Abstract
This study was performed to develop real-time PCR (qPCR) for detection of human seasonal and avian influenza viruses in duplex format. First duplex qPCR detects haemagglutinin (HA) gene of influenza virus A(H1N1)pdm09 and HA gene of influenza virus A(H3N2), the second reaction detects neuraminidase (NA) gene of influenza virus A(H3N2) and NA gene of influenza virus A(H1N1)pdm09 and A(H5N1), and the third reaction detects HA gene of influenza A(H5N1) and nonstructural protein gene of influenza B virus. Primers and probes were designed using multiple alignments of target gene sequences of different reference strains. Assays were optimised for identical thermocycling conditions. Their specificity was confirmed by conventional PCR and monoplex qPCR with nucleic acids isolated from different influenza viruses and other respiratory pathogens. Plasmid constructs with a fragment of specific gene were used to assess sensitivity of the assay. The limit of detection ranged from 27 to 96 cDNA copies/reaction. Clinical specimens (n = 107) have been tested using new assays, immunofluorescence and monoplex qRT-PCR. It has been shown that developed assays have been capable of rapid and accurate simultaneous detection and differentiation of influenza viruses. They are more sensitive than immunofluorescence and at least as sensitive as monoplex qRT-PCR.
Keywords: Influenza, Influenza Virus, Avian Influenza Virus, qPCR Assay, Respiratory Specimen
Introduction
Since 2009, influenza viruses of the A(H1N1)pdm09 and A(H3N2) subtypes as well as influenza B viruses have been responsible for human influenza diseases in the northern hemisphere [11, 12, 33, 35, 37]. Nevertheless, infections with a highly pathogenic avian influenza virus A(H5N1) have been observed in the human population. According to data provided by the World Health Organization (WHO), as of August 10, 2012, a total of 608 human infections caused by A(H5N1) were laboratory confirmed, and 359 fatal cases (59.0 %) were reported since 2003 [36]. Thus, despite the fact that the end of the pandemic caused by A(H1N1)pdm09 was announced in August 2010, there is still a potential threat of another influenza pandemic caused by HPAI A(H5N1) virus [32]. Certainly, the high level of changeability of influenza viruses together with an animal reservoir for these pathogens makes it possible that a completely new virus variant with pandemic potential will emerge [6, 30]. Therefore, high-quality surveillance together with the availability of rapid and accurate diagnostic methods is a big challenge in the case of influenza virus infections. Subtyping of influenza viruses is necessary for surveillance and usually is less important for the diagnosis of an individual patient’s illness, since a clinical diagnosis of influenza is sufficient to commence appropriate treatment [34]. Nevertheless, when a new influenza virus variant is suspected to cause human infections, as was the case with the H1N1 of swine origin in 2009, virus subtyping seems to be justified and necessary to differentiate between the new variant and the older ones [31]. This may be required for appropriate control of infections and case management, especially at the beginning of a new outbreak.
Different laboratory techniques may be used for diagnostics of influenza infections, but nucleic acid amplification techniques have become the most useful, rapid, specific and sensitive when compared to other methods [20, 26, 34]. An improvement in influenza diagnostics depends not only on the technical capabilities of the laboratory equipment but also the skills of the users to apply these pieces of equipment in an optimal way to accomplish specific goals. Therefore, many efforts have been undertaken to develop multiplex PCR or real-time PCR (qPCR) reactions enabling different viruses or different types/subtypes of the same virus to be detected in a single reaction. The development of multiplex qPCR reactions is a difficult task, especially when three or more target genes are to be detected in one reaction. These difficulties are caused by overlapping of fluorescence of fluorophores used for probe labelling. Another difficulty emerges from the changeability of influenza viruses, and this mostly applies to the genes encoding haemagglutinin (HA) and neuraminidase (NA), which determine the subtype of the influenza virus [6]. Thus, the design of appropriate primers and probes enabling the detection of different viruses of a given subtype is a big challenge.
In this paper, we report the development and optimisation of three qualitative two-step duplex qPCR assays for detection of human seasonal and avian influenza viruses, A(H1N1)pdm09, A(H3N2), A(H5N1) and B, and we demonstrate the high specificity, sensitivity and usefulness of these assays.
Materials and methods
Reference strains and specimens
The reference strains of influenza viruses A(H3N2) (A/Moscow/10/1999; A/Christchurch/28/2003; A/California/7/2004; A/Wisconsin/67/2005; A/Brisbane/10/2007; A/Perth/16/2009), A(H1N1)pdm09 (A/California/7/2009, A/Denmark/528/2009), A(H1N1) (A/Salomon Islands/3/2006, A/Brisbane/59/2007, A/Fukushima/141/2006) and B (B/Shanghai/361/2002, B/Malaysia/2506/2004, B/Florida/7/2004, B/Egypt/144/2005, B/Florida/4/2006; B/Brisbane/60/2008) used in the present study were kindly provided by the World Health Organization, Collaborating Centre for Reference and Research on Influenza (London, UK). Viruses were propagated in the allantoic cavities of 11-day-old chicken embryonated eggs. Then, collected fluids were titrated using a haemagglutination test performed with turkey red blood cells and were stored at −70 °C [20]. Inactivated A(H5N1) strains A/Duck/Vietnam/TG24-01/2005 and A/Whooper Swan Germany/R65-2/2006 were kindly provided by the Robert Koch Institut, Nationales Referenzzentrum für Influenza (Berlin, Germany). Other respiratory viruses: human respiratory syncytial virus A and B (RSV-A, -B), human parainfluenza virus 1, 2 and 3 (PIV-1, -2, -3), human adenovirus (hAdV); human metapneumovirus (hMPV), human rhinovirus (hRV), and human coronavirus (hCoV) HKU1and NL63, were kindly provided by the Department of Virology, Erasmus Medical Center (Rotterdam, The Netherlands). The reference strains of Streptococcus pneumoniae (ATCC 6301), Klebsiella pneumoniae (ATCC 4211) and Haemophilus influenzae (ATCC 9006) were provided by the Chair and Department of Medical Microbiology, Medical University of Warsaw, Poland.
The clinical usefulness of the developed assays was confirmed by testing RNA obtained from 57 samples provided by the quality control panels WHO External Quality Assessment Programme (WHO EQAP) and Quality Control for Molecular Diagnostics (QCMD; http://www.qcmd.org) (A(H1N1)pdm09, n = 10; A(H3N2), n = 10; A(H5N1), n = 22; influenza B virus, n = 7; and 8 samples negative for influenza A and B virus). The A(H5N1) RNA samples from EQAP panels belonged to different clades: 1, 2.1, 2.2, 2.3.2., 2.3.4.
A comparison of different laboratory techniques with the newly developed assays was done using 107 respiratory specimens (combined nose and throat swabs) collected from patients with flu symptoms in Poland during the influenza epidemic seasons 2008/2009 (n = 27), 2009/2010 (n = 55) and 2010/2011 (n = 25).
Primer and probe design
Primers and fluorescent probes, based on TaqMan chemistry, were designed to correspond to specific highly conserved regions of the influenza virus A haemagglutinin (H1)pdm09 (but not seasonal influenza A(H1N1) viruses), the H3 and H5 genes, the influenza virus A neuraminidase N1 and N2 genes, and the influenza B virus (both Yamagata and Victoria lineage) nonstructural protein (NS) gene. The most specific and conserved target regions were identified following multiple alignments of the nucleotide sequences of the respective HA/NA/NS genes of different influenza virus strains available from the Influenza Sequence Database (ISD) [19] and GenBank database (National Center of Biotechnology Information). Primer and probe sets were designed according to all basic rules [18, 23], using PrimerQuestSM (Integrated DNA Technologies, Inc. http://eu.idtdna.com/Scitools/Applications/Primerquest/Advanced.aspx) and LightCycler Probe Design2 software (Roche Diagnostics). The reference sequences were as follows: influenza A(H1N1)pdm09 virus, accession no. FJ981613 (HA) and FJ984386 (NA); A(H3N2) virus, accession no. GQ293081 (HA) and CY081429 (NA); A(H5N1) virus, accession no. DQ464354 (HA) and DQ464355 (NA); influenza B virus, accession no. JN992795 (NS).
Primer and probe sequences were analysed for their G + C content and formation of dimers, hairpins and secondary structures using OligoAnalyzer 3.1 (Integrated DNA Technologies, Inc; http://eu.idtdna.com/analyzer/Applications/OligoAnalyzer/). The specificity of each primer and probe set was measured using BLAST against the entire human genome and influenza viruses other than targeted ones to prevent the amplification of non-specific, sequence-related secondary products. All oligonucleotides were synthesised by Genomed S.A. (Warsaw, Poland). The sequences of the primers and probes are shown in Table 1.
Table 1.
Sequences of primers and probes designed and selected for use in the qPCR assays
| Name | Oligonucleotide sequence (5’–3’) | Fluorophore (5’/3’) | Amplicon length (nt) | Target (gene) | Nucleotide positions | GenBank accession no. of reference sequence |
|---|---|---|---|---|---|---|
| H1v_F | GTATTATCATTTCAGATACACCAGTCC | 842-868 | ||||
| H1v_R | GACATTTTCCAATTGTGATCGG | 119 | HA | 961-940 | FJ981613 | |
| H1v_P | AACACCAGCCTCCCATTTCAGAA | JOE/BHQ1 | 910-932 | (H1N1)pdm09 | ||
| H3_F | TTGATTAACAGCACAGGGAATCTA | 778-801 | ||||
| H3_R | TGCATTCAGAATTGCATTTGCC | 114 | HA | 892-821 | GQ293081 | |
| H3_P | AGCTCAATAATGAGATCAGATGCACC | CFR610/BHQ2 | 841-866 | H3N2 | ||
| H5_F | TTTCATTGCTCCAGAATATGCATACA | 786-811 | ||||
| H5_R | GGAATGGCATACTAGAGTTTATCG | 136 | HA | 922-899 | DQ464354 | |
| H5_P | ACTGCAACACCAAGTGTCAAACTCC | JOE/BHQ1 | 865-889 | H5N1 | ||
| B_F | GATCCTCAACTCACTCTTCGA | 717-737 | ||||
| B_R | CTCTTCTGGTGATAATCGGTG | 121 | NS | 837-817 | JN992795 | |
| B_P | ACATTCAAAGCCAATTCGAGCAGCT | CFR610/BHQ2 | 753-777 | B | ||
| N1_F | TAAGACCTTGCTTCTGGGTT | 1253-1272 | ||||
| N1_R | GACCAACCCACAGTGTC | 114 | NA | 1367-1351 | FJ984386 | |
| N1_P | GCAGCATATCCTTTTGTGGTGT | CFR610/BHQ2 | 1321-1342 | (H1N1)pdm09 | ||
| N1(H5)_F | AGTTGGTTGACAATTGGAATTT | 505-526 | ||||
| N1(H5)_R | TTACACATGCACATTCAGACT | 138 | NA | 643-623 | DQ464355 | |
| N1(H5)_P | GCATAATAACAGACACCATCAAGAGTTGGAGGA | CFR610/BHQ2 | 587-601 | H5N1 | ||
| N2_F | CCTTTGATGATGGAAATGACG | 1058-1078 | ||||
| N2_R | CTGTCAACTATGACTTGCCT | 141 | NA | 1199-1180 | CY081429 | |
| N2_P | AAAGTCATTGrAGGCTGGTCCAAmC | JOE/BHQ1 | 1131-1155 | H3N2 |
HA = haemagglutinin; NA = neuraminidase; NS = nonstructural protein; F = forward primer; R = reverse primer; P = probe; r = A, G; m = A, C; CFR610 = California Fluor Red 610, BHQ = Black Hole Quencher
Nucleic acid extraction
A QIAamp Viral RNA Mini Kit (QIAGEN, Germany) was used to purify total RNA from 140 μl of allantoic fluid (reference strain) or clinical specimens. DNA was isolated from 200 μl of bacterial samples using a High Pure Viral Nucleic Acid Kit (Roche Diagnostics). All isolations were performed according to the manufacturer’s instructions, and extracted nucleic acids were eluted in a final volume of 50 μl of elution buffer. Isolated nucleic acids were stored at −70 °C.
Reverse transcription
The viral RNA was reverse-transcribed to cDNA using a Transcriptor First Strand cDNA Synthesis Kit (Roche Diagnostics) with random hexamer primers and 5.0 μl RNA template according to the manufacturer’s instructions. Negative controls were included in each run. The cDNA samples that were obtained were stored at −70 °C until further analysis.
Conventional PCR
The reaction mixture contained Hot Start PCR Buffer (Thermo-Scientific Fermentas), 2.5 μl dNTP mix (0.2 mM each), 2.0 mM MgCl2, 20 μM each primer, 1.0 U Maxima Hot Start Taq DNA polymerase (Thermo-Scientific Fermentas), 2.5 μl of cDNA (influenza B, A(H1N1)pdm09, A(H3N2) or A(H5N1)) and sterile DNase/RNase-free water to a final volume of 25.0 μl. Amplification was performed in a Veriti™ 96-Well Thermal Cycler (Applied Biosystems Inc., USA) as follows: a single cycle of initial denaturation/enzyme activation for 4 min at 95 °C, then 45 cycles of denaturation at 95 °C for 1 min, annealing at 60 °C for 15 s and extension at 72 °C for 20 s. After the last cycle, the reaction was completed by a final extension at 72 °C for 5 min. PCR products in a total volume of 20 μl were separated in a 2.0 % agarose gel stained with GelRedTM 10000× solution in 1× TAE buffer. A GeneRuler 100 bp DNA Ladder was used for estimating the molecular size of the bands obtained (Thermo-Scientific Fermentas).
Real-time PCR
In order to optimise the reactions, the monoplex and duplex reactions were carried out with 1.0, 1.5 or 2.0 μM primers and 100, 150 or 200 nM probes. The assays were performed in a two-step reaction using a LightCycler® TaqMan® Master Kit (Roche Diagnostics) and 5.0 μl of cDNA.
Assays were optimised for using one identical set of thermocycling conditions. Fluorescence levels were measured at the wavelengths 560 nm and 610 nm at the end of the elongation step of each cycle. All qPCR reactions and data analysis were performed using a LightCycler® 2.0 instrument (Roche Diagnostics). Colour compensation software was used in the analysis according to the manual provided by Roche Diagnostics. The crossing point (Cp) value was calculated by determining the point (PCR cycle) at which the fluorescence intensity rises above background (detection limit). Positive and negative controls were included in each run.
Plasmid constructs
In order to assess the sensitivity of the assays, seven plasmid constructs were constructed by cloning a fragment of the HA or NA gene (385 bp) of influenza A(H1N1)09pdm, A(H3N2), and A(H5N1) virus and the NS protein gene of influenza B virus into SmaI-digested pBluescript II SK(-) (Table 2) (Epoch LifeScience, Missouri City, USA). Plasmid DNA was sequenced to confirm correct insertion into the vector. DH5α electrocompetent E. coli cells were transformed with 100-150 ng of each construct using a MicroPulserTM (Bio-Rad) according to the standard protocol. Plasmid DNA was purified using a Plasmid Midi AX Kit (A&A Biotechnology, Gdynia, Poland) according to the manufacturer’s instructions. The concentration of recombinant plasmid DNA was determined spectrophotometrically by measuring the absorbance of UV light at 260 nm and 280 nm. Plasmid DNA was cut with the restriction enzyme PdiI (Thermo-Scientific Fermentas) and serially diluted in sterile, DNase/RNase-free water. The limit of detection (LOD) of each assay was determined by analysis of tenfold serial dilutions of plasmid DNA in the range of 105-101 copies. Each dilution was prepared and analyzed in six independent replicates. Probit analysis was used to calculate the LOD concentration [4].
Table 2.
Characteristic of HA, NA and NS gene fragments included in plasmid constructs
| Nucleotide positions | GenBank accession no. | Strain | Origin |
|---|---|---|---|
| 706-1090 | FJ981613 | A/California/07/2009 (H1N1)09pdm | Segment 4, H1 |
| 1026-1410 | FJ984386 | Segment 6, N1 | |
| 666-1050 | GQ293081 | A/Perth/16/2009 (H3N2) | Segment 4, H3 |
| 936-1320 | CY081429 | Segment 6, N2 | |
| 673-1057 | DQ464354 | A/swan/Germany/R-65/2006 (H5N1) | Segment 4, H5 |
| 376-760 | DQ464355 | Segment 6, N1 | |
| 593-977 | JN992795 | B/Florida/02/2010 | Segment 8, NS |
Comparison with immunofluorescence assay and monoplex RT-PCR
A total of 107 respiratory specimens were tested using the new duplex qPCR assays in comparison with the following standard rapid laboratory methods: immunofluorescence assay (IFA) for influenza A and B viruses and monoplex qPCR for influenza A(H1N1)pdm09 and A(H3N2) viruses and influenza B virus. The IFA was performed using the commercial IMAGENTM Influenza A and B Kit (Oxoid) according to the manufacturer’s instructions. Monoplex qPCR was performed with primers/probe sets from the Centers for Disease Control and Prevention (CDC) RT-PCR Protocol for detection of influenza A(H1N1)pdm09 (WHO, 2009) and influenza A(H3N2) and B viruses (available as part of a material transfer agreement with CDC). Amplification was carried out in a 25.0-μl reaction volume using a SuperScript® III Platinum® One-Step qRT-PCR Kit (Invitrogen Life Technologies) and 5.0 μl of extracted RNA template. The conventional PCR with primers specific for β-actin was performed for all negative samples.
Results
The purpose of the present study was the development of qualitative real-time PCR assays for the detection and differentiation of seasonal and avian influenza viruses.
Assay optimisation
Real-time PCR assays were first performed as monospecific assays with cDNA from targeted viruses and optimised to increase the sensitivity and efficiency of amplification. The monospecific assays were then combined in three duplex reactions and then optimised. The concentrations of primers and probes that gave the best results were as follows: 1.0 μM for influenza virus H3-specific primers and 1.5 μM for influenza virus H1pdm09-, H5-, N1-, N2- and B-specific primers, and 150 nM for each of the probes in a total volume of 20 μl of the reaction mixture. A duplex real-time PCR system was used to identify (i) the (H1)pdm09 and H3 subtypes, (ii) the N2 and N1 subtypes, and (iii) the H5 subtype and the B type. Assays were performed in a two-step reaction and optimised for using the same thermocycling conditions. The best amplification results were obtained with activation of termostable hot-start DNA polymerase for 10 min at 95 °C, followed by 40 cycles comprising denaturation (10 s at 95 °C), annealing (20 s at 60 °C) and strand elongation (5 s at 72 °C). At the end of each strand-elongation step, a single measurement of fluorescence was performed.
Specificity of RT-PCR and duplex qPCR
The selected primers and probes showed no nonspecific homologies in a nucleotide BLAST search including human genomic and influenza viruses other than the targeted ones. To confirm their specificity experimentally, each assay was performed with nucleic acids isolated from the reference strains of different influenza viruses and a panel of common human respiratory pathogens as listed in Materials and methods. All primer/probe sets detected only RNA (cDNA) from the corresponding virus in the conventional PCR as well as in the newly developed qPCR assays (in monoplex and duplex format). No nonspecific signals were observed, and there was no cross-reaction with other common viral or bacterial respiratory pathogens (Fig. 1A, B, C). Duplex qPCR with combined templates from different influenza viruses gave specific and proper results without cross-talk of fluorescence from the 560 nm and 610 channels when the colour compensation software was applied.
Fig. 1.
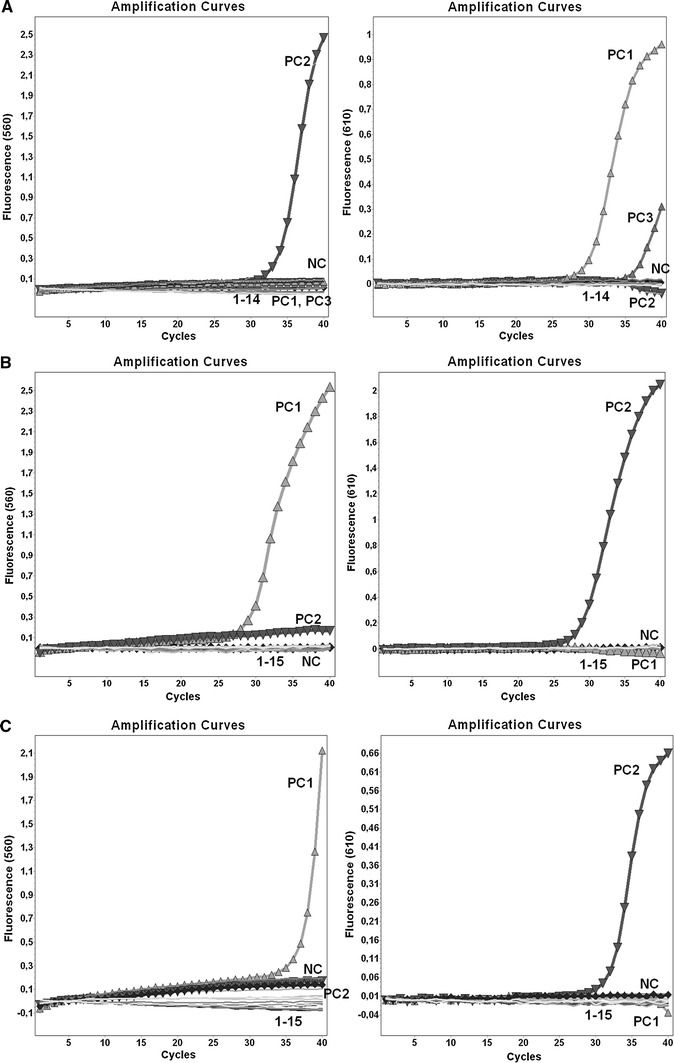
A Specificity of the duplex qPCR assay for the detection of N2 (channel 560) and N1 (channel 610) influenza neuraminidase. NC, negative control; PC1, positive control (H1N1)pdm09; PC2, positive control H3N2; PC3, positive control H5N1; 1, B/Brisbane/60/2008; 2, A/Brisbane/59/2007 (H1N1); 3, RSV-A; 4, RSV-B; 5, PIV-1; 6, PIV-2; 7, PIV-3; 8, hAdV; 9, hMPV; 10, hRV; 11, hCoV HKU1and NL63; 12, H. influenzae ATCC 9006; 13, S. pneumoniae ATCC 6301; 14, K. pneumoniae ATCC 4211. B Specificity of the duplex qPCR assay for the detection of H5 influenza haemagglutinin (channel 560) and influenza B nonstructural protein (channel 610). NC, negative control; PC1, positive control H5N1; PC2, positive control influenza B virus; 1, A/Denmark/528/2009 (H1N1)pdm09; 2, A/Perth/16/2009 (H3N2); 3, A/Solomon Islands/3/2006 (H1N1); 4, RSV-A; 5, RSV-B; 6, PIV-1; 7, PIV-2; 8, PIV-3; 9, hAdV; 10, hMPV; 11, hRV; 12, hCoV HKU1and NL63; 13, H. influenzae ATCC 9006; 14, S. pneumoniae ATCC 6301; 15, K. pneumoniae ATCC 4211. C. Specificity of the duplex qPCR assay for the detection of (H1)pdm09 (channel 560) and H3 (channel 610) influenza haemagglutinin. NC, negative control; PC1, positive control (H1N1)pdm09; PC2, positive control H3N2; 1, A/Whooper Swan Germany/R65-2/2006 (H5N1); 2, A/Solomon Islands/3/2006 (H1N1); 3, B/Brisbane/60/2008; 4, RSV-A; 5, RSV-B; 6, PIV-1; 7, PIV-2; 8, PIV-3; 9, hAdV; 10, hMPV; 11, hRV; 12, hCoV HKU1and NL63; 13, H. influenzae ATCC 9006; 14, K. pneumoniae ATCC 4211; 15, S. pneumoniae ATCC 6301
Clinical performance
In the present study, 57 samples from control panels that were previously diagnosed as positive for influenza A(H1N1)pdm09, A(H3N2), A(H5N1), influenza B or negative for influenza (monoplex one-step qPCR or conventional RT-PCR; data not shown) were used to evaluate the clinical usefulness of the newly developed assays. None of the tested samples showed any false-positive results or nonspecific signals (Fig. 2A, B, C). All positive specimens were found to be positive when tested using our duplex qPCR assays. Despite the high degree of genetic variability and co-circulation of different clades of A(H5N1) subtypes, our assay identified the HA genes of all of the currently circulating clades examined. In eight negative specimens, influenza virus A(H1N1)pdm09, A(H3N2), A(H5N1) and B were not detected, and moreover, no amplified product was visible after agarose gel electrophoresis of qPCR negative samples. These results confirmed, in 100 % of the samples, both the specificity and the clinical usefulness of these assays.
Fig. 2.
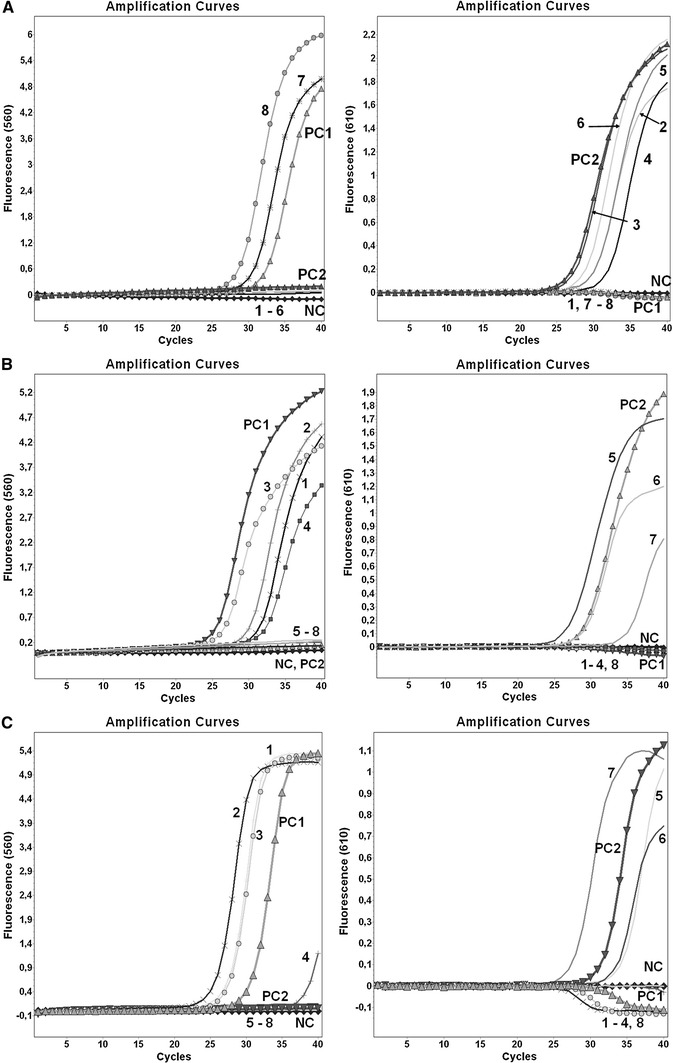
A Detection of N2 (channel 560) and N1 (channel 610) influenza neuraminidase using the duplex qPCR assay. NC, negative control; PC1, positive control H3N2; PC2, positive control (H1N1)pdm09; 1-8, specimens from the quality control panels (EQAP) (1, sample 11/2010 (B); 2, sample 17/2011 (H1N1)pdm09; 3, sample 12/2009 (H1N1)pdm09; 4, sample 20/2011 (H1N1)pdm09; 5, sample 10/2011 (H5N1); 6, sample 16/2009 (H1N1)pdm09; 7, sample 03/2010 (H3N2); 8, sample 14/2010 (H3N2)). B Detection of H5 influenza haemagglutinin (channel 560) and influenza B nonstructural protein (channel 610) using the duplex qPCR assay. NC, negative control; PC1, positive control H5N1; PC2, positive control influenza B virus; 1-8, specimens from the quality control panels (EQAP) (1, sample 04/2009 (H5N1); 2, sample 06/2010 (H5N1); 3, sample 04/2010 (H5N1); 4, sample 17/2009 (H5N1); 5, sample 07/2010 (B); 6, sample 11/2011 (B); 7, sample 11/2010 (B); 8, sample 15/2011 (H3N2)). C Detection of (H1)pdm09 (channel 560) and H3 (channel 610) influenza haemagglutinin using the duplex qPCR assay. 1, negative control; PC1, positive control (H1N1)pdm09; PC2, positive control H3N2; 1-8, specimens from the quality control panels (EQAP) (1, sample 12/2010 (H1N1)pdm09; 2, sample 05/2010 (H1N1)pdm09; 3, sample 04/2011 (H1N1)pdm09; 4, sample 10/2010 (H1N1)pdm09; 5, sample V02/2011 (H3N2); 6, sample 03/2010 (H3N2); 7, sample 15/2011 (H3N2); 8, sample 19/2010 (H5N1))
Sensitivity of duplex qPCR
In the qPCR assay, all serial dilutions of the template, containing 101 to 105 plasmid DNA copies were successfully detected. The results of LOD in the duplex qPCR assays, expressed as DNA copies per reaction tube, are shown in Table 3.
Table 3.
Limits of detection (LOD) of the qPCR assays
| Influenza virus detected in a given qPCR assay | LOD (DNA copies/reaction) | 95 % confidence interval (DNA copies/reaction) |
|---|---|---|
| (H1)pdm09 | 27 | 22-58 |
| H3 | 37 | 29-72 |
| H5 | 33 | 29-67 |
| B | 42 | 31-79 |
| N1 | 96 | 75-137 |
| N2 | 84 | 71-111 |
Comparison of duplex qPCR with standard methods
A total of 107 respiratory specimens were tested using the newly developed assays and the standard rapid laboratory methods IF and monoplex qPCR, as recommended by the CDC. All results of monoplex qPCR were consistent with those obtained by duplex qPCR, i.e., 60 samples were identified as influenza A(H1N1)pdm09, 17 samples as influenza A(H3N2), 18 samples as influenza B and 12 samples were negative for influenza virus. Neither false positive nor false negative results were observed. The results of IFA were consistent with both qPCR assays for 96 specimens (73 influenza A positive, 14 influenza B positive and 9 influenza A and B negative). Eight specimens that were negative for influenza A and B virus by IF assay, were subsequently determined to be positive in both qPCR assays for influenza A(H1N1)pdm09 (n = 3), A(H3N2) (n = 1) and B (n = 4). Three specimens that were positive for influenza A virus in the IF assay were negative in both qPCR assays. Positive results of amplification of the β-actin gene were obtained for all samples.
Discussion
Early detection and virological surveillance of influenza virus strains circulating regionally and globally is a key element in both updating the influenza vaccine composition and epidemic/pandemic prevention and control [29, 34]. The co-circulation of multiple types and subtypes of seasonal influenza viruses increases the difficulty of virus identification and diagnosis. Molecular methods based on PCR allow rapid, accurate, sensitive and direct detection of influenza viruses, including subtyping of influenza virus A, and have become a valuable tool in a routine diagnostics as well as surveillance. However, the high level of genetic variability of influenza viruses, especially among viruses of type A, makes the development of specific molecular assays difficult. Simultaneously, this changeability requires diagnostic methods to be constantly monitored and updated in the event of the emergence of a new genetically distinct strain. Many multiplex qPCR assays for detection of influenza viruses have been developed [2, 5, 7–9, 14, 27, 28]. Nevertheless, to our knowledge, there is only one paper describing multiplex assays for the detection of each of the following influenza viruses: type B, subtypes A(H3N2), A(H5N1) and A(H1N1) but without differentiation between A(H1N1)pdm09 and seasonal A(H1N1) [24]. Thus, three duplex qPCR assays designed in this study for concurrent detection of influenza B, A(H3N2), A(H1N1)pdm09 and A(H5N1) viruses offer a valuable strategic diagnostics approach and could be routinely adapted for the identification of not only seasonal influenza viruses circulating in the human population but also the unusual H5N1 subtype of avian origin.
Genes that encode internal virus proteins such as the non-structural NS protein are highly conserved among different influenza viruses and thus are useful targets for the universal detection of virus type [34]. Since all influenza virus subtypes are defined on the basis of differences in their surface glycoproteins, haemagglutinin and neuraminidase gene targets are the most effective and recommended approach for specific subtyping of influenza A viruses [13]. In the present study, the corresponding genes have been also shown to be useful for differentiation of specific types and subtypes. However, because of the large variability among A(H5N1) strains, two primer/probe sets were selected for the efficient detection of N1. Both probes were labelled with the same fluorophores because the duplex reaction was designed for identification of N1 without differentiation between A(H1N1)pdm09 and A(H5N1).
In multiplex qPCR, amplicons are discriminated by using distinct fluorogenic probes for each nucleic acid target or, alternatively, melting curves when nonspecific DNA-intercalating fluorophores are used as the detection method [18]. The limited number of fluorophores, which show significant overlap in their emission spectra, as well as the finite number of emission channels in a qPCR instrument, limits the number of targets that can be detected a in a single reaction by multiplexing [15, 18]. In the present study, a LightCycler® 2.0 system with an LED light source was used. A duplex format of the reaction was based on hydrolysis of probes labelled with JOE reporter dye and the quencher BHQ-1 or California Fluor Red 610 and the quencher BHQ-2. Despite the two different emission maxima of the fluorophores and measurement of fluorescence in two different detection channels (560 nm for JOE and 610 nm for California Fluor Red 610), the use of color compensation software was necessary due to overlapping of fluorescence. According to literature data [15] as well as our results (data not shown), the LightCycler® 2.0 is an appropriate and well-working platform to detect up to two different fluorophores in a single reaction with TaqMan probes. Designed primer and probe sets labelled in the proposed format can be differently combined and, after validation, used in other duplex formats: (H1)pdm09 with N1, H3 with N2, and H5 with N1. Then, a complete identification of a specific subtype, including haemagglutinin and neuraminidase genes, is possible.
In clinical evaluation based on respiratory specimens collected from patients with influenza symptoms in three epidemic seasons, the concordance rate between the results of the newly developed and validated duplex qPCR and monoplex qPCR assays recommended by CDC was 100.0 %. Nevertheless, comparison with the IF assay showed an advantage of molecular methods. The discrepant results between qPCR and IFA comprised 10.3 % (11/107) of the total. The newly developed assays resulted in eight additional positives that were missed by the routine IF method. Moreover, three specimens that were positive for influenza A virus in IFA were actually negative, as each qPCR assay and also virus isolation (data not shown) gave negative results. These data are consistent with numerous reports showing that PCR-based methods are more sensitive than IFA for the detection of viral infections [3, 10, 16, 17, 22].
Differences in the detection of viruses by various tests can be explained at least partially by the different targets used for each method. The sensitivity of IFA is significantly influenced by the quality of the specimen, since virus-infected cells are very labile and may be easy damaged by inappropriate handling or storage of clinical specimens before processing. Moreover, the analysis and interpretation of results obtained by IFA are often subjective, depending on the experience of the investigator. Molecular methods allow the detection of nucleic acids from viable as well as non-viable viruses, even when the viral copy number is low, and thus exhibit the highest sensitivity. Although PCR methods may be affected by PCR inhibitors, the occurrence of inhibition of amplification in respiratory specimens is rare. Among 545 respiratory specimens tested by Stauffer et al. [25], only 12 (2.2 %) were inhibited in PCR. In addition, RNA/DNA-degrading enzymes present in the specimens, as well as reagent degradation, may reduce the sensitivity of PCR reactions. Therefore, apart from negative and positive controls, an internal control confirming the presence of human nucleic acids and indicating the acceptable quality of specimen and template, should be also used in each run. In the duplex assays described here, the internal control was not included, and thus all negative samples were tested for the presence of inhibitors to eliminate false negative results.
Another limitation is the principle of the PCR and the fact that influenza viruses (especially influenza A) virus evolve constantly [1]. On one hand, high specificity of the primers and probe is crucial for qPCR assays. On the other hand, nucleotide variation in a primer/probe binding region of newly emerging strains may lead to much lower efficiency of hybridisation of a primer or probe with the template. As a consequence of this, a significant decrease in assay sensitivity – or even false results – can be obtained. Thus, the use of different target gene assays is more appropriate for correct detection and identification of circulating influenza viruses than detection of only one gene [31]. The inclusion of highly conserved regions of two different target genes (HA and NA) in the assay described here definitely increases the possibility of detection of a new influenza A strain and significantly decreases the risk that, in the event of a mutation, detection of a specific virus will fail. In the present study, the selection of the target sequences used for the qPCR assays did not contribute to any false negative results.
In conclusion, the three newly developed and validated duplex qPCR assays are capable of rapid and accurate simultaneous detection of influenza viruses, allowing influenza B virus and all currently circulating strains of human seasonal influenza A viruses (H1N1pdm09 and H3N2) as well as avian influenza A(H5N1) to be differentiated. All assays can be run under the same thermal conditions, providing a valuable method for both influenza surveillance and early diagnosis.
Acknowledgments
This study was financed by funds for science in 2007-2012 (scientific project no. NN404 1036 33) received from the Ministry of Science and Higher Education. The authors would like to thank Ms Dorota Urszula Ostapiuk from the Department of Influenza Research, National Influenza Center (NIPH-NIH, Warsaw), for her technical support, and Magdalena Rzewuska, PhD and Mrs. Alicja Grzechnik from the Department of Bacteriology and Molecular Biology (Faculty of Veterinary Medicine, Warsaw University of Life Sciences) for their assistance regarding work on plasmid constructs. We are grateful to Dr. Małgorzata Gieryńska from Department of Immunology (Faculty of Veterinary Medicine, Warsaw University of Life Sciences) and Prof. Marta Wróblewska from the Chair and Department of Medical Microbiology (Medical University of Warsaw) for their kind help during preparation of the manuscript. Acknowledgements are also addressed to sixteen Voivodeship Sanitary Epidemiological Stations for providing some of the clinical specimens.
References
- 1.Arias CF, Escalera-Zamudio M, Soto-Del Río MdeL, Cobián-Güemes AG, Isa P, López S (2009) Molecular anatomy of 2009 influenza virus A (H1N1). Arch Med Res 40:643–654 [DOI] [PubMed]
- 2.Boivin G, Côté S, Déry P, De Serres G, Bergeron MG. Multiplex real-time PCR assay for detection of influenza and human respiratory syncytial viruses. J Clin Microbiol. 2004;42:45–51. doi: 10.1128/JCM.42.1.45-51.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bredius RG, Templeton KE, Scheltinga SA, Claas EC, Kroes AC, Vossen JM. Prospective study of respiratory viral infections in pediatric hemopoietic stem cell transplantation patients. Pediatr Infect Dis J. 2004;23:518–522. doi: 10.1097/01.inf.0000125161.33843.bb. [DOI] [PubMed] [Google Scholar]
- 4.Burns M, Valdiva H. Modelling the limit of detection in real-time quantitative PCR. Eur Food Res Technol. 2008;226:1513–1524. doi: 10.1007/s00217-007-0683-z. [DOI] [Google Scholar]
- 5.Chen Y, Ciu D, Zheng S, Yang S, Tong J, Yang D, Fan J, Zhang J, Lou B, Li X, Zhuge X, Ye B, Chen B, Mao W, Tan Y, Xu G, Chen Z, Chen N, Li L. Simultaneous detection of influenza A, influenza B, and respiratory syncytial viruses and subtyping of influenza A H3N2 virus and H1N1 (2009) virus by multiplex real-time PCR. J Clin Microbiol. 2011;49:1653–1656. doi: 10.1128/JCM.02184-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox NJ, Subbarao K. Global Epidemiology of Influenza: past and present. Ann Rev Med. 2000;51:407–421. doi: 10.1146/annurev.med.51.1.407. [DOI] [PubMed] [Google Scholar]
- 7.van Elden LJ, Nijhuis M, Schipper P, Schuurman R, Loon AM. Simultaneous detection of influenza viruses A and B using real-time quantitative PCR. J Clin Microbiol. 2001;39:196–200. doi: 10.1128/JCM.39.1.196-200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis JS, Curran MD. Simultaneous molecular detection and confirmation of influenza AH5, with internal control. Methods Mol Biol. 2011;665:161–181. doi: 10.1007/978-1-60761-817-1_10. [DOI] [PubMed] [Google Scholar]
- 9.Enders KO, Cheng PPKC, Antia YY, Hoang TL, Lim WWL. Influenza A H5N1 detection. Emerg Infect Dis. 2005;11:1303–1305. doi: 10.3201/eid1108.041317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdman DD, Weinberg GA, Edwards KM, Walker FJ, Anderson BC, Winter J, González M, Anderson LJ. GeneScan reverse transcription-PCR assay for detection of six common respiratory viruses in young children hospitalized with acute respiratory illness. J Clin Microbiol. 2003;41:4298–4303. doi: 10.1128/JCM.41.9.4298-4303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Centre for Disease Prevention and Control (ECDC) (2011). Influenza surveillance in Europe 2010-2011 http://www.ecdc.europa.eu/en/publications/Publications/111209_SUR_Influenza_surveillance_Europe%20_2010_2012.pdf
- 12.European Centre for Disease Prevention and Control (ECDC) (2012). Influenza in Europe—Season 2011-2012. http://www.ecdc.europa.eu/en/publications/Publications/Influenza-Europe-2011-2012-surveillance-report.pdf
- 13.Fereidouni SR, Beer M, Vahlenkamp T, Starick E (2009) Differentiation of two distinct clusters among currently circulating influenza A(H1N1)v viruses, March-September 2009. Euro Surveill 14. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19409. (pii: 19409) [PubMed]
- 14.Huber I, Campe H, Sebah D, Hartberger C, Konrad R, Bayer M, Busch U, Sing A (2011) A multiplex one-step real-time RT-PCR assay for influenza surveillance. Euro Surveill 16. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19798. (pii: 19798) [PubMed]
- 15.Jothikumar P, Hill V, Narayanan J. Design of FRET-TaqMan probes for multiplex real-time PCR using an internal positive control. Biotechniques. 2009;46:519–524. doi: 10.2144/000113127. [DOI] [PubMed] [Google Scholar]
- 16.Kuypers J, Wright N, Ferrenberg J, Huang ML, Cent A, Corey L, Morrow R. Comparison of real-time PCR assays with fluorescent-antibody assays for diagnosis of respiratory virus infections in children. J Clin Microbiol. 2006;44:2382–2388. doi: 10.1128/JCM.00216-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao RS, Tomalty LL, Majury A, Zoutman DE. Comparison of viral isolation and multiplexs real-time reverse transcription-PCR for confirmation of respiratory syncytial virus and influenza virus detection by antigen immunoassays. J Clin Microbiol. 2009;47:527–532. doi: 10.1128/JCM.01213-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacKay I. Real-time PCR in Microbiology: From Diagnosis to Characterization. Australia: Caister Academic Press; 2007. [Google Scholar]
- 19.Macken C, Lu H, Goodman J, Boykin L. The value of a database in surveillance and vaccine selection. In: Osterhaus ADME, Cox N, Hampson AW, editors. Options for the control of influenza IV. Amsterdam: Elsevier; 2001. pp. 103–106. [Google Scholar]
- 20.Manuguerra J-C, Hannoun C. Influenza and other viral respiratory diseases. Surveillance and laboratory diagnosis. Paris: Institute Pasteur; 1999. [Google Scholar]
- 21.Murphy BR, Webster RG. Orthomyxovirueses. In: Fields BN, editor. Fields virology. New York: Raven; 1996. pp. 1397–1445. [Google Scholar]
- 22.van de Pol AC, van Loon AM, Wolfs TF, Jansen NJ, Nijhuis M, Breteler EK, Schuurman R, Rossen JW. Increased detection of respiratory syncytial virus, influenza viruses, parainfluenza viruses, and adenoviruses with real-time PCR in samples from patients with respiratory symptoms. J Clin Microbiol. 2007;45:2260–2262. doi: 10.1128/JCM.00848-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raymaekers M, Smets R, Maes B, Cartuyvels R. Checklist for optimization and validation of real-time PCR assays. J Clin Lab Anal. 2009;23:145–151. doi: 10.1002/jcla.20307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shisong F, Jianxiong L, Xiaowen C, Cunyou Z, Ting W, Xing L, Xin W, Chunli W, Renli Z, Jinquan C, Hong X, Muhua Y. Simultaneous detection of influenza virus type B and influenza A virus subtypes H1N1, H3N2, and H5N1 using multiplex real-time RT-PCR. Appl Microbiol Biotechnol. 2011;90:1463–1470. doi: 10.1007/s00253-011-3192-8. [DOI] [PubMed] [Google Scholar]
- 25.Stauffer F, Haber H, Rieger A, Mutschlechner R, Hasenberger P, Tevere J, Young KK. Genus level identification of mycobacteria from clinical specimens by using an easy-to-handle Mycobacterium-specific PCR assay. J Clin Microbiol. 1998;36:614–617. doi: 10.1128/jcm.36.3.614-617.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefańska I, Romanowska M, Brydak LB. Methods of detection of selected respiratory viruses. Postepy Hig Med Dosw (Online) 2012;66:452–460. doi: 10.5604/17322693.1001898. [DOI] [PubMed] [Google Scholar]
- 27.Suwannakarn K, Payungporn S, Chieochansin T, Samransamruajkit R, Amonsin A, Songserm T, Chaisingh A, Chamnanpood P, Chutinimitkul S, Theamboonlers A, Poovorawan Y. Typing (A/B) and subtyping (H1/H3/H5) of influenza A viruses by multiplex real-time RT-PCR assays. J Virol Methods. 2008;152:25–31. doi: 10.1016/j.jviromet.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Templeton KE, Scheltinga SA, Beersma MF, Kroes AC, Claas EC. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and B viruses, respiratory syncytial virus, and parainfluenza viruses 1, 2, 3, and 4. J Clin Microbiol. 2004;42:1564–1569. doi: 10.1128/JCM.42.4.1564-1569.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R, Taubenberger JK. Methods for molecular surveillance of influenza. Expert Rev Anti Infect Ther. 2010;8:517–527. doi: 10.1586/eri.10.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization (2009) WHO information for laboratory diagnosis of pandemic (H1N1) 2009 virus in humans—revised. http://www.who.int/csr/resources/publications/swineflu/WHO_Diagnostic_RecommendationsH1N1_20090521.pdf
- 32.World Health Organization (2011) Influenza A(H1N1) 2009 virus: current situation and post-pandemic recommendations. Wkly Epidemiol Rec 8(86):61–66. http://www.who.int/wer/2011/wer8608.pdf [PubMed]
- 33.World Health Organization (2011) Review of the 2010–2011 winter influenza season, northern hemisphere. Wkly Epidemiol Rec 22(89):222–227. http://www.who.int/wer/2011/wer8622.pdf
- 34.World Health Organization (2011) Manual for the laboratory diagnosis and virological surveillance of influenza. World Health Organization, Geneva, Switzerland. http://whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf
- 35.World Health Organization (2012) Influenza virus activity in the world. World Health Organization, Geneva, Switzerland. http://www.who.int/influenza/gisrs_laboratory/updates/summaryreport/en/index.html
- 36.World Health Organization (2012) Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2012. World Health Organization, Geneva, Switzerland. http://www.who.int/influenza/human_animal_interface/EN_GIP_20120810CumulativeNumberH5N1cases.pdf
- 37.World Health Organization (2012) Review of the 2011-2012 winter influenza season, northern hemisphere. Wkly Epidemiol Rec 24(87):233–240. http://www.who.int/wer/2012/wer8724.pdf [PubMed]


