Abstract
The advent of immune checkpoint inhibition represents a paradigm shift in the treatment of an increasing number of cancers. However, the incredible therapeutic promise of immunotherapy brings with it the need to understand and manage its diverse array of potential adverse events. The skin is the most common site of immune-related adverse vents (irAEs), which can present with a wide variety of disparate morphologies and severities. These toxicities can endanger patient health and the ability to continue on therapy. This review summarizes our current understanding of the presentation and management of the most common and clinically significant cutaneous irAEs associated with immune checkpoint inhibitor (ICI) therapy. Effective management of these cutaneous irAEs requires an understanding of their morphology, their appropriate clinical characterization, and their potential prognostic significance. Their treatment is additionally complicated by the desire to minimize compromise of the patient’s anti-neoplastic regimen and emphasizes the use of non-immunosuppressive interventions whenever possible. However, though cutaneous irAEs represent a challenge to both oncologist and dermatologist alike, they offer a unique glimpse into the mechanisms that underlie not only carcinogenesis, but many primary dermatoses, and may provide clues to the treatment of disease even beyond cancer.
Keywords: Dermatology, oncology, oncodermatology, supportive oncodermatology, immune checkpoint inhibitor, immune related adverse event, drug toxicity, skin toxicity
Introduction
Immune checkpoint inhibitor (ICI) therapy represents a paradigm shift in immunotherapeutics that has revolutionized the management of cancer patients. Numerous types of cancer, many of which had only minimally effective preexisting therapies, have shown impressive response to immune checkpoint inhibitors (ICIs), and their indications for use continue to expand. However, these powerful medications carry with them the risk for varied and potentially severe toxicities within multiple organ systems. These toxicities have led to the need for specialized and multidisciplinary management of oncologic patients receiving ICIs. The role of the dermatologist is central in this setting, as cutaneous irAEs are among the most frequently encountered and prompt diagnosis and management can profoundly impact a patient’s treatment course. Further, cutaneous irAEs provide fundamental insight into the anti-tumoral response and immunopathogenesis of many prevalent dermatologic conditions.
Biology of Immune Checkpoint Inhibition
The immune system is capable of recognizing tumor cells as non-self and mounting an appropriate response, but often this effort is confounded by immune downregulation, which can occur at many different points in the immune cascade [1]. Immune checkpoint therapy affects the anti-tumor immune response at the level of T cell activation by antigen presenting cells (APCs). APCs load protein fragments onto major histocompatibility complexes (MHCs), which are expressed on the APC surface and interact with uniquely compatible T cell receptors [1]. The resulting activation of the target T cell is mediated by costimulatory interactions between other proteins on the surface of the APC and T cells. One such key costimulatory interaction occurs between the CD28 protein on T cells and the B7 family of proteins on APCs. The CTLA-4 protein is also expressed on T cells, and competes with CD28 for binding to B7 [1]. It is thus a competitive inhibitor of T cell activation. Pharmacologic CTLA-4 inhibition increases binding of CD28 to B7 and thereby promotes T cell activation.
T cells also express the Programmed Death 1 receptor (PD-1), which is activated by PD ligands 1 and 2 (PD-L1 and PD-L2) to decrease T cell activation by inhibiting proliferation, decreasing cytokine production, and promoting apoptosis [1]. Notably, PD-L1 is expressed by somatic cells in peripheral tissue beds, and can also be upregulated by cancer cells [2]. Thus, while CTLA-4 inhibition functions at the level of initial T cell antigen activation, PD-1 axis inhibition stimulates T cell function downstream at sites of immune activity.
ICIs are medications that act at the level of T cell costimulation to increase immune activation, with the goal of promoting an anti-tumor immune response. All ICIs are monoclonal antibodies. Ipilimumab is the lone FDA-approved inhibitor of CTLA-4 [3]; a more recently developed antibody, tremelimumab, is under investigation in clinical trials but is not FDA-approved at this time. In contrast, there is a growing array of FDA-approved inhibitors of the PD-1 axis. Nivolumab and pembrolizumab, both PD-1 receptor antagonists, are the oldest and best studied of these. Combination CTLA-4 and PD-1 axis inhibition has been shown to be more effective than monotherapy in the treatment of metastatic melanoma; however, combination therapy may be substantially more toxic, and so careful patient selection is important [4].
Though immunotherapy was pioneered in melanoma, and ipilimumab is still predominantly used in the treatment of melanoma, inhibition of the PD-1 axis has found broader application in the treatment of a wide variety of cancers. Nivolumab and pembrolizumab are both approved for the treatment of non-small cell lung cancer and a range of other solid organ and hematologic malignancies [5,6]. Cemiplimab is a more recently developed PD-1 inhibitor that is approved specifically for metastatic or unresectable squamous cell carcinoma [7]. In addition, atezolizumab, avelumab, and durvalumab are PD-L1 inhibitors, which are approved for the treatment of several tumor types including small and non-small cell lung carcinoma, urothelial carcinoma, and Merkel cell carcinoma [8-10]. The names of FDA-approved ICIs, their targets, and their indications are summarized in Table 1. There are mounting case series and clinical trials supporting the use of ICIs in other cancer types and at earlier stages.
Table 1. FDA-Approved Immune Checkpoint Inhibitors.
| Generic Name | Trade Name | Target | FDA-Approved Indications |
| ipilimumab | Yervoy | CTLA-4 | • Melanoma (unresectable/metastatic or Stage 3, first-line or adjuvant) |
| • Renal cell carcinoma (combination with nivolumab) | |||
| • Metastatic colorectal cancer (microsatellite instability-high or mismatch repair deficient, second line)(w/ nivolumab) | |||
| pembrolizumab | Keytruda | PD-1 | • Melanoma (unresectable/metastatic or Stage 3, first-line or adjuvant) |
| • Non-small cell lung cancer (first-line or second line, single agent or combination, based on gene expression) | |||
| • Small cell lung cancer (third line) | |||
| • Head and neck squamous cell carcinoma (first-line or second line, single agent or combination, based on gene expression) | |||
| • Classical Hodgkin Lymphoma (fourth-line) | |||
| • Primary mediastinal large B-cell lymphoma (third-line) | |||
| • Urothelial carcinoma (first or second line, based on gene expression) | |||
| • Microsatellite Instability-High cancer (second-line for colorectal, last-line for any other type) | |||
| • Gastric adenocarcinoma (third-line, with compatible gene expression) | |||
| • Cervical carcinoma (second-line, with compatible gene expression) | |||
| • Hepatocellular carcinoma (second-line) | |||
| • Merkel cell carcinoma (recurrent locally advanced or metastatic) | |||
| • Renal cell carcinoma (first line)(combination with axitinib) | |||
| nivolumab | Opdivo | PD-1 | • Melanoma (unresectable/metastatic or Stage 3, first-line or adjuvant) |
| • Non-small cell lung cancer (second- or third-line, based on gene expression) | |||
| • Small cell lung cancer (third-line) | |||
| • Head and neck squamous cell carcinoma (second-line) | |||
| • Classical Hodgkin Lymphoma (third- to fourth-line, post-transplant) | |||
| • Urothelial carcinoma (second-line) | |||
| • Colorectal adenocarcinoma (MSI-high or dMMR, second-line, +/- ipilimumab) | |||
| • Hepatocellular carcinoma (second-line) | |||
| • Renal cell carcinoma (first-line with ipilimumab or second-line) | |||
| cemiplimab | Libtayo | PD-1 | • Cutaneous squamous cell carcinoma (unresectable or metastatic) |
| atezolizumab | Tecentriq | PD-L1 | • Urothelial carcinoma (first- or second-line, based on gene expression) |
| • Non-small cell lung cancer (first-line in combination, second-line single, based on gene expression) | |||
| • Small cell lung cancer (first-line in combination) | |||
| • Breast carcinoma, triple-negative (first-line in combination) | |||
| avelumab | Bavencio | PD-L1 | • Merkel cell carcinoma (metastatic, first-line) |
| • Urothelial carcinoma (second-line) | |||
| • Renal cell carcinoma (first-line in combination with axitinib) | |||
| durvalumab | Imfinzi | PD-L1 | • Urothelial carcinoma (second-line) |
| • Non-small cell lung cancer (patients without progression after first-line therapy) |
Immune-Related Adverse Events
ICIs cause widespread and relatively nonspecific activation of patient T cells, and so it is unsurprising that their use is associated with a number of deleterious phenomena, collectively termed immune-related adverse events (irAEs). The management of irAEs is complex and benefits from a multidisciplinary specialist approach. It is important that clinicians are aware of the spectrum of potential immune-related complications that may arise, which are outlined in Table 2 [11].
Table 2. Immune-related adverse events associated with immune checkpoint inhibitors.
| Organ System | Immune-Related Adverse Event | Symptoms |
| Dermatologic | Pruritus | itch with or without rash |
| Morbilliform exanthem | transient and coalescing pink macules and papules | |
| Vitiligo-like depigmentation | loss of skin pigmentation, halo nevi | |
| Lichenoid dermatitis | pruritic, violaceous papules/plaques, may involve mucosal surfaces | |
| Bullous pemphigoid | tense vesicles/bullae, erosions, urticarial plaques, pruritus | |
| Severe cutaneous adverse reactions (SJS/TEN, AGEP, DRESS) | fever, widespread rash, edema, vesicles/bullae/pustules, skin sloughing, end organ dysfunction | |
| Endocrine | Hypophysitis, adrenal insufficiency | fatigue, weakness, weight change, mood change, temperature sensitivity |
| Primary hypothyroidism | cold intolerance, constipation, change in hunger/thirst/sweating, fatigue, hair loss | |
| Hyperthyroidism | heat intolerance, loose stools, change in hunger/thirst/sweating, fatigue, hair loss | |
| Autoimmune diabetes | polyuria/polydipsia, altered mental status, weight loss, blurry vision | |
| Gastrointestinal | Colitis | abdominal pain, nausea, cramping, diarrhea, bloody stools |
| Hepatitis | jaundice, nausea/vomiting, abdominal pain, altered mental status, urine color change | |
| Pulmonary | Pneumonitis | cough, shortness of breath, fatigue, chest pain |
| Rheumatologic | Inflammatory Arthritis | joint pain, swelling, morning stiffness, weakness |
| Polymyalgia-like Syndrome | pain and stiffness in proximal upper and lower extremities | |
| Myositis | muscle pain, weakness, life-threatening if respiratory/cardiac muscles | |
| Ophthalmologic | Uveitis | blurred vision, double vision, eye pain, redness |
| Cardiac | Myocarditis | cough, shortness of breath, chest pain, palpitations |
| Renal | Nephritis | change in urine color/volume, hematuria, edema/anasarca |
| Neurologic | Myasthenia Gravis | fatigable or fluctuating muscle weakness, ptosis, double vision, dysphagia, dysarthria, facial muscle weakness |
| Guillain-Bare Syndrome | ascending progressive and usually symmetric muscle weakness | |
| Encephalitis | altered mental status, headaches, seizures |
Adverse events are assigned grades based on the degree of severity and associated morbidity. The Common Terminology Criteria for Adverse Events (CTCAE) extensively defines the parameters of these grades for each affected organ system and for each class of reaction type. In general, Grade 1 reactions are asymptomatic, Grade 2 reactions have minor effect on patient quality of life and generally respond to conservative measures, Grade 3 reactions significantly affect a patient’s functionality and may require more aggressive treatment, Grade 4 reactions are potentially life-threatening and require hospitalization, and Grade 5 is reserved for adverse events resulting in patient death [11]. Familiarity with the CTCAE grading system is important in communicating with a patient’s oncology team, as higher grade events are more likely to result in interruption or permanent discontinuation of immunotherapy.
Cutaneous irAEs
Dermatologic toxicities are among the most common complications of ICI therapy [12,13]. Many cutaneous irAEs present similarly to primary dermatoses and may share properties with autoimmune skin disorders [12,14,15]. This report will highlight common, uncommon, and rare cutaneous irAEs, and emphasize how they fit into the overall treatment of patients receiving ICIs.
Common Cutaneous irAEs
Cutaneous adverse events may occur in up to 30 to 50% of patients on ICIs [13,14]. These include pruritus, exanthems, vitiligo, and lichenoid reactions. Fortunately, these cutaneous irAEs are typically mild, and can usually be treated without interruption of immunotherapy.
Pruritus, or itch, in the absence of rash occurs in 11 to 18% of patients treated with PD-1 inhibitors and up to 30% of patients treated with anti-CTLA-4 or dual checkpoint inhibition [16-18]. It is defined as the presence of pruritus without primary dermatologic findings, though secondary findings such as excoriations and lichenification may be present [16]. Importantly, pruritus may accompany cutaneous irAEs as well. It is important to consider prodromal bullous pemphigoid in the evaluation of intense and refractory pruritus, which will be discussed below. Patients are generally managed with topical anti-pruritics such as camphor-menthol, topical steroids, anti-histamines, and occasionally other anti-pruritic drugs such as gabapentin or pregabalin, mu-opioid antagonists such as naloxone and naltrexone, and the neurokinin-1 receptor antagonist aprepitant [18-20]. Phototherapy with narrowband ultraviolet B may be helpful. Only severe and debilitating cases require interruption of immunotherapy and systemic steroids [20].
Morbilliform exanthems occur in about one quarter of patients receiving ipilimumab or combination ICI therapy, and about 15% of patients treated with anti-PD-1 monotherapy [15,17-20]. These exanthems typically present during the first few weeks of treatment and manifest as blanchable pink coalescent macules and papules that typically spare the face and palmoplantar surfaces and are generally pruritic (Figure 1). The presentation is similar to that of a morbilliform drug eruption to antibiotics. While most exanthems are usually low grade, self-limited or respond to topical steroids, patients should be observed for progression to a higher grade reaction such as drug reaction with eosinophilia and systemic symptoms (DRESS, discussed later), which may require interruption of immunotherapy and management with systemic steroids [20].
Figure 1.
Morbilliform exanthem in a patient with metastatic melanoma on anti-CTLA-4 therapy. Morbilliform exanthems in ICI therapy present classically with pruritic, erythematous coalescent macules, and papules favoring the trunk and extremities.
Vitiligo-like depigmentation is a common cutaneous irAE that may be seen in up to a quarter of patients treated for melanoma, and rarely in patients with other malignancies [15,21-24]. Depigmentation typically first presents several months into therapy, and may be preceded by an inflammatory phase [15,25]. The distribution is often symmetric and photodistributed (Figure 2), distinguishing it from the periorificial and acral presentation of classical vitiligo [25]. Coincident poliosis of scalp, eyebrow, eyelash, and body hair may occur. Treatment is not necessary, but topical steroids or calcineurin inhibitors and phototherapy may be attempted.
Figure 2.
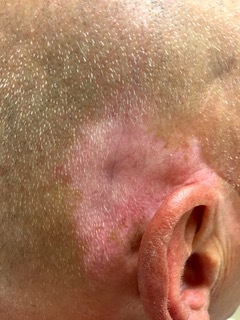
Vitiligo-like depigmentation in a patient with metastatic melanoma on anti-PD-1 therapy. The pattern of depigmentation in ICI therapy is distinct from primary vitiligo and involves different sites such as the ear, seen here.
Lichenoid dermatitis is the most well-characterized cutaneous irAE in patients receiving anti-PD-1 or anti-PD-L1 therapy, and occurs less commonly in CTLA-4 inhibition [15,26]. It affects approximately one-fifth of patients receiving anti-PD-1 therapy, and can develop at any time from weeks to several months after treatment initiation [18,27]. This classically presents as pruritic, polygonal, pink-violaceous papules with an overlying white network of scale (Figure 3); however, a wide variety of morphologies have been reported including hypertrophic, palmoplantar, and mucosal lesions [27]. Other papulosquamous reactions with overlapping features may occur, such as psoriasis as well as eczematous reactions. Treatment for these rashes is usually with topical steroids and anti-pruritic drugs, while more severe presentations may require systemic steroids, phototherapy, or other systemic agents such as acitretin, methotrexate, apremilast, or hydroxychloroquine [18-20,30-32]. Biologic medications approved for psoriasis such as TNF-a, IL-17, and IL-12 and IL-23 inhibitors have historically been avoided due to concerns of immune inhibition and they have not been well studied in oncologic patients, but may be considered in severe and recalcitrant cases. Fortunately, most patients with these rashes are able to remain on immunotherapy [20].
Figure 3.
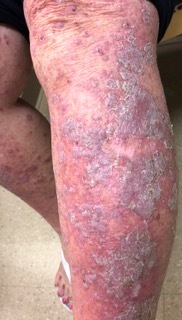
Lichenoid dermatitis in the setting of anti-PD-L1 therapy for lung cancer. Lichen planus classically presents as pruritic, violaceous papules/plaques with scale on the extremities.
Uncommon and Severe Cutaneous irAEs
There are several uncommon and potentially life-threatening dermatologic irAEs that may arise from ICI therapy and must be carefully considered when evaluating patients with severe presentations. One study at our institution suggested that 25% of patients with rashes during ICIs ultimately experience temporary or permanent discontinuation of immunotherapy, and the following eruptions are most likely to disturb ICI therapy [20].
Immunobullous eruptions, usually mimicking bullous pemphigoid (BP), occurs in approximately 1% of patients receiving PD-1 or PD-L1 inhibitors [18,28,29]. It can also appear in the context of sequential treatment with PD-1 and CTLA-4 inhibitors, but is not typically associated with ipilimumab monotherapy [30,31]. Most immunobullous reactions present with tense bullae overlying edematous pink urticarial plaques (Figure 4), and mucosal involvement may occur. Prodromal BP – that is, early BP that has not progressed to bulla formation – may be a cause of intractable pruritus in patients on immunotherapy. Diagnosis is made from skin biopsy and direct immunofluorescence which often demonstrates IgG and C3 deposits along the dermoepidermal junction, and positive BP180 autoantibody on ELISA [28,29]. In addition to BP, other immunobullous irAEs are much more rarely reported, including pemphigus vulgaris, lichen planus pemphigoides, erythema multiforme, and dermatitis herpetiformis [32-35]. Though conservative topical management or doxycycline/niacinamide is sometimes possible for mild presentations, most cases requires treatment interruption and initiation of systemic steroids due to the severity of the rash and impact on patient’s quality of life [34]. ICI-induced BP may persist after drug withdrawal [15,29]. For refractory cases, rituximab may be used, and there is early data suggesting that this monoclonal antibody to CD20 on B cells may not interfere with the antitumoral activity of ICIs [36,37]. In addition, for bullous eruptions with high IgE levels, omalizumab may be effective [38], and has the advantage of being minimally immunosuppressive.
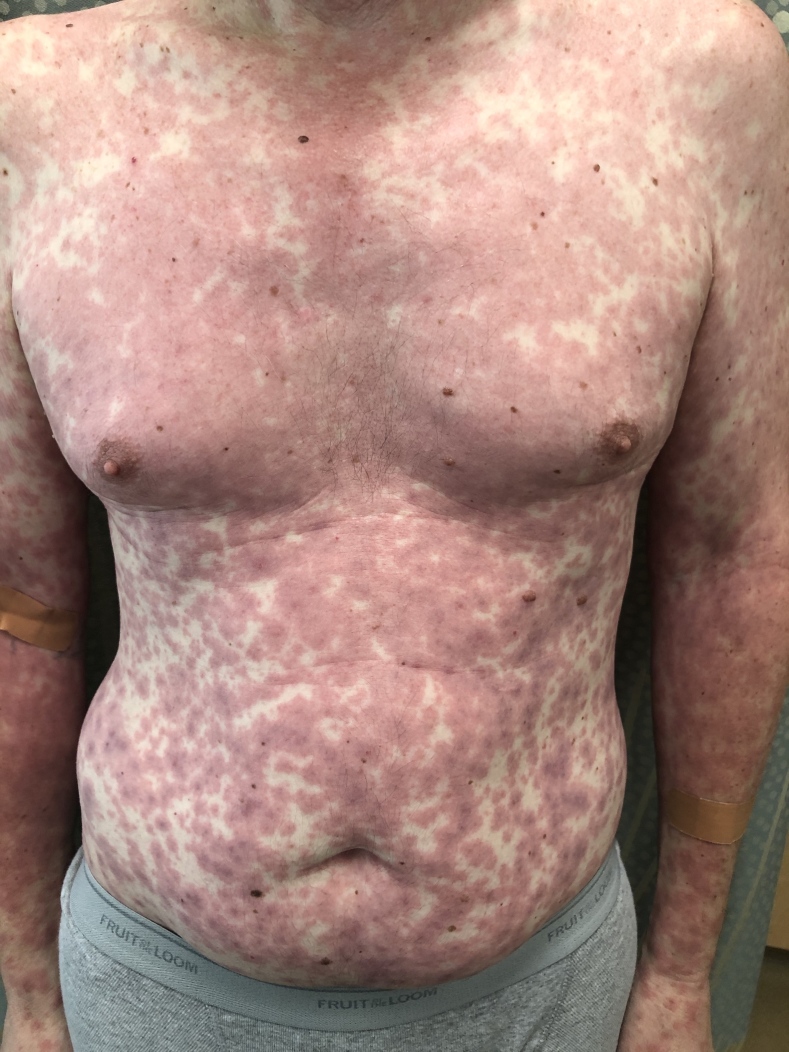
Figure 4.
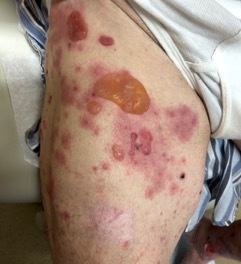
Bullous pemphigoid in the setting of anti-PD-1 therapy for metastatic melanoma. ICI-induced bullous pemphigoid classically presents as tense vesicles/bullae overlying urticarial plaques on the trunk and extremities.
In addition to BP, severe cutaneous adverse reactions (SCARs) can occur in ICI therapy, but are fortunately rare. Acute Generalized Exanthematous Pustulosis (AGEP) presents acutely with the development of erythematous and edematous plaques covered in monomorphic pustules, as well as protracted fever and often facial edema and mucous membrane involvement [12]. AGEP is fortunately a self-resolving condition; however, care must be taken to distinguish it from other dermatoses with which it may share overlapping features. The presence of tiny pustules and surrounding exanthem helps distinguish AGEP from pustular psoriasis.
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) or drug-induced hypersensitivity syndrome (DIHS) presents initially with an extensive morbilliform rash that may initially resemble a simple morbilliform exanthem. However, the exanthem of DRESS may become indurated or purpuric, and is accompanied by other symptoms such as fever, facial edema, or lymphadenopathy, as well as laboratory evidence of eosinophilia and end-organ dysfunction [39-41]. The RegiScar scoring system is based on the presence of suggestive signs and studies, and is often helpful in assessing the likelihood of DRESS in a patient with a morbilliform-appearing exanthem [42]. Once identified, DRESS is treated with permanent ICI discontinuation and an extended prednisone taper, along with studies to assess for long-term end organ dysfunction. Of note, uncommonly patients may present with overlapping features of both DRESS and AGEP.
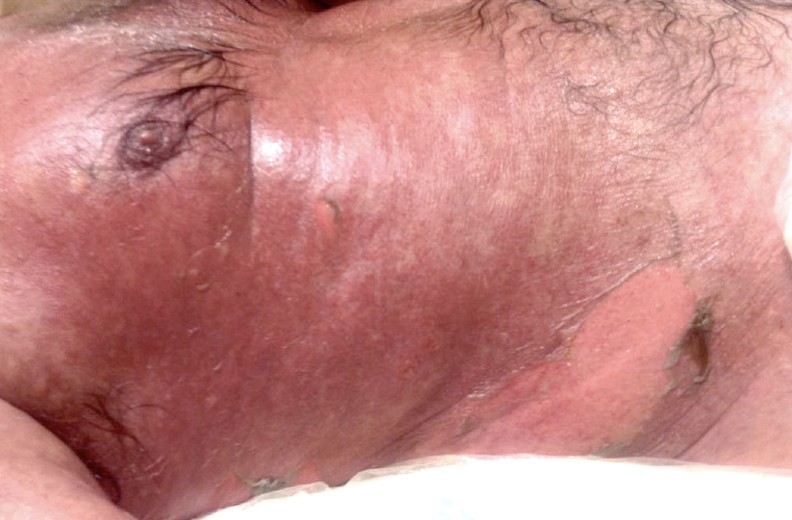
Patients treated with ICIs can develop classic Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis (SJS/TEN), in which dusky patches on the skin and mucous membranes rapidly progress to full thickness epidermal necrosis and sloughing. Alternatively, ICI-induced SJS/TEN-like reactions can present with an atypical, slowly evolving presentation, and can arise in the context of other morphologic presentations such as exanthems or severe lichenoid toxicity (Figure 5), requiring a high level of suspicion and close monitoring [43-45]. SJS/TEN is potentially fatal and treatment discontinuation is mandatory. The mainstay of management is intense supportive care with skilled auxiliary service support and appropriate specialist evaluation. Medical therapy with prednisone, intravenous immunoglobulin, cyclosporine, and biologic TNF-alpha inhibitors may be employed to decrease morbidity and mortality [39,46].
Figure 5.
Toxic Epidermal Necrolysis (TEN)-like lesions in a patient with metastatic melanoma treated with anti-CTLA-4 and anti-PD-1 therapy. This patient experienced full thickness epidermal necrosis leading to the denudation of large areas of skin, typical of TEN.
Other Rare Cutaneous irAEs
ICI therapy causes a wide variety of eruptions even beyond those described above. Neutrophilic dermatoses such as Sweet’s syndrome have been rarely reported with both anti-CTLA-4 and -PD-1 therapy, as have granulomatous reactions including granuloma annulare and sarcoidosis [15]. ICI therapy has also been associated with papulopustular and rosaceiform eruptions, cutaneous lymphoproliferative disorders, autoimmune connective tissue disorders such as lupus, dermatomyositis, and eosinophilic fasciitis, and several kinds of vasculitis [15]. In addition, alopecia areata may develop which can progress to alopecia totalis [15].
The Role of the Dermatologist
Given the frequency and morbidity of cutaneous irAEs, dermatologists can play a crucial role in multidisciplinary care of patients with cancer. The field of “Supportive Oncodermatology” has emerged to address the important need for specialized dermatologic care in cancer patients. Importantly, timely assessment of patients with an acute rash is needed, and this is facilitated by oncodermatology programs associated with cancer centers or oncology practices, where dermatologists who specialize in the management of toxicities to cancer drugs have close communication channels with oncologists.
Fortunately, consensus guidelines and the CTCAE report on irAEs exist that provide recommendations for the management of irAEs based on grade. Oncologists generally manage low grade presentations without the need for consultation. Patients with significant cutaneous irAE should be promptly referred to a dermatologist for diagnosis and management, as recalcitrant and severe cases often pose a therapeutic challenge [20].
Dermatologists may provide an accurate and timely diagnosis of a cutaneous irAE, assess its severity, and communicate a treatment plan with the oncology team. Documentation of the appropriate CTCAE grade is a useful tool to communicate reaction severity to oncologists. Dermatologists may also offer targeted approaches for the specific rash and avoid the unnecessary use of systemic steroids for a familiar rash.
The impact of systemic steroids on survival in patients receiving ICIs is controversial, and a matter of ongoing research. Although there is some suggestion that patients who receive steroids have comparable clinical courses [47], other studies suggest that high dose steroids blunt the efficacy of ICI therapy and lead to worse outcomes [48,49]. While the use of systemic steroids is appropriate in the treatment of severely morbid or life threatening toxicities, it is incumbent upon dermatologists to advocate against their use for less severe conditions that may be better addressed by other modalities. The role of the supportive oncodermatologist is therefore one of diagnosis and finesse, to minimize morbidity, optimize both dermatologic and antineoplastic therapy, and to provide a specialized perspective on the prognosis of these cutaneous reactions.
What Cutaneous irAEs Tell Us About Cancer Response and Dermatologic Conditions
Interestingly, dermatologic irAEs provide us with a window into the mechanism by which ICIs treat cancer, help prognosticate response to therapy, and allow us to better understand the primary dermatoses that develop from immune up-regulation during ICI therapy.
Cutaneous irAEs are generally thought to be due to ICI-mediated overactivation of the immune system, which is the same mechanism by which these therapies exert their anti-tumor effect. Interestingly, the development of certain cutaneous irAEs may be a positive prognostic indicator for the treatment of the underlying malignancy. This is most well established with ICI-induced vitiligo-like depigmentation during the treatment of melanoma, which is strongly correlated with improved progression-free and overall survival [50]. Similarly, other cutaneous reactions such as morbilliform exanthems, lichenoid and eczematous presentations, and immunobullous disease may also be associated with improved prognosis, though the links are not as robustly established [24,28,51]. Further studies are needed to compare the prognostic value of the diverse rash types, and help stratify which patients are at greatest risk for the development of particular irAEs.
One striking quality of cutaneous irAEs is that they recapitulate an incredibly broad range of primary dermatoses. Though many dermatologic diseases are fundamentally caused by misdirected immune response or autoimmunity, the specific nature of that immune response differs by disease. It is therefore surprising that a medication that was designed to activate T cells can cause conditions as disparate as psoriasis, sarcoidosis, and bullous pemphigoid. This argues for a role for T cells in the pathogenesis of all of these diseases, and provides an avenue through which to explore the exact mechanisms underlying their etiology. It is further interesting to note that the CTLA-4 and PD-1 axis inhibitors, which work at different stages of T cell activation, result in a different array of irAEs, suggesting a mechanistic distinction between the pathways downstream of each level of activation. Though cutaneous irAEs are a substantial source of morbidity, they provide an unprecedented opportunity to understand not just the biology of cancer, but that of some of the fundamental diseases of dermatology.
Conclusion
Immune checkpoint inhibition carries enormous potential for the treatment of numerous cancers, but this promise is balanced by an increased need for specialist management of the many toxicities that are immune-related and that may impact patient quality of life and even disrupt cancer therapy in severe instances. The skin is the most common site for irAEs, and skilled dermatologic care can radically alter the course of therapy. Treatment of patients with toxicities to cancer drugs represents an important opportunity both to provide meaningful benefit to patient care and to advance our fundamental understanding of oncology and dermatology.
Glossary
- irAEs
immune-related adverse vents
- ICI
immune checkpoint inhibitor
- APCs
antigen presenting cells
- MHCs
major histocompatibility complexes
- PD-1
Programmed Death 1 receptor
- CTCAE
Common Terminology Criteria for Adverse Events
- DRESS
drug reaction with eosinophilia and systemic symptoms
- BP
bullous pemphigoid
- SCARs
severe cutaneous adverse reactions
- AGEP
Acute Generalized Exanthematous Pustulosis
- DIHS
drug-induced hypersensitivity syndrome
- SJS/TEN
Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis
Author Contributions
IWT prepared the manuscript. JSL provided guidance for the manuscript’s content and structure, and edited the manuscript.
References
- Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. [DOI] [PubMed] [Google Scholar]
- Yao H, Wang H, Li C, Fang JY, Xu J. Cancer Cell-Intrinsic PD-1 and Implications in Combinatorial Immunotherapy. Front Immunol. 2018;9:1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squibb BM. Yervoy (ipilumumab) [package insert]. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125377s104lbl.pdf Revised September 2019. Accessed December 21, 2019.
- McDermott D, Lebbe C, Hodi FS, Maio M, Weber JS, Wolchok JD, et al. Durable benefit and the potential for long-term survival with immunotherapy in advanced melanoma. Cancer Treat Rev. 2014;40(9):1056–64. [DOI] [PubMed] [Google Scholar]
- Merck. Keytruda (pembrolizumab) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125514s065lbl.pdf Revised September 2019. Accessed December 21, 2019.
- Squibb BM. Opdivo (nivolumab) [package insert]. U.S. Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125554s075lbl.pdf Revised September 2019. Accessed December 21, 2019.
- Regeneron. Libtayo (Cemiplimab) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761097s000lbl.pdf Revised March 2019. Accessed December 21, 2019.
- AstraZeneca Imfinzi (durvalumab) [package insert]. U.S. Food and Drug Administration https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761069s013lbl.pdf Revised July 2019. Accessed December 21, 2019.
- Genentech. Tecentriq (atezolizumab) [package insert]. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761034s021lbl.pdf Revised December 2019. Accessed December 21, 2019.
- Serono E. Bavencio (avelumab) [package insert]. U.S. Food and Drug Administration website. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761049s006lbl.pdf Revised May 2019. Accessed December 21, 2019.
- Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry JL, Tetzlaff MT, Nagarajan P, Drucker C, Diab A, Hymes SR, et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. J Cutan Pathol. 2017;44(2):158–76. [DOI] [PubMed] [Google Scholar]
- Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4(5):560–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacouture M, Sibaud V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am J Clin Dermatol. 2018;19 Suppl 1:31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibaud V. Dermatologic Reactions to Immune Checkpoint Inhibitors : Skin Toxicities and Immunotherapy. Am J Clin Dermatol. 2018;19(3):345–61. [DOI] [PubMed] [Google Scholar]
- Ensslin CJ, Rosen AC, Wu S, Lacouture ME. Pruritus in patients treated with targeted cancer therapies: systematic review and meta-analysis. J Am Acad Dermatol. 2013;69(5):708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel JC, Heinzerling L, Aberle J, Bahr O, Eigentler TK, Grimm MO, et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev. 2017;57:36–49. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Carlos G, Wakade D, Byth K, Kong BY, Chou S, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. J Am Acad Dermatol. 2016;74(3):455-61 e1. [DOI] [PubMed] [Google Scholar]
- Gravalos C, Sanmartin O, Gurpide A, Espana A, Majem M, Suh Oh HJ, et al. Clinical management of cutaneous adverse events in patients on targeted anticancer therapies and immunotherapies: a national consensus statement by the Spanish Academy of Dermatology and Venereology and the Spanish Society of Medical Oncology. Clin Transl Oncol. 2019;21(5):556–71. [DOI] [PubMed] [Google Scholar]
- Sanlorenzo M, Vujic I, Daud A, Algazi A, Gubens M, Luna SA, et al. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA Dermatol. 2015;151(11):1206–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Consuegra G, Chou S, Fernandez Penas P. Vitiligo-like depigmentation in oncology patients treated with immunotherapies for nonmelanoma metastatic cancers. Clin Exp Dermatol. 2019;44(6):643–6. [DOI] [PubMed] [Google Scholar]
- Uenami T, Hosono Y, Ishijima M, Kanazu M, Akazawa Y, Yano Y, et al. Vitiligo in a patient with lung adenocarcinoma treated with nivolumab: A case report. Lung Cancer. 2017;109:42–4. [DOI] [PubMed] [Google Scholar]
- Yin ES, Totonchy MB, Leventhal JS. Nivolumab-associated vitiligo-like depigmentation in a patient with acute myeloid leukemia: A novel finding. JAAD Case Rep. 2017;3(2):90–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman-Keller M, Kim Y, Cronin H, Richards A, Gibney G, Weber JS. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res. 2016;22(4):886–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsabal M, Marti A, Jacquemin C, Rambert J, Thiolat D, Dousset L, et al. Vitiligo-like lesions occurring in patients receiving anti-programmed cell death-1 therapies are clinically and biologically distinct from vitiligo. J Am Acad Dermatol. 2017;76(5):863–70. [DOI] [PubMed] [Google Scholar]
- Collins LK, Chapman MS, Carter JB, Samie FH. Cutaneous adverse effects of the immune checkpoint inhibitors. Curr Probl Cancer. 2017;41(2):125–8. [DOI] [PubMed] [Google Scholar]
- Shi VJ, Rodic N, Gettinger S, Leventhal JS, Neckman JP, Girardi M, et al. Clinical and Histologic Features of Lichenoid Mucocutaneous Eruptions Due to Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Immunotherapy. JAMA Dermatol. 2016;152(10):1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo J, Schindler K, Querfeld C, Busam K, Cunningham J, Page DB, et al. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. Cancer Immunol Res. 2016;4(5):383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J, Totonchy M, Damsky W, Berk-Krauss J, Castiglione F, Jr, Sznol M, et al. Bullous disorders associated with anti-PD-1 and anti-PD-L1 therapy: A retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J Am Acad Dermatol. 2018;79(6):1081–8. [DOI] [PubMed] [Google Scholar]
- Hanley T, Papa S, Saha M. Bullous pemphigoid associated with ipilimumab therapy for advanced metastatic melanoma. JRSM Open. 2018;9(10):2054270418793029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwatsuka Y, Iwanaga A, Kuwatsuka S, Okubo Y, Murayama N, Ishii N, et al. Bullous pemphigoid induced by ipilimumab in a patient with metastatic malignant melanoma after unsuccessful treatment with nivolumab. J Dermatol. 2018;45(1):e21–2. [DOI] [PubMed] [Google Scholar]
- Chen WS, Tetzlaff MT, Diwan H, Jahan-Tigh R, Diab A, Nelson K, et al. Suprabasal acantholytic dermatologic toxicities associated checkpoint inhibitor therapy: A spectrum of immune reactions from paraneoplastic pemphigus-like to Grover-like lesions. J Cutan Pathol. 2018;45(10):764–73. [DOI] [PubMed] [Google Scholar]
- Jour G, Glitza IC, Ellis RM, Torres-Cabala CA, Tetzlaff MT, Li JY, et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: a report on bullous skin eruptions. J Cutan Pathol. 2016;43(8):688–96. [DOI] [PubMed] [Google Scholar]
- Mochel MC, Ming ME, Imadojemu S, Gangadhar TC, Schuchter LM, Elenitsas R, et al. Cutaneous autoimmune effects in the setting of therapeutic immune checkpoint inhibition for metastatic melanoma. J Cutan Pathol. 2016;43(9):787–91. [DOI] [PubMed] [Google Scholar]
- Schmidgen MI, Butsch F, Schadmand-Fischer S, Steinbrink K, Grabbe S, Weidenthaler-Barth B, et al. Pembrolizumab-induced lichen planus pemphigoides in a patient with metastatic melanoma. J Dtsch Dermatol Ges. 2017;15(7):742–5. [DOI] [PubMed] [Google Scholar]
- Damsky W, Jilaveanu L, Turner N, Perry C, Zito C, Tomayko M, et al. B cell depletion or absence does not impede anti-tumor activity of PD-1 inhibitors. J Immunother Cancer. 2019;7(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowerby L, Dewan AK, Granter S, Gandhi L, LeBoeuf NR. Rituximab Treatment of Nivolumab-Induced Bullous Pemphigoid. JAMA Dermatol. 2017;153(6):603–5. [DOI] [PubMed] [Google Scholar]
- Kremer N, Snast I, Cohen ES, Hodak E, Mimouni D, Lapidoth M, et al. Rituximab and Omalizumab for the Treatment of Bullous Pemphigoid: A Systematic Review of the Literature. Am J Clin Dermatol. 2019;20(2):209–16. [DOI] [PubMed] [Google Scholar]
- Bolognia JL, Schafer JV, Cerroni L. Dermatology. Philadelphia: Elsevier Saunders; 2017. [Google Scholar]
- Lu J, Thuraisingam T, Chergui M, Nguyen K. Nivolumab-associated DRESS syndrome: A case report. JAAD Case Rep. 2019;5(3):216–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza S, Hill E, Ludlow SP, Nanjappa S. Checkpoint inhibitor-associated drug reaction with eosinophilia and systemic symptom syndrome. Melanoma Res. 2017;27(3):271–3. [DOI] [PubMed] [Google Scholar]
- Cacoub P, Musette P, Descamps V, Meyer O, Speirs C, Finzi L, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124(7):588–97. [DOI] [PubMed] [Google Scholar]
- Coleman E, Ko C, Dai F, Tomayko MM, Kluger H, Leventhal JS. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: A single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. J Am Acad Dermatol. 2019;80(4):990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dika E, Ravaioli GM, Fanti PA, Piraccini BM, Lambertini M, Chessa MA, et al. Cutaneous adverse effects during ipilimumab treatment for metastatic melanoma: a prospective study. Eur J Dermatol. 2017;27(3):266–70. [DOI] [PubMed] [Google Scholar]
- Saw S, Lee HY, Ng QS. Pembrolizumab-induced Stevens-Johnson syndrome in non-melanoma patients. Eur J Cancer. 2017;81:237–9. [DOI] [PubMed] [Google Scholar]
- Wang CW, Yang LY, Chen CB, Ho HC, Hung SI, Yang CH, et al. Randomized, controlled trial of TNF-alpha antagonist in CTL-mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128(3):985–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015;33(28):3193–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol. 2018;36(28):2872–8. [DOI] [PubMed] [Google Scholar]
- Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124(18):3706–14. [DOI] [PubMed] [Google Scholar]
- Teulings HE, Limpens J, Jansen SN, Zwinderman AH, Reitsma JB, Spuls PI, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol. 2015;33(7):773–81. [DOI] [PubMed] [Google Scholar]
- Min Lee CK, Li S, Tran DC, Zhu GA, Kim J, Kwong BY, et al. Characterization of dermatitis after PD-1/PD-L1 inhibitor therapy and association with multiple oncologic outcomes: A retrospective case-control study. J Am Acad Dermatol. 2018;79(6):1047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]