Abstract
Kinins are proinflammatory peptides that are formed in the skin by the enzymatic action of tissue kallikrein (KLK1) on kininogens. Tissue kallikrein is produced by eccrine sweat glands and also by cells of the stratum granulosum and other skin appendages. Kinin formation may be favored during inflammatory skin disorders when plasma constituents, including kininogens, extravasate from venules and capillaries, which have increased permeability in response to the plethora of inflammatory mediators generated in the course of acute inflammation. By activating either kinin B1 or B2 receptors, kinins modulate keratinocyte differentiation, which relays on activation of several signaling systems that follows receptor stimulation. Participation of the kinin B1 receptor in wound healing is still a matter of controversy though some studies indicate that B1 receptor stimulation regulates keratinocyte migration by controlling metalloproteases 2 and 9 production and by improving wound closure in a mouse model. Development of more stable kinin B1 receptor agonists may be beneficial to modulate wound healing, especially if we take into account that the B1 receptor is up-regulated by inflammation and by cytokines generated in the inflamed microenvironment.
Keywords: tissue kallikrein, KLK1, kinin B1 receptor, kinin B2 receptor, kallikrein-related peptidases, skin, wound healing
Introduction
Kinins are bioactive peptides produced by the enzymatic action of two serine proteases (kininogenases), plasma and tissue kallikreins. These kininogenases are proteases that release the kinin molecule from two endogenous and multifunctional protein substrates known as high and low molecular weight kininogens [1]. Plasma kallikrein participates in surface-dependent activation of blood coagulation, fibrinolysis, and inflammation and is encoded by a single gene (KLKB1) of approximately 31 kb in length that is located on chromosome 4q34–35. KLKB1 was thought traditionally to occur only in the liver, but subsequent studies using quantitative RT-PCR showed that several non-hepatic tissues also synthesized plasma kallikrein [2].
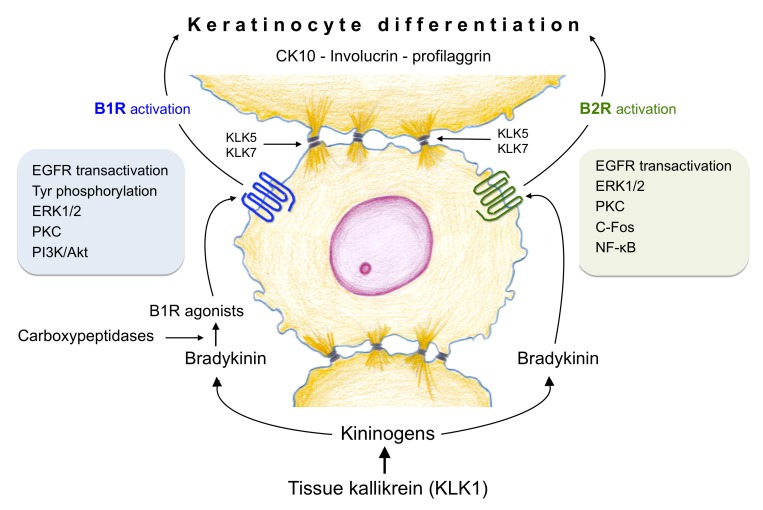
By comparison, tissue kallikrein (KLK1) is the oldest member of a family that comprises a multigene group of 15 serine proteases designated as KLK1 to KLK15 located in tandem within chromosome 19 q13.3-13.4. The other 14 members, referred to as kallikrein-related peptidases, are characterized by their trypsin- or chymotrypsin-like enzymatic activity. In the skin, KLK5 and KLK7 (Figure 1) have been shown to participate in keratinization, hydrolysis of desmosomal adhesion molecules and terminal keratinocyte differentiation [3,4]. So far, tissue kallikrein KLK1 is the only member of the family that exhibits kininogenase activity both in vitro and in vivo. Previous reports have shown that tissue kallikrein, kininogens, and kinin receptors are expressed in normal and pathological human skin suggesting that kinin peptides may be formed in their microenvironments so as to modulate important skin functions that could be of relevance to the pathogenesis of some skin disorders. Actually, kinins are proinflammatory peptides with the capacity to mimic the four clinical signs of inflammation including pain when they are in contact with a denuded epithelial surface.
Figure 1.
Interactions of tissue kallikrein (KLK1), kallikrein-related peptidases (KLK5 and KLK7) and kinin receptors in the human epidermis. Kinins are formed in the skin by the enzymatic action of tissue kallikrein (KLK1) on kininogens; once formed, bradykinin, a kinin B2 receptor (B2R) agonist, is hydrolyzed by carboxypeptidases N and M to produce kinin B1 receptor (B1R) agonists. Both type of receptors activate signaling pathways (protein tyrosine phosphorylation, ERK1/2, PKC, cFos, NF-κB and/or PI3K/Akt) associated to the differentiation program of human keratinocytes; epidermal growth factor receptor (EGFR) transactivation is also involved. Keratinocyte differentiation can be monitored by the appearance of several protein markers such as involucrin, cytokeratin 10 and profilaggrin. KLK5 and KLK7 participate in desquamation of the superficial epidermal layers.
In this review, we summarize the role of kinins and their receptors in skin homeostasis and how they contribute to keratinocyte differentiation and wound healing.
The Kinin System in the Human Skin
Tissue Kallikrein (KLK1)
The presence of a kinin-releasing enzyme in human sweat was first reported by Fox and Hilton [5]. The occurrence of tissue kallikrein in human sweat was determined in experiments in which a specific immunoassay was used [6]. Subsequently, the amount of immunoreactive tissue kallikrein was measured in sweat obtained from different regions of the body; the highest levels of the enzyme were found in samples taken from the trunk and forehead [7]. Additional studies described the major biochemical properties of the tissue kallikrein present in human sweat and showed that its relative molecular mass and inhibitor profile were identical to those described previously for KLK1 [8]. Immunocytochemical procedures performed on human skin tissue sections localized tissue kallikrein in the secretory granules of the “dark cells” in the fundus of eccrine sweat glands [9]. Expression of KLK1 mRNA in eccrine sweat glands was later confirmed by in situ hybridization techniques [10]. Interestingly, KLK1 expression was also found in the stratum granulosum of normal epidermis and in appendageal structures such as the inner root sheath of hair follicular epithelium [10]. Immunohistochemical procedures also localized the kinin-forming substrates (kininogens) in the interstitial tissue space and in the space between keratinocytes, making viable the hypothesis that kinins are formed in the skin [11]. High levels of kininogens occur during inflammatory skin disorders when plasma constituents extravasate from venules in response to the different mediators generated in the inflammatory milieu. Thus, the formation of kinins may be favored during some inflammatory skin diseases.
It has also been suggested that tissue kallikrein may promote skin wound healing since the active form of the enzyme induces keratinocyte migration and proliferation by a mechanism that is mediated by protease-activated receptor-1 and epidermal growth factor receptor (EGFR) activation, and independent of kinin receptors activation and nitric oxide (NO) formation [12]. In fact, tissue kallikrein-induced migration of wounded keratinocyte monolayers was associated with increased phosphorylation of EGFR, extracellular signal regulated kinases 1/2 (ERK1/2) mitogen-activated protein kinase (MAPK) and release of heparin-binding EGF-like growth factor (HB-EGF) and amphiregulin, two EGFR ligands [12].
Kinin Receptors
Once formed, kinin peptides exert their effects by activating two G protein-coupled receptors characterized by 7-transmembrane spanning helices; these receptors are known as B1 (BDKR1 gene, B1R) and B2 (BDKR2 gene, B2R). The human kinin B2R is preferentially activated by bradykinin and it mediates most of the physiological effects produced by kinins in different tissues/cells throughout the body including the keratinocyte (Figure 1). Bradykinin and its parent molecule Lys-bradykinin have a short half-life (15 to 30 seconds in plasma) because they are rapidly hydrolyzed by several peptidases known as kininases [1]. Two of these kininases, carboxypeptidases N and M, cleave both kinin molecules at the C-terminal Arg converting them into Lys-des[Arg9]bradykinin or des[Arg9]bradykinin, both agonists of the kinin B1R [1]. Of the two B1R ligands described so far the human B1R has greater affinity for Lys-des[Arg9]bradykinin than for des[Arg9]bradykinin; the opposite occurs with the rodent B1R [13,14].
The kinin B1R is usually expressed at low levels but is rapidly up-regulated during inflammation or after exposure to noxious stimuli such as lipopolysaccharide and proinflammatory cytokines (TNF-ɑ, IL-1β, IL-2, IFN-ɣ). Kinin B1R up-regulation in different systems is correlated with nuclear translocation of NF-κB, a process that can be blocked by inhibitors of NF-κB stimulation. In addition, glucocorticoids and protein synthesis inhibitors are able to block B1R up-regulation. Up-regulation of the B2R by inflammatory cytokines such as IFN-ɣ, IL-1, and TNF-ɑ has also been reported (reviewed in [13]). Both kinin B1 and B2 receptor agonists favor nociception and pain, vasodilatation, and vascular permeability [1,15]; B1R has also been shown to facilitate the chronic itching sensation in a diphenylcyclopropenone-induced model of chronic inflammation, an experimental model in which kinin B1R mRNA and protein levels are increased [16].
In general, stimulation of both kinin B1 and B2 receptors trigger a number of common intracellular signaling pathways that include calcium mobilization, phospholipase C, arachidonic acid release, inositol 3-phosphate, MAPK phosphorylation, and EGFR transactivation, among others. Nevertheless, activation of specific intracellular routes depends on both the stimulus and the biological effect that is characteristic for each cell type.
Keratinocyte Proliferation or Differentiation?
The expression of both kinin B1R and B2R (mRNA, protein and binding sites) has been observed in normal human skin and in tissues obtained from patients suffering various skin disorders. By using in situ hybridization, RT-PCR and immunohistochemistry we and others have shown the expression of both kinin receptors in the human epidermis, in primary cultures of human keratinocytes and in HaCaT cells, an immortalized keratinocytes cell line [17-20].
The first functional studies reported that bradykinin induced phosphoinositide turnover and 1,2-diglyceride formation and tyrosine phosphorylation of several proteins in cultured human keratinocytes [21,22]. Our group later demonstrated that the in vitro stimulation of B2R induced ERK1/2 MAPK phosphorylation, an event that is partially dependent on EGFR transactivation. ERK1/2 MAPK phosphorylation was also dependent on protein kinase C (PKC) activation since the PKC inhibitor GF109203X abolished it [19]. Similar observations were recorded following stimulation of the kinin B1R in human keratinocytes; transactivation of EGFR was visualized as phosphorylation of a band of 170 kDa. Additional experiments showed that EGFR transactivation resulted in phosphorylation of residues Tyr845, Tyr992, and Tyr1068 of EGFR [20].
Several studies had reported that kinins increased DNA synthesis and cell proliferation in different cell systems (reviewed in [1]). However, neither bradykinin [23-25] nor Lys-bradykinin [19] stimulates keratinocyte proliferation when compared with the effect produced by EGF. Similar results were observed when keratinocytes were stimulated with the natural kinin B1R agonist, Lys-des[Arg9]bradykinin and 5-bromo-2’-deoxyuridine (BrdU) incorporation was assessed [20,26]. Moreover, after kinin stimulation, BrdU incorporation rate was lower than that observed on non-stimulated keratinocytes [20]. This finding contrasted with the fact that when the same keratinocytes were stimulated with EGF or fetal calf serum they exhibited a high BrdU incorporation rate [20].
On the contrary, stimulation of human keratinocytes with the kinin B2R agonist Lys-bradykinin produced a rapid increase in [Ca2+]i, c-Fos expression, nuclear translocation of NF-κB and a moderate (pro)filaggrin synthesis indicating that it modulates keratinocyte differentiation [19]. Interestingly, the kinin B1R agonist Lys-des[Arg9]bradykinin also stimulated the synthesis of (pro)filaggrin, cytokeratin-10 and involucrin, three protein markers of keratinocyte differentiation [20] (Figure 1). PKC is a key component of the signaling route that triggers keratinocyte differentiation since its inhibition by GF109203X alters the expression of several differentiation markers [27]. Nevertheless, it is likely that the mechanisms involved in keratinocyte differentiation may not be identical because kinin B2R stimulation produces an increase in [Ca2+]i whereas kinin B1R stimulation does not [20]. The fact that kinin B1R activation does not result in an increase of [Ca2+]i mobilization suggests that keratinocyte differentiation may involve a Ca2+-independent PKC, a type of activity that represents 95% of total PKC activity [28]. On the other hand, the calcium increase induced by bradykinin is potentiated by a parathyroid hormone-related peptide, a fragment that has been shown to regulate keratinocyte proliferation and differentiation [29]. Whether any of the parathyroid hormone-related peptides can also potentiate the keratinocyte differentiation induced by kinin B2R agonists needs to be investigated.
Thus, by triggering specific intracellular signaling pathways kinin peptides may produce growth arrest and activation of keratinocyte differentiation to generate a cellular phenotype that can be identified by detecting specific differentiation markers.
Does Activation of the Kinin B1R Favor Wound Healing?
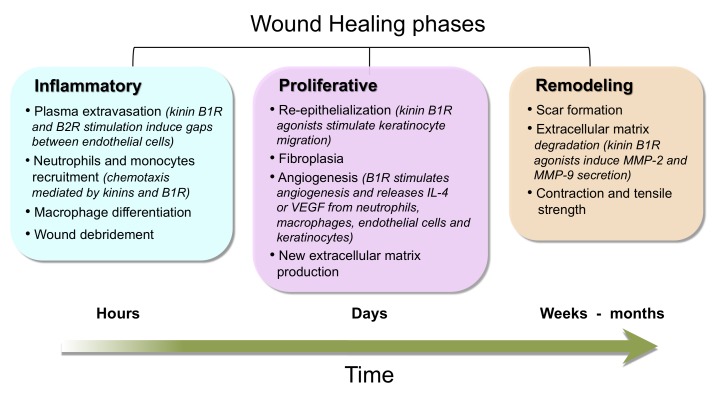
Wound healing is a complex cascade of events, orchestrated by growth factors and proteases; this process involves several phases: i) an inflammatory response, ii) wound re-epithelialization, angiogenesis and iii) granulation tissue formation, wound contraction, scar formation, and tissue remodeling [30] (Figure 2). As a whole, activation and acceleration of healing require the interaction of different cellular types such as leukocytes, fibroblasts, endothelial cells, and keratinocytes.
Figure 2.
Wound healing phases. Major characteristics of the three wound healing phases and the periods of time involved in each of them are depicted. Participation of kinins and kinin receptors during these healing phases is also included.
Diverse in vitro and in vivo studies have demonstrated the expression of kinin B1R on several cellular players of wound healing. Kinins are important inflammatory mediators and can modulate keratinocyte differentiation and proliferation/migration of endothelial cells. However, the role of kinin B1R in wound healing has been scarcely investigated. So far, only three groups have addressed this topic, but have reported contradictory results. The recent study performed by Soley et al. [31] using kinin B1R knockout mice showed a delay of the skin healing process; in fact, wild-type mice showed a complete resolution of wound healing at day 12 whereas kinin B1R knockout mice resolved lesions at day 17, demonstrating that kinin B1R is an important player in this process. The results obtained by this group are in agreement with our results in which topical administration of the kinin B1R agonist, des[Arg9]bradykinin accelerated wound closure supporting participation of kinin B1R in wound healing [32]. On the contrary, Desposito et al. [33] observed that systemic treatment of mice wounds with the stable B1R agonist SarLys[Hyp3,Igl5,DPhe8]desArg9-bradykinin had no effect on wound closure. However, the extremely high EC50 (400 ± 46 nM) of this agonist in the mouse when compared with that of the natural agonist des[Arg9]bradykinin (EC50= 21 ± 3 nM) [34] may explain the lack of effect reported by them in this species. Moreover, Desposito et al. [33], performed 8 mm diameter full-thickness wounds on the dorsal skin of agonist-treated mice and the results obtained were compared with those observed in similar wounds made on untreated mice. This type of comparison is difficult because there are different healing rates in different mice even when they come from the same litter. By comparison, our model considered a topical treatment and two full-thickness 6 mm punch wounds performed on the back of each mouse in such a way that comparison between wounds was performed in the same animal, avoiding animal variability.
The Kinin B1R in the Inflammatory, Proliferative, and Remodeling Wound Healing Phases
Inflammatory Phase
In this phase, migration of neutrophils and monocytes from blood compartment to the wound removes blood clot and cell debris from damaged tissue (Figure 2). Leukocytes are recruited by multiple released vasoactive mediators such as kinins, histamine, prostaglandins, leukotrienes, thrombin, IL-8, monocyte chemoattractant protein-1 (MCP-1), or bacterial lipopolysaccharides and chemotactic peptides [30,35]. At the wound site, neutrophils are considered to be primarily bactericidal, killing microorganisms by means of reactive oxygen species and neutrophil extracellular traps [36]. On the other hand, monocytes are recruited by specific chemoattractants such as transforming growth factor-β (TGF-β) and MCP-1, and then differentiate into M1 pro-inflammatory macrophages that later acquire a M2 phenotype (anti-inflammatory and tissue repair activities). Macrophages have an essential role because macrophage-depleted wounds show defective wound repair [30]. M1 macrophages secrete MCP-1 that is crucial for wound healing since MCP-1 deficient mice have an anomalous re-epithelialization [37]. Another important factor is TGF-β1 because knockout animals or inhibition of the major signaling pathways activated by TGF-β1 show an accelerated epithelialization and impaired inflammatory response [38,39]. By comparison, M2 macrophages acquire the capacity to produce platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF), two mediators that initiate granulation tissue formation.
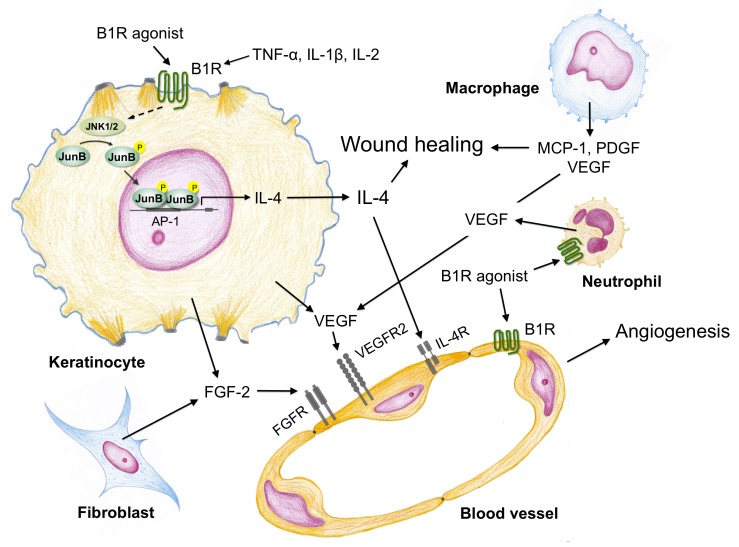
Schremmer-Danninger et al. [35] showed that B1R is increased in human skin biopsies obtained following surgery whereas kinin B2R expression did not change in the traumatized skin. Furthermore, using a murine model of thermal injury Rawlingson et al. [40] reported an early involvement of both kinin B1 and B2 receptors in plasma extravasation into the burn wound suggesting an important regulatory role for kinin receptors at the beginning of the wound healing process. Actually, kinin B1R agonists increase venular permeability by inducing contraction of endothelial cells and hence producing intercellular gaps through which plasma diffuse freely. Further, B1R agonists activate phospholipase C and NO generation in endothelial cells of precapillary vessels producing arteriolar dilatation [41]. On the other hand, the kinin B1R is an important player for recruitment of both neutrophils and macrophages at the site of injury and the high level of cytokines (TNF-α, IL-1β, IL-2, and IL-4), present in the inflammatory milieu up-regulate the expression of B1R in these leukocytes [41,42] (Figure 3). Stimulation of kinin B1R in human neutrophils results in chemotaxis, release of several proteases and up-regulation of CD11b/CD18 integrins [42-44]. Interestingly, kinin B1R agonists also induce the expression of intercellular adhesion molecule, ICAM-1 in endothelial cells [44]. The interaction between both neutrophils and endothelial cells facilitates neutrophil migration into the injury site. In addition, kinin B1R activation modulates the release of prostaglandins, TNF-α, IL-1β and chemokines [41]. Importance of kinin B1R on leukocytes recruitment is supported by studies showing that kinin B1R knockout mice exhibit lower numbers of neutrophils and mononuclear cells than wild-type animals at the wound site [31]. Moreover, our results show that topical application of a kinin B1R agonist onto the wounds increases recruitment of CD68 immunoreactive macrophages (unpublished results). Only a few studies have focused on the consequence of kinin B1R activation in macrophages, but early studies showed that stimulation of macrophages with a kinin B1R agonist induces TNF-α and IL-1 release, and increases NO levels [13,45,46].
Figure 3.
Major signaling pathways triggered by kinin B1 receptor (B1R) agonists in the human keratinocyte and its cross-talk with endothelial cells, fibroblasts, neutrophils and macrophages. Stimulation of kinin B1R in the human keratinocyte results in phosphorylation (P) of JunB that translocates into the nucleus to bind AP-1 sites and activate interleukin-4 (IL-4) synthesis. Release of IL-4 and also vascular endothelial growth factor (VEGF) from keratinocytes induces angiogenesis on blood vessels that expose VEGF receptors (VEGFR2) and IL-4 receptors (IL-4R) on the surface of endothelial cells. In addition, fibroblasts produce fibroblast growth factor-2 (FGF-2) and neutrophils and macrophages release VEGF that enhances the angiogenic response. Cytokines generated in the inflammatory milieu (TNF-ɑ, IL-1β, IL-2) may up-regulate the kinin B1R expressed by keratinocytes, neutrophils, macrophages and endothelial cells.
In mouse models, neutrophil depletion does not negatively affect wound healing as profoundly as macrophage depletion. However, in diabetes where infection risk is high, neutrophils are clearly required [30]. Thus, the kinin B1R is a key molecule for cell recruitment, as confirmed in a skin healing study, where the absence of the B1R produced a significant reduction of leukocytes infiltration and delay in resolution of the tissue repair process [31] (Figure 3).
Proliferative and Remodeling Phase
This phase is characterized by angiogenesis, migration of keratinocytes, and fibroblast proliferation that produces new extracellular matrix. Angiogenesis provides new blood vessels that deliver oxygen and nutrients for successful healing whereas migration of keratinocytes is a critical step for wound re-epithelialization. Keratinocytes receive signals to proliferate, migrate, and finally differentiate to restore the injured epidermis. For this purpose, keratinocytes express and/or activate surface exposed integrins (ɑ3β1, ɑvβ6, ɑvβ5, and a9β1), growth factor receptors and some of their ligands (TGF-ɑ, TGF-β, amphiregulin, epiregulin, HB-EGF, neuregulins 1 and 2 and keratinocyte growth factor). When receptors are activated, they trigger a number of signaling cascades that lead to reorganization of the cytoskeleton and phosphorylation of transcription factors involved in the expression of proteins/proteases required for keratinocyte migration, proliferation, and differentiation [47]. Wound healing experiments performed on TGF-ɑ deficient-mice show that the early phase of re-epithelialization is delayed [48] whereas a lack of HB-EGF produces a marked setback in wound closure as a consequence of severe delay in keratinocyte migration [49]. As mentioned before TGF-β is an important factor for re-epithelialization because it initiates keratinocyte migration.
The activation of kinin B1R regulates positively keratinocyte differentiation, but does not increase keratinocyte proliferation in vitro or in the margin of mice wounds treated with a B1R agonist [19,20,32]. Apparently, kinin B1R stimulation produces opposite effects on cell migration depending on the cell type involved; our in vitro approach showed that kinin B1R agonists produce a weak keratinocyte migration whereas its topical application onto wounds in an in vivo mouse model significantly reduced the wound area, probably by augmenting keratinocyte migration [32]. The in vivo microenvironment is much more complex than the in vitro situation and possibly the topical application of a kinin B1R agonist induces the release of cytokines (IL-4) and growth factors (HB-EGF) [32,50] or activates metalloproteases like MMP-2 and MMP-9, key players of keratinocyte migration [51] (Figure 3). Coincidently, stimulation of kinin B1R produces a transient c-JunN-terminal kinase phosphorylation and JunB nuclear translocation, transcription factor, which is known to regulate IL-4 expression [50]. Using a mouse model of wound healing, we observed that immunoreactivity for both MMP-2 and MMP-9 gelatinases was concentrated around wound borders and that cells expressed both MMPs in a cytoplasm area that was in close contact with the extracellular matrix [32] suggesting an association with extracellular matrix degradation or cleavage of growth factors/cytokines sited throughout the matrix.
An essential requirement for keratinocyte adhesion and migration is to change the integrins profile to allow its release from tight and adherens junctions. Integrins mediate cell-matrix interactions (cell polarity and migration) and act as signaling molecules across the plasma membrane that transduce both “inside-out” and “outside-in”. The integrin repertoire in basal keratinocytes is restricted to ɑ2β1, ɑ3β1, ɑ9β1, and ɑ6β4, whereas during wound healing ɑ3β1 and ɑ9β1 are up-regulated. Integrins can also regulate the balance between cell proliferation and differentiation to produce an effective re-epithelialization and a firm attachment of the new epithelial layer [47]. Thus, lack of integrins ɑ3, ɑ6, β4, β1, and β6 results in a disorganized basement membrane and abnormal cell adhesion, proliferation and differentiation [52]. There are few studies concerning the role of kinin B2R on integrin expression/activation [53,54], but there are no studies that analyze the effect of kinin B1R agonists on the expression/activation of integrins in keratinocytes and during wound healing.
When formation of new stroma or granulation tissue begins macrophages, fibroblast and blood vessels move into the wound at the same time. In this phase, macrophages provide a continuing source of growth factors, like PDGF and TGF-β1, necessary to stimulate fibroplasia and angiogenesis. In the wound, and influenced by the local microenvironment, macrophages undergo phenotypic switching from M1 to M2 phenotype, an event that depends on down-regulation of IL-10 and up-regulation of IL-4 and IL-13 [30]. Likewise, fibroblasts, activated by PDGF and TGF-β1 in concert with extracellular matrix molecules, proliferate, migrate, and produce the new matrix necessary to support cell ingrowth. Studies on the effect of kinin B1R agonists on fibroblasts are contradictory; in human embryonic lung fibroblasts they stimulate type I collagen synthesis, whereas in rat cardiac myofibroblast they decrease collagen secretion [55,56]. Further, kinin B1R agonists have been reported to have no effect on mouse fibroblast migration and proliferation [33]. In alliance with macrophages and fibroblasts, the new vessels move into the wound to initiate formation of granulation tissue. Endothelial cells initiate angiogenesis in response to growth factors like FGF-2 and VEGF, which are partially secreted by macrophages. The importance of VEGF-A for an adequate wound healing (Figure 3) has been demonstrated by using neutralizing VEGF-A antibodies onto porcine wounds, treatment that strongly impaired angiogenesis and formation of granulation tissue [57,58]. Several reports deal with participation of kinin peptides in angiogenesis; they produce an angiogenic effect on endothelial cells, by up-regulating FGF-2 expression, potentiating migration and cell growth or by stimulating VEGF synthesis and release [59]. We have shown that B1R stimulation produced significant endothelial cell migration and release of both MMP-2 and MMP-9, but did not increase endothelial cell proliferation [50]. Our in vitro studies so far indicate that kinin B1R agonists stimulate keratinocytes to release VEGF and IL-4, growth factors that promote endothelial cell migration and release of MMP-2 and MMP-9, two crucial events during angiogenesis (Figure 3).
Participation of Kinins and their Receptors in Other Skin Disorders
Psoriasis
Early studies showed that human biopsies obtained from patients suffering basal cell carcinoma, lichenificated atopic eczema, and psoriasis have expression levels of tissue kallikrein (KLK1) and kinin receptors that are similar to those observed in normal skin [18,35]. On the other hand, several reports have indicated that angiotensin-converting enzyme inhibitors (ACEI) may induce and/or exacerbate psoriasis, an effect that may be due to inhibition of kinins degradation by ACEI; then, the increased levels of kinins in the skin might increase inflammation and make psoriasis worse [60]. Interestingly, presence of ACE insertion polymorphism has been associated to occurrence of psoriasis. This allele has been associated to low ACE activity, a quality that results in reduced kinin degradation [61]. In agreement with this idea is the fact that psoriasis patients have elevated plasma levels of kininogens, the substrates required for kinin release [62]. However, the vascular response to kinins when they are injected intradermally into psoriasis patients is not altered when compared to normal volunteers [63]. Another source of kinins in psoriasis patients may come from circulating neutrophils, which infiltrate the lesional epidermis in these patients. It is important to mention that human neutrophils contain the components, which are needed to form kinins, tissue kallikrein (KLK1) and kininogens [1,42,44]. Moreover, elevated levels of all KLKs have been found in serum and in the lesional stratum corneum of patients with psoriasis [64].
In addition to their actions as proinflammatory peptides, kinins have also been associated to keratinocyte differentiation. Actually, kinin B2R agonists do not increase cell proliferation, but they induce keratinocyte differentiation as established by the expression of the differentiation markers cytokeratin 10, involucrin, and profilaggrin [19,20]. Coincidentally, experiments performed on B2 knockout mice show that these animals have epidermal cellular hyperproliferation and acanthosis when compared with wild type mice [64]. Whether the increased proliferation of keratinocytes, which speeds up their cell cycle, results from B2R malfunction in the microenvironment of lesional skin in psoriasis patients remains to be investigated.
Atopic Dermatitis
Bradykinin has been described as a potent histamine-independent pruritogen in lesional skin of atopic dermatitis. This peptide induces intense itch and pain in lesional skin and the increase in pain does not suppress itch feeling [65]. Notably, bradykinin produced weak itch and pain, of almost identical strength, in non-lesional skin of patients with atopic dermatitis and in healthy volunteers.
Experimental studies using animal models of itch-related scratching show that pretreatment of mice with a kinin B1R antagonist reduces this response when inflammation is induced with complete Freund’s adjuvant [66]. Another mouse model, which uses oxazolone to induce atopic dermatitis, results in up-regulation of B1 and B2 receptors in the skin. Both B1 and B2 receptor antagonists partially reduced the pruritus produced by oxazolone suggesting that participation of kinins and their receptors may have an important role in this model of atopic dermatitis. In fact, knockout mice, which are deficient in kinin B1 or B2 receptors display reduced pruritus following intradermal injection of trypsin, a situation that is also observed when mice are intraperitoneally injected with B1 or B2 receptor antagonists prior challenge [67].
It is important to consider that in addition to their direct effects on pain and pruritus, kinins can increase the release of substance P, calcitonin gene-related peptide, and prostaglandin E2, three major mediators of pruritus and key players of atopic dermatitis and psoriasis. In the skin, neuropeptides are located in nerve fibers of the papillary layer, around skin appendages and blood vessels. Future interdisciplinary studies should focus on the intricate network of interactions that exist between different mediators, their receptors and the cells which are responsible for their production.
Conclusion
Biological actions of kinins range from increase in vascular permeability to angiogenesis and keratinocyte differentiation. In the skin, kinins and other members of the kallikrein system have been investigated for their participation in several physiological and pathological processes. Kinins, and in particular kallikrein-related peptidases (KLK5 and KLK7), modulate keratinocyte differentiation and precise steps of wound healing such as plasma extravasation, leukocytes chemotaxis, keratinocyte migration, and angiogenesis. In addition, kinins can enhance their effects by inducing the release of angiogenic molecules (IL-4 and VEGF) from keratinocytes, endothelial cells, neutrophils, and macrophages.
The complexity of wound healing is amplified by local factors, such as ischemia and infection, also by systemic factors such as age, nutritional status, and pathologies such as diabetes mellitus. The final result is the formation of a scar, which is sufficiently functional. However, in some cases, the repair process is disorganized or insufficient resulting in hypertrophic scars, keloids, or chronic wounds that do not heal. Therefore, new studies could help us to establish the role of kinin peptides and especially of kinin B1R agonists in wound healing, allowing us in the future to identify new molecular targets that contribute to re-epithelialization and wound closure during chronic wound healing as it occurs in diabetic patients.
Acknowledgments
This work was supported by grant Nº DI19-0053 from Universidad de la Frontera, Temuco, Chile.
Glossary
- ACEI
Angiotensin Converting Enzyme Inhibitors
- BDKR1
Kinin B1 Receptor gene
- BDKR2
Kinin B2 Receptor gene
- B1R
B1 Receptor
- B2R
B2 Receptor
- BrdU
5-bromo-2’-deoxyuridine
- CD68
Cluster Differentiation 68
- c-Fos
proto-oncogen encoded by fos gene
- EGFR
Epidermal Growth Factor Receptor
- EC50
drug concentration required to produce 50% of maximal effect
- ERK1/2
Extracellular Signal Regulated Kinases 1 and 2
- FGF-2
Fibroblast Growth Factor-2
- HB-EGF
heparin-binding EGF-like growth factor
- IFN-γ
Interferon-gamma
- GF109203X
Protein Kinase C inhibitor
- IL
Interleukin
- KLKB1
Plasma Kallikrein gene
- KLK1
Tissue Kallikrein 1
- KLK5
Kallikrein-related peptidase 5
- KLK7
Kallikrein Related Peptidase 7
- MAPK
Mitogen-Activated Protein Kinases
- MCP-1
Monocyte Chemoattractant Protein-1
- MMP
Metalloprotease
- mRNA
messenger Ribonucleic Acid
- NF-κB
Nuclear Factor-kappa B
- PDGF
Platelet-Derived Growth Factor
- PKC
Protein Kinase C
- RT-PCR
Reverse Transcription-Polymerase Chain Reaction
- TGF-β
Transforming Growth Factor-beta
- TNF-α
Tumor Necrosis Factor-alpha
- Tyr
Tyrosine residue
- VEGF
Vascular Endothelial Growth Factor
Author Contributions
CEM and CDF designed and wrote the paper, CEM and KDB performed the literature search and edited the paper, CDF created the figures.
Additional Information
Co-author Kanti D. Bhoola, MD, PhD, died December 18, 2019.
References
- Bhoola KD, Figueroa CD, Worthy K. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol Rev. 1992;44(1):1–80. [PubMed] [Google Scholar]
- Fink E, Bhoola KD, Snyman C, Neth P, Figueroa CD. Cellular expression of plasma prekallikrein in human tissues. Biol Chem. 2007;388(9):957–63. [DOI] [PubMed] [Google Scholar]
- Simon M, Jonca N, Guerrin M, Haftek M, Bernard D, Caubet C, et al. Refined characterization of corneodesmosin proteolysis during terminal differentiation of human epidermis and its relationship to desquamation. J Biol Chem. 2001;276(23):20292–9. [DOI] [PubMed] [Google Scholar]
- Sotiropoulou G, Pampalakis G, Diamandis EP. Functional roles of human kallikrein-related peptidases. J Biol Chem. 2009;284(48):32989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RH, Hilton SM. Bradykinin formation in human skin as a factor in heat vasodilatation. J Physiol. 1958;142(2):219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Lipp B, Grunst J, Geiger R, Karl HJ. Determination of kallikrein by radioimmunoassay in human body fluids. Agents Actions. 1980;10(4):329–34. [DOI] [PubMed] [Google Scholar]
- Mayfield RK, Sens DA, Jaffa A, Margolius S. Studies of sweat kallikrein in normal human subjects. Adv Exp Med Biol. 1989;247B:649–55. [DOI] [PubMed] [Google Scholar]
- Hibino T, Takemura T, Sato K. Human eccrine sweat contains tissue kallikrein and kininase II. J Invest Dermatol. 1994;102(2):214–20. [DOI] [PubMed] [Google Scholar]
- Poblete MT, Reynolds NJ, Figueroa CD, Burton JL, Müller-Esterl W, Bhoola KD. Tissue kallikrein and kininogen in human sweat glands and psoriatic skin. Br J Dermatol. 1991;124(3):236–41. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Takata M, Otsuki N, Toyama T, Ohka R, Takehara K, et al. Expression and localization of tissue kallikrein mRNAs in human epidermis and appendages. J Invest Dermatol. 2003;121(3):542–9. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Tsuruta J, Kambara T. Interstitial-tissue localization of high-molecular-weight kininogen in guinea-pig skin. Biochim Biophys Acta. 1987;916:332–42. [DOI] [PubMed] [Google Scholar]
- Gao L, Chao L, Chao J. A novel signaling pathway of tissue kallikrein in promoting keratinocyte migration: activation of proteinase-activated receptor 1 and epidermal growth factor receptor. Exp Cell Res. 2010;316(3):376–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeb-Lundberg LM, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57(1):27–77. [DOI] [PubMed] [Google Scholar]
- Campos MM, Leal PC, Yunes RA, Calixto JB. Non-peptide antagonists for kinin B1 receptors: new insights into their therapeutic potential for the management of inflammation and pain. Trends Pharmacol Sci. 2006;27(12):646–51. [DOI] [PubMed] [Google Scholar]
- Marceau F, Regoli D. Bradykinin receptor ligands: therapeutic perspectives. Nat Rev Drug Discov. 2004;3(10):845–52. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu J, Li M, Dai S, Liang J, Ji W. The effect of kinin B1 receptor on chronic itching sensitization. Mol Pain. 2015;11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schremmer-Danninger E, Heinz-Erian P, Töpfer-Petersen E, Roscher AA. Autoradiographic localization and characterization of bradykinin receptors in human skin. Eur J Pharmacol. 1995;283(1-3):207–16. [DOI] [PubMed] [Google Scholar]
- Schremmer-Danninger E, Hermann A, Fink E, Fritz H, Roscher AA. Identification and occurrence of mRNAs for components of the kallikrein-kinin system in human skin and in skin diseases. Immunopharmacol. 1999;43(2-3):287–91. [DOI] [PubMed] [Google Scholar]
- Vidal MA, Astroza A, Matus CE, Ehrenfeld P, Pavicic F, Sanchez T, et al. Kinin B2 receptor-coupled signal transduction in human cultured keratinocytes. J Invest Dermatol. 2005;124(1):178–86. [DOI] [PubMed] [Google Scholar]
- Matus CE, Ehrenfeld P, Pavicic F, Sarmiento JM, Astroza A, Sanchez T, et al. Activation of kinin B1 receptor triggers differentiation of cultured human keratinocytes. Br J Dermatol. 2008;159(4):792–803. [DOI] [PubMed] [Google Scholar]
- Talwar HS, Fisher GJ, Voorhees JJ. Bradykinin induces phosphoinositide turnover, 1,2 diglyceride formation and growth in cultured adult human keratinocytes. J Invest Dermatol. 1990;95(6):705–10. [DOI] [PubMed] [Google Scholar]
- Schremmer-Danninger E, Töpfer-Petersen E, Fritz H, Roscher AA. Bradykinin-induced tyrosine phosphorylation of proteins in cultured human keratinocytes. Biol Res. 1998;31(3):189–98. [PubMed] [Google Scholar]
- Johnson RM, King KL, Morhenn VB. Comparison of second messenger formation in human keratinocytes following stimulation with epidermal growth factor and bradykinin. Second Messengers Phosphoproteins. 1992;14(1-2):21–37. [PubMed] [Google Scholar]
- Coutant KD, Ryder NS. Bradykinin upregulates immediate-early gene mRNA in human keratinocytes. Arch Dermatol Res. 1996;288(1):2–6. [DOI] [PubMed] [Google Scholar]
- Jung EM, Betancourt-Calle S, Mann-Blakeney R, Griner RD, Bollinger Bollag W. Sustained phospholipase D activation is associated with keratinocyte differentiation. Carcinogenesis. 1999;20(4):569–76. [DOI] [PubMed] [Google Scholar]
- Matus CE, Bhoola KD, Figueroa CD. Kallikreins and Kinin Receptors. Modulators of Skin Homeostasis In: Kinins M, Bader M. Berlin: De Gruyter; 2012. pp. 155–70. [Google Scholar]
- Papp H, Czifra G, Lázár J, Gönczi M, Csernoch L, Kovács L, et al. Protein kinase C isozymes regulate proliferation and high cell density-mediated differentiation in HaCaT keratinocytes. Exp Dermatol. 2003;12(6):811–24. [DOI] [PubMed] [Google Scholar]
- Le Panse R, Coulomb B, Mitev V, Bouchard B, Lebreton C, Dubertret L. Differential modulation of human fibroblast and keratinocyte growth by the protein kinase C inhibitor GF 109203X. Mol Pharmacol. 1994;46(3):445–51. [PubMed] [Google Scholar]
- Burrell HE, Simpson AW, Mehat S, McCreavy DT, Durham B, Fraser WD, et al. Potentiation of ATP- and bradykinin-induced [Ca2+]c responses by PTHrP peptides in the HaCaT cell line. J Invest Dermatol. 2008;128(5):1107–15. [DOI] [PubMed] [Google Scholar]
- Ellis S, Lin EJ, Tartar D. Immunology of wound healing. Curr Dermatol Rep. 2018;7(4):350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soley Bda S, Morais RL, Pesquero JB, Bader M, Otuki MF, Cabrini DA. Kinin receptors in skin wound healing. J Dermatol Sci. 2016;82(2):95–105. [DOI] [PubMed] [Google Scholar]
- Matus CE, Ehrenfeld P, Pavicic F, Gonzalez CB, Concha M, Bhoola KD, et al. Activation of the human keratinocyte B1 bradykinin receptor induces expression and secretion of metalloproteases 2 and 9 by transactivation of EGFR. Exp Dermatol. 2016;25(9):694–700. [DOI] [PubMed] [Google Scholar]
- Desposito D, Chollet C, Taveau C, Descamps V, Alhenc-Gelas F, Roussel R, et al. Improvement of skin wound healing in diabetic mice by kinin B2 receptor blockade. Clin Sci (Lond). 2016;130(1):45–56. [DOI] [PubMed] [Google Scholar]
- Côté J, Savard M, Bovenzi V, Belanger S, Morin J, Neugebauer W, et al. Novel kinin B1 receptor agonists with improved pharmacological profiles. Peptides. 2009;30(4):788–95. [DOI] [PubMed] [Google Scholar]
- Schremmer-Danninger E, Naidoo S, Neuof C, Valeske K, Snyman C, Sander C, et al. Visualisation of tissue kallikrein, kininogen and kinin receptors in human skin following trauma and in dermal diseases. Biol Chem. 2004;385(11):1069–76. [DOI] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. [DOI] [PubMed] [Google Scholar]
- Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, et al. Wound healing in MIP-1alpha(-/-) and MCP-1(-/-) mice. Am J Pathol. 2001;159(2):457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe MJ, Doetschman T, Greenhalgh DG. Delayed wound healing in immunodeficient TGF-β 1 knockout mice. J Invest Dermatol. 2000;115(1):3–11. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1(5):260–6. [DOI] [PubMed] [Google Scholar]
- Rawlingson A, Gerard NP, Brain SD. Interactive contribution of NK(1) and kinin receptors to the acute inflammatory oedema observed in response to noxious heat stimulation: studies in NK(1) receptor knockout mice. Br J Pharmacol. 2001;134(8):1805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri F, Bader M. Kinin B1 receptors as a therapeutic target for inflammation. Expert Opin Ther Targets. 2018;22(1):31–44. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld P, Millan C, Matus CE, Figueroa JE, Burgos RA, Nualart F, et al. Activation of kinin B1 receptors induces chemotaxis of human neutrophils human. J Leukoc Biol. 2006;80(1):117–24. [DOI] [PubMed] [Google Scholar]
- Ehrenfeld P, Matus CE, Pavicic F, Toledo C, Nualart F, Gonzalez CB, et al. Kinin B1 receptor activation turns on exocytosis of matrix metalloprotease-9 and myeloperoxidase in human neutrophils: involvement of mitogen-activated protein kinase family. J Leukoc Biol. 2009;86(5):1179–89. [DOI] [PubMed] [Google Scholar]
- Figueroa CD, Matus CE, Pavicic F, Sarmiento J, Hidalgo MA, Burgos RA, et al. Kinin B1 receptor regulates interactions between neutrophils and endothelial cells by modulating the levels of Mac-1, LFA-1 and intercellular adhesion molecule-1. Innate Immun. 2015;21(3):289–304. [DOI] [PubMed] [Google Scholar]
- Böckmann S, Paegelow I. Kinins and kinin receptors: importance for the activation of leukocytes. J Leukoc Biol. 2000;68(5):587–92. [PubMed] [Google Scholar]
- Catanzaro OL, Dziubecki D, Labal E, Sirois P. Activation of peritoneal macrophages during the evolution of type 1 diabetes (insulitis) in streptozotocin-treated mice. Peptides. 2010;31(10):1884–7. [DOI] [PubMed] [Google Scholar]
- Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, et al. Epithelialization in wound healing: a comprehensive review. Adv Wound Care (New Rochelle). 2014;3(7):445–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Mogford JE, Chao JD, Mustoe TA. Wound epithelialization deficits in the transforming growth factor-alpha knockout mouse. Wound Repair Regen. 2001;9(5):386–90. [DOI] [PubMed] [Google Scholar]
- Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 2005;118(Pt 11):2363–70. [DOI] [PubMed] [Google Scholar]
- Mejia AJ, Matus CE, Pavicic F, Concha M, Ehrenfeld P, Figueroa CD. Intracellular signaling pathways involved in the release of IL-4 and VEGF from human keratinocytes by activation of kinin B1 receptor: functional relevance to angiogenesis. Arch Dermatol Res. 2015;307(9):803–17. [DOI] [PubMed] [Google Scholar]
- Bauvois B. New facets of matrix metalloproteinases MMP-2 and MMP-9 as cell surface transducers: outside-in signaling and relationship to tumor progression. Biochim Biophys Acta. 2012;1825(1):29–36. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, Zheng R, Kenney J, Van De Water L. Integrin-mediated regulation of epidermal wound functions. Cell Tissue Res. 2016;365(3):467–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Chang JT, Hsu SF, Li TM, Cho DY, Huang CY, et al. Bradykinin enhances cell migration in human chondrosarcoma cells through BK receptor signaling pathways. J Cell Biochem. 2010;109(1):82–92. [DOI] [PubMed] [Google Scholar]
- Kramarenko II, Bunni MA, Raymond JR, Garnovskaya MN. Bradykinin B2 receptor interacts with integrin alpha5beta1 to transactivate epidermal growth factor receptor in kidney cells. Mol Pharmacol. 2010;78(1):126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricupero DA, Romero JR, Rishikof DC, Goldstein RH. Des-Arg(10)-kallidin engagement of the B1 receptor stimulates type I collagen synthesis via stabilization of connective tissue growth factor mRNA. J Biol Chem. 2000;275(17):12475–80. [DOI] [PubMed] [Google Scholar]
- Catalán M, Smolic C, Contreras A, Ayala P, Olmedo I, Copaja M, et al. Differential regulation of collagen secretion by kinin receptors in cardiac fibroblast and myofibroblast. Toxicol Appl Pharmacol. 2012;261(3):300–8. [DOI] [PubMed] [Google Scholar]
- Howdieshell TR, Callaway D, Webb WL, Gaines MD, Procter CD., Jr Antibody neutralization of vascular endothelial growth factor inhibits wound granulation tissue formation. J Surg Res. 2001;96(2):173–82. [DOI] [PubMed] [Google Scholar]
- Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152(6):1445–52. [PMC free article] [PubMed] [Google Scholar]
- Bader M. Kallikrein-kinin system in neovascularization. Arterioscler Thromb Vasc Biol. 2009;29(5):617–9. [DOI] [PubMed] [Google Scholar]
- Wolf R, Tamir A, Brenner S. Psoriasis related to angiotensinconverting enzyme inhibitors. Dermatologica. 1990;181(1):51–3. [DOI] [PubMed] [Google Scholar]
- Ozkur M, Erbagci Z, Nacak M, Tuncel AA, Alasehirli B, Aynacioglu AS. Association of insertion/deletion polymorphism of the angiotensin-converting enzyme gene with psoriasis. Br J Dermatol. 2004;151(4):792–5. [DOI] [PubMed] [Google Scholar]
- Winkelmann RK. Total plasma kininogen in psoriasis and atopic dermatitis. Acta Derm Venereol. 1984;64(3):261–3. [PubMed] [Google Scholar]
- Marshman G, Burton GL, Archer CB. Comparison of the actions of kallidin and bradykinin in the skin of normal and psoriatic subjects. Clin Exp Dermatol. 1996;21(2):112–5. [PubMed] [Google Scholar]
- Komatsu N, Saijoh K, Kuk C, Shirasaki F, Takehara K, Diamandis EP. Aberrant human tissue kallikrein levels in the stratum corneum and serum of patients with psoriasis: dependence on phenotype, severity and therapy. Br J Dermatol. 2007;156(5):875–83. [DOI] [PubMed] [Google Scholar]
- Pietrovski EF, Paludo KS, Mendes DA, Guimaraes F S, Veiga SS, Buchi D F, et al. B1 and B2 kinin receptor participation in hyperproliferative and inflammatory skin processes in mice. J Dermatol Sci. 2011;64(1):23–30. [DOI] [PubMed] [Google Scholar]
- Hosogi M, Schmelz M, Miyachi Y, Ikoma A. Bradykinin is a potent pruritogen in atopic dermatitis: a switch from pain to itch. Pain. 2006;126(1-3):16–23. [DOI] [PubMed] [Google Scholar]
- Feng J, Chen Y, Xiong J, Chen X, Liang J, Ji W. The kinin B1 receptor mediates alloknesis in a murine model of inflammation. Neurosci Lett. 2014;560:31–5. [DOI] [PubMed] [Google Scholar]
- Costa R, Manjavachi MN, Motta EM, Marotta DM, Juliano L, Torres HA, et al. The role of kinin B1 and B2 receptors in the scratching behaviour of induced by proteinase-activated receptor-2 agonists in mice. Br J Pharmacol. 2010;159(4):888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]