Abstract
The role of angiotensin II (Ang II) in dengue virus infection remains unknown. The aim of this study was to determine the effect of losartan, an antagonist of the angiotensin II type 1 receptor (AT1 receptor), and enalapril, an inhibitor of angiotensin I-converting enzyme (ACE), on viral antigen expression and IL-1β production in peritoneal macrophages infected with dengue virus type 2. Mice treated with losartan or enalapril and untreated controls were infected intraperitoneally with the virus, and macrophages were analyzed. Infection resulted in increased IL-1β production and a high percentage of cells expressing viral antigen, and this was decreased by treatment with anti-Ang II drugs, suggesting a role for Ang II in dengue virus infection.
Keywords: Dengue virus, Angiotensin II, Enalapril, Losartan, Macrophages
Introduction
Dengue is the most frequent human vector-borne viral disease [1]. Dengue virus (DENV), a mosquito-borne virus belonging to the family Flaviviridae, is an enveloped, single-stranded positive-sense RNA virus. There are four genetically distinct DENV serotypes (DENV1 to 4) that cause disease in humans [2–4]. During DENV infection, levels of several cytokines, cytokine receptors, and apoptosis have been found to be increased in humans and in experimental infections [5]. Increased expression of IL-1 in infected DENV cells probably plays an important role in the pathogenesis of dengue [5, 6]. The renin–angiotensin system is involved in the regulation of blood pressure, vasoconstriction, sodium intake, and potassium excretion. Angiotensin II (Ang II) is one of the main effector molecules in this system, and its pro-inflammatory properties have reported recently. After binding of Ang II to its receptors, in particular AT1 (angiotensin II type 1 receptor: AT1 receptor), intracellular translocation of NF-kB to the nucleus occurs, inducing transcription of pro-inflammatory cytokines and production of oxygen-reactive species. Ang II-mediated inflammatory events can be inhibited by using AT-1 receptor antagonists or by inhibition of angiotensin I converting enzyme (ACE) [7–11]. Ang II is involved in the production of several cytokines, including interleukin-1 (IL-1) [12], and it is also involved in virus-induced respiratory pathogenesis [13–15]. This information suggests that Ang II is an important factor in inflammatory events during virus infection. There is no available information regarding the role of Ang II in DENV infection. Therefore, the aim of this study was to determine the effect of losartan, an angiotensin II AT1 receptor antagonist, and enalapril, an inhibitor of ACE, on the cellular expression of viral antigens and on IL-1β production by peritoneal macrophages infected by DENV2.
Materials and methods
Animals
Adult male NMRI mice weighing 25-30 g (Central Animal Facility, Instituto de Investigaciones Científicas, IVIC, Venezuela) were used. Animals were housed at room temperature (24 °C) with water and food (Ratarina, Proteinal, Valencia, Venezuela) ad libitum and a natural day-night light cycle.
Preparation of virus stock and virus titration
DEN2 virus strain New Guinea C was propagated in C6/36HT mosquito cells that were cultured in Eagle’s MEM medium containing 10 % FBS prior to infection of mice. The virus-containing culture medium was harvested after 5 days of incubation, and after removal of cell debris by centrifugation, the supernatant was aliquoted and stored at -70 °C until used. Virus was titrated by plaque formation assays on Vero cells. Cells were seeded at 1 × 106 cells/well in 24-well plates, and subsequently, serial dilutions of virus were added and the mixtures were incubated at 37 °C for 7 days. Afterwards, the plaques were visualized by staining with a dye solution containing 1 % crystal violet. Virus concentrations are given as plaque-forming units (PFU)/ml.
Experimental design
Mice were left untreated or treated with losartan or enalapril (Merck & Co., Whitehouse Station, NJ, USA) by gavage at 25 mg/kg/day during the entire experimental period. After one week of treatment, untreated and treated mice were injected intraperitoneally (ip) with 2 mL of 3 % thioglycollate solution (Difco Laboratories,Detroit, MI, USA) to elicit inflammatory macrophages. Four days later, mice were infected ip with a suspension of DENV2 (50 μl) at an MOI of 1. This was based on the assumption that, under these conditions, approximately 1 × 107 peritoneal cells/ mL are usually obtained. Six groups of animals were studied (10 per group): losartan-treated, enalapril-treated, and untreated infected animals and losartan-treated, enalapril-treated and untreated uninfected animals. After 12 hours of intraperitoneal incubation, 5 mL of cold sterile PBS was injected ip, and macrophages and peritoneal fluid were obtained after centrifugation (1500 rpm × 10 min) from all of the animals. A portion of macrophage samples was homogenized, and supernatants (peritoneal fluid) and homogenates were kept at -20 °C until needed for IL-1β determination. Another portion of the macrophage preparation (0.5 × 106 cells/mL) was cytocentrifuged to measure cellular DENV2 expression and identify CD14-positive cells.
DENV2, IL-1β, CD14 and protein determinations
Macrophages were fixed for 5 minutes with cold acetone or with 2 % of paraformaldehyde to determine viral antigen or macrophage antigen (CD14), respectively. Intracellular viral antigens were detected in cytocentrifuged macrophages by a direct immunofluorescence assay using a fluorescein-conjugated anti-DENV2-specific monoclonal antibody (Chemicon International Inc. MA, USA). Macrophages were also identified in separate samples using a fluorescein-conjugated monoclonal anti-murine CD14 antibody (eBioscience, Inc. San Diego, CA. USA). Two hundred cells were counted, and the percentage of positive cells for each monoclonal antibody was determined. The IL-1β content was determined by ELISA (Biotra Amersham, Int. England) and expressed as pg/mgs of cellular protein or as pg/mL of peritoneal fluid. The total protein content in the macrophage homogenates was measured using the bicinchoninic acid assay [16].
Statistical analysis
Data were expressed as mean ± standard deviation and analyzed by ANOVA followed by Bonferroni’s test. P-values less than 0.05 were considered statistically significant.
Results
Peritoneal macrophages were obtained from the peritoneal cavity after 12 hours of viral interaction to determine the presence of viral antigen in the cells. At this time, a high percentage of peritoneal macrophages from untreated infected mice were positive for DENV2, as revealed by immunofluorescence assay with an anti-DENV monoclonal antibody, indicating that penetration of the cells had occurred. Infected cells that had been treated with losartan or enalapril showed reduced expression of viral antigens (Fig. 1). In addition, the peritoneal fluid and macrophage contents (homogenates) were tested for the presence of IL-1β, a cytokine related to viral infection. Viral expression was accompanied by increased of IL-1 β levels in the peritoneal fluid and in peritoneal macrophages, suggesting that macrophages were involved in the increased production of this cytokine. Losartan treatment resulted in decreased IL-1 production in dengue-virus-infected cells, but enalapril did not affect IL-1 production (Fig. 2). Resident macrophages had lower IL-1β levels (81 ± 23 pg/mg of protein and 72 ± 13 pg/ml; cellular and peritoneal fluid contents, respectively) than that observed in macrophages elicited by thioglycollate treatment. A high percentage (92 % ± 7 %) of CD14 cells was observed, suggesting that macrophages were the main cell type analyzed in this study.
Fig. 1.
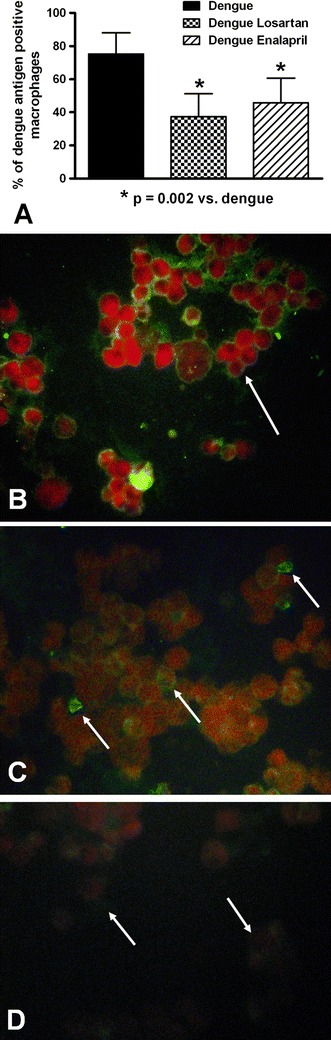
Effects of losartan and enalapril on dengue virus expression in peritoneal macrophages. A) Losartan and enalapril treatment resulted in a decrease in the percentage of macrophages expressing type 2 dengue virus antigens. (B and C) Immunofluorescence staining for dengue virus antigens in untreated (B) and losartan-treated (C) infected peritoneal macrophages at 12 h after infection (arrows indicate positive cells). D) Macrophages from an uninfected animal treated with losartan (arrows indicate negative cells). Positive infected cells were detected using a fluorescein-conjugated anti-DENV2-specific monoclonal antibody. Original magnification: x400
Fig. 2.
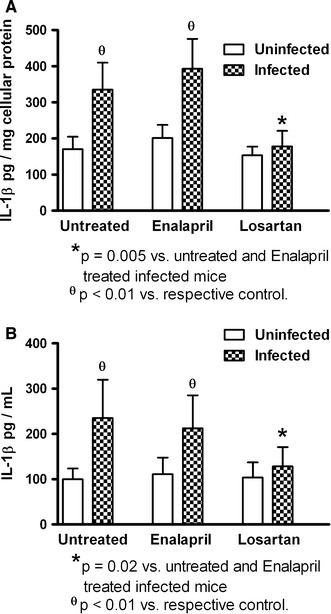
Effects of losartan and enalapril on interleukin-1 beta (IL-1β) production by peritoneal macrophages infected with dengue virus. Cellular (A) and peritoneal fluid (B) IL-1β concentrations were reduced by losartan treatment
Discussion
During dengue infection, the interaction between monocytes/macrophages and DENV occurs in an inflammatory environment [5, 6]. Generally, the normal peritoneal cavity has mature, resident macrophages, and the inflamed peritoneal cavity has immature, inflammatory macrophages recruited from the circulating and marginal pool [17]. Therefore, inflammatory macrophages obtained after peritoneal thioglycollate stimulation were challenged with DENV2 in order to examine expression of viral antigens and IL-1β production and the effect of anti-Ang II drugs on those parameters.
Studies in the last few years have documented new roles for Ang II as a pro-inflammatory molecule. Binding of Ang II to its receptors (in particular, AT1) mediates intracellular translocation of NF-kB to the nucleus, with further production of oxygen-reactive species and pro-inflammatory molecules such as IL-1 that contribute to tissue damage. Blocking Ang II signaling by AT-1 antagonists or by inhibition of ACE protects against those effects [7, 8]. Together, these findings suggest the possibility of targeting Ang II signaling in therapeutic interventions in inflammatory diseases. Since dengue is an inflammatory disease, it is justified to study the effect of anti-Ang II drugs on this disease. In addition, anti-Ang II drugs in several experimental models of inflammatory diseases have been reported [9–11].
The effect of Ang II on viral infection has not been extensively studied. The role of Ang II on viral respiratory pathogenesis has been documented recently. In this regard, H7N9 influenza A virus infection is accompanied by an increase in the Ang II level, inflammatory events, and high viral titers in the lung tissue, which are associated with disease progression and mortality, which are reduced by treatment with losartan and ACE2 [14, 18]. Proteolytic processing of ACE2 promotes SARS coronavirus entry into host cells and induces lung pathogenesis [15, 19, 20]. In addition, enhanced ACE activity with high levels of Ang II in parallel with reduced ACE2 in viral acute respiratory distress syndrome has also been documented [21]. In vitro studies have shown decreased superoxide overproduction in rhinovirus-infected cells treated with losartan [22]. All of this information indicates a possible role of Ang II in viral pathogenesis. Accordingly, blocking of AT1 receptors with losartan and inhibition of ACE with enalapril reduced the percentage of macrophages expressing DENV2 antigens, suggesting a decrease in cell penetration by the virus and a role of Ang II in dengue infection. The mechanism by which Ang II affects dengue virus penetration regulated by remains unclear; however, the interaction of virus with AT1 receptors or phagocytosis receptors could be involved.
High levels of cytokine production in patients with severe dengue and in dengue-virus-infected monocyte/macrophage cultures have been reported [5]. IL-1β is a pro-inflammatory cytokine that plays an important role in dengue [5, 6]. In this study, increased levels of L-1β were found in macrophages and peritoneal fluid after peritoneal DENV2 infection. Treatment with losartan, but not with enalapril, decreased the L-1β levels in both compartments, suggesting a role of AT-1 receptor in IL-1β production and a possible role of Ang II in dengue infection. Ang II is capable of inducing the production of L-1β after its interaction with AT1 receptors via the translocation of NF-κB to the nucleus [12]. However, we cannot rule out an effect of DENV on macrophage biology. In this regard, peritoneal macrophage functions could be affected by DENV-induced cytotoxic factor, producing immunosuppression [23, 24] and affecting IL-1 production. Decreased IL-1β production was observed only when AT1 receptors were blocked, and no decrease was observed in infected untreated and enalapril-treated animals, suggesting a role of AT1 receptor activation instead of a cytotoxic effect induced by DENV2 infection.
There are no reports of the relationship between Ang II and dengue fever in humans or in experimental studies. The current experimental protocol does not represent a model for dengue disease in humans; however, it allows the interactions of DENV with macrophages to be studied in vivo in an inflammatory environment. This study shows that Ang II could be important in the pathogenesis of dengue infection, inducing increased viral infection and cytokine production. Further investigations regarding the role of Ang II in the pathogenesis of dengue in humans are needed.
Acknowledgments
This work was supported by Instituto de Investigaciones Clínicas “Dr. Américo Negrette”, Facultad de Medicina, Universidad del Zulia, Maracaibo, Venezuela, and Consejo de Desarrollo Científico, Humanístico y Tecnológico de la Universidad del Zulia (CONDES/LUZ): VAC-CONDES-CC-0569-13.
References
- 1.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10:100–103. doi: 10.1016/S0966-842X(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 2.Levy A, Valero N, Espina LM, Añez G, Arias J, Mosquera J. Increment of interleukin 6, tumour necrosis factor alpha, nitric oxide, C-reactive protein and apoptosis in dengue. Trans R Soc Trop Med Hyg. 2010;104:16–23. doi: 10.1016/j.trstmh.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Gupta N, Chaturvedi UC. Can helper T-17 cells play a role in dengue haemorrhagic fever? Indian J Med Res. 2009;130:5–8. [PubMed] [Google Scholar]
- 4.Pawitan JA. Dengue virus infection: predictors for severe dengue. Acta Med Indones. 2011;43:129–135. [PubMed] [Google Scholar]
- 5.Arias J, Valero N, Mosquera J, Montiel M, Reyes E, Larreal Y, Alvarez-Mon M. Increased expression of cytokines, soluble cytokine receptors, soluble apoptosis ligand and apoptosis in dengue. Virology. 2014;452–453:42–51. doi: 10.1016/j.virol.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Silva MM, Gil LH, Marques ET, Jr, Calzavara-Silva CE. Potential biomarkers for the clinical prognosis of severe dengue. Mem Inst Oswaldo Cruz. 2013;108:755–762. doi: 10.1590/0074-0276108062013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2:247–257. doi: 10.1002/emmm.201000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruiz-Ortega M, Esteban V, Suzuki Y, Ruperez M, Mezzano S, Ardiles L, Justo P, Ortiz A, Egidio J. Renal expression of angiotensin type 2 (AT2) receptors during kidney damage. Kidney Int Suppl. 2003;86:S21–S26. doi: 10.1046/j.1523-1755.64.s86.5.x. [DOI] [PubMed] [Google Scholar]
- 9.Peña C, Hernández-Fonseca JP, Rincón J, Pedreañez A, Viera N, Mosquera J. Proinflammatory role of angiotensin II in mercuric induced nephropathy in rats. J Immunotoxicol. 2013;10:125–132. doi: 10.3109/1547691X.2012.699478. [DOI] [PubMed] [Google Scholar]
- 10.Vargas R, Rincón J, Pedreañez A, Viera N, Hernández-Fonseca JP, Peña C, Mosquera J. Role of Angiotensin II in the brain inflammatory events during experimental diabetes in rats. Brain Res. 2012;1453:64–76. doi: 10.1016/j.brainres.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz M, Rincón J, Pedreañez A, Viera N, Hernández-Fonseca JP, Mosquera J. Proinflammatory role of angiotensin II in a rat nephrosis model induced by adriamycin. J Renin Angiotensin Aldosterone Syst. 2011;12:404–412. doi: 10.1177/1470320311410092. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y, Wei W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin Exp Immunol. 2015;179:137–145. doi: 10.1111/cei.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang P, Gu H, Zhao Z, Wang W, Cao B, Lai C, Yang X, Zhang L, Duan Y, Zhang S, Chen W, Zhen W, Cai M, Penninger JM, Jiang C, Wang X. Angiotensin-converting enzyme 2 (ACE2) mediates influenza H7N9 virus-induced acute lung injury. Sci Rep. 2014;4:7027–7032. doi: 10.1038/srep07027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiehelman K, Braun R, Fitzpatrick J. Investigation of the bicin choninic acid protein assay. Identification of the groups responsible for color formation. Anal Biochem. 1988;175:231–237. doi: 10.1016/0003-2697(88)90383-1. [DOI] [PubMed] [Google Scholar]
- 17.Michl J, Pieczonka MM, Unkeless JC, Silverstein SC. Effects of immobilized immune complexes on Fc- and complement-receptor function in resident and thioglycollate-elicited mouse peritoneal macrophages. J Exp Med. 1979;150:607–621. doi: 10.1084/jem.150.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang F, Guo J, Zou Z, Liu J, Cao B, Zhang S, Li H, Wang W, Sheng M, Liu S, Pan J, Bao C, Zeng M, Xiao H, Qian G, Hu X, Chen Y, Chen Y, Zhao Y, Liu Q, Zhou H, Zhu J, Gao H, Yang S, Liu X, Zheng S, Yang J, Diao H, Cao H, Wu Y, Zhao M, Tan S, Guo D, Zhao X, Ye Y, Wu W, Xu Y, Penninger JM, Li D, Gao GF, Jiang C, Li L. Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients. Nat Commun. 2014;5:3595. doi: 10.1038/ncomms4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang P, Chen J, Zheng A, Nie Y, Shi X, Wang W, Wang G, Luo M, Liu H, Tan L, Song X, Wang Z, Yin X, Qu X, Wang X, Qing T, Ding M, Deng H. Expression cloning of functional receptor used by SARS coronavirus. Biochem Biophys Res Commun. 2004;315:439–444. doi: 10.1016/j.bbrc.2004.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simmons G, Zmora P, Gierer S, Heurich A, Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: Cutting enzymes at the cutting edge of antiviral research. Antiviral Res. 2013;100:605–614. doi: 10.1016/j.antiviral.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wösten-van Asperen RM, Bos AP, Bem RA, Dierdorp BS, Dekker T, van Goor H, Kamilic J, van der Loos CM, van den Berg E, Bruijn M, van Woensel JB, Lutter R. Imbalance between pulmonary angiotensin-converting enzyme and angiotensin-converting enzyme 2 activity in acute respiratory distress syndrome. Pediatr Crit Care Med. 2013;14:e438–e441. doi: 10.1097/PCC.0b013e3182a55735. [DOI] [PubMed] [Google Scholar]
- 22.Ang LT, Tan LY, Chow VT, Sim MK. Des-aspartate-angiotensin I exerts antiviral effects and attenuates ICAM-1 formation in rhinovirus-infected epithelial cells. Eur J Pharmacol. 2012;683:310–315. doi: 10.1016/j.ejphar.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 23.Gulati I, Chaturvedi UC, Mathur A. Depressed macrophage functions in dengue virus infected mice: role of the cytotoxic factor. Br J Exp Pathol. 1982;63:194–201. [PMC free article] [PubMed] [Google Scholar]
- 24.Gulati L, Chaturvedi UC, Mathur A. Production of dengue virus-induced macrophage cytotoxin in vivo. Br J Exp Pathol. 1986;67:269–277. [PMC free article] [PubMed] [Google Scholar]


