Abstract
Respiratory syncytial virus (RSV) infection is associated with chronic respiratory morbidity in infants born very prematurely. Our aims were to determine if infants born moderately prematurely (32–35 weeks of gestation) who had had an RSV hospitalisation, compared to those who had not, had greater healthcare utilisation and related cost of care in the first 2 years. Two thousand and sixty-six eligible infants’ records were examined to identify three groups: 20 infants admitted for an RSV lower respiratory tract infection (RSV), 30 admitted for another respiratory problem (other respiratory) and 108 admitted for a non-respiratory problem/never admitted (non-respiratory). Healthcare utilisation was assessed by examining hospital and general practitioner records and cost of care calculated using the National Scheme of Reference costs and the British National Formulary prices. The mean cost of care in the RSV group (£12,505) was greater than the non-respiratory (£1,178) (95% CI for difference £5,015 to £17,639, p = 0.002) and the other respiratory (£3,356) groups (95% CI for difference £2,963 to £15,606, p < 0.001). The adjusted mean differences in the cost of care were £11,186 between the RSV and non-respiratory groups (95% CI £4,763 to £17,609) and £9,076 (95% CI £2,515 to £15,637) between the RSV and the other respiratory groups. Forty-two of 2,066 eligible infants had an RSV hospitalisation (2%); thus, assuming prophylaxis would reduce the hospitalisation rate by 50%, the number needed to treat was 98. In conclusion, RSV hospitalisation in moderately prematurely born infants is associated with increased health-related cost of care. Nevertheless, if RSV prophylaxis is to be cost-effective, a high risk group of moderately prematurely born infants needs to be identified.
Keywords: Prematurity, Respiratory syncytial virus, Cost of care
Introduction
Respiratory syncytial virus (RSV) infection is associated with chronic respiratory morbidity in infants born very prematurely, that is, before 32 weeks of gestation [5–7]. In those who had developed bronchopulmonary dysplasia (BPD), healthcare utilisation and related cost of care were increased up to 8 years of age following RSV hospitalisation in the first 2 years after birth [5–7]. In addition, at 8 years of age, the children who had had an RSV hospitalisation during infancy had worse lung function [5]. Other studies [2, 3] have highlighted that prematurely born infants who had an RSV lower respiratory tract infection (LRTI) regardless of a past history of BPD compared to those who had not were more likely at follow-up to cough and wheeze, had more general practitioner (GP) attendances [2] and worse lung function abnormalities [3]. In those studies [2, 3, 5, 7], RSV infection was confirmed by identifying RSV by immunofluorescence and/or positive culture from nasopharyngeal aspirates. RSV hospitalisation may also increase subsequent healthcare utilisation in infants born between 32 and 35 weeks of gestation [16, 17, 19]. In one study [19], however, the International Classification of Diseases was used and infants with probable as well as confirmed RSV hospitalisations were included. Probable RSV hospitalisations were defined as those with non-specific pneumonia or bronchiolitis, when RSV was reported to be circulating in the community. In two other studies [16, 17] which included infants born between 33 and 36 weeks of gestation, ICD-9 codes were also used and it was not possible to record whether the diagnosis of RSV was confirmed by culture or other investigation. It is, therefore, uncertain whether proven RSV hospitalisation increases subsequent healthcare utilisation and associated cost of care in infants born between 32 and 35 weeks of gestation. It is important to determine whether such infants do have increased morbidity at follow-up, as they may have worse acute hospital outcomes for bronchiolitis or RSV pneumonia than more prematurely born infants [8]. In addition, infants born between 32 and 35 weeks of gestation represent the majority of prematurely born infants. Hence, the results would be important for the planning of healthcare resource utilisation and the use of RSV prophylactic agents. The aims, therefore, of this study were to examine healthcare utilisation and the related cost of care in the first 2 years after birth following maternity/neonatal unit discharge in infants born between 32 and 35 weeks gestation to determine if it differed according to whether the infants had had an RSV hospitalisation or not. We chose to study the impact of RSV hospitalisation as prematurely born infants who require hospitalisation for an RSV LRTI rather than remain in the community suffer greater morbidity at follow-up [2]. In addition, we wished to determine whether infants admitted for a respiratory cause other than a proven RSV LRTI differed in outcome compared to those who were admitted for an RSV LRTI. A further aim was to calculate, using the data generated from this study, the number needed to treat assuming a 50% reduction in the hospitalisation rate if RSV prophylaxis had been given [9] and hence whether the cost of prophylaxis might be justified in moderately prematurely born infants.
Materials and methods
A review was undertaken of infants born between 32 and 35 weeks of gestation at two hospitals (King’s College and Guy’s and St Thomas’ NHS Foundation Trusts) between 2000 and 2007. The hospitals’ maternity and neonatal databases were interrogated to identify infants born between 32 and 35 weeks of gestation. The neonatal records were then examined, and infants found to be born prior to 32 weeks or later than 35 weeks of gestation, had major congenital abnormalities (e.g. major congenital heart abnormalities) or died before discharge from the hospital were excluded. The hospital records were then examined to determine whether the infant had been admitted to a hospital following maternity/neonatal unit discharge and the diagnosis of any admission. The GPs of all infants who had an RSV hospitalisation, all those who had been admitted for another respiratory problem and the first 200 who had had no admission or an admission for a non-respiratory problem were contacted to determine whether the infants were still alive and to confirm their home address. The parents of surviving infants with known home addresses were then sent information about the study and asked to send back signed written consent if they agreed to their infant’s hospital and primary care medical records being examined to determine their infant’s healthcare utilisation and health-related cost of care during the first 2 years after birth (infancy).
Healthcare utilisation during the first 2 years was reviewed for those infants whose parents gave informed written consent. If the infant had been admitted to the neonatal unit, the following data were retrieved: birth weight; use of antenatal steroids and postnatal surfactant; duration of ventilatory support and supplementary oxygen; and use of high frequency oscillation and/or nitric oxide. From the GPs’ records, the following data were retrieved: venue of all hospital readmissions, number of GP consultations, all medication prescribed, use and duration of home oxygen, number of referrals to a health visitor or community paediatric nurse and use of community support services. For each hospital admission, the following information was recorded: diagnosis leading to the admission; duration of stay, whether the infant was admitted to a paediatric ward, high dependency unit (HDU), or intensive care unit (ICU); days of supplementary oxygen and intravenous fluids; and duration and frequency of all medication. The cost of care for each admission was calculated as the number of days the infant spent at each level of care, that is in a paediatric ward, HDU or ICU multiplied by the cost of care of that level of care. The costs of each admission at each level of care and outpatient attendance were calculated using data from the National Scheme of Reference Costs (2003). Each infant’s hospital records were examined to ascertain the number of outpatient attendances. Costs were calculated over the 2-year period following maternity/neonatal unit discharge. Medication costs were calculated from the British National Formulary prices. All primary care costs were those reported by Netten et al. [15]. The data are presented for all admissions, outpatient attendances, etc and then separately for those admissions, outpatient attendances, etc due to a respiratory illness.
Analysis
Three groups of infants were studied:
Infants who had at least one hospitalisation for a proven RSV LRTI (RSV identified on a nasopharyngeal aspirate (NPA) by immunofluorescence, culture and/or real-time reverse transcription polymerase chain reaction (rt PCR) (RSV group)
Infants who had at least one admission for another respiratory problem, that is other than proven RSV LRTI (other respiratory group)
Infants who had at least one admission for a non-respiratory cause or no admission (non-respiratory group)
Statistical methods
The three groups of infants were compared using one-way analysis of variance where the data were normally distributed and by Kruskal–Wallis one-way analysis of variance by ranks where data were ordinal, but not normally distributed. Post hoc comparisons of pairs of groups were made when the overall variability between the three groups was statistically significant. A modified p value (below 0.017) was considered significant to allow for multiple testing.
The cost data were highly skewed; however, we have reported the main results as arithmetic means rather than medians as these can be used to provide the total cost of treating all patients. If a median cost is applied to all patients, the total calculated cost would be different to the actual total cost and so be misleading [23]. The approach previously advocated [1] was followed, that is, fitting a generalised linear model. Using a gamma distribution and identity link, a model whose deviance residuals were very close to normal was obtained (a standard normal distribution model gave a marked skewness and larger standard errors and thus was not used). Results of these models were presented as differences in arithmetic means with 95% CIs. In order to account for any differences in the demographics of the three groups, a principal components analysis was first used to reduce the birthweight, gestational age, antenatal steroid use and surfactant data to three principal components that explained almost 90% of the total variability in these factors. These three components were then used as covariates in a further generalised linear model to obtain adjusted estimates. The number needed to treat (NNT) was calculated assuming a 50% reduction in the hospitalisation rate if RSV prophylaxis had been given [8].
Sample size calculation
We planned to recruit 30 infants with an RSV respiratory admission, 30 with another respiratory admission and 90 with non-respiratory/no admission. Comparison of 30 infants with an RSV hospitalisation to at least 30 infants in each of the other two groups would allow us to detect with 80% power at the 5% level a difference equivalent to 0.75 standard deviation in healthcare utilisation and cost of care. It was planned to recruit 90 infants into the non-respiratory/no admission group to ensure sufficient data for regression analysis.
Results
Two thousand, five hundred possible infants were identified from the antenatal and neonatal records, but 434 were found to be ineligible as they met one or more of the exclusion criteria (Fig. 1). Twenty parents of infants who had had an RSV hospitalisation and 30 parents of infants in the other respiratory group gave informed consent for their infants’ medical records to be examined and it was possible to locate all the records. As it was not possible to predict whether all the medical records would be available, we approached 200 parents of infants in the non-respiratory group; 108 parents gave informed written consent for their infants’ medical records to be examined and all their records were available. The demographics of the three groups were similar (Table 1). The diagnoses of the other respiratory group were RSV negative bronchiolitis (n = 20), pneumonia (n = 7) and shortness of breath with wheeze (n = 3). In the non-respiratory group, 20 patients had been admitted: four with gastroenteritis, two with upper respiratory tract infections, one with a urinary tract infection, one with a head injury, one with an apnoeic episode possibly due to choking, four with poor feeding and jaundice, four for surgery and three for investigations. None of the infants in any of the groups developed BPD defined as oxygen dependency beyond 28 days, had received RSV prophylaxis or went home in supplementary oxygen.
Fig. 1.
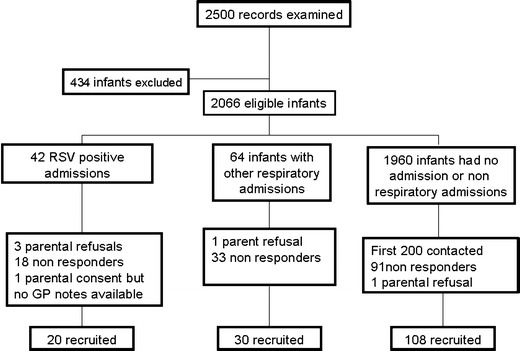
Consort diagram of recruitment
Table 1.
Demographics by hospital readmission status
| RSV (n = 20) | Other respiratory (n = 30) | Non-respiratory (n = 108) | p value | |
|---|---|---|---|---|
| Gestational age (weeks) | 33.3 (0.16) | 33.6 (0.97) | 33.3 (0.95) | 0.40 |
| Birthweight (g) | 2,033 (394) | 2,146 (444) | 2,105 (456) | 0.68 |
| Antenatal steroids | 14 (75%) | 14 (47%) | 64 (59.3%) | 0.24 |
| Surfactant | 1 (10%) | 2 (6.6%) | 7 (6.4%) | 0.87 |
| Admission to neonatal unit | 15 (75%) | 29 (97%) | 95 (88%) | 0.07 |
| Duration of ventilation (days) | 0.25 (0.55) | 0.13 (0.57) | 0.38 (1.17) | 0.49 |
Data are presented as mean (SD) or n (%)
Healthcare utilisation was significantly greater in the RSV compared to the non-respiratory group for respiratory outpatient visits, hospital admissions, respiratory admission, duration of hospital admission, PICU admission, respiratory GP visits, accident and emergency (A&E) visits and respiratory A&E visits (Table 2). Healthcare utilisation was not significantly greater in the RSV compared to the other respiratory group, although the duration of hospital admission tended to be longer in the RSV group (p = 0.06) (Table 2). The health-related cost of care was significantly greater in the RSV compared to the non-respiratory group for outpatient visits, A&E visits, prescriptions and total cost. The health-related cost of care was greater in the RSV compared to the other respiratory group for the total costs (Table 3). The differences in the total mean costs between groups remained significant after adjustment (Table 4).
Table 2.
Healthcare utilisation by hospital readmission status
| RSV (n = 20) | Other respiratory (n = 30) | Non-respiratory (n = 108) | Overall p value | Post hoc testsa | p for post hoc comparison | |
|---|---|---|---|---|---|---|
| Hospital admissions | 2.3 (2; 1–12) | 1.8 (1; 1–6) | 0.3 (0; 0–3) | p < 0.001 | RSV vs non-resp | <0.001 |
| RSV vs other resp | 0.56 | |||||
| Respiratory hospital admission | 1.3(1; 1–2) | 1.2 (1; 1–4) | 0 (0; all = 0) | p < 0.001 | RSV vs non-resp | <0.001 |
| RSV vs other resp | 0.21 | |||||
| Duration of hospital admission (days) | 9.6 [4.5; 1–110] | 3.3 [2; 1–13] | 0.4 [0; 0–10] | p < 0.001 | RSV vs non-resp | <0.001 |
| RSV vs other resp | 0.06 | |||||
| PICU admission (days) | 1.6 [0; 0–29] | 0.03 [0; 0–1] | 0 [0; 0–0] | p < 0.001 | RSV vs non-resp | <0.001 |
| RSV vs other resp | 0.6 | |||||
| GP visits (n) | 12.4 (12; 1–27) | 14.4 (12; 1–50) | 9.4 (8; 0–34) | p = 0.016 | RSV vs non-resp | 0.07 |
| RSV vs other resp | 0.80 | |||||
| Respiratory GP visits (n) | 5.0 (4; 0–14) | 5.9 (5; 0–29) | 2.9 (2; 0–17) | p = 0.002 | RSV vs non-resp | 0.010 |
| RSV vs other resp | 0.98 | |||||
| A&E visits | 3.0 (3; 1–6) | 3.4 (3; 1–14) | 0.7 (0; 0–9) | p < 0.001 | RSV vs non-resp | <0.001 |
| RSV vs other resp | 0.90 | |||||
| Respiratory A&E visits | 1.6 (2; 1–4) | 2.2 (2; 0–7) | 0.1 (0; 0–7) | p < 0.001 | RSV vs non-resp | <0.001 |
| RSV vs other resp | 0.25 | |||||
| OPD visits | 6.1 (5; 1–24) | 5.8 (3; 0–20) | 3.8 (3; 0–13) | p = 0.146 | N/A | |
| Respiratory OPD | 0.6 (0; 0–4) | 0.9 (0; 0–7) | 0.1 (0; 0–6) | p < 0.001 | RSV vs non-resp | <0.001 |
| RSV vs other resp | 0.21 |
Data are demonstrated as mean (median; range)
aIn the final column: for statistical significance, p must be less than 0.017 to account for multiple testing
Table 3.
Health-related cost of care in UK pounds by hospital readmission status
| RSV (n = 20) | Other respiratory (n = 30) | Non-respiratory (n = 108) | Overall p value | Post hoc tests of significant pairs | p for post hoc comparison | |
|---|---|---|---|---|---|---|
| Hospital admission | 10,936 (31,928) [1,895, 956–142,584] | 1,705 (1,073) [1,097, 478–5,887] | 230 (562) [0, 0–3,304] | p = 0.019 | RSV vs non-resp | 0.06 |
| RSV vs other resp | 0.10 | |||||
| Respiratory admission | 8,878 (31,385) [1,123, 956–142,051] | 1,211 (506) [1,087, 478–3,117] | 0 (0) [0, 0–0] | p = 0.09 | RSV vs other resp | 0.09 |
| OPD visit | 931 (885) [695,126–4,092] | 900 (916) [530, 0–3,929] | 579 (435) [501, 0–2,186] | p = 0.017 | RSV vs non-resp | 0.05 |
| RSV vs other resp | 0.89 | |||||
| Respiratory OPD visit | 95 (221) [0, 0–882] | 180 (301) [0, 0–1,182] | 25 (157) [0, 0–1,542] | p = 0.56 | RSV vs non-resp | n/a |
| RSV vs other resp | ||||||
| A&E visit | 284 (158) [224, 71–606] | 273 (232) [227, 71–1,170] | 52 (102) [0, 0–694] | p = 0.003 | RSV vs non-resp | 0.03 |
| RSV vs other resp | 0.93 | |||||
| Respiratory A&E visit | 149 (69) [137, 46–296] | 178 (148) [149, 0–616] | 13 (61) [0, 0–585] | p = 0.38 | RSV vs non-resp | n/a3 |
| RSV vs other resp | 0.38 | |||||
| Prescription | 74 (102) [42, 1.7–426] | 118 (249) [38, 7.6–1,109] | 29 (28) [21, 0–125] | p = 0.001 | RSV vs non-resp | 0.04 |
| RSV vs other resp | 0.22 | |||||
| Respiratory prescription | 17 (22) [14, 0–90] | 63 (195) [16, 0–1,070] | 5 (12) [1.4, 0–109] | p = 0.05 | RSV vs non-resp | 0.21 |
| RSV vs other resp | 0.11 | |||||
| Total costs | 12,505 (32,137) [2,939, 1,648–144,034] | 3,356 (2,121) [2,410, 1,279–9,111] | 1,178 (940) [900, 32–5,066] | p < 0.001 | RSV vs non-resp | <0.001 |
| RSV vs other resp | 0.005 | |||||
| Total respiratory admission costs | 9,273 (31,409) [1,570, 1,088–142,498] | 1,815 (1,192) [1,472, 515–5,896] | 80 (186) [0, 0–1,565] | p = 0.011 | RSV vs non-resp | 0.05 |
| RSV vs other resp | 0.12 | |||||
| Total respiratory minus index respiratory admission | 1,342 (3,239) [459, 0–14,858] | 647 (977) [359, 0–4,940] | 80 (186) [0, 0–1,565] | p = 0.016 | RSV vs non-resp | 0.06 |
| RSV vs other resp | 0.33 |
Data are demonstrated as mean (SD) [median, range]. All analyses use a generalised linear model with gamma distribution and identity link. In the final column: for statistical significance, p must be less than 0.017 to account for multiple testing. For 97/128 subjects in the ‘non-respiratory’ group, the total cost was zero and so the model would not permit this group to be included. Hence, the comparison RSV vs other respiratory only has been included for this variable. Mean costs should be used in any calculations to obtain total costs and not medians
Table 4.
Unadjusted and adjusted mean total costs according to hospital readmission status
| Unadjusted mean differencea (£) | 95% CI for difference | p value | Adjusted mean differenceb (£) | 95% CI for difference | p value | |
|---|---|---|---|---|---|---|
| Other—non-respiratory | 2,178 | 772 to 3,583 | 0.002 | 2,110 | 708 to 3,512 | 0.003 |
| RSV—non-respiratory | 11,327 | 5,015 to 17,639 | <0.001 | 11,186 | 4,763 to 17,609 | 0.001 |
| RSV—other respiratory | 9,149 | 2,693 to15,606 | 0.005 | 9,076 | 2,515 to 15,637 | 0.007 |
Both models use a gamma distribution and identity link function
aCalculated using a generalised linear model without covariates
bCalculated using a generalised linear model with covariates representing birthweight, gestation, antenatal steroid use and surfactant use
Forty-two of the 2,066 eligible infants had an RSV hospitalisation, giving an RSV admission rate of 2%. Assuming prophylaxis would reduce the hospitalisation rate by 50%, the NNT was 98.
Discussion
We have demonstrated that infants born between 32 and 35 weeks of gestation had an increased health-related cost of care in the first 2 years after birth compared to either infants who had no admission/non-respiratory admission or those admitted with another respiratory diagnosis. The higher cost of care in the RSV group compared to the other two groups was mainly due to the index RSV hospitalisation, as shown in Table 3 by the magnitude of change when comparing the total respiratory admission costs to the total respiratory minus the index respiratory admission. As in our previous studies [2, 4, 5, 7], we applied the same strict definition of an RSV hospitalisation, that is RSV had to be identified from a NPA by either immunofluorescence or culture and in this study or by rt PCR. In the other respiratory group, two thirds of the infants had RSV-negative bronchiolitis, which can result from a variety of other viruses. The significant difference in health-related cost of care between the RSV and the other respiratory group suggests that other respiratory viruses may not be associated with chronic morbidity of the same magnitude as RSV.
There are some limitations to our study. The data were collected retrospectively, but all possible eligible infants were identified from comprehensive maternal and neonatal databases. We were not able to obtain data on all infants who had an RSV hospitalisation or another respiratory admission, but this was usually due to being unable to trace the infants, which seems unlikely to have biased our sample. We had planned to recruit 30 infants with RSV hospitalisations, but only 20 patients gave informed written consent. Comparison of 20 infants, however, gave us 80% power at the 5% level to detect a difference equivalent to one standard deviation in healthcare utilisation and the related cost of care. We did not have information as to whether the infants had had other viral infections. There have been studies suggesting that other viruses may be important in the development of subsequent wheezing/asthma in infants born at term. In the COAST study, rhinovirus was the most important association of development of wheezing at 3 [14] and 6 [10] years in children with a strong family history of atopy, and in another study [18], human coronavirus NL63 was associated with asthma at 6 years. Other studies have suggested that other viruses in combination with RSV may increase subsequent morbidity. For example, rhinovirus and RSV were demonstrated to interact with atopy in infancy to promote later asthma [11], exposure to parainfluenza virus and RSV in the first year after birth was associated with possible asthma at 2 years of age [13] and human metapneumovirus and RSV in infancy were associated with asthma at age 5 years [4]. It would be important to determine in a future prospective study the relative contributions of RSV and other viral infections to the chronic respiratory morbidity seen in prematurely born infants.
The incidence of hospitalisation for RSV infection among prematurely born infants has been reported to vary between 2.8% and 37%. In a previous UK study [22] assessing infants born prior to 32 weeks of gestation, a 4% rate was noted. In the PICNIC study, the overall hospitalisation rate was 3.6% for infants of 33–35 weeks of gestational age [24]. In a multicentre Italian birth cohort study [12], 4.5% of infants born between 33 and 37 weeks of gestation were admitted during a 6-month period for an LRTI, including RSV LRTIs. The lower admission rate (2%) in this study likely reflects differences in admission policies. None of the infants in this study received RSV prophylaxis. Palivizumab (MedImmune Inc/Abbott Laboratories) is a monoclonal antibody preparation against the F glycoprotein of RSV. Its licensed indication is for the prevention of serious LRT disease requiring hospitalisation in children who are born at ≤35 weeks of gestation and ≤6 months of age at the onset of the RSV season, as well as infants ≤2 years of age with BPD who had required treatment within the last 6 months. In a multicentre randomised trial [9], prophylaxis with palivizumab was associated with a 55% reduction in RSV hospital admission, and the greatest reduction occurred in the infants who did not have BPD. In our cohort, the RSV admission rate of 2% meant that the NNT was 98 assuming a 50% reduction in hospitalisation following prophylaxis and hence the cost of RSV prophylaxis would exceed the savings made by any reduction in RSV hospitalisation. There is a caveat in interpreting these data, for although we documented all hospital admissions not only those which occurred at the two hospitals of birth, not all of the hospitals had the range of virological tests which were available at the two main sites and thus some RSV hospitalisations may have been missed.
There are data [20, 21] highlighting that RSV prophylaxis might reduce chronic respiratory morbidity following RSV LRTI. In a prospective, matched, double cohort, multicentre study of prematurely born infants, later recurrent wheezing was 58% lower and physician diagnosed that recurrent wheezing was 65% lower in infants who received palivizumab compared to the controls [21]. It was subsequently highlighted [20] that RSV prophylaxis was associated with an 80% reduction in the relative risk of recurrent wheezing in non-atopic children, but had no significant effect in infants with an atopic family history. The study, however, excluded infants who had received RSV prophylaxis and were subsequently hospitalised, and such infants might have been expected to do worse at follow-up. A randomised trial is clearly needed to test the effect of RSV prophylaxis on longer term outcomes. Nevertheless, if an assumption is made that RSV prophylaxis reduces the health-related cost of care during infancy following RSV hospitalisation by 60%, the cost of prophylaxis would still exceed any savings made.
In conclusion, RSV hospitalisation was associated with a significant increase in the health-related cost of care in the first 2 years after birth in infants born between 32 and 35 weeks of gestation. Nevertheless, if RSV prophylaxis is to be cost-effective, a high risk group of moderately prematurely born infants needs to be identified.
Acknowledgements
We thank Mrs Deirdre Gibbons for secretarial support and research nurse Esther Bowden who took part in the data collection.
Conflict of interest
MedImmune who market palivizumab (an RSV prophylaxis agent) funded this study.
References
- 1.Barber J, Thompson S. Multiple regression of cost data: use of generalized linear models. J Health Serv Res Policy. 2004;9:197–204. doi: 10.1258/1355819042250249. [DOI] [PubMed] [Google Scholar]
- 2.Broughton S, Roberts A, Fox G, Pollina E, Zuckerman M, Chaudhry S, Greenough A. Prospective study of health care utilisation and respiratory morbidity due to RSV infection in prematurely born infants. Thorax. 2005;60:1039–1044. doi: 10.1136/thx.2004.037853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broughton S, Sylvester KP, Fox G, Zuckerman M, Smith M, Milner AD, Rafferty GF, Greenough A. Lung function in prematurely born infants following viral LRTI. Pediatr Infect Dis J. 2007;26:1019–1024. doi: 10.1097/INF.0b013e318126bbb9. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Garcia ML, Calvo C, Casas I, Bracamonte T, Rellán A, Gozalo F, Tenorio T, Pérez-Breña P. Human metapneumovirus bronchiolitis in infancy is an important risk factor for asthma at age 5. Pediatr Pulmonol. 2007;42:458–464. doi: 10.1002/ppul.20597. [DOI] [PubMed] [Google Scholar]
- 5.Greenough A, Alexander J, Boit P, Boorman J, Burgess S, Burke A, Chetcuti PA, Cliff I, Lenney W, Lytle T, Morgan C, Raiman C, Shaw NJ, Sylvester KP, Turner J. School age outcome of hospitalisation with respiratory syncytial virus infection of prematurely born infants. Thorax. 2009;64:490–495. doi: 10.1136/thx.2008.095547. [DOI] [PubMed] [Google Scholar]
- 6.Greenough A, Alexander J, Burgess S, Bytham J, Chetcuti PA, Hagan J, Lenney W, Melville S, Shaw NJ, Boorman J, Coles S, Turner J, Pang F. Health care utilisation of prematurely born, preschool children related to hospitalisation for RSV infection. Arch Dis Child. 2004;89:673–678. doi: 10.1136/adc.2003.036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenough A, Alexander J, Burgess S, Lenney W, Turnbull F, Burgess S, Chetcuti PA, Shaw NJ, Woods A, Boorman J, Coles S, Turner J. Health care utilisation of chronic lung disease infants related to hospitalisation for respiratory syncytial virus infection. Arch Dis Child. 2001;85:463–468. doi: 10.1136/adc.85.6.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horn SD, Smout RJ. Effect of prematurity on respiratory syncytial virus hospital resource use and outcomes. J Pediatr. 2003;143:S133–S141. doi: 10.1067/S0022-3476(03)00509-2. [DOI] [PubMed] [Google Scholar]
- 9.The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–537. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 10.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF., Jr Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanari M, Adorni F, Silvestri M, Coscia A, Musicco M, ‘Italian Study Group on Risk Factors for RSV-Related Hospitalization’ The multicenter Italian birth cohort study on incidence and determinants of lower respiratory tract infection hospitalization in infants at 33 weeks GA or more: preliminary results. Early Hum Dev. 2011;875:S43–S46. doi: 10.1016/j.earlhumdev.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Lee KK, Hegele RG, Manfreda J, Wooldrage K, Becker AB, Ferguson AC, Dimich-Ward H, Watson WT, Chan-Yeung M. Relationship of early childhood viral exposures to respiratory symptoms, onset of possible asthma and atopy in high risk children: the Canadian Asthma Primary Prevention Study. Pediatr Pulmonol. 2007;42:290–297. doi: 10.1002/ppul.20578. [DOI] [PubMed] [Google Scholar]
- 14.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, Kirk CJ, Reisdorf E, Roberg KA, Anderson EL, Carlson-Dakes KT, Adler KJ, Gilbertson-White S, Pappas TE, Dasilva DF, Tisler CJ, Gern JE. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Netten A, Dennett J, Knight J. Unit costs of health and social care. Canterbury: University of Kent, Personal Social Services Research Unit; 2000. [Google Scholar]
- 16.Palmer L, Hall CB, Katkin JP, Shi N, Masaquel AS, McLaurin KK, Mahadevia PJ. Healthcare costs within a year of respiratory syncytial virus among Medicaid infants. Pediatr Pulmonol. 2010;45:772–781. doi: 10.1002/ppul.21244. [DOI] [PubMed] [Google Scholar]
- 17.Palmer L, Hall CB, Katkin JP, Shi N, Masaquel AS, McLaurin KK, Mahadevia PJ. Respiratory outcomes, utilization and costs 12 months following a respiratory syncytial virus diagnosis among commercially insured late-preterm infants. Curr Med Res Opin. 2011;27:403–412. doi: 10.1185/03007995.2010.542744. [DOI] [PubMed] [Google Scholar]
- 18.Pappas T, Sullivan Dille K, Lee W, Grindle K, Roberg K, Da Silva D, Tisler C, Anderson E, Hansen K, Grabher R, Pleiss Salazar L, Evans M, Gagnon R, Gern J, Lemanske R., Jr Coronavirus NL63 illnesses in infancy are a risk factor for asthma at age six. J Allergy Clin Immunol S. 2007;146:577. [Google Scholar]
- 19.Sampalis JS. Morbidity and mortality after RSV-associated hospitalizations among premature Canadian infants. J Pediatr. 2003;143:S150–S156. doi: 10.1067/S0022-3476(03)00513-4. [DOI] [PubMed] [Google Scholar]
- 20.Simoes EAF, Carbonell Estrany X, Rieger CHL, Mitchell I, Fredrick L, Groothuis JR, Palivizumab Long-Term Respiratory Outcomes Study Group The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and non atopic children. J Allergy Clin Immunol. 2010;126:256–262. doi: 10.1016/j.jaci.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simoes EAF, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, Kimpen JL, Palivizumab Long-Term Respiratory Outcomes Study Group Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. doi: 10.1016/j.jpeds.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 22.Thomas M, Bedford-Russell A, Sharland M. Hospitalisation for RSV infection in ex-preterm infants—implications for use of RSV immune globulin. Arch Dis Child. 2000;83:122–127. doi: 10.1136/adc.83.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ. 2000;320(7243):1197–2000. doi: 10.1136/bmj.320.7243.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang EE, Law BJ, Stephens D. Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) prospective study of risk factors and outcomes in patients hospitalized with respiratory syncytial viral lower respiratory tract infection. J Pediatr. 1995;126:212–219. doi: 10.1016/S0022-3476(95)70547-3. [DOI] [PubMed] [Google Scholar]


