Abstract
The aim of this study was to establish a multiplex PCR (mPCR) method that can simultaneously detect canine parvovirus (CPV-2), canine coronavirus (CCoV) and canine adenovirus (CAV), thereby eliminating the need to detect these pathogens individually. Based on conserved regions in the genomes of these three viruses, the VP2 gene of CPV-2, the endoribonuclease nsp15 gene of CCoV, and the 52K gene of CAV were selected for primer design. The specificity of the mPCR results showed no amplification of canine distemper virus (CDV), canine parainfluenza virus (CPIV), or pseudorabies virus (PRV), indicating that the method had good specificity. A sensitivity test showed that the detection limit of the mPCR method was 1 × 104 viral copies. A total of 63 rectal swabs from dogs with diarrheal symptoms were evaluated using mPCR and routine PCR. The ratio of positive samples to total samples for CPV-2, CCoV, and CAV was 55.6% (35/63) for mPCR and 55.6% (35/63) for routine PCR. Thirty-five positive samples were detected by both methods, for a coincidence ratio of 100%. This mPCR method can simultaneously detect CCoV (CCoV-II), CAV (CAV-1, CAV-2) and CPV-2 (CPV-2a, CPV-2b, CPV-2c), which are associated with viral enteritis, thereby providing an efficient, inexpensive, specific, and accurate new tool for clinical diagnosis and laboratory epidemiological investigations.
Introduction
Recently, pet dogs have become more common in people’s daily lives. Pet dogs often bring joy and abate loneliness and, more importantly, provide some people with emotional sustenance. Viral enteritis is a major threat to pet dogs. Canine parvovirus (CPV-2), canine coronavirus (CCoV) and canine adenovirus (CAV) are the main pathogens that cause canine viral enteritis.
CPV-2 is a member of the genus Protoparvovirus, family Parvoviridae, and is an important, highly contagious and potentially lethal enteric pathogen in dogs. It can infect dogs of any age, resulting in enteritis symptoms that include hemorrhagic diarrhea, vomiting, loss of appetite, and depression [2, 8, 10]. CCoV, a member of the family Coronaviridae, often causes mild diarrhea and is characterized by high morbidity and low mortality in canids; however, in puppies, the disease is more serious, with high morbidity and high mortality. If CCV and CPV-2 coinfection occurs, it often leads to lethal diarrhea [14, 15]. CAV is a member of the genus Mastadenovirus, family Adenoviridae. Although CAV is not primarily responsible for enteritis, it can also cause diarrheal clinical symptoms [5, 7]. These three viral infections are often mixed in the clinic, and their clinical symptoms of diarrhea are similar; therefore, it is often difficult to distinguish between them during clinical diagnosis and epidemiological investigations [9, 11].
PCR combined with sequencing is the most widely used detection method in the field of pathogen detection. Due to its specificity and sensitivity, this method is gradually becoming the gold standard for pathogen identification. The multiplex PCR (mPCR) method is based on a single PCR that can simultaneously detect a variety of pathogens and simplify the operation steps, thereby saving time and expense [3]. Recently, mPCR has been widely used in the field of pathogen detection because it has advantages of speed, specificity and efficiency [1, 4, 16]. In this study, an mPCR method was established and applied for the detection of CPV-2, CAV and CCoV. The mPCR method was validated as an easy and effective method for the detection of these three viruses in rectal swab samples.
Materials and methods
Viruses
The CCoV (CCoV-II), CAV (CAV-1, CAV-2), CPV-2 (CPV-2a, CPV-2b, CPV-2c), pseudorabies virus (PRV), canine distemper virus (CDV), and canine parainfluenza virus (CPIV) strains used in this study were maintained in our laboratory.
Primer design
Primers for the conserved regions of CPV-2, CCoV, and CAV were designed using Oligo 7.0 software. The specificity of the primers was verified using BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The optimal combination of the three pairs of primers (Table 1) was synthesized by Sangon Biotech Company (Beijing, China).
Table 1.
Primers for the multiplex and routine PCRs
| Test | 5’-3’ Sequence | Position | Reference or source |
Amplicon size (bp) |
|---|---|---|---|---|
|
CPV-F CPV-R |
ATCACAGCAAACTCAAGCAGACTT AAATGGTGGTAAGCCCAATGCTC |
2695-2718a 3384-3362a |
This study | 690 |
|
CCoV-F CCoV-R |
ACATGGTATATCTATGTGCGCAA TGCAAGGCGCACTTGAGAT |
18902-18924b 19153-19135b |
This study | 252 |
|
CAV-F CAV-R |
TGTGCCCATCGACAAGGAA CTAATAGAAGCGGCCCAACTG |
10454-10472c 10886-10866c |
This study | 433 |
|
CPV1 CPV2 |
GAAGAGTGGTTGTAAATAATT CCTATATAACCAAAGTTAGTAC |
3017-3037d 3698-3677d |
[13] | 682 |
|
CCoV1 CCoV2 |
TCCAGATATGTAATGTTCGG TCTGTTGAGTAATCACCAGCT |
6729-6748e 7138-7118e |
[12] | 212 |
| CCoV3 | GGTGTCACTCTAACATTGCTT | 6927-6947e | ||
|
CAV1 CAV2 |
CGCGCTGAACATTACTACCTTGTC CCTAGAGCACTTCGTGTCCGCTT |
CAVI: 769-790f CAVII: 1387-1408g CAVI: 1273-1253f CAVII: 2413-2392g |
[6] |
508 (CAVI) 1030 (CAVII) |
aOligonucleotide position in reference to the canine parvovirus 2a strain CPV/CN/SD6/2014 sequences (GenBank accession no. KR002801.1)
bOligonucleotide position in reference to the canine coronavirus strain K378 sequence (GenBank accession no. KC175340.1)
cOligonucleotide position in reference to the canine adenovirus sequence (GenBank accession no. NC_001734.1)
dOligonucleotide position in reference to the CPV-2 strain CPV-b sequence (GenBank accession no. JQ268283.1)
eOligonucleotide position in reference to the CCoV strain Insavc-1 sequence (GenBank accession no. D13096.1)
fOligonucleotide position in reference to the canine adenovirus type 1 E3 region sequence (GenBank accession no. S38238.1)
gOligonucleotide position in reference to the Cav-2 strain Toronto A26-61 sequence (GenBank accession no. U77082.1)
gOligonucleotide position in reference to the sequence of the Cav-2 strain Toronto A26-61 (GenBank accession no. U77082.1)
Nucleic acid extraction
Nucleic acids were extracted from CPV-2, CAV and CCoV using a MiniBEST Viral RNA/DNA Extraction Kit (TaKaRa, Japan) according to the manufacturer’s protocol. The RNA extracted from CCoV was reverse transcribed to cDNA using the specific primer CCoV-R (Table 1) and Moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega, USA) according to the manufacturer’s protocol prior to its use as a template. In contrast, the nucleic acid extraction products of CPV-2 and CAV were used directly as PCR templates. Viruses obtained from cell culture were used directly for nucleic acid extraction, but clinical samples were first diluted and centrifuged at 5000 rpm for 15 min before nucleic acid extraction to remove impurities.
Establishment of the mPCR method
A mixture containing proviral DNA and cDNA in equal amounts was used as a template to optimize the annealing temperature and primer concentration. The reaction was performed in a 25-μl volume containing 0.25 μl of Ex Taq (TaKaRa, Japan), 3 μl of 10× PCR buffer (20 mM Mg2+ Plus), 3 μl of dNTP mixture (2.5 mM each), 300 ng of both template proviral DNA and cDNA, 0.7 μl each of CCoV-F and CCoV-R, 0.45 μl each of CAV-F and CAV-R, 0.35 μl each of CPV-F and CPV-R, and distilled water. The reaction procedure was as follows: initial denaturation at 94°C for 4 min; 35 cycles of denaturation at 98°C for 10 s, annealing at 63°C for 30 s, and extension at 72°C for 30 s; and a final extension at 72°C for 5 min. The mPCR products were evaluated by 1.0% agarose gel electrophoresis.
Standard plasmid preparation
To determine the sensitivity of the mPCR and to obtain positive controls, the specific PCR products of CCoV (252 bp), CPV-2 (690 bp) and CAV (433 bp) were ligated to the pMD-18T vector (TaKaRa, Japan) to construct the recombinant plasmids pMD-C (CCoV), pMD-P (CPV-2) and pMD-A (CAV), respectively. The plasmid copy number was calculated according to the following formula: copy number (copies/μl) = NA (copies/mol) × concentration (g/μl)/MW (g/mol) [17].
Specificity of the mPCR method
The mPCR method was used to detect CCoV, CPV-2, CAV, PRV, CDV and CPIV. Nucleic acids were extracted from CAV, CPV-2, CPIV, CDV, CCoV and PRV, and the extraction products of CDV, CCoV and CPIV were reverse transcribed into cDNA using the special primer CCoV-R (Table 1) and M-MLV reverse transcriptase (Promega, USA) according to the manufacturer’s protocol. The nucleic acid extraction products of CPV-2, PRV, and CAV were used directly as PCR templates. In contrast, the viral RNA extraction products of CCoV, CDV, and CPIV required reverse transcription prior to use as templates. Deionized water was used as a negative control.
Sensitivity of the mPCR method
The concentrations of the three plasmids (pMD-C, pMD-P and pMD-A) were measured using a NanoDrop 2000 Spectrophotometer (Thermo Scientific, USA). The copy number was calculated based on the plasmid concentration. The mixture of the three plasmids (pMD-C/pMD-P/pMD-A) and each individual plasmid (pMD-C, pMD-P and pMD-A) were diluted from 1 × 108 to 1 × 101 copies/μl and used to determine the minimum detection limit of the mPCR method.
The broad-spectrum ability of the mPCR method
This experiment was used to verify the broad-spectrum ability of this method to detect the different genotypes of the three viruses. In this experiment, we detected CPV-2a, CPV-2b, CPV-2C, CCoV-II, CAV-1 and CAV-2, respectively. The mPCR products were evaluated by 1.0% agarose gel electrophoresis.
Routine PCR
A nested PCR method for the detection of CCoV [12], a PCR method for the detection of CPV-2 [13], and a PCR method for the detection and differentiation of CAV [6] were used as the routine PCR methods for comparison with the mPCR method. The details of the primers used are provided in Table 1. The protocols were the same as the protocols described in the references.
Evaluation of clinical samples
During the period from April to September 2017, a total of 63 rectal swabs (3 ml) from dogs with diarrheal symptoms were collected from five pet hospitals in Jilin Province, China. All swab samples were used for the primary application of the mPCR method. Each swab sample was also evaluated using the routine PCR methods for the three viruses. The viral swab storage solutions were centrifuged at 6,000 rpm for 30 min, and the nucleic acids in the supernatant were extracted for detection via mPCR and routine PCR in triplicate. To confirm the results, all specific fragments amplified by mPCR were sequenced by the Sangon Biotech Company (Beijing, China).
Results
Establishment of the mPCR method
The best reaction conditions were determined by optimizing the annealing temperature, primer concentration, extension time, and cycle number. Seven mixed combinations of the three viral templates were used to verify the mPCR, and the products were visualized by 1.0% agarose gel electrophoresis (Fig. 1).
Fig. 1.
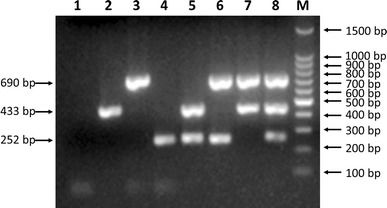
Agarose gel electrophoresis (1.0%) of specific fragments amplified by mPCR from proviral DNA and cDNAs of specific CPV-2, CAV, and CCoV isolates. Lane 1, negative control; lane 2, CAV (433-bp fragment); lane 3, CPV-2 (690-bp fragment); lane 4, CCoV (252-bp fragment); lane 5, CCoV/CAV; lane 6, CPV-2/CCoV; lane 7, CAV/CPV-2; lane 8, CPV-2/CAV/CCoV; lane M, 100-bp DNA ladder marker
Specificity of the mPCR method
The specificity of the mPCR method was determined by detecting CCoV, CPV-2, CAV, PRV, CDV and CPIV. The results showed amplified bands only in the three lanes for CCoV, CPV-2 and CAV, whereas the lanes for PRV, CDV and CPIV did not show amplified bands (Fig. 2). These findings indicated that the mPCR method has good specificity.
Fig. 2.
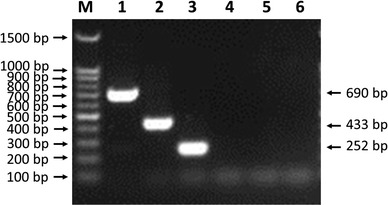
Specificity of the mPCR method. Agarose gel electrophoresis (1.0%) of specific fragments amplified by mPCR from the proviral DNA and cDNAs of specific CPV-2, CAV, CCoV, CDV, PRV, and CPIV isolates. Lane M, 100-bp DNA ladder marker; lane 1, CPV-2; lane 2, CAV; lane 3, CCoV; lane 4, CDV; lane 5, PRV; lane 6, CPIV
Sensitivity of the mPCR method
The sensitivity test results showed that the minimum detection limit for mPCR was 1 × 104 viral copies of each virus when the mixed plasmids (pMD-C/pMD-P/pMD-A) were used as the template (Fig. 3). When only a single plasmid was used for detection, the minimum detection limits for pMD-C, pMD-P and pMD-A were 1×102, 1×103, and 1×102 viral DNA copies, respectively (Fig. 3).
Fig. 3.
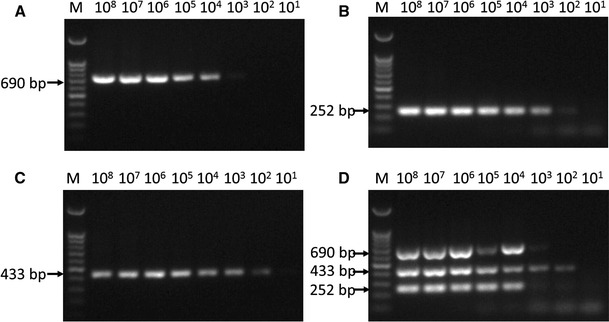
Sensitivity of the mPCR method. The mixed plasmids (pMD-C/pMD-P/pMD-A) and three single plasmids (pMD-C, pMD-P and pMD-A) were diluted from 1 × 108 to 1 × 101 copies/μl to determine the minimum detection limit of the mPCR method. (A) The sensitivity of pMD-P; (B) the sensitivity of pMD-C; (C) the sensitivity of pMD-A; (D) the sensitivity of pMD-C/pMD-P/pMD-A. M, 100-bp DNA ladder marker
The broad-spectrum ability of the mPCR method
The broad-spectrum ability of this method to detect specific genotypes of these three viruses was tested (Fig. 4), and it was found that CPV-2a, CPV-2b, CPV-2C, CCoV-II, CAV-1 and CAV-2 all can be detected by the mPCR method.
Fig. 4.
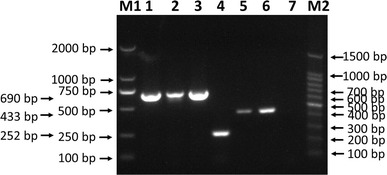
The broad-spectrum ability of the mPCR method. CPV-2a, CPV-2b, CPV-2C, CCoV-II, CAV-1 and CAV-2 were detected by the mPCR method. Lane M1, 2000-bp DNA marker; lane 1, CPV-2a; lane 2, CPV-2b; lane 3, CPV-2C; lane 4, CCoV-II; lane 5, CAV-1; lane 6, CAV-2; lane 7, negative control; lane M2, 100-bp DNA ladder marker
Evaluation of clinical samples
The statistics of the clinical samples evaluated using mPCR and routine PCR are shown in Table 2. The ratio of positive samples to total samples for CPV-2, CCoV and CAV was 55.6% (35/63) by mPCR and 55.6% (35/63) by routine PCR. The 35 positive samples were all detected by both methods, for a coincidence ratio of 100%. The sequencing results were consistent with the mPCR results.
Table 2.
Statistics for the clinical samples detected by mPCR and routine PCR
| mPCR | Routine PCR | |||
|---|---|---|---|---|
| Number positive | Positive ratio | Number positive | Positive ratio | |
| CCoV | 16 | 25.4% | 16 | 25.4% |
| CPV-2 | 9 | 14.3% | 9 | 14.3% |
| CAV | 0 | 0% | 0 | 0% |
| CCoV/CPV-2 | 7 | 11.1% | 7 | 11.1% |
| CCoV/CAV | 1 | 1.6% | 1 | 1.6% |
| CPV-2/CAV | 0 | 0% | 0 | 0% |
| CCoV/CPV-2/CAV | 2 | 3.2% | 2 | 3.2% |
| TOTAL | 63 | 55.6% | 63 | 55.6% |
Discussion
Recently, animal hospitals have experienced more cases of viral enteritis in dogs, and these cases have primarily been caused by CCoV, CAV and CPV-2. The clinical symptoms of these three viruses are similar, and cases of illness are often caused by a mixed infection. Several detection methods for these three viruses exist [6, 12, 13], but no single method can detect the presence of all three viruses together. As a result, the CCoV, CPV-2 and CAV must be detected individually, which is not only cumbersome but also a waste of time and reagents. In this study, an mPCR method for CPV-2, CCoV and CAV was established and applied. This method can detect all three viruses together, therefore simplifying the operation steps, shortening the detection time, and lowering the cost.
Primer design is the most important step in the process of establishing the method and must satisfy the following conditions: the use of conserved sequences, the appropriate combination of amplicons, similar annealing temperatures, and the lack of dimers or CARD structures. In this method, the primer combination produced amplicons of 690 bp, 433 bp and 252 bp, which were easy to distinguish from one another. In addition, the three pairs of primers had similar annealing temperatures, which needed to be sufficiently high to avoid nonspecific bands, and 63°C was found to be sufficient. The specificity of the primers was verified using NCBI Primer-BLAST and, the DNASTAR software was used to check whether dimers or hairpin structures could form between the three primer pairs.
The specificity of the mPCR method was evaluated using other common canine viruses (PRV, CDV and CPIV) and found not to produce cross-reactions or nonspecific reactions with these viruses. The mPCR method used a specific primer as the reverse transcription primer, which improved the specificity of the method.
The sensitivity of the mPCR results was 1×104 viral copies, which is less than that of the routine PCR method. The mPCR method is based on a single PCR method. Therefore, although this method has the advantages of simple operation, time savings, and low cost, there are inherent weaknesses of sensitivity due to the amount of competitive inhibition between the amplicons, which renders the sensitivity of the mPCR lower than that of single PCR [1, 4, 16]. Therefore, our expectation of the mPCR effect was that when the viral titer of each virus in the sample exceeded the sensitivity of the mPCR method, all viruses would be detected. A total of 63 rectal swabs from dogs with diarrheal symptoms were evaluated using mPCR and routine PCR; the results obtained by sequencing are shown in Table 2. The ratio of positive samples to total samples for CPV-2, CCoV and CAV was 55.6% (35/63) by mPCR and 55.6% (35/63) by routine PCR. Thirty-five positive samples were detected by both methods, for a coincidence ratio of 100%, indicating that the specificity and sensitivity of the method was sufficient for testing clinical specimens.
In summary, the mPCR established in this study is an efficient, inexpensive, specific, and accurate method for detecting CCoV (CCoV-II), CAV (CAV-1, CAV-2) and CPV-2 (CPV-2a, CPV-2b, CPV-2c). This study provides a new tool for clinical diagnosis and laboratory epidemiological investigations.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2016YFD0501001). We express our sincere gratitude to Jia-Li Zhang at the Institute of Animal Hospital of Jinlin Agricultural University for assistance with clinical sample collection.
Compliance with ethical standards
Funding
This study was funded by the National Key Research and Development Program of China (2016YFD0501001).
Conflict of interest
The authors declare no conflicts of interest. The authors have declared that no competing interests exist.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
All the authors have agreed that Xijun Yan may act on their behalf regarding any subsequent processing of the paper.
Xiaoyu Deng and Jiali Zhang contributed to the work equally and should be regarded as co-first authors.
References
- 1.Cargnelutti JF, Weiblen R, Flores EF. A multiplex PCR for viruses associated with exanthematic and vesicular disease in cattle. J Virol Methods. 2017;239:38–41. doi: 10.1016/j.jviromet.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Carman PS, Povey RC. Pathogenesis of canine parvovirus-2 in dogs: haematology, serology and virus recovery. Res Vet Sci. 1985;38:134–140. [PubMed] [Google Scholar]
- 3.Elnifro EM, Ashshi AM, Cooper RJ, Klapper PE. Multiplex PCR: optimization and application in diagnostic virology. Clin Microbiol Rev. 2000;13:559–570. doi: 10.1128/CMR.13.4.559-570.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Q, Yun BL, Wang Q, Jiang LL, Zhu HB, Gao YN, Qin LT, Wang YQ, Qi XL, Gao HL, Wang XM, Gao YL. Development and application of a multiplex PCR method for rapid differential detection of subgroup A, B, and J avian leukosis viruses. J Clin Microbiol. 2014;52:37–44. doi: 10.1128/JCM.02200-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamelin C, Jouvenne P, Assaf R. Association of a type-2 canine adenovirus with an outbreak of diarrhoeal disease among a large dog congregation. J Diarrhoeal Dis Res. 1985;3:84–87. [PubMed] [Google Scholar]
- 6.Hu RL, Huang G, Qiu W, Zhong ZH, Xia XZ, Yin Z. Detection and differentiation of CAV-1 and CAV-2 by polymerase chain reaction. Vet Res Commun. 2001;25:77–84. doi: 10.1023/A:1006417203856. [DOI] [PubMed] [Google Scholar]
- 7.Macartney L, Cavanagh HM, Spibey N. Isolation of canine adenovirus-2 from the faeces of dogs with enteric disease and its unambiguous typing by restriction endonuclease mapping. Res Vet Sci. 1988;44:9–14. [PubMed] [Google Scholar]
- 8.Miranda C, Thompson G. Canine parvovirus: the worldwide occurrence of antigenic variants. J Gen Virol. 2016;97:2043–2057. doi: 10.1099/jgv.0.000540. [DOI] [PubMed] [Google Scholar]
- 9.Mochizuki M, Hashimoto M, Ishida T. Recent epidemiological status of canine viral enteric infections and Giardia infection in Japan. J Vet Med Sci. 2001;63:573–575. doi: 10.1292/jvms.63.573. [DOI] [PubMed] [Google Scholar]
- 10.Parrish CR. Pathogenesis Of Feline Panleukopenia Virus And Canine Parvovirus. Bailliere Clin Haem. 1995;8:57–71. doi: 10.1016/S0950-3536(05)80232-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pratelli A, Martella V, Elia G, Tempesta M, Guarda F, Capucchio MT, Carmichael LE, Buonavoglia C. Severe enteric disease in an animal shelter associated with dual infections by canine adenovirus type 1 and canine coronavirus. J Vet Med B. 2001;48:385–392. doi: 10.1046/j.1439-0450.2001.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pratelli A, Tempesta M, Greco G, Martella V, Buonavoglia C. Development of a nested PCR assay for the detection of canine coronavirus. J Virol Methods. 1999;80:11–15. doi: 10.1016/S0166-0934(99)00017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senda M, Parrish CR, Harasawa R, Gamoh K, Muramatsu M, Hirayama N, Itoh O. Detection by Pcr Of wild-type canine parvovirus which contaminates dog vaccines. J Clin Microbiol. 1995;33:110–113. doi: 10.1128/jcm.33.1.110-113.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tennant BJ, Gaskell RM, Kelly DF, Carter SD, Gaskell CJ. Canine coronavirus infection in the dog following oronasal inoculation. Res Vet Sci. 1991;51:11–18. doi: 10.1016/0034-5288(91)90023-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Li C, Guo D, Wang X, Wei S, Geng Y, Wang E, Wang Z, Zhao X, Su M, Liu Q, Zhang S, Feng L, Sun D. Co-Circulation of Canine Coronavirus I and IIa/b with High Prevalence and Genetic Diversity in Heilongjiang Province, Northeast China. PloS One. 2016;11:e0146975. doi: 10.1371/journal.pone.0146975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng ZY, Liu ZJ, Wang WC, Tang DY, Liang HY, Liu Z. Establishment and application of a multiplex PCR for rapid and simultaneous detection of six viruses in swine. J Virol Methods. 2014;208:102–106. doi: 10.1016/j.jviromet.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Zhou G, Cai WB, Liu XL, Niu CM, Gao CX, Si CD, Zhang W, Qu LD, Han LX. A duplex real-time reverse transcription polymerase chain reaction for the detection and quantitation of avian leukosis virus subgroups A and B. J Virol Methods. 2011;173:275–279. doi: 10.1016/j.jviromet.2011.02.017. [DOI] [PubMed] [Google Scholar]


