Abstract
There is a dearth of information on the seroprevalence of bovine norovirus (BoNoV) and nebovirus in cattle of the US. In this retrospective study, serum IgG antibodies to two bovine enteric caliciviruses, GIII.2 BoNoV (Bo/CV186-OH/00/US) and genetically and antigenically distinct nebovirus (Bo/NB/80/US), were evaluated in feedlot and veal calves from different regions of the US during 1999-2001. Three groups of 6- to 7-month-old feedlot calves from New Mexico (NM) (n=103), Arkansas (AR) (n=100) and Ohio (OH) (n=140) and a group of 7- to 10-day-old Ohio veal calves (n=47) were studied. Serum samples were collected pre-arrival or at arrival to the farms for the NM, AR and OH calves and 35 days after arrival for all groups for monitoring seroconversion rates during the period. Virus-like particles of Bo/CV186-OH/00/US and Bo/NB/80/US were expressed using the baculovirus expression system and were used in ELISA to measure antibodies. A high seroprevalence of 94-100 % and 78-100 % was observed for antibodies to GIII.2 BoNoV and nebovirus, respectively, in the feedlot calves tested. In the Ohio veal farm, an antibody seroprevalence of 94-100 % and 40-66 % was found for GIII.2 BoNoV and nebovirus, respectively. Increased seropositive rates of 38-85 % for GIII.2 BoNoV and 26-83 % for nebovirus were observed at 35 days after arrival and commingling on farms for all groups. Infection of calves with either GIII.2 BoNoV or nebovirus, or both viruses, appeared to be common in the regions studied in the US during 1999-2001. These two viruses likely remain endemic because no commercial vaccines are available.
Keywords: Antibody Titer, Fourfold Increase, Seroprevalence Rate, Seropositive Rate, Geometric Mean Titer
Introduction
Caliciviruses are non-enveloped, single-stranded RNA viruses of positive polarity. They are divided into five genera: Vesivirus, Lagovirus, Norovirus, Sapovirus, and Nebovirus [3]. Their genomes range from 7.3 to 8.3 kb in size, and these viruses have a diameter between 27 and 40 nm [4, 9]. Among them, noroviruses (NoVs) are known to be infectious pathogens in a variety of mammal species (humans, pigs, ruminants, rodents, dogs, and cats) [19, 26, 27, 30, 33]. In humans, they are the leading cause worldwide of epidemic, nonbacterial gastroenteritis in all ages [8]. Phylogenetically, the genus Norovirus is classified into five genogroups (GI-V). Genogroups I, II and IV infect humans [2, 15], whereas GIII and GV infect ruminants and rodents, respectively.
Bovine NoVs (BoNoVs) comprise two distinct genotypes: GIII.1 (prototype Bo/Jena/80/DE) and GIII.2 (prototype Bo/Newbury-2/76/UK). They were initially identified in fecal samples from calves (under 7 days of age) in farms with histories of diarrhea in England (Newbury-2 strain) and Germany (Jena strain) in 1978 and 1980, respectively [10, 34]. In the US, two distinct bovine enteric calicivirus strains were discovered by electron microscopy and RT-PCR and later characterized genetically by sequence analysis. Bo/CV186-OH/00/US is a GIII.2 BoNoV that was identified in a stool sample from veal calves in Ohio in 2000 [29]. Bo/NB/80/US, detected in a fecal sample from a diarrheic calf from Nebraska in 1980, is a nebovirus that is genetically unrelated to the BoNoVs [28, 29]. Notably, the NB strain was the first nebovirus to be completely sequenced, and based on sequence data, it represented a potentially new calicivirus genus [28]. It is noteworthy that Newbury-1 virus, which was detected from diarrheic calves in the UK in 1978, also belongs to the genus Nebovirus, as confirmed by sequence analysis in subsequent studies [22]. In the US, other GIII.1 BoNoVs were later detected and characterized molecularly from calves in Michigan and Wisconsin [32]. Previous study showed no antigenic relatedness between the Bo/NoV/GIII.2/Newbury-2 and Bo/Nebovirus/Newbury-1 strains [5]. Another study has shown that BoNoV GIII.1 (Jena) and GIII.2 (Newbury-2) are also antigenically unrelated, suggesting that they are distinct serotypes [23].
A high seroprevalence (93-98 %) for either the GIII.1 or GIII.2 BoNoVs has been detected in cattle in Germany and the UK, indicating that both groups of viruses are endemic in the bovine population in these countries [24]. In another study, a 93 % seroprevalence for GIII.2 BoNoVs was observed for cattle in Belgium [21]. Currently, there is little information about the seroprevalence of BoNoVs in the US, and no information for nebovirus. In this retrospective study, the seroprevalence of two bovine enteric caliciviruses, BoNoV GIII.2 and nebovirus, was evaluated in sera of feedlot and veal calves from different regions of the US, sampled during 1999-2001.
Materials and methods
Study population
This study included three groups of feedlot calves from New Mexico, Arkansas and Ohio. The animals were commingled from different regions of the US. Also, a veal farm from Ohio with animals collected from New York, Pennsylvania, West Virginia, Virginia and Ohio was studied. These groups are described in detail as follows.
New Mexico (NM) – Arkansas (AR) feedlot group
In the fall of 2001, two groups of feedlot calves (NM and AR) between 6 and 7 months old from the southwestern and south-central United States were shipped to a feedlot research station in NM where they were commingled and distributed in pens for a feedlot study. The calves from NM (n=103) originated from a ranch with predominantly Red Angus with some Hereford crosses. The AR calves (n=100) were assembled from three local sale barns and were already commingled for at least 3-4 days prior to shipping to the NM feedlot. These animals were predominantly beef breed crosses representing Charolais, Angus, Hereford and Brahman.
Ohio (OH) feedlot groups
In the fall of 1999 (n=56) and 2000 (n=84), two groups of crossbred beef steers between 6 and 7 months old were randomly selected from an incoming group of more than 200 feedlot calves. The source of these animals was a mixed-provider livestock auction market in West Virginia. The animals were transported to a feedlot at the Ohio Agricultural Research and Development Center in Wooster, OH.
Ohio (OH) veal calf group
A fourth group of animals consisted of 47 Holstein 7- to 10-day-old bull calves from a commercial veal farm in Ohio. These animals were systematically selected from a population of 360 bull calves during the fall of 1999. The calves were provided by a bull calf supplier who obtained calves from different regions representing several US states (Ohio, West Virginia, Virginia, Pennsylvania, and New York). The calves remained in their assigned stalls throughout their production period and were individually fed with a milk replacement diet twice daily using a bucket. These calves had no contact with adult cattle during the study period, and stringent biosecurity measures were implemented by researchers and farm workers.
Collection of serum samples
Serum samples were collected before arrival (3-22 days pre-arrival) at the feedlot and at day 35 post-arrival for the NM and AR groups, as well as at arrival and at day 35 for the 2000 OH feedlot group and the OH veal-calf group. Samples were only collected at arrival for the 1999 OH feedlot group. Blood was collected by jugular venipuncture, and about 2 ml of serum per animal was obtained and placed in plastic vials. All samples were frozen at −20 °C, shipped to the Food Animal Health Research Program at The Ohio State University, aliquoted, heat inactivated at 56 °C for 30 min and stored at −70 °C until tested.
Expression and purification of virus-like particles (VLPs) of Bo/CV186-OH/00/US and Bo/NB/80/US strains
A recombinant baculovirus containing the capsid protein (VP1) gene (ORF2) of the CV186-OH/00/US strain was generated by using a Bac-N-Blue Transfection Kit (Invitrogen, Carlsbad, CA, USA), as described previously [11]. Similarly, recombinant baculovirus carrying the VP1 gene of the nebovirus NB/80/US strain was also generated. Briefly, the NB/80/US capsid gene was ligated into the pBlueBac4.5 baculovirus transfer vector (Invitrogen) to create the pBac-NB vector, which was then introduced into Spodoptera frugiperda 9 (Sf9) cells by transfection using a Bac-N-Blue Transfection Kit (Invitrogen) according to the manufacturer’s instructions. Recombinant clones (rBac-NB) were plaque purified and confirmed to contain the NB/80/US capsid gene by PCR.
Sf9 cells were infected with the recombinant baculoviruses at a multiplicity of infection of 5 to 10 and were incubated at 27 °C for 7 to 10 days. Cell lysates and cell culture supernatants were harvested and centrifuged (2000×g for 30 min at 4 °C) to remove cell debris. VLPs were purified by CsCl gradient ultracentrifugation as described previously [11]. Purified VLPs were analyzed for their particle sizes and morphologies by electron microscopy (EM) as described previously [11]. The protein concentrations of the VLPs were determined using a Protein Assay Kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
VLP-based enzyme-linked immunosorbent assay (ELISA)
CV186-OH/00/US and NB/80/US VLPs, semi-purified by ultracentrifugation through a 40 % (w/v) sucrose cushion, were used as antigens in an ELISA test to investigate the antigenic relationships between these two viruses, as described previously, with slight modifications [11]. Briefly, 96-well microtiter plates (Nalgene Nunc, Rochester, NY, USA) were coated overnight at 4 °C with 1 μg/ml of either CV186-OH/00/US or NB/80/US VLPs or with semi-purified Sf9 cell supernatant as a negative control in 0.05M carbonate-bicarbonate buffer (pH 9.6). The VLPs were incubated for 2 h at room temperature (RT) with two Gn calf hyperimmune antisera, Bo9114 (1:500) and Bo12611 (1:500), against CV186-OH/00/US and NB/80/US, respectively. A goat polyclonal antibody to bovine IgG (whole IgG) and conjugated to horseradish peroxidase (1:1000) (KPL, Gaithersburg, MD, USA) was diluted in 0.01 M phosphate-buffered saline (PBS) (pH 7.4) containing 0.05 % Tween-20. The antibody-containing plates were incubated 1.5 h at RT. Color development was done by adding tetramethylbenzidine (KPL) and incubating the samples for 15 to 20 min at RT, and the reaction was stopped by adding 1.8 N H2SO4. The absorbance at 450 nm was measured using an Emax microplate reader (Molecular Devices Co., Sunnyvale, CA, USA).
For serum antibody detection, semi-purified CV186-OH/00/US and NB/80/US VLPs were used as antigens in an antibody capture ELISA test as described previously [11]. Briefly, 96-well microtiter plates (Nalgene Nunc) were coated overnight at 4 °C with either CV186-OH/00/US or NB/80/US VLPs or with semi-purified Sf9 cell proteins as a negative control in 0.05 M carbonate-bicarbonate buffer (pH 9.6). Serial twofold dilutions of serum samples in 0.01 M PBS (pH 7.4) (ranging from 1:25 to >1:51,200) were applied, and plates were incubated for 1 h at 37 °C. One positive serum (for either CV186-OH/00/US or NB/80/US) from convalescent gnotobiotic calves and five negative control sera (feedlot and veal calf sera testing negative) were used for each microtiter plate. Horseradish-peroxidase-labeled goat anti-bovine IgG (KPL) was added to each well of the microtiter plate and incubated for 1 h at 37 °C. The substrate 2,2’-azino-di (3-ethylbenzthiazoline-6-sulfonate) (ABTS) plus 1:1000 hydrogen peroxide was applied to each well, and the absorbance at 405 nm was measured using an Emax microplate reader (Molecular Devices Co.). Absorbance values were calculated by subtracting the absorbance of sera in wells coated with Sf9 cell proteins from the absorbance of sera in VLP-coated wells. Paired positive and negative wells with a resulting difference in absorbance greater than the cutoff value (average value for absorbance of negative control samples plus three times the standard deviation) were considered positive for CV186-OH/00/US or NB/80/US virus antibodies. The antibody titers were calculated and expressed as the reciprocal of the highest serum dilution positive for CV186-OH/00/US or NB/80/US virus IgG antibodies.
Results
Antigenic relationships of Bo/CV186-OH/00/US and Bo/NB/80/US strains
CV186-OH/00/US VLPs have been described previously [11]. The capsid proteins of the NB/80/US strain were expressed and self-assembled into VLPs, which were 36-42 nm in diameter as visualized by EM (Fig. 1). By two-way ELISA, CV186-OH/00/US VLPs reacted only with CV186-OH/00/US antisera, whereas NB/80/US VLPs reacted only with NB/80/US antisera. Based on the ELISA results, the CV186-OH/00/US and NB/80/US strains were antigenically unrelated.
Fig. 1.
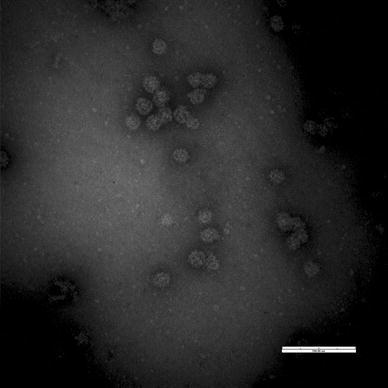
Electron micrograph of VLPs of nebovirus Bo/NB/80/US strain. Nebovirus VLPs were purified by CsCl-gradient ultracentrifugation from the cell culture supernatants of recombinant baculovirus-infected Sf9 cells. For visualization of VLPs, EM was performed without incubation with antiserum. Bar, 200 nm
Antibody titers, seroprevalence rates and increased seropositive rates for GIII.2 BoNoV
As summarized in Table 1 and described in Fig. 2A, 97 % (100/103) of the NM calves had serum antibodies against GIII.2 BoNoV before arrival at the feedlot, with an antibody geometric mean titer (GMT) of 457 (range: 0-12,800). At day 35 after arrival, 75 % (77/103) of all calves in this group showed at least a fourfold increase in antibody titer. The GIII.2 BoNoV antibody GMT at this time was 5,949 (range: 100-25,600) and increased by approximately 13 times compared to the pre-arrival GMT (Fig. 2A), and the seroprevalence rate increased to 100 % (103/103). Ninety-four percent (94/100) of the AR calves had antibodies against GIII.2 BoNoV before arrival at the feedlot with an antibody GMT of 381 (range: 0-6,400) (Table 1; Fig. 2B). At day 35 after arrival, 85 % (85/100) of the AR calves showed at least a fourfold increase in antibody titer. The GIII.2 BoNoV antibody GMT was 4,707 (range: 0-25,600) and increased by approximately 12 times compared to the pre-arrival GMT (Fig. 2B), and the seroprevalence rate increased to 99 % (99/100).
Table 1.
Seroprevalence and seropositivity increases (≥4-fold) of GIII.2 BoNoV and nebovirus in 6- to 7-month-old feedlot calves from New Mexico (NM), Arkansas (AR) and Ohio (OH) and 7- to 10-day-old veal calves from OH pre- and post-arrival on farms
| Region (years) | GIII.2 BoNoV | Nebovirus | |||||
|---|---|---|---|---|---|---|---|
| 3-22 days pre-arrival | 35 days post-arrival (or post-commingling) | 3-22 days pre-arrival | 35 days post-arrival (or post-commingling) | ||||
| Seroprevalence rate | Seropositive increase ratea | Seroprevalence rate | Seroprevalence rate | Seropositive increase ratea | Seroprevalence rate | ||
| 6-7-month-old feedlot calves | MN (2001) | 97 % (100/103) | 75 % (77/103) | 100 % (103/103) | 78 % (80/103) | 65 % (67/103) | 90 % (93/103) |
| AR (2001) | 94 % (94/100) | 85 % (85/100) | 99 % (99/100) | 100 % (100/100) | 83 % (83/100) | 100 % (100/100) | |
| OH (1999-2000) | 94 % (132/140) | 38 % (32/84)b | 100 % (84/84)b | 99 % (83/84)c | 32 % (27/84) | 97 % (81/84) | |
| Total | 95 % (326/343) | 68 % (194/287) | 100 % (286/287) | 92 % (263/287) | 62 % (177/287) | 95 % (274/287) | |
| 7-10-day-old veal calves | OH (1999) | 94 % (44/47) | 49 % (23/47) | 100 % (47/47) | 40 % (19/47) | 26 % (12/47) | 66 % (31/47) |
aSeropositive increase rate was defined as a fourfold increase in antibody titer
bFor the 1999 OH feedlot group, samples (n=56) were collected only at arrival, but not at 35 days post-arrival
cOnly the 2000 OH feedlot-group samples (n=84) were tested for nebovirus antibody
Fig. 2.
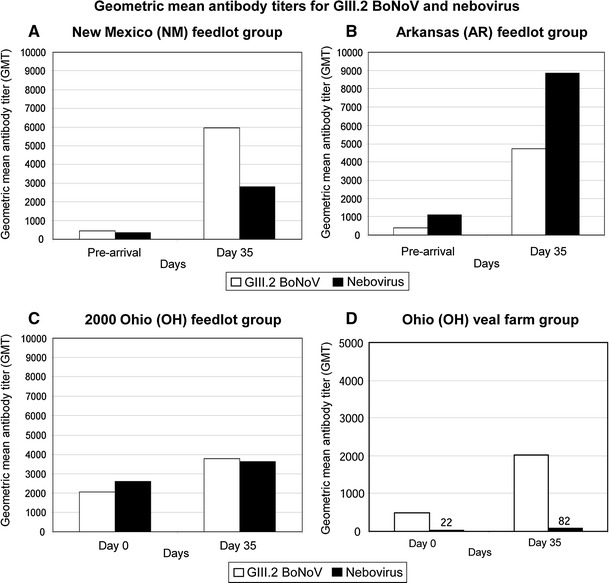
Geometric mean antibody titers for GIII.2 BoNoV and nebovirus in the feedlot and veal calves from different regions of the US during 1999-2001. Three groups of 6- to 7-month-old feedlot calves from New Mexico (NM) (A), Arkansas (AR) (B), and Ohio (OH) (C) and a group of 7- to 10-day-old Ohio veal calves (D), comprising animals commingled from different regions of the US, were studied. Serum samples were collected pre-arrival or on arrival at the farms for the NM, AR and OH calves, and 35 days after arrival for all groups to monitor seroprevalence and seropositive increase rates during the period. The VLPs of Bo/CV186-OH/00/US and Bo/NB/80/US strains were expressed using the baculovirus expression system and were used in ELISA to measure IgG antibodies
Eighty-nine percent (50/56) of the 1999 OH feedlot calves had antibodies against GIII.2 BoNoV on arrival at the feedlot with an antibody GMT of 386 (range: 0-25,600). This feedlot group was tested only for antibodies to GIII.2 BoNoV on the day of arrival and not tested post-arrival. Ninety-eight percent (82/84) of the 2000 OH feedlot calves had antibodies against GIII.2 BoNoV on arrival at the feedlot, with an antibody GMT of 2,045 (range: 0-25,600) (Table 1; Fig. 2C). At day 35 after arrival, 38 % (32/84) of calves in this group showed at least a fourfold increase in antibody titer. The GIII.2 BoNoV antibody GMT at this time was 3,770 (range: 200-25,600) and increased only approximately two times compared to the pre-arrival GMT (Fig. 2C), and the seroprevalence rate increased to 100 % (84/84).
A similar trend was observed in the younger OH veal calves, 94 % (44/47) of which had antibodies against GIII.2 BoNoV on arrival at the farm, with an antibody GMT of 492 (range: 0-12,800) (Table 1; Fig. 2D). At day 35 after arrival, 49 % (23/47) of all calves in this group showed at least a fourfold increase in antibody titer. The GIII.2 BoNoV antibody GMT was 2,016 (range: 100-12,800) and increased approximately fourfold compared to the pre-arrival GMT (Fig. 2D), and the seroprevalence rate increased to 100 % (47/47).
Antibody titers, seroprevalence rates and increased seropositive rates for nebovirus
Seventy-eight percent (80/103) of the NM calves had antibodies against nebovirus before arrival at the feedlot, with an antibody GMT of 356 (range: 0-12,800) (Table 1; Fig. 2A). At day 35 after arrival, 65 % (67/103) of the NM calves showed at least a fourfold increase in antibody titer. The nebovirus antibody GMT was 2,806 (range: 0-25,600) and increased approximately eightfold compared to the pre-arrival GMT (Fig. 2A), and the seroprevalence rate increased to 90 % (93/103). One hundred percent (100/100) of the AR calves had antibodies against nebovirus before arrival at the feedlot, with an antibody GMT of 1,112 (range: 200-6,400) (Table 1; Fig. 2B). At day 35 after arrival, 83 % (83/100) of the AR calves showed at least a fourfold increase in antibody titer. The nebovirus antibody GMT at this day was 8,849 (range: 1,600-25,600) and increased approximately eightfold compared to the pre-arrival GMT (Fig. 2B), and the seroprevalence rate remained 100 %.
Ninety-nine percent (83/84) of the 2000 OH feedlot calves had antibodies against nebovirus on arrival at the feedlot, with an antibody GMT of 2,596 (range: 0-25,600). At day 35 after arrival, 32 % (27/84) of calves in this group showed at least a fourfold increase in antibody titer, with a nebovirus antibody GMT of 3,632 (range: 0-25,600) (Fig. 2C), but the seroprevalence rate decreased slightly to 97 % (81/84).
Only 40 % (19/47) of the OH veal calves had antibodies against nebovirus on arrival at the farm, with an antibody GMT of 22 (range: 0-12,800) (Table 1; Fig. 2D). At day 35 after arrival, 26 % (12/47) of all calves in this group showed at least a fourfold increase in antibody titer. The nebovirus antibody GMT at day 35 was 82 (range: 0-25,600) (Fig. 2D), and the seroprevalence rate increased to 66 % (31/47).
Discussion
There have been detailed studies on the seroprevalence of BoNoVs in Europe [6, 21, 24]; however, to our knowledge, no such studies have been performed in the US. Furthermore, there have been no studies on the seroprevalence of nebovirus. Our retrospective serological study demonstrates a high seroprevalence (up to 100 %) of antibodies to GIII.2 BoNoV and nebovirus in cattle from 1999 to 2001 in the US, indicating that these two viruses were endemic in cattle during the period tested. However, further serological surveys of contemporary serum samples are needed to determine if these viruses remain endemic in US cattle. The distribution of nebovirus is unknown, and these viruses have been detected in the US, UK, France, Italy, and Tunisia [7, 13, 18, 22, 28]. In contrast, the distribution of GIII.1 and GIII.2 BoNoVs appears to be worldwide. Bovine NoVs have been detected in North and South America, Asia, and Europe [1, 14, 16, 18, 21, 25, 29, 34]. The prevalence is likely to be variable, depending on the location and the diagnostic test used. In addition, our study demonstrates that the GIII.2 BoNoV CV186-OH/00/US strain and the nebovirus NB/80/US strain are antigenically unrelated, which coincides with data reported previously showing no antigenic relatedness between the GIII.2 BoNoV Newbury-2/76/UK strain and the nebovirus Newbury-1/76/UK strain [5].
The seroprevalence rates for GIII.2 BoNoV and nebovirus in the feedlot calves tested ranged from 78 to 100 % on arrival at the feedlots, which indicates that most animals had been exposed to this virus previously. In the US, feedlot calves are usually assembled in order-buyer barns. After the animals are purchased, they may be transported long distances and commingled with animals assembled from other farms into feedlots. During this shipping and commingling process, calves become naturally infected with many pathogens whose spread may be enhanced by stressful conditions during the transport and close confinement. Based on this calf collection system practiced in auction barns in the US, where animals are purchased from different regions of the country, it can be inferred from the samples tested that both GIII.2 BoNoV and nebovirus infections are widespread in the feedlot calves in the areas of the US represented. It is notable that a majority of feedlot calves tested had antibodies to both viruses, indicating that infection of US calves with both of these viruses is common. Consistent with our current findings of archival samples (1999-2001), recent studies in Germany and in the UK have shown that GIII.1 (Jena) and GIII.2 (Newbury-2) BoNoVs are endemic in cattle in those countries, with seroprevalence rates ranging from 66 to 99 % [6, 24]. Likewise, a recent study in Belgium has shown that GIII.2 BoNoVs are endemic in the cattle of that country, with a detected seroprevalence rate of 93 % for the group of calves studied [21]. Such high seroprevalence rates are expected for BoNoVs because these viruses are stable, highly contagious, and easily transmitted [12, 17]. Human NoVs are also stable in the environment, and the infectious dose may be as low as 10-100 virus particles [15]. The high seroprevalence rates may also reflect the prolonged shedding of BoNoVs in the feces as detected in conventional or gnotobiotic calves infected with GIII.2 BoNoVs [12, 16, 17].
The high level of antibodies (range of GMTs: 381-2,596) and seropositive increase rates (range: 32-85 %) of the feedlot calves tested, in which a majority of animals were already seropositive at arrival, indicates that animals might be infected with these viruses at auctions barns or re-infected in feedlots. On the other hand, the OH veal calves had lower titers and seropositive increase rates than the feedlot calves; this could reflect the influence of repeated exposures in older cattle. Similar results were observed in a recent study in Belgium, where antibody levels against GIII.2 BoNoV were higher in adult cattle (older than 1 year) than in younger calves [21]. Repeated exposures have been thought to be necessary in humans for longer-lasting immunity against norovirus infections [20], and evidence for high rates of infection and re-infections of humans with NoVs has been reported [20].
Based on the young age of the veal calves from our study at entry to the farm (7-14 days), it can also be inferred that the high seroprevalence of BoNoVs (94 %) observed in these animals is a reflection of passive antibodies acquired in colostrum from the dams of these calves, and these antibodies likely reflect passive transfer of colostral antibodies to serum and high seropositive rates in the dams. Indeed, several of these calves had declining titers by day 35 after arrival. However, 49 % of these calves developed more than a fourfold increase in antibody titer to GIII.2 BoNoV, indicating that they were exposed at the veal calf farm and later developed an active immune response to the virus.
We further observed that, in the veal farm group, the seroprevalence (40 %) and increased seropositive rates (26 %) for nebovirus were considerably lower than those for GIII.2 BoNoV. This coincided with our shedding data from an earlier study of this same group of veal calves [29], where it was shown that the viral RNA prevalence in feces, as determined by RT-PCR, was considerably lower for nebovirus (29 %) than for GIII.2 BoNoV (60 %). The data suggest that the rate of infection by nebovirus was lower than that by GIII.2 BoNoV in this group of animals.
The increased seropositive rates for the OH feedlot calves (32-38 %) were considerably lower than the increased seropositive rate for the AR and NM calves (65-85 %). This difference among groups of feedlot calves can be explained by the fact that the OH 2000 feedlot group had a higher antibody GMT (2,596 for nebovirus and 2,045 for GIII.2 BoNoV) at arrival than the AR (1,112 for nebovirus and 389 for GIII.2 BoNoV) and NM (356 for nebovirus and 457 for GIII.2 BoNoV) groups. This trend has also been observed for other viruses such as bovine coronavirus [31]. Field studies of bovine coronavirus in feedlot cattle showed that animals arriving at the feedlots with higher antibody GMTs were less likely to become infected and seroconvert for bovine coronavirus than animals arriving with lower antibody GMT [31].
In conclusion, infection of calves with either nebovirus or GIII.2 BoNoV, or both viruses, appeared to be common in the US during the period surveyed (1999-2001). To date, no commercial vaccines for these two viruses are available in the US. Thus, the seropositive rates reflect natural field exposures. The increased seropositive rates of calves to nebovirus or GIII.2 BoNoV also indicate that cattle might be infected at a young age and re-exposed to either virus at auction barns or in feedlots. However, further serological surveys of contemporary serum samples are needed to determine if these viruses remain endemic in US cattle. Although GIII.1 BoNoVs have also been detected in US cattle in the limited surveys conducted [29, 32], future GIII.1 BoNoV seroprevalence studies in the US are also needed.
Acknowledgments
This study was supported by a grant to LJ Saif (PI) from the National Institute of Allergy and Infectious Diseases (Grant AI-49742). Salaries and research support were provided by state and federal grants provided to the Ohio Agricultural Research and Development Center (OARDC) of The Ohio State University. We thank Dr. Robert E. Briggs from the United States Department of Agriculture (USDA) and Dr. Glenn C. Duff from The University of Arizona, Department of Animal Sciences, for providing us with the Arkansas and New Mexico feedlot serum samples. We also thank Susan Wang and Thavamathi Annamalai for technical assistance with VLP production.
Footnotes
C. Thomas and K. Jung contributed equally to this work.
References
- 1.Alcala AC, Hidalgo MA, Obando C, Vizzi E, Liprandi F, Ludert JE. Molecular identification of bovine enteric calciviruses in Venezuela. Acta Cient Venez. 2003;54:148–152. [PubMed] [Google Scholar]
- 2.Ando T, Noel JS, Fankhauser RL. Genetic classification of Norwalk-like viruses. J Infect Dis. 2000;181(Suppl 2):S336–S348. doi: 10.1086/315589. [DOI] [PubMed] [Google Scholar]
- 3.Carstens EB. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2009) Arch Virol. 2010;155:133–146. doi: 10.1007/s00705-009-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke IN, Lambden PR. Organization and expression of calicivirus genes. J Infect Dis. 2000;181(Suppl 2):S309–S316. doi: 10.1086/315575. [DOI] [PubMed] [Google Scholar]
- 5.Dastjerdi AM, Snodgrass DR, Bridger JC. Characterisation of the bovine enteric calici-like virus, Newbury agent 1. FEMS Microbiol Lett. 2000;192:125–131. doi: 10.1111/j.1574-6968.2000.tb09370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng Y, Batten CA, Liu BL, Lambden PR, Elschner M, Gunther H, Otto P, Schnurch P, Eichhorn W, Herbst W, Clarke IN. Studies of epidemiology and seroprevalence of bovine noroviruses in Germany. J Clin Microbiol. 2003;41:2300–2305. doi: 10.1128/JCM.41.6.2300-2305.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Martino B, Di Profio F, Martella V, Ceci C, Marsilio F. Evidence for recombination in neboviruses. Vet Microbiol. 2011;153:367–372. doi: 10.1016/j.vetmic.2011.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N Engl J Med. 2009;361:1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Green KY, Ando T, Balayan MS, Berke T, Clarke IN, Estes MK, Matson DO, Nakata S, Neill JD, Studdert MJ, Thiel HJ. Taxonomy of the caliciviruses. J Infect Dis. 2000;181(Suppl 2):S322–S330. doi: 10.1086/315591. [DOI] [PubMed] [Google Scholar]
- 10.Gunther H, Otto P. Diarrhea in young calves. 7. “Zackenvirus” (Jena agent 117/80)—a new diarrhea pathogen in calves. Arch Exp Veterinarmed. 1987;41:934–938. [PubMed] [Google Scholar]
- 11.Han MG, Wang Q, Smiley JR, Chang KO, Saif LJ. Self-assembly of the recombinant capsid protein of a bovine norovirus (BoNV) into virus-like particles and evaluation of cross-reactivity of BoNV with human noroviruses. J Clin Microbiol. 2005;43:778–785. doi: 10.1128/JCM.43.2.778-785.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han MG, Cheetham S, Azevedo M, Thomas C, Saif LJ. Immune responses to bovine norovirus-like particles with various adjuvants and analysis of protection in gnotobiotic calves. Vaccine. 2006;24:317–326. doi: 10.1016/j.vaccine.2005.07.071. [DOI] [PubMed] [Google Scholar]
- 13.Hassine-Zaafrane M, Kaplon J, Sdiri-Loulizi K, Aouni Z, Pothier P, Aouni M, Ambert-Balay K. Molecular prevalence of bovine noroviruses and neboviruses detected in central-eastern Tunisia. Arch Virol. 2012;157:1599–1604. doi: 10.1007/s00705-012-1344-5. [DOI] [PubMed] [Google Scholar]
- 14.Herbst W, Lange H, Krauss H. Electron microscopic demonstration of calicivirus-like particles in the feces of diarrheic calves. Dtsch Tierarztl Wochenschr. 1987;94:406–407. [PubMed] [Google Scholar]
- 15.Hutson AM, Atmar RL, Estes MK. Norovirus disease: changing epidemiology and host susceptibility factors. Trends Microbiol. 2004;12:279–287. doi: 10.1016/j.tim.2004.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jor E, Myrmel M, Jonassen CM. SYBR Green based real-time RT-PCR assay for detection and genotype prediction of bovine noroviruses and assessment of clinical significance in Norway. J Virol Methods. 2010;169:1–7. doi: 10.1016/j.jviromet.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung K, Scheuer K, Zhang Z, Wang QH, Saif LJ (2012) Pathogenesis of bovine norovirus GIII.2 strain CV186-OH/00/US in gnotobiotic calves. In: The American Society for Virology (ASV) 31th Annual Meeting, University of Wisconsin-Madison, Madison, Wisconsin, USA, p 295
- 18.Kaplon J, Guenau E, Asdrubal P, Pothier P, Ambert-Balay K. Possible novel nebovirus genotype in cattle, France. Emerg Infect Dis. 2011;17:1120–1123. doi: 10.3201/eid1706.100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martella V, Lorusso E, Decaro N, Elia G, Radogna A, D’Abramo M, Desario C, Cavalli A, Corrente M, Camero M, Germinario CA, Banyai K, Di Martino B, Marsilio F, Carmichael LE, Buonavoglia C. Detection and molecular characterization of a canine norovirus. Emerg Infect Dis. 2008;14:1306–1308. doi: 10.3201/eid1408.080062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsui SM, Greenberg HB. Immunity to calicivirus infection. J Infect Dis. 2000;181(Suppl 2):S331–S335. doi: 10.1086/315587. [DOI] [PubMed] [Google Scholar]
- 21.Mauroy A, Scipioni A, Mathijs E, Saegerman C, Mast J, Bridger JC, Ziant D, Thys C, Thiry E. Epidemiological study of bovine norovirus infection by RT-PCR and a VLP-based antibody ELISA. Vet Microbiol. 2009;137:243–251. doi: 10.1016/j.vetmic.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oliver SL, Asobayire E, Dastjerdi AM, Bridger JC. Genomic characterization of the unclassified bovine enteric virus Newbury agent-1 (Newbury1) endorses a new genus in the family Caliciviridae. Virology. 2006;350:240–250. doi: 10.1016/j.virol.2006.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oliver SL, Batten CA, Deng Y, Elschner M, Otto P, Charpilienne A, Clarke IN, Bridger JC, Lambden PR. Genotype 1 and genotype 2 bovine noroviruses are antigenically distinct but share a cross-reactive epitope with human noroviruses. J Clin Microbiol. 2006;44:992–998. doi: 10.1128/JCM.44.3.992-998.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver SL, Wood E, Asobayire E, Wathes DC, Brickell JS, Elschner M, Otto P, Lambden PR, Clarke IN, Bridger JC. Serotype 1 and 2 bovine noroviruses are endemic in cattle in the United kingdom and Germany. J Clin Microbiol. 2007;45:3050–3052. doi: 10.1128/JCM.02015-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SI, Jeong C, Kim HH, Park SH, Park SJ, Hyun BH, Yang DK, Kim SK, Kang MI, Cho KO. Molecular epidemiology of bovine noroviruses in South Korea. Vet Microbiol. 2007;124:125–133. doi: 10.1016/j.vetmic.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pinto P, Wang Q, Chen N, Dubovi EJ, Daniels JB, Millward LM, Buonavoglia C, Martella V, Saif LJ. Discovery and genomic characterization of noroviruses from a gastroenteritis outbreak in domestic cats in the US. PLoS One. 2012;7:e32739. doi: 10.1371/journal.pone.0032739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scipioni A, Mauroy A, Vinje J, Thiry E. Animal noroviruses. Vet J. 2008;178:32–45. doi: 10.1016/j.tvjl.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Smiley JR, Chang KO, Hayes J, Vinje J, Saif LJ. Characterization of an enteropathogenic bovine calicivirus representing a potentially new calicivirus genus. J Virol. 2002;76:10089–10098. doi: 10.1128/JVI.76.20.10089-10098.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smiley JR, Hoet AE, Traven M, Tsunemitsu H, Saif LJ. Reverse transcription-PCR assays for detection of bovine enteric caliciviruses (BEC) and analysis of the genetic relationships among BEC and human caliciviruses. J Clin Microbiol. 2003;41:3089–3099. doi: 10.1128/JCM.41.7.3089-3099.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith DB, McFadden N, Blundell RJ, Meredith A, Simmonds P. Diversity of murine norovirus in wild-rodent populations: species-specific associations suggest an ancient divergence. J Gen Virol. 2012;93:259–266. doi: 10.1099/vir.0.036392-0. [DOI] [PubMed] [Google Scholar]
- 31.Thomas CJ, Hoet AE, Sreevatsan S, Wittum TE, Briggs RE, Duff GC, Saif LJ. Transmission of bovine coronavirus and serologic responses in feedlot calves under field conditions. Am J Vet Res. 2006;67:1412–1420. doi: 10.2460/ajvr.67.8.1412. [DOI] [PubMed] [Google Scholar]
- 32.Wise AG, Monroe SS, Hanson LE, Grooms DL, Sockett D, Maes RK. Molecular characterization of noroviruses detected in diarrheic stools of Michigan and Wisconsin dairy calves: circulation of two distinct subgroups. Virus Res. 2004;100:165–177. doi: 10.1016/j.virusres.2003.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolf S, Williamson W, Hewitt J, Lin S, Rivera-Aban M, Ball A, Scholes P, Savill M, Greening GE. Molecular detection of norovirus in sheep and pigs in New Zealand farms. Vet Microbiol. 2009;133:184–189. doi: 10.1016/j.vetmic.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Woode GN, Bridger JC. Isolation of small viruses resembling astroviruses and caliciviruses from acute enteritis of calves. J Med Microbiol. 1978;11:441–452. doi: 10.1099/00222615-11-4-441. [DOI] [PubMed] [Google Scholar]


