Abstract
Red foxes (Vulpes vulpes) are susceptible to viral diseases of domestic carnivores. In this study, by screening rectal swabs collected from 34 red foxes in Italy, we identified kobuvirus RNA in five samples. Based on analysis of partial RdRp and full-length VP1 genes, all of the strains shared the highest identity with canine kobuviruses (CaKVs) recently detected in the US, the UK and Italy. These findings provide the first evidence of the circulation of these novel viruses in foxes.
Keywords: Canine Distemper Virus, Rectal Swab, Vulpes Vulpes, Aichi Virus, Porcine Kobuvirus
Picornaviruses are small, non-enveloped viruses of approximately 27–30 nm in diameter with single-stranded, positive-polarity RNA genomes of 8.2–8.4 kb. According to the International Committee on Taxonomy of Viruses (http://ictvonline.org/virusTaxonomy.asp?version=2012), the family Picornaviridae is currently divided into 17 genera: Aphthovirus, Aquamavirus, Avihepatovirus, Cardiovirus, Cosavirus, Dicipivirus, Enterovirus, Erbovirus, Hepatovirus, Kobuvirus, Megrivirus, Parechovirus, Salivirus, Sapelovirus, Senecavirus, Teschovirus and Tremovirus.
The genus Kobuvirus consists of three species [1], Aichivirus A (formerly Aichi virus), Aichivirus B (formerly Bovine kobuvirus) and Aichivirus C (porcine kobuvirus).
Human Aichi virus (AiV) was first recognized in 1989 as the cause of oyster-associated nonbacterial gastroenteritis in humans in Aichi Prefecture, Japan [27]. Several investigations worldwide have revealed that AiVs are involved in 0.9-4.1 % of sporadic cases of pediatric gastroenteritis [2, 10, 11, 21].
Novel kobuviruses that are genetically and antigenically closely related to human AiVs have been found recently in domestic carnivores [5, 6, 12, 14], raising public health concerns of potential cross-species transmission. Carnivore kobuviruses were first discovered in diarrhoeic and asymptomatic dogs [12, 14]. Based on sequence analysis of the complete genome, the novel virus designated canine kobuvirus (CaKV) was found to be genetically closest to the recently identified mouse kobuvirus M-5/USA/2010 (84.0 % amino acid [aa] sequence identity) [17] and to human AiVs (80.0 % aa sequence identity). Accordingly, CaKV may be considered a distinct genotype (CaKV type 1) from both murine kobuvirus (MuKV type 1) and human AiV (AiV type 1), within the species Aichivirus A (http://ictvonline.org/proposals/2012.014aV.A.v1.Kobuvirus-Sp,Ren.pdf).
Subsequently, kobuviruses that are genetically related to CaKVs were identified in the faecal samples of cats with diarrhoea [6]. Nucleotide sequence identity between CaKVs and feline kobuviruses in the RdRp region ranged from 82.2 % to 89.3 %, while identity to MuKV and to human AiV was 82.4-85.5 % and 81.9-82.4 %, respectively.
Also, a novel kobuvirus more close related to bovine and ovine kobuviruses within the species Aichivirus B has been identified recently in a rectal swab from a ferret [23], demonstrating that non-domestic carnivores are susceptible to kobuvirus infections.
Here we report the detection of kobu-like viruses in faecal samples obtained from Italian red foxes (Vulpes vulpes) that are genetically very similar to CaKVs.
Between September 2009 and May 2013, individual rectal swabs were collected from 34 red foxes in northern Italy (Valle d’Aosta and Piemonte regions) submitted to the National Reference Center for Wild Animal Diseases (CeRMAS). The samples were obtained from animals of both genders (14 females and 20 males) found dead or shot during the regular hunting season. Twelve foxes were under 1 year of age, and 22 were adults. Fresh faecal samples were placed into sterile containers and stored at −80 °C until tested.
RNA was extracted from 200 μl of 10 % (wt/vol) faecal suspension using TRIzol LS (Invitrogen, Ltd, Paisley, UK) procedure. The final RNA pellet was resuspended in 50 μl of RNase-free water and used directly in RT-PCR assays or stored at −80 °C. Viral DNA was extracted from the supernatants of faecal homogenates by boiling for 10 min and chilling on ice as described previously [7].
Kobuvirus RNA was detected by RT-PCR using a broadly reactive primer pair, UNIV-kobu-F/UNIV-kobu-R, which amplifies a 217-bp fragment of the 3D gene of all kobuviruses, following reaction conditions described previously [20].
The samples were also tested by RT-PCR for canine coronaviruses (CCoVs) [18] and canine noroviruses (NoVs) [16, 25], and by PCR for canine parvovirus (CPV-2) [4].
Novel primers (VP1rf-F, 5′-GCGGGCGAATCCTTCAAC-3, and VP1rf-R, GCGACCTTTCGGAGCGCC-3′) to amplify the complete VP1 gene were designed by visual inspection of an alignment containing the kobu-like sequences obtained in this study and the corresponding conserved regions of the CaKVs and AiVs available on the NCBI website.
All of the amplicons were purified using a QIAquick Gel Extraction Kit (QIAGEN GmbH, Hilden, Germany) and sequenced directly using BigDye Terminator cycle chemistry and a 3730 DNA analyzer (Applied Biosystems, Foster City, CA). RT-PCR products for which no sequences were obtained by direct sequencing were cloned into the pCR2.1 vector (Invitrogen, Ltd, Paisley, UK). Plasmid DNA was purified using a Miniprep Kit (QIAGEN GmbH, Hilden, Germany); three clones per product were sequenced. Basic Local Alignment Search Tool (BLAST; http://www.ncbi.nlm.nih.gov) and FASTA (http://www.ebi.ac.uk/Tools/sss/fasta/) with default values were used to find homologous hits. Sequence editing and multiple alignments were performed with the BioEdit software package, version 2.1 [9]. A phylogenetic tree (neighbor joining and p-distance model) with bootstrap analysis (1,000 replicates) was constructed using the MEGA software package, version 3.0 [13].
Out of 34 faecal specimens, five (14.7 %) were found to be positive for kobuvirus RNA, and two (5.8 %) for CCoV RNA. Canine NoVs and CPV-2 were not detected. Kobuvirus was the single identified enteric virus in four specimens, while a mixed infection with both pathogens (CCoV + kobuvirus, n = 1) was found in one sample. All of the samples were obtained from adult animals. None of the foxes that were positive for CCoV or kobuvirus RNA showed pathological lesions indicative of enteritis.
By FASTA and BLAST analysis of the partial 3D region amplified with the primers UNIV-kobu-F/UNIV-kobu-R, the five fox strains (GenBank accession no. KF781168-KF781172) shared 95.4-96.8 % nt sequence identity with each other and displayed the closest relatedness (92.7-96.2 % nt) with the CaKVs previously found in the US, the UK and Italy [5, 8, 12, 14]. Identity to feline kobuviruses [6] ranged from 81.1 % to 87.7 %, while nt sequence identity to human AiVs was 81.6-85.0 %.
The full-length sequence of the VP1 gene was determined for each of the five kobuvirus strains (GenBank accession no. KF781163-KF781167). Sequence analysis indicated that the fox kobuviruses were highly related to each other (91.7-98.5 % nt and 97.8-100 % aa identities) and to the CaKV strain UK/003 (accession no. KC161964) (92.6-96.4 % nt and 98-99 % aa identities) recently identified in a healthy dog in the UK [5]. Neighbor-joining phylogenetic analysis based on the VP1 sequences was carried out with a selection of strains representative of the genus Kobuvirus, including murine kobuvirus [17], human AiVs, wild-boar kobuvirus [22], the ferret kobuvirus strain MpKoV32 [23], and the prototype strains porcine kobuvirus S-1 [19] and bovine kobuvirus U1 [26]. Based on inspection of the tree (Fig. 1), all of the fox kobuvirus sequences formed a tight cluster with the CaKVs recently identified in the UK and in the US [5, 12, 14], sharing a common root with murine kobuvirus and human AiVs.
Fig. 1.
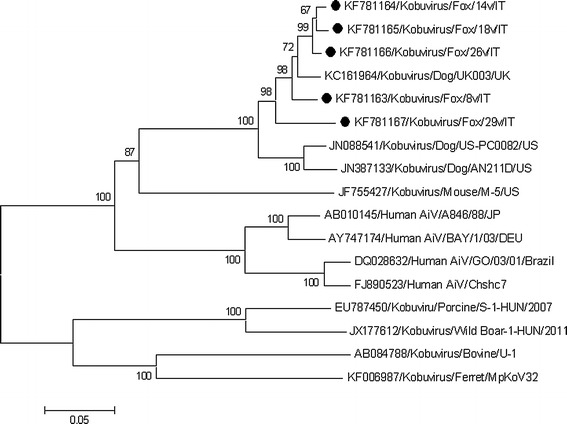
Neighbor-joining phylogenetic tree based on the VP1 gene (834 bp) of the kobuvirus strains detected in red foxes. The tree was generated using the neighbor-joining method and p-distance correction, supplying a statistical support with bootstrapping of 1000 replicates. The scale bar indicates nucleotide substitutions per site. The markers indicate the sequences detected in this study
Historical evidence shows that foxes are susceptible to viral diseases of domestic carnivores, including infections with canine distemper virus [15, 24], CPV-2 [24], canine adenoviruses [3, 24], CCoV [28], and canine herpesvirus [24].
In the present study, we detected kobuviruses in free-ranging red foxes with a prevalence rate of 14.7 % (5/34). Investigations based on the molecular analysis of the full-length VP1 gene revealed that the five fox strains were most closely related to the CaKVs (97.8-100 % aa) recently identified in dogs [5, 12, 14]. Therefore, the fox kobuviruses detected in this study may be considered members of genotype CaKV-1 within the species Aichivirus A. In addition, this is the first study demonstrating the presence of CCoVs in free-ranging red foxes in Italy.
Although CaKVs were found in both asymptomatic and diarrhoeic dogs, the pathogenic potential of these novel kobuviruses in carnivores remains to be elucidated. In this preliminary study, all of the CaKV-like viruses detected were identified in adult foxes found dead or shot during the regular hunting season without apparent pathological enteric lesions. Accordingly, it is unclear if these viruses may have a role in the aetiology of diarrhoea in foxes. Future large-scale virologic investigations by screening faecal samples collected from both diarrhoeic and healthy animals are needed to assess the distribution and pathogenic potential of CaKV-like viruses in foxes.
In conclusion, this study provides direct evidence of the circulation of CaKV-like viruses in red foxes, reinforcing the previous observations that kobuvirus infections are probably as frequent in wild animals as they are in domestic animals [22, 23]. Additional studies are warranted in order to determine the significance of these findings and to evaluate the epidemiological and clinical importance of these novel viruses in wild carnivores. Furthermore, as fox kobuviruses are newly recognized viruses and information on their diversity is still limited, a definitive characterization should rely on sequence analysis of the complete genome.
Acknowledgments
This work was financed by grants from the University of Teramo, Italy, and from the Italian Ministry of University and Research.
Conflict of interest
All authors declare that there are no financial or other relationships that might lead to a conflict of interest. All authors have seen and approved the manuscript and have contributed significantly to the work.
References
- 1.Adams MJ, King AM, Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2013) Arch Virol. 2013;158:2023–2030. doi: 10.1007/s00705-013-1688-5. [DOI] [PubMed] [Google Scholar]
- 2.Ambert-Balay K, Lorrot M, Bon F, Giraudon H, Kaplon J, Wolfer M, Lebon P, Gendrel D, Pothier P. Prevalence and genetic diversity of Aichi virus strains in stool samples from community and hospitalized patients. J Clin Microbiol. 2008;46:1252–1258. doi: 10.1128/JCM.02140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balboni A, Verin R, Morandi F, Poli A, Prosperi S, Battilani M. Molecular epidemiology of canine adenovirus type 1 and type 2 in free-ranging red foxes (Vulpes vulpes) in Italy. Vet Microbiol. 2013;162:551–557. doi: 10.1016/j.vetmic.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Buonavoglia D, Cavalli A, Pratelli A, Martella V, Greco G, Tempesta M, Buonavoglia C. Antigenic analysis of canine parvovirus strains isolated in Italy. New Microbiol. 2000;23:93–96. [PubMed] [Google Scholar]
- 5.Carmona-Vicente N, Buesa J, Brown PA, Merga JY, Darby AC, Stavisky J, Sadler L, Gaskell RM, Dawson S, Radford AD. Phylogeny and prevalence of kobuviruses in dogs and cats in the UK. Vet Microbiol. 2013;164:246–252. doi: 10.1016/j.vetmic.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung JY, Kim SH, Kim YH, Lee MH, Lee KK, Oem JK. Detection and genetic characterization of feline kobuviruses. Virus Genes. 2013 doi: 10.1007/s11262-013-0953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decaro N, Elia G, Martella V, Desario C, Campolo M, Di Trani L, Tarsitano E, Tempesta M, Buonavoglia C. A real-time PCR assay for rapid detection and quantitation of canine parvovirus type 2 in the feces of dogs. Vet Microbiol. 2005;105:19–28. doi: 10.1016/j.vetmic.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Di Martino B, Di Felice E, Ceci C, Di Profio F, Marsilio F. Canine kobuviruses in diarrhoeic dogs in Italy. Vet Microbiol. 2013;166:246–249. doi: 10.1016/j.vetmic.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall TA. BioEdit: a user-friendly biological sequence alignment and analysis program for Windows 95/98/NT. Nucleic Acids Symp. 1999;41:95–98. [Google Scholar]
- 10.Jonsson N, Wahlström K, Svensson L, Serrander L, Lindberg AM. Aichi virus infection in elderly people in Sweden. Arch Virol. 2012;157:1365–1369. doi: 10.1007/s00705-012-1296-9. [DOI] [PubMed] [Google Scholar]
- 11.Kaikkonen S, Räsänen S, Rämet M, Vesikari T. Aichi virus infection in children with acute gastroenteritis in Finland. Epidemiol Infect. 2010;138:1166–1171. doi: 10.1017/S0950268809991300. [DOI] [PubMed] [Google Scholar]
- 12.Kapoor A, Simmonds P, Dubovi EJ, Qaisar N, Henriquez JA, Medina J, Shields S, Lipkin WI. Characterization of a canine homolog of human Aichi virus. J Virol. 2011;85:11520–11525. doi: 10.1128/JVI.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Tamura K, Nei M. MEGA3: integrated software for molecular evolutionary genetics analysis and sequence alignment. Brief Bioinform. 2004;5:153–163. doi: 10.1093/bib/5.2.150. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Pesavento PA, Shan T, Leutenegger CM, Wang C, Delwart E. Viruses in diarrhoeic dogs include novel kobuviruses and sapoviruses. J Gen Virol. 2011;92:2534–2541. doi: 10.1099/vir.0.034611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martella V, Bianchi A, Bertoletti I, Pedrotti L, Gugiatti A, Catella A, Cordioli P, Lucente MS, Elia G, Buonavoglia C. Canine distemper epizootic among red foxes. Italy, 2009. Emerg Infect Dis. 2010;16:2007–2009. doi: 10.3201/eid1612.100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mesquita JR, Barclay L, Nascimento MS, Vinjé J. Novel norovirus in dogs with diarrhea. Emerg Infect Dis. 2010;16:980–982. doi: 10.3201/eid1606.091861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phan TG, Kapusinszky B, Wang C, Rose RK, Lipton HL, Delwart EL. The fecal viral flora of wild rodents. PLoS Pathog. 2011;7:e1002218. doi: 10.1371/journal.ppat.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pratelli A, Tempesta M, Greco G, Martella V, Buonavoglia C. Development of a nested PCR assay for the detection of canine coronavirus. J Virol Methods. 1999;80:11–15. doi: 10.1016/S0166-0934(99)00017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reuter G, Boldizsár A, Kiss I, Pankovics P. Candidate new species of kobuvirus in porcine hosts. Emerg Infect Dis. 2008;14:1968–1970. doi: 10.3201/eid1412.080797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuter G, Boldizsár A, Pankovics P. Complete nucleotide and amino acid sequences and genetic organization of porcine kobuvirus, a member of a new species in the genus Kobuvirus, family Picornaviridae. Arch Virol. 2009;154:101–108. doi: 10.1007/s00705-008-0288-2. [DOI] [PubMed] [Google Scholar]
- 21.Reuter G, Boldizásr A, Papp G, Pankovics P. Detection of Aichi virus shedding in a child with enteric and extraintestinal symptoms in Hungary. Arch Virol. 2009;154:1529–1532. doi: 10.1007/s00705-009-0473-y. [DOI] [PubMed] [Google Scholar]
- 22.Reuter G, Nemes C, Boros A, Kapusinszky B, Delwart E, Pankovics P. Porcine kobuvirus in wild boars (Sus scrofa) Arch Virol. 2013;158:281–282. doi: 10.1007/s00705-012-1456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smits SL, Raj VS, Oduber MD, Schapendonk CM, Bodewes R, Provacia L, Stittelaar KJ, Osterhaus AD, Haagmans BL. Metagenomic analysis of the ferret fecal viral flora. PLoS One. 2013;8(8):e71595. doi: 10.1371/journal.pone.0071595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truyen U, Müller T, Heidrich R, Tackmann K, Carmichael LE. Survey on viral pathogens in wild red foxes (Vulpes vulpes) in Germany with special emphasis on parvoviruses and analysis of a DNA sequence from a red fox parvovirus. Epidemiol Infect. 1998;121:433–440. doi: 10.1017/S0950268898001319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vennema H, de Bruin E, Koopmans M. Rational optimization of generic primers used for Norwalk-like virus detection by reverse transcriptase polymerase chain reaction. J Clin Virol. 2002;25:233–235. doi: 10.1016/S1386-6532(02)00126-9. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita T, Ito M, Kabashima Y, Tsuzuki H, Fujiura A, Sakae K. Isolation and characterization of a new species of kobuvirus associated with cattle. J Gen Virol. 2003;84:3069–3077. doi: 10.1099/vir.0.19266-0. [DOI] [PubMed] [Google Scholar]
- 27.Yamashita T, Kobayashi S, Sakae K, Nakata S, Chiba S, Ishihara Y, Isomura S. Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J Infect Dis. 1991;164:954–957. doi: 10.1093/infdis/164.5.954. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Ma G, Lu C, Wen H. Detection of canine coronaviruses genotype I and II in raised Canidae animals in China. Berl Munch Tierarztl Wochenschr. 2006;119:35–39. [PubMed] [Google Scholar]


