and the positive rate decreased with age without gender difference. Furthermore, IFV, PIV, EV and RSV were most frequently observed in children with ARTIs and displayed seasonal epidemic patterns.
Materials and methods
Study population and sample collection
From August 2011 to December 2014, 2819 children with ARTIs who had been admitted to Shanghai Nanxiang Hospital, a district-level general hospital in Shanghai, China, were recruited in this study. The study was approved by the Medical Ethics Committee of Shanghai Nanxiang Hospital. Demographic information and clinical characteristics were recorded for each patient. Nasopharyngeal swabs were collected from these patients after obtaining informed consent from their parents or guardians, and the samples were transported to the laboratory at Institut Pasteur of Shanghai, Chinese Academy of Sciences, in virus transport medium (including Hank’s buffer, BSA, HEPES and antibiotics) within 24 hours for the detection of respiratory viruses.
RNA extraction and detection of respiratory viruses by multiplex RT-PCR
Viral RNA was extracted from each clinical sample using a QIAamp Viral RNA Mini Kit (QIAGEN, Germany). Respiratory viruses were detected by a multiplex RT-PCR assay as described previously [6, 14]. In brief, 17 viruses were detected in a five-tube mRT-PCR assay. Tube 1 targeted IFV-A, IFV-B, RSV, HMPV; tube 2, PIV1–4; tube 3, EV, HRV and IFV-C; tube 4, CoV-229E, CoV-OC43, CoV-NL63 and CoV-HKU1; and tube 5, ADV and BoV. For each tube, we selected one of the targeted viruses and used the culture supernatant of the standard strain of the selected virus as a positive control for RNA extraction. As a result, RSV, PIV-3, HRV, Co-229E and ADV were selected for tubes 1-5, respectively. The amplification reaction was performed using a QIAGEN OneStep RT-PCR Kit (QIAGEN, Germany). For quality control in the multiple RT-PCR, a mixture of plasmid templates was used as a positive control, and distilled water was used as a negative control. A 25-µL PCR mix including 5× QIAGEN OneStep RT-PCR Buffer, dNTP Mix (final concentration of each dNTP, 400 µM), primers F and R (final concentration, 0.6 µM), QIAGEN OneStep RT-PCR Enzyme Mix (1 µL), RNA template (2.5 µL), and RNase-free water was prepared. The reaction steps included reverse transcription at 50 °C for 30 min, initial PCR activation at 95 °C for 15 min, 40 cycles at 94 °C for 30 s, 55 °C (tube 1 and tube 2) or 50 °C (tube 3, tube 4 and tube 5) for 30 s, and 72 °C for 1 min, and a final extension at 72 °C for 10 min. To increases the amplification in tube 3 and tube 4, an additional eight cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 1 min were added after the initial PCR activation. The respiratory viruses were distinguished according to the product sizes in 2 % agarose gel electrophoresis.
Meteorological data collection
The data on mean monthly temperature (°C), rainfall (mm) and relative humidity (%) of Jiading district of Shanghai were obtained from the Jiading Weather Bureau of Shanghai. The meteorological station is located at 31° 22’ N, 121° 15’ E.
Statistical analysis
Statistical analysis was performed using SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA). Statistical comparisons of viral incidence between different groups (i.e., genders, ages and seasons) were performed using the chi-square (χ2) test. Bivariate correlation and multiple stepwise regression analysis were used to analyze the associations between viral infection and meteorological parameters. A probability (p) value less than 0.05 was considered statistically significant.
Results
Patient characteristics
From August 2011 to December 2014, nasopharyngeal swabs were collected from 2819 children with ARTIs at Shanghai Nanxiang Hospital. Their ages ranged from 2 months to 12 years. The majority of them (1189, 67.4 %) were between 1 and 6 years old. The ratio of boys to girls was 1.32 (Table 1). Among 2819 clinical samples from the recruited children with ARTIs, 691 (24.5 %) samples were found to be respiratory virus positive. There was no significant difference in the incidence of respiratory viral infection between boys (392/1603; 24.5 %) and girls (299/1216; 24.6 %) (χ2 = 0.007, p = 0.934). The respiratory virus positive rate was significantly different among the different age groups (χ2 = 10.967, p = 0.012). The positive rate appeared to decrease with age. Children less than one year old had the highest positive rate of 29.0 % (94/324). The positive rate decreased to 25.9 % (197/762) and 24.7 % (281/1137) for the age groups of 1-3 and 3-6 years old, respectively. The lowest positive rate was observed in the age group of more than 6 years old (119/596; 20.0 %) (Table 1). A statistically significant difference was observed between the age group of less than 1 year old and the age group of more than 6 years old (χ2 = 9.653, p = 0.002). No statistically significant difference was observed among the other age groups.
Table 1.
Patient characteristics of 2819 children with ARTIs in Shanghai Nanxiang Hospital from 2011 to 2014
| Characteristic | Number (%) | Number virus positive (%)a | Virus-positive rate in each groupb |
|---|---|---|---|
| Sex | |||
| Male | 1603 (56.9 %) | 392 (56.7 %) | 392/1603 (24.5 %) |
| Female | 1216 (43.1 %) | 299 (43.3 %) | 299/1216 (24.6 %) |
| Age group (year) | |||
| ≤1 | 324 (11.5 %) | 94 (13.6 %) | 94/324 (29.0 %) |
| 1-≤3 | 762 (27.0 %) | 197 (28.5 %) | 197/762 (25.9 %) |
| 3-≤6 | 1137 (40.3 %) | 281 (40.7 %) | 281/1137 (24.7 %) |
| >6 | 596 (21.1 %) | 119 (17.2 %) | 119/596 (20.0 %) |
| Total | 2819 | 691 |
aProportion of each group in virus-positive samples
bProportion of each group in all the samples
The clinical symptoms of virus-positive ARTIs children included cough, fever, sore throat, expectoration, runny rose, rales, diarrhea, rash, headache, muscular soreness, stomachache, dyspnea, and chest pain (Table 2). Of these, cough (89.9 %), fever (81.2 %), and sore throat (73.2 %) were the most commonly observed symptoms. These virus-positive children were often diagnosed with tonsillitis (37.2 %), bronchitis (34.3 %), upper respiratory tract infections (URTIs, 26.1 %) or pneumonia (15.5 %) (Table 2).
Table 2.
Clinical symptoms and diagnoses of virus-positive patients
| Clinical symptom | Number | Percentage (%) |
|---|---|---|
| Cough | 621 | 89.9 % |
| Fever | 564 | 81.2 % |
| Sore throat | 506 | 73.2 % |
| Running nose | 277 | 40.1 % |
| Expectoration | 300 | 43.4 % |
| Rales | 197 | 28.5 % |
| Diarrhea | 41 | 5.9 % |
| Rash | 36 | 5.2 % |
| Headache | 30 | 4.3 % |
| Muscular soreness | 28 | 4.1 % |
| Stomachache | 17 | 2.5 % |
| Dyspnea | 16 | 2.3 % |
| Chest pain | 11 | 1.6 % |
| Diagnosis | ||
| Tonsillitis | 257 | 37.2 % |
| Bronchitis | 237 | 34.3 % |
| Upper respiratory tract infections | 180 | 26.1 % |
| Pneumonia | 107 | 15.5 % |
| Total | 691 |
Viral etiology
Seventeen respiratory viruses, including IFV (IFV-A/B/C), PIV 1-4, EV, RSV, CoV (229E, HKU1, OC43, NL63), ADV, HMPV, BoV, and HRV were detected among 691 of 2819 children with ARTIs in Nanxiang District of Shanghai (Table 3). Multiple viral infections were detected in 60 (2.1 %) patients (46 with two pathogens and 14 with three pathogens). Influenza viruses, including IFV-A, IFV-B and IFV-C, were the most frequently detected viruses (163/2819, 5.8 %), followed by PIV (161/2819, 5.7 %), EV (120/2819, 4.3 %), and RSV (100/2819, 3.6 %). The detection rate of CoV, ADV, HMPV, BoV and HRV was 2.3 %, 1.5 %, 1.5 %, 1.2 %, and 0.6 %, respectively.
Table 3.
Viral etiologies for all participants (N = 2819)
| Virus detected | Number | Percentage (%) | |
|---|---|---|---|
| IFV | 163 | 5.9 % | |
| IFV-A | 87 | 3.1 % | |
| IFV-B | 78 | 2.8 % | |
| IFV-C | 3 | 0.1 % | |
| PIV | 161 | 5.7 % | |
| PIV-1 | 62 | 3.1 % | |
| PIV-2 | 25 | 1.2 % | |
| PIV-3 | 52 | 1.8 % | |
| PIV-4 | 24 | 0.9 % | |
| EV | 120 | 4.3 % | |
| RSV | 100 | 3.6 % | |
| CoV | 65 | 2.3 % | |
| HMPV | 49 | 1.5 % | |
| ADV | 41 | 1.5 % | |
| BoV | 36 | 1.2 % | |
| HRV | 20 | 0.6 % | |
| Co-infection | 60 | 2.1 % | |
| Total | 691 |
Among these respiratory viruses, most had a similar distribution among the different age groups (Fig.1). Interestingly, the prevalence of IFV appeared to increase with age, while the prevalence of PIV appeared to decrease with age.
Fig. 1.
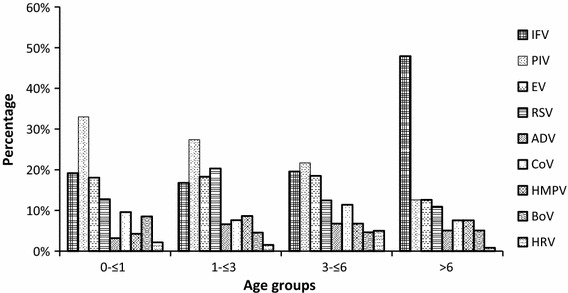
The distribution of respiratory viruses in different age groups
Seasonal distribution of respiratory viruses
We further investigated the seasonal distribution of the respiratory viruses (Table 4). Of the analyzed viruses, IFV, PIV and EV appeared to have seasonal patterns (p < 0.05) (Table 4 and Supplemental Figure 1). For IFV, the highest prevalence was observed in winter (72/607, 11.9 %), while the lowest prevalence occurred in summer (22/797, 2.8 %). Distinct from IFV, the highest prevalence of PIV occurred in summer (76/797, 9.5 %), and it was only 1.7 % (10/607) in winter. The prevalence of EV was similar in spring (38/668, 5.7 %), summer (36/797, 4.5 %), and autumn (32/747, 4.3 %), substantially higher than that in winter (15/607, 2.5 %) (Table 4). RSV and CoV were relatively active throughout the whole year and have similar prevalence in different seasons (Table 4 and Supplemental Figure 1). ADV, HMPV, BoV, and HRV were excluded from the analysis because their sample sizes were too small.
Table 4.
Seasonal distribution of respiratory viruses
| IFV | PIV | EV | RSV | CoV | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| + | - | + | - | + | - | + | - | + | - | |
| Spring | 34 | 634 | 49 | 619 | 38 | 630 | 19 | 649 | 14 | 654 |
| Summer | 22 | 775 | 76 | 721 | 36 | 761 | 23 | 774 | 21 | 776 |
| Autumn | 35 | 712 | 25 | 722 | 32 | 715 | 26 | 721 | 7 | 740 |
| Winter | 72 | 535 | 10 | 597 | 15 | 592 | 30 | 577 | 17 | 590 |
| χ2 | 56.776 | 51.585 | 8.169 | 5.511 | 7.496 | |||||
| p | <0.001* | <0.001* | 0.043* | 0.138 | 0.058 | |||||
+, number of positive samples; -, number of negative samples
The chi-square (χ2) test was performed using SPSS. A P-value < 0.05 is indicated by * and is considered statistically significant (in bold)
Correlation of the prevalence of various respiratory viruses with meteorological variables
The seasonal patterns of IFV, PIV and EV prevalence (Table 4 and Supplemental Figure 1) suggest a correlation between the respiratory virus epidemic and meteorological variables. We performed regression analysis to determine whether meteorological factors (including mean monthly temperature, humidity, and rainfall) were associated with prevalence of various respiratory viruses. The Nanxiang district of Shanghai has a mean monthly temperature of 17.5 ± 9.2 °C, humidity of 69.7 ± 6.2 %, and rainfall of 90.8 ± 79.1 mm. Bivariate correlation showed that the incidence of influenza virus infection was significantly negatively correlated with mean monthly temperature (Spearman’s rho: -0.472, p = 0.002), but it was not correlated with mean monthly humidity (Spearman’s rho: -0.103, p = 0.521) or mean monthly relative rainfall (Spearman’s rho: -0.223, p = 0.162) (Table 5). When multiple stepwise regression analysis was performed, mean monthly temperature appeared to be the only factor associated with influenza virus infection. In contrast, the incidence of PIV infection was significantly positively correlated with mean monthly temperature (Spearman’s rho: 0.545, p < 0.001) and mean monthly rainfall (Spearman’s rho: 0.415, p = 0.007). Multiple stepwise regression analysis showed that mean monthly temperature was the only factor associated with PIV infection. No significant correlation was observed between the prevalence of RSV, EV, and CoV and meteorological variables (Table 5).
Table 5.
Correlations between viral incidence and meteorological parameters
| Meteorological parameter | IFV | PIV | EV | RSV | CoV | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | r-value | p-value | |
| Mean monthly temperature | -0.472 | 0.002* | 0.545 | <0.001* | 0.14 | 0.382 | -0.205 | 0.198 | -0.106 | 0.51 |
| Mean monthly humidity | -0.103 | 0.521 | 0.134 | 0.402 | -0.055 | 0.733 | -0.054 | 0.737 | 0.066 | 0.681 |
| Mean monthly rainfall | -0.223 | 0.162 | 0.415 | 0.007* | 0.022 | 0.891 | -0.242 | 0.127 | 0.047 | 0.769 |
Bivariate correlation analysis was performed using SPSS. A probability (p) value < 0.05 is indicated by * and is considered statistically significant (in bold)
Discussion
Infections with respiratory viruses are the most common causes of ARTIs in children. They can lead to serious diseases such as bronchiolitis and pneumonia and sometimes even cause death in infants and children worldwide [15]. In China, the positive rates of respiratory viruses in children with ARTIs or influenza-like illness (ILI) range from 37.6 to 78.7 % [5–13]. The positive rates appear to be slightly different in different regions, which may be attributed to different years and regional variation, as well as a lack of unified methodology. However, a large-scale surveillance based on 39,756 children with ARTIs in central China showed that the prevalence was only 13.1 %, substantially lower than that found in other studies [16]. Similarly, in this study, we found that the prevalence of respiratory viruses was 24.5 % among 2891 children with ARTIs from August 2011 to December 2014 in Jiading district of Shanghai, significantly lower than those (59.5 % and 78.7 %) obtained from two previous studies based on 817 children with ARTIs during 2006 to 2008 [17] (χ2=353.907, p<0.001), and 164 children with ARTIs from May 2009 to July 2010 [20] (χ2=227.960, p<0.001) in Shanghai. This may imply that it is important to have a large sample size in the investigation.
We found that the prevalence of respiratory viruses was similar between boys and girls, suggesting that there was no bias for respiratory viral infection with regard to gender. However, the prevalence appeared to decrease with age, with the highest prevalence of 29.0 % in the age group of less than 1 year old (Table 1). One possible reason is that children younger than 1 year old are more susceptible to infection with respiratory viruses than older children are. Another reason might be that children younger than 1 year old are more likely to be brought to the hospital when exhibiting symptoms of ARTIs than older children are.
Cough and fever were the two most common symptoms in our enrolled patients with ARTIs. The majority of febrile infants in Shanghai are hospitalized and prescribed antibiotics without accurate identification of bacterial infections, which may delay treatment and cause unnecessary side-effects. For precise treatment, it is urgently required that doctors know which kind of pathogens the children are infected with in order to give them proper medical treatment while avoiding the overuse of antibiotics.
The proliferation of respiratory viruses among children with ARTIs differed among different regions and often varied over time. IFV, RSV and HRV were the most commonly detected respiratory viruses among children with ARTIs or ILI in most regions of China. In Shanghai, a previous study based on 817 cases showed that PIV, HRV, IFV and RSV were the top four common respiratory viruses during 2006-2008 [6]. In this study, we investigated 2819 children with ARTIs and found that IFV, PIV and RSV were still the most common viruses, together with EV. Compared to the previous study [6], we detected a decreasing prevalence (0.6 %) of HRV. One possible reason was that relatively severe cases (e.g., sudden onset of fever >38, cough or sore throat and difficulty breathing) were enrolled in the previous study [6, 7], which resulted in a higher detection rate. On the other hand, the genotypes of certain respiratory viruses also vary over time. A genotype shift of RSV was observed in Chongqing and Shanghai from 2006 to 2012 [14, 17, 18]. For PIV, the proportions of four genotypes (PIV-1, -2, -3, -4) were consistent with those reported previously [7, 11, 19, 20].
Some previous studies have shown that the prevalence of certain respiratory viruses (e.g. HMPV, RSV) was correlated with meteorological variables [21, 22]. We observed seasonal variations for IFV, PIV and EV. The prevalence of IFV appeared to be significantly negatively correlated with mean monthly temperature, while the prevalence of PIV was significantly positively correlated with mean monthly temperature. Since 2000, a nationwide surveillance network for influenza has been implemented in the People’s Republic of China. Sentinel hospitals of the network are required to upload their daily influenza-like illness case numbers to the database of Chinese Centers for Disease Control and Prevention. The national data will help to draw a definite conclusion on the correlation of the prevalence of respiratory viruses with the meteorological variables.
Winter and spring are the high-incidence season of RSV. Here, we detected a total of 100 RSV-positive samples. The prevalence of RSV in autumn (3.5 %) and winter (4.9 %) was slightly higher than that in spring (2.8 %) and summer (2.9 %) (Table 4). The high prevalence of RSV in summer differed from what has been observed in previous studies [11], and the majority of the cases detected in summer were from July 2012, suggesting a partial outbreak of RSV during a short period. Because RSV sequences were not obtained from the samples from July 2012 [14], we were unable to determine the reason for this unusual RSV outbreak.
Conclusion
In summary, we performed an epidemiological investigation on respiratory viruses in children with ARTIs in Jiading district of Shanghai from 2011 to 2014. We found that the prevalence of respiratory viruses decreased with age and that the highest prevalence (29.0 %) occurred in children less than 1 year old, suggesting that children younger than 1 year old are the most susceptible to infection with respiratory viruses. Multiple respiratory viruses were detected in children with ARTIs in Jiading district of Shanghai. IFV, PIV, EV, and RSV were the dominant respiratory viruses, with prevalence of 5.8 %, 5.7 %, 4.3 %, and 3.6 %, respectively. The prevalence of IFV, PIV and EV exhibited distinct seasonal variation. The prevalence of IFV and PIV was significantly negatively and positively correlated with mean monthly temperature, which was the only meteorological factor associated with IFV and PIV infection.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1: Fig. 1 Seasonal distribution of respiratory viruses (DOCX 13 kb)
Financial support
This work was supported by grants from the China National Mega-projects for Infectious Diseases (2012ZX10004211-002 and 2013ZX10004101-005), and Health Bureau Key Disciplines in Jiading District of Shanghai-Pediatric Respiratory Specialty (ZD03).
Compliance with ethical standards
Conflict of interest
None.
Footnotes
W. Dong and Q. Chen contributed equally to this work.
Contributor Information
Ke Lan, Phone: +8618918100087, Email: lanke@sibs.ac.cn.
Chiyu Zhang, Phone: +8615800781776, Email: zhangcy1999@ips.ac.cn.
Reference
- 1.Williams BG, Gouws E, Boschi-Pinto C, Bryce J, Dye C. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect Dis. 2002;2:25–32. doi: 10.1016/S1473-3099(01)00170-0. [DOI] [PubMed] [Google Scholar]
- 2.Sloots TP, Whiley DM, Lambert SB, Nissen MD. Emerging respiratory agents: new viruses for old diseases? J Clin Virol. 2008;42:233–243. doi: 10.1016/j.jcv.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 4.Brittain-Long R, Nord S, Olofsson S, Westin J, Anderson LM, Lindh M. Multiplex real-time PCR for detection of respiratory tract infections. J Clin Virol. 2008;41:53–56. doi: 10.1016/j.jcv.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng J, Kong W, Guo D, Liu M, Wang Y, Zhu H, Pang B, Miao X, Yu B, Luo T, Hu Q, Zhou D. The epidemiology and etiology of influenza-like illness in Chinese children from 2008 to 2010. J Med Virol. 2012;84:672–678. doi: 10.1002/jmv.22247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, Cavailler P, Ren P, Zhang J, Dong W, Yan H, Mardy S, Cailhol J, Buchy P, Sheng J, Fontanet A, Deubel V. Molecular monitoring of causative viruses in child acute respiratory infection in endemo-epidemic situations in Shanghai. J Clin Virol. 2010;49:211–218. doi: 10.1016/j.jcv.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang G, Hu Y, Wang H, Zhang L, Bao Y, Zhou X. High incidence of multiple viral infections identified in upper respiratory tract infected children under three years of age in Shanghai, China. PLoS One. 2012;7:e44568. doi: 10.1371/journal.pone.0044568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang HY, Li ZM, Zhang GL, Diao TT, Cao CX, Sun HQ. Respiratory viruses in hospitalized children with acute lower respiratory tract infections in harbin, China. Jpn J Infect Dis. 2009;62:458–460. [PubMed] [Google Scholar]
- 9.Huang G, Yu D, Mao N, Zhu Z, Zhang H, Jiang Z, Li H, Zhang Y, Shi J, Zhang S, Wang X, Xu W. Viral etiology of acute respiratory infection in Gansu Province, China, 2011. PLoS One. 2013;8:e64254. doi: 10.1371/journal.pone.0064254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Y, Zhang RF, Xie ZP, Yan KL, Gao HC, Song JR, Yuan XH, Cheng WX, Hou YD, Duan ZJ. Newly identified respiratory viruses associated with acute lower respiratory tract infections in children in Lanzou, China, from 2006 to 2009. Clin Microbiol Infect. 2012;18:74–80. doi: 10.1111/j.1469-0691.2011.03541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai XY, Wang Q, Lin GY, Cai ZW, Lin CX, Chen PZ, Zhou XH, Xie JC, Lu XD. Respiratory virus infections among children in South China. J Med Virol. 2014;86:1249–1255. doi: 10.1002/jmv.23931. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Wei Q, Tan A, Wang L. Epidemiological analysis of respiratory viral etiology for influenza-like illness during 2010 in Zhuhai, China. Virol J. 2013;10:143. doi: 10.1186/1743-422X-10-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huo X, Qin Y, Qi X, Zu R, Tang F, Li L, Hu Z, Zhu F. Surveillance of 16 respiratory viruses in patients with influenza-like illness in Nanjing, China. J Med Virol. 2012;84:1980–1984. doi: 10.1002/jmv.23401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, Mu Y, Dong W, Yao F, Wang L, Yan H, Lan K, Zhang C. Genetic variation of human respiratory syncytial virus among children with fever and respiratory symptoms in Shanghai, China, from 2009 to 2012. Infect Genet Evol. 2014;27:131–136. doi: 10.1016/j.meegid.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Tsukagoshi H, Ishioka T, Noda M, Kozawa K, Kimura H. Molecular epidemiology of respiratory viruses in virus-induced asthma. Front Microbiol. 2013;4:278. doi: 10.3389/fmicb.2013.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Ai H, Xiong Y, Li F, Wen Z, Liu W, Li T, Qin K, Wu J, Liu Y. Prevalence and correlation of infectious agents in hospitalized children with acute respiratory tract infections in Central China. PLoS One. 2015;10:e0119170. doi: 10.1371/journal.pone.0119170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin X, Zhang C, Zhao Y, Zhao X. Genetic variability of subgroup A and B respiratory syncytial virus strains circulating in southwestern China from 2009 to 2011. Arch Virol. 2013;158:1487–1495. doi: 10.1007/s00705-012-1552-z. [DOI] [PubMed] [Google Scholar]
- 18.Zhang ZY, Du LN, Chen X, Zhao Y, Liu EM, Yang XQ, Zhao XD. Genetic variability of respiratory syncytial viruses (RSV) prevalent in Southwestern China from 2006 to 2009: emergence of subgroup B and A RSV as dominant strains. J Clin Microbiol. 2010;48:1201–1207. doi: 10.1128/JCM.02258-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henrickson KJ. Parainfluenza viruses. Clin Microbiol Rev. 2003;16:242–264. doi: 10.1128/CMR.16.2.242-264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Zhu N, Xie Z, Lu R, He B, Liu C, Ma X, Tan W. Viral etiology and clinical profiles of children with severe acute respiratory infections in China. PLoS One. 2013;8:e72606. doi: 10.1371/journal.pone.0072606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Martinez CE, Sossa-Briceno MP, Acuna-Cordero R. Relationship between meteorological conditions and respiratory syncytial virus in a tropical country. Epidemiol Infect. 2015;143:2679–2686. doi: 10.1017/S0950268814003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noyola DE, Mandeville PB. Effect of climatological factors on respiratory syncytial virus epidemics. Epidemiol Infect. 2008;136:1328–1332. doi: 10.1017/S0950268807000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1: Fig. 1 Seasonal distribution of respiratory viruses (DOCX 13 kb)


