Abstract
We investigated the antiviral activity of Arbidol, an antiviral chemical compound, against Coxsackie virus B5 (CVB5) in vitro and in vivo. Arbidol not only prevented the cytopathic effect (CPE) of CVB5, as demonstrated in an MTT colorimetric assay, when added during or after viral infection, with a 50% inhibitory concentration (IC50) from 2.66 to 6.62 μg/ml, but it also decreased the CVB5-RNA level in infected host cells, as shown in semi-quantitative RT-PCR. BALB/c mice were used as an animal model to test the Arbidol activity in vivo. Orally administered Arbidol at 50 mg/kg body weight/day (once a day) significantly reduced mean virus yields in the lungs and heart as well as mortality after infection for 6 days. Our results demonstrate that in vitro and in vivo infection with CVB5 can be effectively treated by Arbidol.
Keywords: Myocarditis, Antiviral Activity, Cidofovir, Infectious Bronchitis Virus, Virus Yield
Introduction
Coxsackieviruses are members of the genus Enterovirus, family Picornaviridae, and are divided into two groups on the basis of pathogenicity in mice: 23 serotypes of Coxsackie A viruses (CAV) and 6 serotypes of Coxsackie B viruses (CVB). CVB had been implicated in childhood myocarditis by the end of the 1950s. Since then, six CVB serotypes (CVB1-6) have been shown to cause not only myocarditis but also a wide variety of human diseases, including common colds, cardiomyopathy, diabetes, neurological disorders, and inflammation [1–4]. Numerous outbreaks of CVB infections have been noted at the community level, but only Coxsackie virus B5 (CVB5) is known to have caused widespread nationwide epidemics in the United States, particularly in 1961, 1972, and 1983 [5]. Moreover, CVB5 is associated with several human diseases, including encephalitis and myocarditis in immunocompromised children and CNS disease in older adults.
Since direct viral cytotoxicity is one possible cause of damage, targeting the causative agent should limit the extent of damage, especially with early treatment. Until now, there has been no enterovirus-specific vaccine or therapeutic reagent available for clinical use [6], although some reports have presented effective candidates for CVB3 in a murine model [1, 7, 8]. A great number of picornavirus replication inhibitors have been described in vitro, but few of them have shown effectiveness in vivo [9], and none has been approved for clinical use. Gancyclovir and cidofovir have been studied in myocarditis caused by cytomegalovirus in mice [10], but there is no evidence that it also works for enterovirus-induced myocarditis. Acyclovir has been successfully used to treat myocarditis associated with Epstein-Barr virus [11, 12] and varicella [13], but there are no reports demonstrating that acyclovir is effective in CVB myocarditis.
Arbidol is an antiviral molecule first developed in Russia. Its chemical name is ethyl-6-bromo-4-[(dimethylamino)-methyl]-5-hydroxy-1-methyl-2-[(phenylthio) methyl]-indole-3-carboxylate hydrochloride monohydrate. Arbidol was first found to have wide-spectrum antiviral activity against human influenza virus. It was shown to inhibit the reproduction of rimantadine-resistant human influenza virus A, avian viruses H5N1 and H9N2, and influenza viruses B and C [14]. In many studies, Arbidol also exhibited broad and potent antiviral activity against a number of viruses including respiratory syncytial virus [15], adenovirus [16], parainfluenza virus type 5, rhinovirus type 14, avian coronavirus, infectious bronchitis virus and Marek disease virus [17], hepatitis B virus [18], and hepatitis C virus [19]. In our previous study, Arbidol showed broad-spectrum antiviral activity against a number of respiratory viruses, including FLU-A, RSV, HRV 14, and CVB3 in vitro [20]. The extensive list of Arbidol-sensitive viruses includes RNA and DNA viruses, enveloped and non-enveloped viruses, and pH-dependent and -independent viruses, emphasizing a broad spectrum of Arbidol antiviral activity. At the same time, the wide spectrum of Arbidol’s activity suggests that Arbidol targets common critical step(s) in virus–cell interaction. In this study, we systematically investigated the antiviral activity of Arbidol against CVB5 in vitro and in vivo.
Materials and methods
Chemicals and reagents
Arbidol was synthesized at Qianjiang Pharmaceutical Co. Ltd, Hubei, China. The compound was initially dissolved in dimethyl sulfoxide (DMSO) and further diluted with complete test medium. The final maximum DMSO concentration was 0.05%, which showed no effect on cell cultures. Therefore, 0.05% DMSO was also added to all no-compound control samples. The efficacy of these preparations did not appear to change upon freezing and short-term storage (1 month at 4°C). For the in vivo experiment, the compound was dissolved in 0.5% methylcellulose solution.
Cell cultures and viruses
HEp-2 (human laryngeal carcinoma) cells were maintained in our laboratory and were routinely grown in Eagle’s minimal essential medium plus nonessential amino acids (Eagle’s MEM; Sigma) supplemented with 10% fetal bovine serum, 0.1% l-glutamine, 100 U/ml penicillin, and 0.1 mg/ml streptomycin. The test medium used for the cytotoxic assay as well as for antiviral assays contained 2% of the appropriate serum.
CVB5 was maintained in our laboratory and propagated in HEp-2 cells. The virus was stored in small aliquots at −80°C until use.
Virus titration
Virus titration was performed by the limited dilution method, using a 96-well plate with six wells per dilution. The virus titer was estimated from cytopathogenicity of cells induced by viral infection and expressed as 50% tissue culture infectious doses/ml (TCID50/ml) [21].
MTT assays
The cytotoxicity and antiviral activity of the compound were determined by quantitative colorimetric MTT [(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide)] assay [13, 22]. Briefly, HEp-2 cells were seeded at 2 × 104 cells per well in 96-well plates and grown to subconfluence. After removal of the growth medium, serial twofold dilutions of the compound in 200 μl test medium were added. At each dilution, four wells were infected with 100 TCID50/0.1 ml of virus, while four wells were left uninfected for toxicity determination. The same dose of Arbidol was added to the plate daily, since its half-life in cultured cells is about 18 h [23]. The plates were incubated at 37°C, and the development of cytopathic effect (CPE) was monitored daily by light microscopy until the virus-infected, untreated cells showed CPE up to 80%. At this time point, the culture medium was removed and 25 μl of the MTT solution (5 mg/ml in phosphate-buffered saline, PBS) was added to each well. The plate was further incubated for 4 h to allow MTT formazan formation. After removal of the supernatant, 50 μl of DMSO was added for solubilization of formazan crystals, which were homogenized on a microplate shaker for 15 min. The optical densities (ODs) were then read using a microplate spectrophotometer at double wavelengths of 540 and 690 nm. Results were expressed as a percentage of OD value of treated cell cultures with respect to untreated ones. All data were analyzed with SPSS 11.5, and the 50% cytotoxic (CC50) and 50% inhibitory (IC50) concentrations of the agent were determined. Thus, the therapeutic index (TI) for the compound was also determined from CC50/IC50. Each dilution was tested in triplicate, and in each set of experiments, three control wells without drug were included.
Antiviral activity in vitro
Compound treatment before virus infection
Serial twofold dilutions of the compound were dissolved in MEM and incubated with HEp-2 cells in 96-well microtiter plates for 24 h at 37°C in a 5% CO2 atmosphere. After removal of the compound, the cells were washed twice with PBS and challenged with 100 TCID50/0.1 ml of CVB5. After a 1-h incubation, the cells were washed twice with PBS and further incubated with test medium until typical CPE was visible. The inhibition of the virus-induced CPE was scored by light microscopy and measured by the MTT assay. Four untreated virus controls and four uninfected, untreated cell controls were included in all assays. All data presented are results of experiments performed in triplicate.
Virucidal assay
Viral suspensions were cocultured with serial twofold dilutions of the compound at 37°C for 1 h and then added to confluent cells. After a 1-h incubation, the solutions containing both compound and viruses were removed; the cells were rinsed carefully with PBS and further incubated with test medium. The assay was performed following the protocol described above.
Compound treatment after virus infection
The experiment was carried out as above with the following difference: confluent cell monolayers were infected with 100TCID50/0.1 ml viruses for 1 h. The cell sheets were washed with PBS and overlaid with different doses of the compound in 200 μl test medium.
Semi-quantitative detection of viral RNA by RT-PCR
RNA was extracted from both supernatants and cells after compound treatment and virus infection using Trizol reagent (Invitrogen). We also purified the RNA of the uninfected cells, the infected cells without treatment and the test medium as negative, positive and blank controls. RNA was dissolved in RNase-free water, and the concentration was measured using a spectrophotometer (Bio-Rad). All samples were adjusted to 1 μg/μl according to the method of Yamamoto et al. [24]. Reverse transcription was carried out at 42°C for 45 min, then at 95°C for 5 min. Random primers were used for the preparation of complementary DNA templates for PCR. The forward and reverse primers used for RT-PCR were 5′-CCCCGGACTGAGTATCAATA-3′ (180–199) and 5′-GCAGTTAGGATTAGCCGCAT-3′ (479–460), respectively.
β-actin was used as internal control, and the sequences were 5′-GGCGGGACCACCATGTACCCT-3′ and 5′-AGGGGCCGGACTCGTCATACT-3′.
Twenty-eight cycles of PCR were carried out in 50-μl reaction mixtures containing 10 μl synthesized cDNA, 5 μl 10X PCR buffer, 10 μl 1 M dNTPs, 1 μl of primer 1, 1 μl of primer 2, 1 μl β-actin 220 P1, 1 μl β-actin 220 P2, 1 μl of Taq DNA polymerase, and 30 μl of mineral oil, which was placed on top of the PCR mixture to prevent evaporation. A programmed thermal cycler was set as follows: 94°C for 300 s, 94°C for 300 s, and 53°C for 45 s, 72°C for 45 s, and 72°C for 5 min. Then, 5 μl of each PCR product was loaded onto an agarose gel containing ethidium bromide and separated by electrophoresis. The gel was scanned using the SX-300 photoanalysis system and analyzed using Four Stars Bioimage software, which calculates the ratio of peak value adsorption of CVB to that of β-actin.
Coxsackie virus B5 infections in mice
Specific-pathogen-free male BALB/c mice, 3–4 weeks old (13–15 g), obtained from Animal Center of Wuhan University, were used in all experiments. Sixty-four BALB/c mice (16 mice/group, four groups) were used in this experiment. Forty-eight mice were infected intraperitoneally with CVB5 (105TCID50/0.1 ml), and the remaining 16 were used as normal control and injected intraperitoneally with the same volume of PBS. After infection for 24 hours, mice were treated by oral gavage (p.o.) with 0.1 ml Arbidol, corresponding to approximately 25 and 50 mg/kg body weight/day, and treatment was continued daily for 6 days unless otherwise indicated. The virus control group and the normal (PBS) control group received 0.5% methylcellulose solution instead of the compound.
Eight mice from each group were sacrificed by cervical dislocation on day 6 after viral exposure. The body weights of the mice were recorded daily until the animals were killed. The lungs and hearts were harvested and weighed. The organs from four mice of each group were subsequently homogenized to 10% (weight/volume) suspensions in test medium. The homogenates were frozen and thawed twice to release the virus and centrifuged at 3,000 rpm for 10 min. Virus titration was determined by plaque assay, and the organs from another four mice were used for pathological examination (HE staining).The lung index was expressed as the ratio of mean lung weights to mean body weights [25]. The remaining eight animals of each group were observed daily for changes in body weight and for any deaths.
Viral plaque assay of lung and heart samples
The titers of infectious virus particles were determined by the standard plaque formation assay. Replicate aliquots (500 μl/well) of serial 10-fold dilutions were inoculated onto HEp-2 cell monolayers in 6-well plates and incubated at 37°C in 5% CO2. Two days later, the CPE was read; results were expressed as PFUs.
Results
Antiviral effect of Arbidol against Coxsackie virus B5 in vitro
Arbidol showed significant inhibitory activity against CVB5 when added after infection, with an IC50 of 6.62 μg/ml, TI of 12.45, and mild inhibitory activity against CVB when added before infection, with an IC50 of 30.34 μg/ml, which is close to the CC50 of the compound (82.12 μg/ml), and TI of 2.7 (see Table 1).
Table 1.
Mode of action of Arbidol against CVB5
| IC50 (μg/ml)a | TI | |
|---|---|---|
| Drug added before infection | 30.34 ± 1.6 | 2.7 |
| Drug added after infection | 6.62 ± 1.1 | 12.45 |
| Virucidal assay | 2.66 ± 0.4 | 27.56 |
IC50 is the inhibitory concentration required to reduce viral replication by 50%
TI is expressed as the ratio of CC50/IC50
a Mean ± SD values are shown from three independent experiments
To investigate the direct inactivating effect of Arbidol, virus was treated for 1 h with concentrations of Arbidol ranging from 0.5 to 20 μg/ml. As shown in Table 1, Arbidol was virucidal for CVB5, with an IC50 of 2.66, and it inhibited the CPE of CVB5 on HEp-2 cells, with a TI of 27.56.
Semiquantitative detection of viral RNA by RT-PCR
RNA was extracted from the supernatants and cells in each well of the plates that were treated with the compound after virus infection, using Trizol (Invitrogen) according to the manufacturer’s protocol. Specific amplified products of CVB5 (280 bp) and β-actin (220 bp) by the two pairs of primers were identified as described in “Materials and methods”. Figure 1a is the gel picture of the PCR products. The gel was scanned using the SX-300 photoanalysis system and analyzed using Four Stars Bioimage software. The ratio of peak value adsorption of CVB to that of β-actin was calculated by the software (shown in Fig. 1b). There was a quantitative relationship between the ratio and dose of Arbidol. The ratio decreased with increasing dose of Arbidol, suggesting that the synthesis of CVB5 RNA decreased with the increasing dose of compound when treatment was done after infection.
Fig. 1.
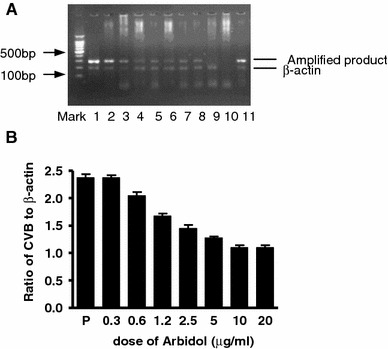
Arbidol prevents CVB5 RNA synthesis in vitro. RNA extracted from cells treated with Arbidol after infection was used for RT-PCR. a RT-PCR products of CVB5 RNA and β-actin. Lanes 1–8 cells treated with increasing concentrations of Arbidol (0.3, 0.6, 1.2, 2.5, 5, 10, 20, 40 μg/ml, respectively). Lanes 9, 10, and 11 are negative (uninfected cell), blank (test medium as PCR template), and positive controls (infected but untreated cells), respectively. b The ratio of CVB5 to β-actin in different groups
Antiviral effects of arbidol against Coxsackie virus B5 in mice
Clinical observations
In the viral control group, animals showed a tendency to huddle; diminished vitality, ruffled fur, and weight loss (Fig. 2a) were observed on day 6 after infection. On day 8, this group of animals began to die, and by 18 day, all of them had died (Fig. 2b). In the compound-treated group, the animals receiving 25 mg/kg body weight/day began to show behavior changes and lose weight by day 9 (Fig. 2a) and began to die on day 12 after challenge. By day 30, half of them had survived (Fig. 2b). The animals receiving 50 mg/kg body weight/day and the normal control animals did not show abnormalities during the 30-day period, except that the former group had a slight weight loss (Fig. 2a). The i.p. infection with CVB5 led to an increase in mean lung weight and a decrease in mean body weight on day 8 after virus exposure. Lung indexes of the 25- and 50-mg/kg body weight/day groups were significantly lower than that of the viral control group, with the 50-mg group almost the same as the normal group. There was no significant difference between the 50-mg and normal group in lung index (Fig. 4c), and there was no significant difference among the groups of animals in mean heart weight (data not shown).
Fig. 2.
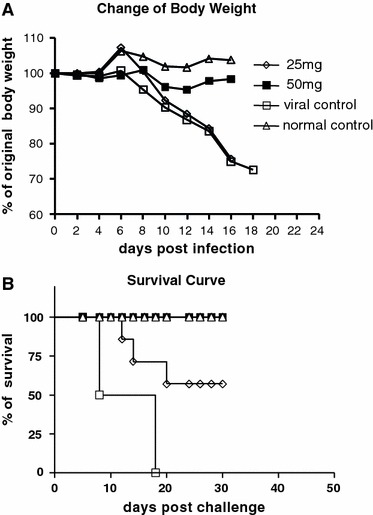
Orally administered Arbidol at 50 mg/kg day prevents weight loss and prolongs survival. BALB/c mice (eight mice per group) were infected intraperitoneally with Coxsackie virus B5 (105 TCID50/0.1 ml). Twenty-four hours after infection, mice were treated by oral gavage with 25 or 50 mg/kg body/day Arbidol for 6 days. The virus control group and the normal control group received placebo instead of Arbidol. Body weight (a) and survival (b) were recorded daily
Fig. 4.
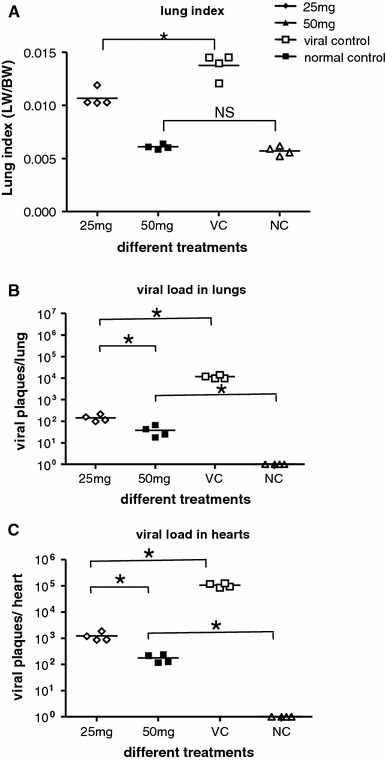
Arbidol prevents the increase of both lung index and viral titer in CBV5-infected mice. Four mice from each group were sacrificed on day 6 post-infection, and body weight and lung weight were used to calculate lung index (a). Viral titer of lungs (b) and hearts (c) were determined by plaque assay. Asterisk means P < 0.05, NS no statistical difference
Pathological evaluation
Four animals of each group were sacrificed on day 8 after viral exposure, and tissue samples were obtained from the lungs, kidneys, liver, heart, and spleen for pathological examination. As shown microscopically, the lungs of the viral control animals had thickened alveoli walls due to edema of alveolar epithelia, proliferation of interstitial cells, interstitial lymphocytes and macrophage inflammatory infiltration, and hemorrhage (Fig. 3a). The hearts of the viral control animals had denaturation of cardiac muscle cells (Fig. 3c). Tissue samples from the remaining animals did not have changes, except that the lungs of animals receiving 25 mg/kg body weight/day showed local hemorrhage (data not shown).
Fig. 3.
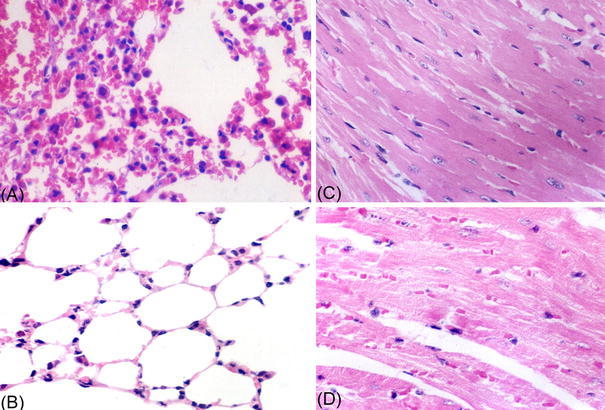
Interstitial pneumonia and myocarditis caused by CVB5 infection Four mice of each group were sacrificed on day 6 after viral exposures, and tissue samples were obtained for pathological examination. a Lungs from infected mice: the walls of the pulmonary alveoli were obviously thickened due to edema of alveolar epithelia, proliferation of interstitial cells and interstitial lymphocytes, and macrophage inflammatory infiltration. b Normal pulmonary alveoli and epithelia. c Hearts from infected mice: isotropic denaturation of cardiac muscle cell; striation of cardiac muscle absent in viral control. d Normal cardiac muscle. HE stain 400×
Virological evaluation
Four animals of each group were sacrificed on day 8 after viral exposure, and tissue samples were obtained from the lungs and hearts for virological examination. The virus titers of lung and heart were significantly lower in mice receiving oral administration of Arbidol daily for 6 days than in the viral control group. In the groups treated with Arbidol at 25 and 50 mg/kg body weight/day, the mean virus yields of lungs were around 102 and 101 viral plaques/lung, respectively, whereas the yields in virus control mice were 104 viral plaques/lung (Fig. 4a). The mean virus yields of hearts were reduced to 103 and 102 viral plaques/heart, respectively, whereas the yields in virus-control mice were 105 viral plaques/heart (Fig. 4b).
Discussion
Arbidol is a Russian-made broad-spectrum antiviral compound that has been shown to inhibit the fusion of influenza A and B viruses within endosomes [26]. Influenza-virus-induced fusion needs an acidic environment (pH 5.0). A slight increase in pH can abolish the fusion process [27]. Since Arbidol is a weak base, it may elevate endosomal pH and abrogate virus-endosome fusion. Arbidol has also been shown to exert antiviral activity against other pH-dependent viruses, such as hepatitis B virus [18] and rhinovirus 14 [17].
Moreover, Arbidol has demonstrated low toxicity upon single-dose oral administration in many animal models. The animal LD50 (50% lethal dose) was 687 mg/kg for mice, 3,000 mg/kg for rats, and 4,000 mg/kg for guinea pigs [28]. A long-term Arbidol intake study also did not show pathologic changes in animals, as confirmed by clinical, hematological, biochemical, and pathological data. At therapeutic doses, Arbidol possessed no mutagenic or teratogenic activity [28].
In this study, we tested the antiviral activity of Arbidol against CVB5, both in vitro and in vivo. Like other enteroviruses, CVB is a small RNA virus that replicates at 37°C, lacks a lipid envelope, and is stable at acid pH. When primarily cultured HEp-2 cells infected with CVB3 were treated with Arbidol by different methods, arbidol was found to present potent antiviral activity against CVB5 when added before and after infection. In a virucidal assay, Arbidol exhibited a strong effect against CVB5, and inhibited the CPE of CVB5 on HEp-2 cells with a TI of 27.56, which is higher than other treatments. Those data suggested that Arbidol had a strong antiviral effect against CVB5 during the adsorption step. We also cocultured HEp-2 cells with different doses of Arbidol after infection to study whether it still had an antiviral effect after virus entry into the host cell. The MTT assay showed that Arbidol had inhibitory activity against CVB5, with an IC50 of 6.62 μg/ml and a TI of 12.45 (see Table 1). Semiquantitative RT-PCR data suggested that Arbidol prevented the synthesis of CVB5 RNA in a dose-dependent manner. In short, our data demonstrate that Arbidol can induce durable antiviral activity in host cells, not only blocking the adsorption of viral particles onto cells but also inhibiting the late stage of the viral replication cycle.
To study further whether Arbidol has antiviral ability against CVB5 in vivo, we established a mouse model of infection. Mice infected with CVB5 had some symptoms of circulatory failure such as cyanosis and lack of blood perfusion in the tail and auricle, and there was evidence of virus replication in the heart and lungs. Pathological findings were consistent with the results from virus isolation, and all viral control mice died by day 18 after infection. Thus, CVB5 infection in BALB/c mice is a suitable in vivo model to study in vivo antiviral activity.
Orally administered Arbidol at 50 mg/kg body weight/day (once a day) after infection with Coxsackievirus B5 for 6 days significantly enhanced survival rate, reduced virus yields and released pathological abnormalities in lungs and hearts.
In summary, our results suggest that Arbidol can elicit protective antiviral activity against CVB5 in vitro and in vivo. The exact antiviral mechanism of Arbidol is still unknown. Arbidol may play a significant role in medical countermeasures against CVB5 infections.
Acknowledgments
We thank Drs. Wei Wang and Linming Zhao for primers and reagents, Dr. Po Ye for pathological analysis, and Dr. Gang Zheng for critical reading of the manuscript.
References
- 1.Fohlman J, Pauksen K, Morein B, Bjare U, Ilback NG, Friman G. High yield production of an inactivated coxsackie B3 adjuvant vaccine with protective effect against experimental myocarditis. Scand J Infect Dis Suppl. 1993;88:103–108. [PubMed] [Google Scholar]
- 2.Galbraith DN, Nairn C, Clements GB. Evidence for enteroviral persistence in humans. J Gen Virol. 1997;78(Pt 2):307–312. doi: 10.1099/0022-1317-78-2-307. [DOI] [PubMed] [Google Scholar]
- 3.Muir P, Nicholson F, Illavia SJ, McNeil TS, Ajetunmobi JF, Dunn H, Starkey WG, Reetoo KN, Cary NR, Parameshwar J, Banatvala JE. Serological and molecular evidence of enterovirus infection in patients with end-stage dilated cardiomyopathy. Heart. 1996;76:243–249. doi: 10.1136/hrt.76.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramsingh AI, Chapman N, Tracy S. Coxsackieviruses and diabetes. Bioessays. 1997;19:793–800. doi: 10.1002/bies.950190909. [DOI] [PubMed] [Google Scholar]
- 5.Hamby BB, Pallansch MA, Kew OM. Reemergence of an epidemic coxsackievirus B5 genotype. J Infect Dis. 1987;156:288–292. doi: 10.1093/infdis/156.2.288. [DOI] [PubMed] [Google Scholar]
- 6.Rotbart HA. Treatment of picornavirus infections. Antiviral Res. 2002;53:83–98. doi: 10.1016/S0166-3542(01)00206-6. [DOI] [PubMed] [Google Scholar]
- 7.Henke A, Wagner E, Whitton JL, Zell R, Stelzner A. Protection of mice against lethal coxsackievirus B3 infection by using DNA immunization. J Virol. 1998;72:8327–8331. doi: 10.1128/jvi.72.10.8327-8331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henke A, Zell R, Stelzner A. DNA vaccine-mediated immune responses in Coxsackie virus B3-infected mice. Antiviral Res. 2001;49:49–54. doi: 10.1016/S0166-3542(00)00132-7. [DOI] [PubMed] [Google Scholar]
- 9.Carrasco L. Picornavirus inhibitors. Pharmacol Ther. 1994;64:215–290. doi: 10.1016/0163-7258(94)90040-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenzo JC, Shellam GR, Lawson CM. Ganciclovir and cidofovir treatment of cytomegalovirus-induced myocarditis in mice. Antimicrob Agents chemother. 2001;45:1444–1449. doi: 10.1128/AAC.45.5.1444-1449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baykurt C, Caglar K, Ceviz N, Akyuz C, Secmeer G. Successful treatment of Epstein-Barr virus infection associated with myocarditis. Pediatr Int. 1999;41:389–391. doi: 10.1046/j.1442-200x.1999.01077.x. [DOI] [PubMed] [Google Scholar]
- 12.Rich R, McErlean M. Complete heart block in a child with varicella. Am J Emerg Med. 1993;11:602–605. doi: 10.1016/0735-6757(93)90011-Y. [DOI] [PubMed] [Google Scholar]
- 13.Garozzo A, Cutri CC, Castro A, Tempera G, Guerrera F, Sarva MC, Geremia E. Anti-rhinovirus activity of 3-methylthio-5-aryl-4-isothiazolecarbonitrile derivatives. Antiviral Res. 2000;45:199–210. doi: 10.1016/S0166-3542(00)00072-3. [DOI] [PubMed] [Google Scholar]
- 14.Leneva IA, Fediakina IT, Gus’kova TA, Glushkov RG. Sensitivity of various influenza virus strains to arbidol. Influence of arbidol combination with different antiviral drugs on reproduction of influenza virus A. Ter Arkh. 2005;77:84–88. [PubMed] [Google Scholar]
- 15.Leneva IA, Sokolova MV, Fediakina IT, Khristova ML, Fadeeva NI, Gus’kova TA. Study of the effect of antiviral drugs on the reproduction of the respiratory syncytial virus by enzyme immunoassay. Vopr Virusol. 2002;47:42–45. [PubMed] [Google Scholar]
- 16.Boriskin YS, Leneva IA, Pécheur EI, Polyak SJ. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr Med Chem. 2008;15:997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 17.Brooks MJ, Sasadeusz JJ, Tannock GA. Antiviral chemotherapeutic agents against respiratory viruses: where are we now and what's in the pipeline? Curr Opin Pulm Med. 2004;10:197–203. doi: 10.1097/00063198-200405000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Chai H, Zhao Y, Zhao C, Gong P. Synthesis and in vitro anti-hepatitis B virus activities of some ethyl 6-bromo-5-hydroxy-1H-indole-3-carboxylates. Bioorg Med Chem. 2006;14:911–917. doi: 10.1016/j.bmc.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 19.Boriskin YS, Pecheur EI, Polyak SJ. Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection. Virol J. 2006;3:56. doi: 10.1186/1743-422X-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi L, Xiong H, He J, Deng H, Li Q, Zhong Q, Hou W, Cheng L, Xiao H, Yang Z. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch Virol. 2007;152:1447–1455. doi: 10.1007/s00705-007-0974-5. [DOI] [PubMed] [Google Scholar]
- 21.Reed AF. A left superior vena cava draining the blood from a closed coronary sinus. J Anat. 1938;73:195–197. [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, But PP, Ooi VE. Antiviral activity and mode of action of caffeoylquinic acids from Schefflera heptaphylla (L.) Frodin. Antiviral Res. 2005;68:1–9. doi: 10.1016/j.antiviral.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Guskova TA, Leneva IA, Fedyakina IT, Chistyakov VV, Glushkov RG. Arbidol kinetics and its effect on influenza A virus replication in MDCK cell culture. Chemico Pharmaceut J. 1999;6:14–17. [Google Scholar]
- 24.Yamamoto H, Yoshida K, Kondo Y, Inoue K. Production of cornoside in Abeliophyllum distichum cell suspension cultures. Photochemistry. 1998;48:273–277. doi: 10.1016/S0031-9422(97)01134-5. [DOI] [Google Scholar]
- 25.Schulman JL. Effect of l-amantanamine hydrochloride (amantadine HCl) and methyl-l-adamantanethylamine hydrochloride (rimantadine HCl) on teansmission of influenza virus infection in mice. Proc Soc Exp Biol Med. 1968;128:1173–1178. [Google Scholar]
- 26.Anonymous Arbidol. Drugs R D. 1999;2:171–172. doi: 10.2165/00126839-199902030-00003. [DOI] [PubMed] [Google Scholar]
- 27.White J, Kartenbeck J, Helenius A. Membrane fusion activity of influenza virus. Embo J. 1982;1:217–222. doi: 10.1002/j.1460-2075.1982.tb01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guskova TA, Glushkov RG. Arbidol: a new antiviral, immunomodulator and interferon-inducer. Moscow: Timotech; 1999. [Google Scholar]


