Abstract
Infectious bronchitis virus (IBV) is one of the most critical pathogens in the poultry industry, causing serious economic losses in all countries including Iraq. IBV has many genotypes that do not confer any cross-protection. This virus has been genotyped by sequence analysis of the S1 glycoprotein gene. A total of 100 tracheal and kidney tissue specimens from different commercial broiler flocks in the middle and south of Iraq were collected from September 2013 to September 2014. Thirty-two IBV-positive samples were selected from among the total and were further characterized by nested PCR. Phylogenetic analysis revealed that isolates belong to four groups (group I, variant 2 [IS/1494-like]; group II, 793/B-like; group III, QX-like; group IV, DY12-2-like). Sequence analysis revealed nucleotide sequence identities within groups I, II, and III of 99.68 %-100 %, 99.36 %-100 %, and 96.42 %-100 %, respectively. Group I (variant 2) was the dominant IBV genotype. One Chinese-like recombinant virus (DY12-2-like) that had not been reported in the Middle East was detected. In addition, the presence of QX on broiler chicken farms in the area studied was confirmed. This is the first comprehensive study on the genotyping of IBV in Iraq with useful information regarding the molecular epidemiology of IBV. The phylogenetic relationship of the strains with respect to different time sequences and geographical regions displayed complexity and diversity. Further studies are needed and should include the isolation and full-length molecular characterization of IBV in this region.
Keywords: Avian Influenza Virus, Infectious Bronchitis Virus, Infectious Bronchitis Virus Strain, Kurdistan Region, High Nucleotide Sequence Identity
Introduction
Coronaviruses are an important group of viruses that cause highly contagious respiratory and enteric diseases in animals and humans [13]. Avian corona viruses can infect a wide range of avian species, particularly those reared in close proximity to domesticated poultry, such as fowl, partridge, geese, pigeons, guinea fowl, teal, ducks, and pea fowl [8]. Infectious bronchitis virus (IBV) causes enormous problems, causing a highly contagious disease in chickens. Respiratory, reproductive, digestive, and renal infections are the primary clinically important types of IBV infections in domestic chickens [15]. IBV belongs to the genus Gammacoronavirus, along with other avian coronaviruses. It is an enveloped, positive-stranded RNA virus with a genome of about 27 kb containing 5’ and 3’ untranslated regions (UTRs) and a poly(A) tail [5]. A major part of the genome is composed of two overlapping open reading frames (ORFs), 1a and 1b, which are translated into large polyproteins 1a and 1ab, respectively, through a ribosomal frameshift mechanism. The primary structural proteins spike (S), envelope (E), membrane (M), and nucleocapsid (N) are encoded in the remaining part of the RNA genome [20, 24]. The glycoprotein S consists of two subunits (S1 and S2) and contains a wide variety of antigenic determinants that may induce the production of specific neutralizing antibodies. Mutations within this genomic region may result in the emergence of new viral variants [19]. In recent years, genotyping has been performed using S1 gene sequencing [17], which has become the procedure of choice for differentiating between vaccine and field viruses.
More than 20 IBV serotypes have been recognized worldwide [30]. IB still causes serious problems in the Iraqi poultry industry due to the inability of vaccines to provide cross-protection between different genotypes. Due to the limited network of poultry diagnostic laboratories in Iraq, differential diagnosis is can only be done based on clinical signs and gross lesions. The characterization of IBV has raised additional problems in terms of both epidemiology and control. Although IBV on the poultry farms in Iraq (with H120 and 4/91 strains) is presently controlled by inactivated and live attenuated vaccines, outbreaks of IB have nevertheless been observed on broiler farms [1, 21]. Thus, the spread of viral respiratory diseases has become the most commonly reported condition in commercial broiler flocks in Iraq; however, there have been no studies on the detection and genotyping of these viruses using molecular techniques and sequencing. In this study, genotyping of IBV isolates from broiler farms in the south and middle of Iraq was carried out based on partial S1 sequencing.
Materials and methods
Sampling
Samples were collected from 100 broiler chicken farms in the south and middle (five governorates) of Iraq (Al-Kut, Al-Najaf, Thi-Qar, Al-Muthanna and Al-Basrah; 20 farms per governorate; Fig. 1) from September 2014 to December 2015. The samples (trachea and kidney) were taken from chickens that showed clinical signs suggesting IB (respiratory problems such as gasping, sneezing and bronchial rales, and nephritis lesions such as enlargement, and congestion in kidneys). The samples were collected with aseptic technique and frozen at -70 °C. The details of positive samples are shown in Table 1.
Fig. 1.
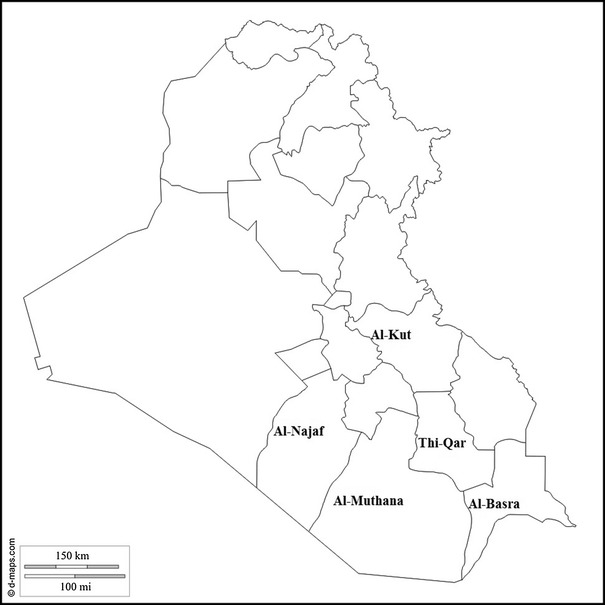
The sample-collection areas for genotyping of infectious bronchitis viruses from broiler farms in Iraq between 2014 and 2015
Table 1.
Sample data for genotyping of infectious bronchitis viruses from broiler farms in Iraq, 2014-2015
| No. | Isolate name | Genotype | Organ | Year | Governorate | Accession number |
|---|---|---|---|---|---|---|
| 1 | SGK-34 | IS-1494 | Trachea | 2015 | Al-Kut | KU143883 |
| 2 | SGK-33 | IS-1494 | Trachea | 2015 | Al-Najaf | KU143884 |
| 3 | SGK-25 | IS-1494 | Trachea | 2014 | Al-Kut | KU143885 |
| 4 | SGK-24 | IS-1494 | Trachea | 2015 | Al-Najaf | KU143886 |
| 5 | SGK-20 | IS-1494 | Trachea | 2015 | Al-Muthana | KU143887 |
| 6 | SGK-19 | IS-1494 | Kidney | 2014 | Al-Najaf | KU143888 |
| 7 | SGK-17 | IS-1494 | Kidney | 2015 | Al-Kut | KU143889 |
| 8 | SGK-16 | IS-1494 | Kidney | 2014 | Al-Kut | KU143890 |
| 9 | SGK-14 | IS-1494 | Kidney | 2014 | Al-Najaf | KU143891 |
| 10 | SGK-13 | IS-1494 | Trachea | 2014 | Al-Muthana | KU143892 |
| 11 | SGK-12 | IS-1494 | Kidney | 2014 | Thi-Qar | KU143893 |
| 12 | SGK-9 | IS-1494 | Trachea | 2014 | Al-Basra | KU143894 |
| 13 | SGK-7 | IS-1494 | Kidney | 2015 | Al-Basra | KU143895 |
| 14 | SGK-8 | IS-1494 | Kidney | 2014 | Al-Basra | KU143896 |
| 15 | SGK-5 | IS-1494 | Trachea | 2015 | Thi-Qar | KU143897 |
| 16 | SGK-21 | QX | Trachea | 2015 | Al-Najaf | KU143898 |
| 17 | SGK-6 | QX | Kidney | 2014 | Al-Kut | KU143899 |
| 18 | SGK-11 | QX | Trachea | 2015 | Al-Najaf | KU143900 |
| 19 | SGK-2 | DY12-2 | Kidney | 2015 | Al-Najaf | KU143901 |
| 20 | SGK-1 | 793/B | Kidney | 2014 | Al-Kut | KU143902 |
| 21 | SGK-4 | 793/B | Kidney | 2014 | Al-Najaf | KU143903 |
| 22 | SGK-10 | 793/B | Kidney | 2014 | Al-Muthana | KU143904 |
| 23 | SGK-15 | 793/B | Kidney | 2014 | Thi-Qar | KU143905 |
| 24 | SGK-18 | 793/B | Kidney | 2014 | Thi-Qar | KU143906 |
| 25 | SGK-22 | 793/B | Kidney | 2015 | Al-Kut | KU143907 |
| 26 | SGK-23 | 793/B | Kidney | 2015 | Al-Kut | KU143908 |
| 27 | SGK-26 | 793/B | Trachea | 2015 | Al-Najaf | KU143909 |
| 28 | SGK-27 | 793/B | Kidney | 2015 | Al-Muthana | KU143910 |
| 29 | SGK-28 | 793/B | Kidney | 2015 | Thi-Qar | KU143911 |
| 30 | SGK-29 | 793/B | Kidney | 2015 | Al-Basra | KU143912 |
| 31 | SGK-30 | 793/B | Kidney | 2015 | Al-Basra | KU143913 |
| 32 | SGK-31 | 793/B | Kidney | 2015 | Al-Muthana | KU143914 |
RNA extraction and cDNA synthesis
RNA was extracted from tissue samples using CinnaPure RNA Extraction Kit (SinaClone, Iran). For cDNA synthesis, 1 µL (0.2 µg) of random hexamer primer (SinaClon, Iran) was added to 5 µL of extracted RNA, and the mixture was heated at 65 °C for 5 minutes. Fourteen µL of cDNA master mix containing 7.25 µL of DEPC-treated water (SinaClon, Iran), 2 µL of dNTP mix (SinaClon, Iran), 0.25 µL of RiboLock RNase Inhibitor (Thermo Fisher Scientific, USA), 0.5 µL of Revert Aid Reverse Transcriptase (Thermo Fisher Scientific, USA), and 4 µL of 5X RT reaction buffer was added to each tube, resulting in a final volume of 20 µL. Then, the mixture was incubated at 25 °C for 5 min, 42 °C for 60 min, 95 °C for 5 min, and 4 °C for 1 min. The cDNA was stored at −20 °C until use. Sampling, RNA extraction, and cDNA synthesis were conducted in Iraq, and the remainder of the work was carried out in Iran.
Real-time PCR for IBV detection
Real-time PCR for IBV detection based on the 5′ UTR was chosen for this study. The amplification was performed by using an amplification kit (Bioneer, South Korea) with the forward primer 5′GCTTTTGAGCCTAGCGTT3′, reverse primer 5′GCCATGTTGTCACTGTCTATTG3′ and TaqMan® dual-labeled probe FAM-CACCACCAGAACCTGTCACCTC-BHQ1 as described by Callison et al. [7].
Nested PCR for genotyping
Nested PCR was performed using spike gene primers that were designed to amplify a ~390-bp fragment of the gene [16]. First-round amplification (494 bp) was performed in a final volume of 20 µL containing 2 μl of distilled water, 13 μl of SinaClon 2 × PCR master mix (SinaClon, Iran), 2 μl of SX1 (5′CACCTAGAGGTTTGYTWGCATG3′) and SX2 (5′TCCACCTCTATAAACACCYTTAC3′) primers (10 µM), and 3 μl of cDNA . The amplification was performed with a 35-cycle thermal profile (94 °C for 2 min, 94 °C for 30 s, 58 °C for 30 s, 72 °C for 30 s, 72 °C for 10 min). In the second round of the nested PCR, SX3 (5′TAATACTGGYAATTTTTCAGATGG3′) and SX4 (5′AATACAGATTGCTTACAACCACC3′) primers were used. The second round of amplification was performed in a volume of 20 µL (4.5 µL of distilled water, 13 µL of SinaClon 2 × PCR master mix (SinaClon, Iran), 2 µL of SX3 and SX4 primers (10 µM), and 0.5 µL of the first-round PCR product). The reaction was carried out under the same cycling conditions. The PCR product was analyzed by electrophoresis on a 1.5 % agarose gel and visualized under UV light.
Phylogenetic analysis
An AccuPrep® PCR Purification Kit (Bioneer Co., Korea) was used for the purification of the PCR products. Sequencing was performed with the primers (both directions) that were used in the second step of nested PCR (Bioneer Co., Korea). Chromatograms were evaluated with CromasPro (CromasPro Version 1.5). A phylogenetic tree was constructed by the neighbor-joining method, using MEGA 5.1 software, and each tree was produced using a consensus of 1000 bootstrap replicates [31]. The nucleotide sequences of a partial segment of the S1 gene were compared with several S1 sequences from GenBank, including H120 (JN600610), Sul/01/09 (GQ281656), GRAY (L14069), Connecticut (IBAS1A), Kurdistan-Erbil 12VIR10065-16-2012 (KF153245), IR-Razi-HKM1-2010(JN600609), IR-Razi-HKM3-2010 (JN600612), PCRLab/06/2012 (JX477827), QX (AF193423), 4/91pathogen (AF093794), 4/91 vaccine (KF377577), and IS/1494 (Table 3). The S1 gene sequences of the IBVs were submitted to the NCBI GenBank database with the accession numbers KU143883-KU143914.
Table 3.
Nucleotide sequence similarity of the eight Iraqi IBV isolates (SGK32, SGK4, SGK2, SGK6, SGK11, SGK13, SGK33, SGK9) (numbers 1 to 8), and reference strains (number 9 to 18)
| Name of strain | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 SGK32 | 100 | 89.4 | 80.9 | 80.4 | 81.2 | 79.9 | 79.9 | 81.7 | 80.5 | 79.9 | 80.4 | 75.8 | 81.3 | 100 | 100 | 100 | 81.3 | |
| 2 SGK4 | 89.4 | 80.9 | 80.4 | 81.2 | 79.9 | 79.9 | 81.7 | 80.5 | 79.9 | 80.4 | 80.5 | 75.8 | 100 | 100 | 100 | 81.3 | ||
| 3 SGK2 | 91.9 | 91.6 | 82.4 | 82.4 | 82.4 | 82.4 | 81.3 | 80.8 | 81.1 | 79.2 | 90.9 | 89.4 | 89.4 | 89.6 | 91.6 | |||
| 4 SGK6 | 99.6 | 82.9 | 82.9 | 82.9 | 82.9 | 81.7 | 79.9 | 80.4 | 81.3 | 98.3 | 80.9 | 80.9 | 81.8 | 99.6 | ||||
| 5 SGK11 | 82.4 | 83.3 | 83.3 | 82.4 | 82.1 | 79.5 | 79.9 | 80.9 | 98.0 | 80.4 | 80.4 | 81.8 | 99.3 | |||||
| 6 SGK13 | 98.0 | 98.0 | 99.6 | 99.0 | 86.4 | 86.7 | 82.9 | 82.9 | 81.2 | 81.2 | 80.2 | 82.4 | ||||||
| 7 SGK33 | 100 | 97.7 | 97.6 | 85.6 | 85.9 | 82.9 | 82.9 | 79.9 | 79.9 | 79.3 | 82.4 | |||||||
| 8 SGK9 | 97.7 | 97.6 | 85.6 | 85.9 | 82.9 | 82.9 | 79.9 | 79.9 | 80.2 | 82.4 | ||||||||
| 9 IS/1494 | 99.3 | 99.2 | 87.1 | 82.9 | 82.9 | 81.7 | 81.7 | 81.0 | 82.4 | |||||||||
| 10 Kurdistan-Erbil | 85.7 | 86.1 | 81.7 | 80.5 | 80.5 | 80.5 | 79.8 | 81.3 | ||||||||||
| 11 SUL/O1/09 | 99.6 | 79.4 | 80.8 | 79.9 | 79.9 | 81.2 | 79.5 | |||||||||||
| 12 IR612 | 79.2 | 80.0 | 80.4 | 81.2 | 80.4 | 81.6 | ||||||||||||
| 13 H120 | 82.6 | 75.8 | 75.4 | 75.4 | 81.3 | |||||||||||||
| 14 QXF423 | 81.3 | 81.3 | 82.3 | 98.0 | ||||||||||||||
| 14 4/91 PATH | 100 | 100 | 81.3 | |||||||||||||||
| 16 IR609 | 100 | 81.3 | ||||||||||||||||
| 17 4/91 Vaccine | 81.3 | |||||||||||||||||
| 18 PCR Lab |
Results
Real-time PCR for IBV detection (IBV incidence rate)
Real-time PCR for IBV detection based on the 5′ UTR was used in this study. Of the 100 samples tested, 32 were positive for IBV by real-time PCR. The number of positive samples from each governorate is presented together with the sample size from each governorate. The total incidence rate for the middle and south Iraqi governorates was 32 %, where the highest incidence rate of IBV in broiler chickens was detected in Al-Najaf governorate 10/20 (50 %), while the lowest rate 4/20 (20 %) was detected in Al-Muthana governorate (Table 2).
Table 2.
Number of identified IBV genotypes among 32 positive samples from each of the governorates of Iraq during 2014–2015
| IS/1494 | 793/B | QX | DY 12-2 | Total positive | |
|---|---|---|---|---|---|
| Al-Kut | 4 (12.5 %) | 3 (9.37 %) | 1 (3.12 %) | 0 | 8 |
| Al-Najaf | 4 (12.5 %) | 3 (9.37 %) | 2 (6.25 %) | 1 (3.12 %) | 10 |
| Al-Muthana | 2 (6.25 %) | 2 (6.25 %) | 0 | 0 | 4 |
| Thi-Qar | 2 (6.25 %) | 3 (9.37 %) | 0 | 0 | 5 |
| Al-Basra | 3 (9.37 %) | 2 (6.25 %) | 0 | 0 | 5 |
The percentage is indicated in parentheses
Nested PCR and IBV genotyping
To carry out IBV genotyping, all 32 positive samples were subjected to amplification of a portion of the S1 gene for sequencing. All samples that were positive in real-time PCR were positive in nested PCR. Four genotypes were detected after sequencing. The percentage of IS/1494, 793/B, QX, and DY12-2 genotypes in five Iraqi governorates was 46.87 %, 40.62 %, 9.37 % and 3.12 %, respectively (Table 2 and Fig. 2). The QX genotype was detected in Al-Kut and Al-Najaf governorates, while the DY12-2 genotype was only detected in Al-Najaf governorate. IS/1494 and 793/B genotypes were detected in all governorates (Table 2).
Fig. 2.
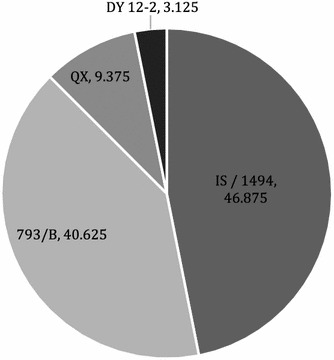
Prevalence of different IBV genotypes circulating on Iraqi broiler farms, 2014-2015
Phylogenetic analysis
The nucleotide sequences of the S1 gene from 32 Iraqi IBV isolates obtained in this study were aligned and compared with those of previously identified isolates from Iraq, neighboring countries, and worldwide reference IBV strains, as shown in Table 3. The analysis revealed that all sequences obtained in this study were genetically different. Phylogenetic analysis of the 32 strains (Fig. 2) revealed that Iraqi IBV strains could be classified into four genetic groups or genotypes: group I, variant 2 IS/1494-like viruses, including 15 field isolates (46.87 %); group II, 793/B-like viruses, including 13 field strains (40.62 %); group III, QX-like viruses, including three field strains (9.37); and group IV, DY12-2-like viruses, including one field strain (3.12). The strains from this study are indicated by black circles, while white dots (Iranian strains) and black triangles (Iraqi strains) refer to reference strains. The sequence identities within the sequenced groups were 99.36 %–100 % for group 1, 96.42 %–100 % for group 2 and 99.68 %–100 % for group 3 (Table 3). IS/1494-like viruses (group I) shared high nucleotide sequence identity (99.67 %) with IS/1494/06, Kurdistan-Erbil 12VIR10065-16, Kurdistan-Sulaymania 12VIR10065-5, and Eg/CLEVB-2/IBV 012, while showing 98 % similarity to CH/Turkey 10RS-3161. In 793/B-like viruses (group II), the partial S1 sequences of the Iraqi strains showed 100 % identity to the pathogenic 4/91 strain. In addition, the sequence analysis of group II revealed high levels of nucleotide sequence similarity (96.39 % and 100 % identity) to the IR-1062-GA and IR-Razi-HKM1-2010 strains, respectively. The QX-like viruses (group III) were 98.39 % identical to the Chinese QX (AF193423) strain. This group shared 99.68 % identity with the Iranian QX IBV strain (PCR Lab/06/2012). Group III viruses were 99.68 % to 100 % identical to CH/AH 2010, HC10 and CH /JS/YC11-1. SGK-2 (DY12-2 like virus) was related to CK/CH/SC/DY12-2, CH/Guangdong Xindadi 0903 and CK/CH/ZJ QZ12-2 strains, with 98.72 % identity.
Discussion
Avian infectious bronchitis (IB) is caused by IBV and is an acute and highly contagious disease of chickens that leads to complex respiratory diseases. Respiratory diseases caused by bacteria and mycoplasmas have already been detected on broiler farms located in the central and southern parts of Iraq. However, the molecular detection studies (infection rate and genotyping) of respiratory viral diseases such as IB in broilers in Iraq are limited (personal communication with veterinary organizations). We conducted the first study to determine the infection rate and IBV genotypes on broiler farms, using molecular and phylogenetic techniques. The approved vaccine strains used in Iraq are based on serotypes Ma5, H120, and 793/B [21]. IB still causes serious problems in the Iraqi poultry industry due to the inability of the vaccines to provide cross-protection between different genotypes [1]. The genotyping of IBV is necessary not only for understanding virus evolution but also for effective modification of the vaccination programs [10]. In this study, the infection rate of IBV was 32 %, which indicated widespread distribution of IBV in the southern and middle parts of Iraq. The highest infection rate of IBV was observed in Al-Najaf (50 %) governorate, while the lowest (20 %) was observed in Al-Muthana (Fig. 3). The results of the present work are in line with a study by Al-Dabhawi et al., who reported an IBV prevalence of 92.1 % in the samples collected from central Iraq, using quantitative PCR. In the latter study, 20 % were infected with IBV alone, 45.71 % were infected with IBV and Mycoplasma gallisepticum and/or avian influenza virus (subtype H9), and 25.71 % were positive for both IBV and avian influenza virus [1]. The current result is comparable to what has been observed in Saudi Arabia, where 35.36 % of tested commercial poultry farms were positive for IBV [32]. The infection rate was lower than the rate previously reported on commercial farms within neighboring countries: 58.8 % and 42.8 % in Jordan and Iran, respectively [2]. Even where there were no poultry farms in Iraq, the infection rate was as high as 32 %. This may be attributed to poor biosecurity on the farms, resulting in the spread of infection between and within the flocks [22].
Fig. 3.
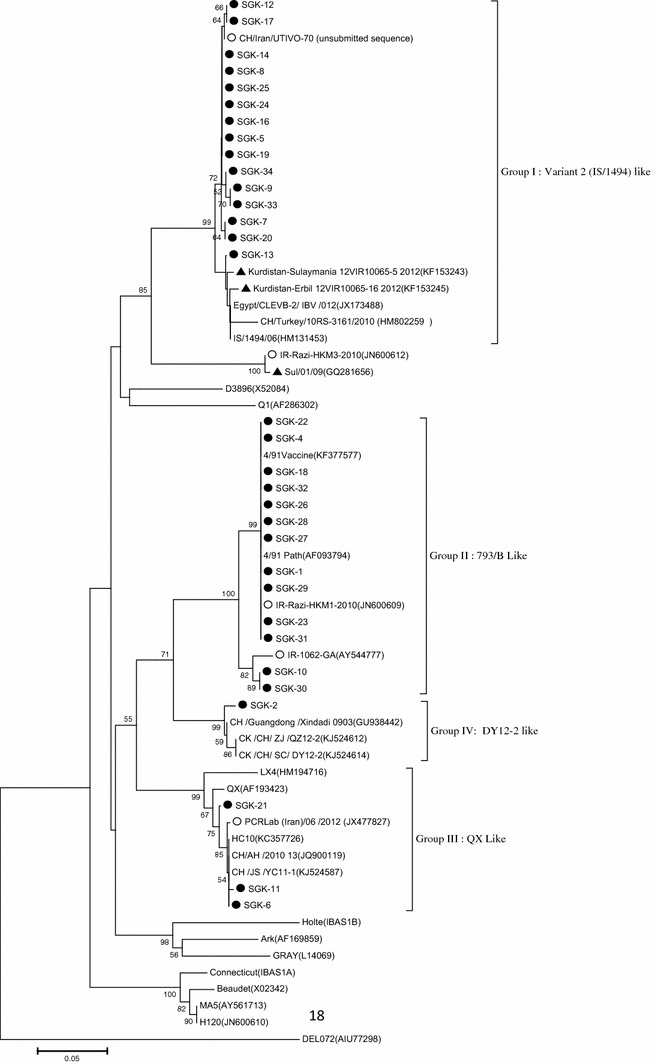
Phylogenetic tree of 32 Iraqi isolates and 24 reference strains of infectious bronchitis virus based on the nucleotide sequences of the S1 gene. The phylogenetic tree was constructed, using MEGA version 5, by the neighbor-joining method with 1000 bootstrap replicates (bootstrap values are shown on the tree). The isolates from this study are indicated by black circles. White dots indicate Iranian reference strains, and black triangles indicate Iraqi reference strains
Phylogenetic analysis showed that the Iraqi isolates clustered into four genetic groups (group I, variant 2 (IS/1494)-like; group II, 793/B-like; group III, QX-like; and group IV, DY12-2-like). Variant 2 (IS/1494-like) viruses were the dominant IBV genotype infecting broiler chickens, with an overall prevalence of 46.87 % in IBV-positive samples. They shared high nucleotide sequence identity with IBV isolates from Iran, Israel, Egypt, Turkey, and Kurdistan. The isolation of IS/1494/06 (EU780077) IBV, one of two Israeli variant strains, was first reported by Meier and Mahar in Israel [23]. IBV variants have been recognized in Iraq since 2012 (Iraqi Veterinary Directorate/Ministry of Agriculture). The present work is similar to another study in which three IBV genotypes were found in Slemani-Kurdistan, Iraq, including group A (very similar to Iranian isolates), group B (closely related to Chinese isolates) and group C (similar to IS/1494 and Egypt/Beni-Seuf/01 isolates) [26]. These findings are in agreement with those of Kanan et al., who reported that IS/1494/06 IBV was the most common genotype in Egypt (2010), Lebanon (2010-2012), Jordan (2011-2012), Kuwait (2012), and Oman (2013) [12]. The current study is similar to another study on an IS/1494 strain in Turkey [18].
The second group consisted of 793/B-like viruses that were detected in different Iraqi governorates at the high prevalence of 40.62 %. The present study revealed the presence of 793/B on the broiler farms. The IBV strains of the 793/B serotype were first identified in France in 1985, followed by Great Britain in 1991. Subsequently, these strains spread to other countries in Europe, Asia (Particularly to Iran, Turkey), and North America and are some of the most common IBV serotypes in some countries [9]. Our result were in line with those of Mahmood et al., who conducted the first study of identification and genotyping of IBV isolates and indicated that the 4/91-like virus is circulating on vaccinated broiler farms of the Kurdistan region of Iraq [21]. The detection of 793/B in commercial flocks was previously reported in Iran, Jordan, and Israel [23, 27, 29]. However, because all flocks have been vaccinated, we concluded that the detected viruses probably belonged to the vaccine strains. Therefore, the full-length S1 gene of the isolates should be determined to differentiate field and vaccine isolates. In this study, another important IBV variant (QX-like) known to cause respiratory, renal, and reproductive problems [12] was detected with a prevalence of 9.37 %. In 1996, QX was first described and identified in China, after which the prevalence of the so-called QX-like IBV genotype was reported, and it became one of the most dominant genotypes in many countries [28]. The genotype, designated as QX IBV, has spread from Asia, where it was described for the first time in 1996 in China, and spread to Europe and recently to the southern part of the African continent [4]. The QX-like genotype was also previously detected in central Iraq by the Iraqi veterinary directorate (official report). This finding was in line with another study in the Kurdistan region of Iraq, which showed that the phylogenetic aspects of the Kurdistan viruses were closely related (98.9 %) to the QX strain reported in China from 2009 to 2010 [4]. QX-type virus was isolated in Israel in 2004 [4]. The PCR Lab/06/2012 (JX477827) strain was isolated in Iran, which is genetically (99.36 %–99.68 %) related to strains of the present study [6]. This finding revealed that the QX strain has been widely transferred from other countries (probably Iran) to Iraq. Although no information is currently available about the introduction of the QX strain from China into other countries, it has been hypothesized that wild birds may be the source of introduction based on the evidence that IBV may replicate in members of the order Anseriformes [25].
It is of interest that that DYI2-2-like IBV genotype was detected in the present study. This is the first report of this genotype in Iraq, and it has not yet been reported in other countries in the Middle East. The virus appeared following the recombination of CK/CH/GD/LZ09 and TA09 IBV in China and is currently a circulating IBV genotype in China [11]. It is also fair to say that the origin of this virus and the means by which it was introduced to Iraq are not clear. Based on the persistence of the DY12-2 like genotype in China, it could be predicted that this genotype might spread in Iraq and the Middle East in the future. In this survey, some IBV genotypes, such as IS/720, Massachusetts, D274, and Q1, were not detected. These IBV genotypes were detected previously and reported in Iraq, Iran, the United Arab Emirates and Saudi Arabia [3, 12, 14, 21].
New serotypes or variant strains may emerge because of only a few changes in the amino acid sequence of the S1 protein. These changes could be due to immunological pressure caused by the extensive use of vaccines, recombination as the outcome of mixed infections, or a decrease in the prevalence of dominant serotypes as a result of vaccination, allowing other field strains to emerge [25]. Not much is known about the mode of spread of IBV between the countries in the Middle East. However, cross-border movements of poultry and poultry-related products are likely to be important factors [21].
In summary, our study demonstrated a high rate of infection with IBV (32%) in central and southern Iraq. This is the first report indicating the presence of an IBV DY12-2-like strain in the Middle East. It is an updated and comprehensive study of genotyping of IBV in Iraq and completes the IBV puzzle in the region. Phylogenetic analysis showed that the detected strains are closely related to other IBV strains infecting broilers, pullets, and layers in the region. Finally, it could be suggested that 1) work be done on whole-genome sequencing, 2) cross-protection studies be conducted for designing the best vaccination program in the Iraqi poultry industry, 3) molecular surveillance in other governorates in Iraq be continued, and 4) the pathogenesis of different Iraqi IBV isolates be studied.
Acknowledgments
The authors gratefully acknowledge Dr. Hamideh Najafi, Dr. Iraj Ashrafi, and Mr. Behrooz Asadi for their extensive technical support.
Compliance with ethical standards
Funding
Financial support for this study was provided by the Ministry of Higher Education and Scientific Research, Iraq, University of Basrah (7.30.4603), and the Research Council of the University of Tehran under Grant No. 28692-6-2.
Conflict of interest
No conflict of interest.
Ethical approval
This article does not contain any studies of animals performed by any of the authors.
References
- 1.Al-Dabhawe A, Kadhim H, Samaka H. Molecular detection of infectious bronchitis virus and it is relation with avian influenza virus (H9) and Mycoplasma gallisepticum from different geographical regions in Iraq. Iraqi J Vet Sci. 2013;27:97–101. [Google Scholar]
- 2.Al-Shekaili T, Baylis M, Ganapathy K. Molecular detection of infectious bronchitis and avian metapneumoviruses in Oman backyard poultry. Res Vet Sci. 2015;99:46–52. doi: 10.1016/j.rvsc.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Shekaili T, Baylis M, Ganapathy K. Molecular detection of infectious bronchitis and avian metapneumoviruses in Oman backyard poultry. Res Vet Sci. 2015;99:46–52. doi: 10.1016/j.rvsc.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin O, Valastro V, Salviato A, Drago A, Cattoli G, Monne I. Circulation of QX-like infectious bronchitis virus in the Middle East. Pathology. 2012;40:223–235. doi: 10.1136/vr.100896. [DOI] [PubMed] [Google Scholar]
- 5.Boursnell ME, Brown TD, Foulds IJ, Green PF, Tomley FM, Binns MM. Completion of the sequence of the genome of the coronavirus avian infectious bronchitis virus. J Gen Virol. 1987;68(Pt 1):57–77. doi: 10.1099/0022-1317-68-1-57. [DOI] [PubMed] [Google Scholar]
- 6.Bozorgmehri-Fard M, Charkhkar S, Hosseini H. Detection of the Chinese genotype of infectious bronchitis virus (QX-type) in Iran. Iran J Virol. 2014;7:21–24. [Google Scholar]
- 7.Callison SA, Hilt DA, Boynton TO, Sample BF, Robison R, Swayne DE, Jackwood MW. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J Virol Methods. 2006;138:60–65. doi: 10.1016/j.jviromet.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavanagh D. Coronaviruses in poultry and other birds. Avian Pathol. 2005;34:439–448. doi: 10.1080/03079450500367682. [DOI] [PubMed] [Google Scholar]
- 9.Cavanagh D, Picault J-P, Gough RE, Hess M, Mawditt K, Britton P. Variation in the spike protein of the 793/B type of infectious bronchitis virus, in the field and during alternate passage in chickens and embryonated eggs. Avian Pathol. 2005;34:20–25. doi: 10.1080/03079450400025414. [DOI] [PubMed] [Google Scholar]
- 10.de Wit JJ, Cook JK. Factors influencing the outcome of infectious bronchitis vaccination and challenge experiments. Avian Pathol. 2014;43:485–497. doi: 10.1080/03079457.2014.974504. [DOI] [PubMed] [Google Scholar]
- 11.Feng K, Xue Y, Wang F, Chen F, Shu D, Xie Q. Analysis of S1 gene of avian infectious bronchitis virus isolated in southern China during 2011–2012. Virus Genes. 2014;49:292–303. doi: 10.1007/s11262-014-1097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganapathy K, Ball C, Forrester A. Genotypes of infectious bronchitis viruses circulating in the Middle East between 2009 and 2014. Virus Res. 2015;210:198–204. doi: 10.1016/j.virusres.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 13.Guy JS. Turkey coronavirus is more closely related to avian infectious bronchitis virus than to mammalian coronaviruses: a review. Avian Pathol. 2000;29:207–212. doi: 10.1080/03079450050045459. [DOI] [PubMed] [Google Scholar]
- 14.Hashemzadeh M, Karimi V, Masoudi S, Shoushtary A, Langeroudi A, Momayez R, Shirazi M, Maghsodloo H, Hasanzadeh R, Eshratabadi F. Phylogenetic study of Iranian infectious bronchitis virus isolates during 2010–2011 using glycoprotein S1 gene. J Vet Res. 2013;68:135–141. [Google Scholar]
- 15.Jackwood MW. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56:634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- 16.Jones RC, Worthington KJ, Capua I, Naylor CJ. Efficacy of live infectious bronchitis vaccines against a novel European genotype, Italy 02. Vet Record. 2005;156:646–647. doi: 10.1136/vr.156.20.646. [DOI] [PubMed] [Google Scholar]
- 17.Jones RM, Ellis RJ, Cox WJ, Errington J, Fuller C, Irvine RM, Wakeley PR. Development and validation of RT-PCR tests for the detection and S1 genotyping of infectious bronchitis virus and other closely related gammacoronaviruses within clinical samples. Transbound Emerg Dis. 2011;58:411–420. doi: 10.1111/j.1865-1682.2011.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahya S, Coven F, Temelli S, Eyigor A, Carli KT. Presence of IS/1494/06 genotype-related infectious bronchitis virus in breeder and broiler flocks in Turkey. Ankara Üniv Vet Fak Derg. 2013;60:27–31. doi: 10.1501/Vetfak_0000002549. [DOI] [Google Scholar]
- 19.Kamble NM, Pillai AS, Gaikwad SS, Shukla SK, Khulape SA, Dey S, Mohan CM. Evolutionary and bioinformatics analysis of the spike glycoprotein gene of H120 vaccine strain protectotype of infectious bronchitis virus from India. Biotechnol Appl Biochem. 2014 doi: 10.1002/bab.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maclachlan NJ, Dubovi EJ. Fenner’s veterinary virology. New York: Academic press; 2010. [Google Scholar]
- 21.Mahmood ZH, Sleman RR, Uthman AU. Isolation and molecular characterization of Sul/01/09 avian infectious bronchitis virus, indicates the emergence of a new genotype in the Middle East. Vet Microbiol. 2011;150:21–27. doi: 10.1016/j.vetmic.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matilda A-A, Cornelius A, Ohene A, Kwaku B. Infectious Bronchitis Virus: a major cause of respiratory disease outbreaks in chickens in Ghana. J Biol Agric Healthcare. 2013;3:56–60. [Google Scholar]
- 23.Meir R, Rosenblut E, Perl S, Kass N, Ayali G, Hemsani E, Perk S. Identification of a novel nephropathogenic infectious bronchitis virus in Israel. Avian Dis. 2004;48:635–641. doi: 10.1637/7107. [DOI] [PubMed] [Google Scholar]
- 24.Mo M, Huang B, Wei P, Wei T, Chen Q, Wang X, Li M, Fan W. Complete genome sequences of two Chinese virulent avian coronavirus infectious bronchitis virus variants. J Virol. 2012;86:10903–10904. doi: 10.1128/JVI.01895-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pohuang T, Chansiripornchai N, Tawatsin A, Sasipreeyajan J. Detection and molecular characterization of infectious bronchitis virus isolated from recent outbreaks in broiler flocks in Thailand. J Vet Sci. 2009;10:219–223. doi: 10.4142/jvs.2009.10.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauf HS (2014) Investigation and molecular characterization of avian infectious bronchitis virus in suspected broiler farms in Slemani Governorate. Council of College of Veterinary Medicine University of Sulaimani in Partial Fulfillment of the Requirements for the Degree of Master in Veterinary Medicine/Clinical Pathology By Hana Sherzad Rauf BVM &S., University of Sulaimani
- 27.Roussan D, Totanji W, Khawaldeh G. Molecular subtype of infectious bronchitis virus in broiler flocks in Jordan. Poultry Sci. 2008;87:661–664. doi: 10.3382/ps.2007-00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sasipreeyajan TPJ. The pathogenesis of a new variant genotype and QX-like infectious bronchitis virus isolated from chickens in Thailand. Thai J Vet Med. 2012;42:51–57. [Google Scholar]
- 29.Seyfi Abad Shapouri M, Mayahi M, Assasi K, Charkhkar S. A survey of the prevalence of infectious bronchitis virus type 4/91 in Iran. Acta Vet Hungarica. 2004;52:163–166. doi: 10.1556/AVet.52.2004.2.4. [DOI] [PubMed] [Google Scholar]
- 30.Sjaak de Wit JJ, Cook JK, van der Heijden HM. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40:223–235. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yousef MAA. Molecular survey and phylogenic analysis of infectious bronchitis virus (IBV) circulating among chicken flocks in Riyadh Province, Saudi Arabia. J Anim Vet Adv. 2014;13:1002–1008. [Google Scholar]


