Abstract
Ultraviolet radiation (UVR) exposure is well established as the major environmental risk factor for the development of melanoma, cutaneous squamous cell carcinoma (cSCC), and basal cell carcinoma (BCC). Additional risk factors including genetic mutations, other environmental agents, and immune status are important in modulating the effects of UVR. Dermatologists advocate a multi-pronged approach to minimizing UVR exposure including lifestyle modifications, UVR protective clothing, and topically applied sun-protective products, i.e. sunscreen. New Federal Drug Administration (FDA) regulations on sunscreen have brought certain long-standing ingredients in sunscreen products under scrutiny. The FDA’s proposed rule for over the counter (OTC) monograph states that the inorganic sunscreens, zinc oxide and titanium dioxide, were found to be “generally recognized as safe and effective,” but cite insufficient evidence to grant organic sunscreens the same designation. This proposed rule by the FDA and our increasing understanding of multifactorial mechanisms of UVR damage are an impetus for innovation and advances in sun protective technology. A complete set of strategies designed to limit the risk of UV-induced skin cell malignant transformation and tumor development must address the fuller consideration of genetic, environmental, and immune factors that cooperatively drive cutaneous carcinogenesis. Recent advances in our understanding of the biochemical processes underpinning UVR associated cutaneous cellular damage, genotoxicity, and clonal expansion provide investigators with a spectrum of opportunities for technologic innovation in the prevention of skin cancer. Strategies to improve upon current topical sunscreen formulations have strived for broader UVR spectral coverage, more favorable aesthetics, increased adherence, and minimal penetration into the living epidermis. In addition to improved sunscreens, future topical therapies may target processes within the epidermis that contribute to carcinogenesis. These include reactive species quenching, delivery of DNA repair enzymes, and targeting of cytokines essential to the proliferation of mutant keratinocytes.
Keywords: Sunscreen, skin cancer, ultraviolet radiation, photobiology
Ultraviolet Radiation as a Risk Factor for Skin Cancer
Cancers that derive from the skin’s keratinocytes and melanocytes comprise by far the most commonly diagnosed malignancies in the United States [1]. There are an estimated one million cases of the keratinocyte-derived skin cancers (KDSC), including cSCC and BCC, and seventy-six thousand cases of cutaneous melanoma detected each year, with rates of each increasing annually [2,3]. Although the root causes of these increased rates have not been fully established, investigators have implicated an aging population, lifestyle changes, increased cumulative UVR exposure, and depletion of the atmosphere’s ozone layer as potential contributors [1-3].
KDSCs have relatively low rates of metastasis and subsequent mortality, but their high prevalence and potential for local invasion results in over one million procedures in the United States each year, costing the economy an estimated six hundred million dollars [4]. Additionally, despite low rates of metastasis, the high prevalence of cSCC results in an estimated three to eight thousand deaths in the United States annually [5]. Melanoma has higher patient mortality, with over ten thousand deaths each year, and an additional estimated financial burden of one hundred million dollars annually [2].
Exposure to ultraviolet radiation (UVR) in varying forms has been shown to be the primary environmental risk factor for the development of these malignancies. Chronic exposure to UVR is strongly associated with the most common forms of KDSCs in a dose-dependent fashion, while intermittent high doses of UVR (i.e. sunburns) have been more strongly linked to the development of melanoma and BCC [1,6].
UVR may be separated into four ranges – UVA1 (340-400 nm), UVA2 (320-340 nm), UVB (280-320 nm), and UVC (200-280 nm) – with the majority of UVR reaching the skin’s surface falling into the former three categories due to the filtering effects of atmospheric ozone [7]. Exposure to UVR damages epidermal DNA through multiple mechanisms [8]. Direct damage occurs when DNA itself acts as a photophore and absorbs energy from incident UVR. DNA has an absorption maximum in the UVC region at approximately 260nm, with substantial absorption in the UVB region and in the UVA regions as well [9]. Because minimal UVC reaches the Earth’s surface, the majority of direct DNA damage is attributed to radiation in the UVB spectrum [10]. Absorption of energy in these wavelengths induces characteristic photoproducts, the most common of which are the cyclobutane pyrimidine dimers (CPDs) [11]. CPDs are formed between C-4 and C-5 carbon atoms of two adjacent pyrimidines, with double bonds becoming saturated to produce a four-member ring [9]. Subtypes of CPDs include thymine-thymine (T=T), cytosine-cytosine (C=C), thymine-cytosine (T=C), and cytosine-thymine (C=T). When nucleotide excision enzymes fails to repair these alterations and DNA polymerases attempt to replicate the structurally altered DNA, the polymerases insert adenines opposite these bulky photoproducts [12]. In the case of T=T dimers, there are no resulting mutations, as A is normally paired with T. However, in the case of C=C CPDs, a CC>TT transition occurs, resulting in a mutated DNA sequence. Thus, areas of the genome with a high frequency of adjacent pyrimidines are considered “UV hotspots” and show high rates of C>T and CC>TT “UV signature” mutations. The p53 tumor suppressor gene, for example, is mutated in up to 90% of human cSCCs, with the predominant alterations being C>T and CC>TT alterations [13]. While UVA is a less potent mutagen, natural sunlight contains 20-100 fold more UVA leading to increased dose compared to UVB. In addition, UVA is less filtered by car windows and protective clothing and penetrates deeper into the epidermis due to its longer wavelength. Increased penetration also contributes to dermal changes that result in photoaging of the skin.
Recent work by Martincorena et al. showed that such cutaneous mutations are common even in healthy-appearing sun-exposed skin. Ultradeep sequencing of eyelid biopsies revealed a somatic mutation burden of two to six mutations per megabase per cell, similar to that seen in many cancers [14]. Furthermore, the data suggests that keratinocytes with tumor driver mutations (TP53, NOTCH1, NOTCH2, FAT1, and RBM10) are positively selected for in these skin samples [10]. Of note, direct absorption of UVR by DNA can also result in the less common 6,4-photoproduct, which is more readily repaired by mammalian cells [15].
Indirect damage, in contrast, begins when a photophore in the epidermis other than DNA is excited by UVR. Such endogenous molecules include tryptophan, riboflavin, porphyrins, and melanin, among others [16,17]. When these molecules transition from a UVR-excited state back to resting state, energy may be transferred to DNA, causing structural changes indistinguishable from those of direct DNA damage. Alternatively, energy may be transferred to other molecules in the epidermis, ultimately generating reactive oxygen species (ROS) [18]. ROS are highly reactive derivatives of oxygen that include singlet oxygen, hydrogen peroxide, and the superoxide radical ion, among others [11]. These highly reactive molecules, in addition to altering proteins and lipids in the epidermis, are capable of causing single-strand DNA breaks and the oxidized guanine product 8-hydroxydeoxyguanosine [19].
Recently, D. Brash and colleagues described a novel mechanism of indirect DNA damage mediated by melanin [9]. This group showed that following UVR exposure, CPDs continue to form hours after the initial UVR insult, a phenomenon they term “dark CPD formation.” UVR results in increases in superoxide and nitric oxide, which degrade the polymer melanin into high-energy monomers that may enter the nucleus and transfer their triplet excitation state energy to DNA, inducing CPDs, long after the initial exposure event. Quenchers of these excited melanin monomers, including ethyl sorbate and tocopherol-alpha, may help limit this pathway of DNA damage in the hours following exposure [16].
Other Environmental Risk Factors for Skin Cancer
Strong consideration should also be given to the potential of exogenous chemical agents present in the environment to find their way into the skin and potentiate UV-damage pathways. Most relevant is the diverse class of the industrial byproduct family of polyaromatic hydrocarbon (PAH) compounds that are byproducts of incomplete combustion [20]. These include benzo[a]pyrene and benzathracene and their derivatives that are ubiquitous in air and ground water of industrialized regions as well as in cigarette smoke and charbroiled meats [20]. Laboratory evidence strongly suggests that systemic and transcutaneous absorption may lead to low levels of PAHs in the skin that synergistically with UV provoke direct mutagenesis, and/or enhance indirect cellular damage and genotoxicity via UV potentiation of PAH chemoexcitation states and generation of ROS intermediates [21-23]. While clinical evidence for the fuller effects of exogenous PAH in exacerbating skin cancer risk have not been fully substantiated, the historical landmark finding of Percival Pott’s identification of the association of chimney soot exposure with scrotal cSCC in chimney sweeps over 200 years ago may yet prove relevant today [24]. Certainly, animal studies abound in the carcinogenic synergy of UV exposure and mutagenic PAHs [21-23]. For example, smoking simulation has synergistically provoked UV-induced human skin carcinogenesis in xeno-transplanted mice [25,26]. An estimated forty-two million individuals in the United States currently smoke cigarettes [27], the smoke of which contain sixty known carcinogens, ten of which are polyaromatic hydrocarbons [28]. According to the largest meta-analysis to date, cigarette smoking is a significant risk factor for the development of cSCC (OR 1.52, 95% CI 1.15-2.01) [29].
Interestingly, the vast majority of chemical UV filters also owe their UV protective capacity to aromatic hydrocarbons where energy absorption results in excitation states of electrons. Therefore, such agents that penetrate into skin cells may also generate reactive species after UV exposure, similar to other PAHs with established risk profiles [30-33]. Titanium dioxide also has the potential to release reactive oxygen species into tissue when exposed to UVR. The titanium dioxide nanoparticles used in sunscreen products are coated with a nonreactive chemical to prevent the release of ROS and prevent oxidative damage to the tissue.
Genetic Risk Factors for Skin Cancer
Genome-wide associated studies (GWAS) have also implicated a number of inherited genetic factors in the development of cutaneous malignancies. Genes associated with nevus count (CDKN2A0MTAP, PLA2G6, and TERT) and pigmentation (SLC45A2, TYR, MC1R, and ASIP) have been tied to melanoma risk [34]. Many of the same pigmentation and nevus count associated genes have been linked to BCC risk, as have the hedgehog pathway genes PTCH1, PTCH2, and SUFU, which are known to cause Gorlin Syndrome (basal cell nevus syndrome) [35]. Recent GWAS studies have also implicated MYCN, ZFHX4, CASP8, and GATA3 as risk factors for BCC development [35]. Genetic factors increasing the risk of cSCC include MC1R, DEF8, IRF4, TYR, XRCC1, CTLA-4, UBAC2, and EXOC2 [36]. Inactivating MC1R polymorphisms, known to increase the risk of BCC, cSCC, and melanoma, increase the concentration of pheomelanin, which is a potent generator of the high-energy melanin monomers responsible for “dark dimer” formation [16].
Several prescription medications have also been linked to DNA damage and photocarcinogenesis. Voriconazole induces facial erythema and actinic cheilitis in 8-10 percent of patients and has been linked to increased risk of both cSCC and melanoma [37]. Both voriconazole and its primary metabolite voriconazole N-oxide induce free radicals following UVR excitation, representing a potential mechanism for photocarcinogenesis facilitation [38]. Calcineurin inhibitors promote the progression of cSCC and BCC through immunosuppression as well as inhibit CPD repair and apoptosis by human keratinocytes following UVB exposure [39].
Approaches to Minimizing UVR Exposure
Given that UVR is the major environmental risk factor for the development of skin cancers – as well as for sunburn and signs of photoaging that include rhytides, telangiectasia, and dyspigmentation – dermatologists employ a multi-pronged approach to minimizing UVR exposure [40]. Strategies to minimize UVR exposure include lifestyle modifications, UVR protective clothing, and topically applied sun-protective products, i.e. sunscreen.
Behavior Modification
In 2009, the World Health Organization’s (WHO) International Agency for Research on Cancer (IARC) reclassified indoor tanning as a group 1 carcinogen, placing it alongside cigarette smoke within the most potent group of carcinogens [41]. A recent meta-analysis found that more than 450,000 NMSC cases and more than 10,000 melanoma cases can be attributed to indoor tanning in the United States, Europe, and Australia [42]. Although indoor tanning rates have decreased over recent years, there were nonetheless an estimated 7.8 million women and 1.9 million men engaged in indoor tanning in the United States in 2015 [43]. Rates are disproportionally high in some of the most vulnerable populations in the United States, including those demonstrating tobacco and alcohol dependence [43-45]. In addition to being a carcinogen, tanning has been implicated as an addictive disorder. Some groups have suggested tanning is a type of substance-related disorder, adapting scales for alcohol dependence to study the phenomenon [46]. Other groups have begun to study the genetic factors that may predispose individuals to tanning addiction [47]. Though the precise physiology underlying UV addiction is an area of active research, UVR-induced synthesis of beta-endorphin (a cleavage product of keratinocyte-produced proopiomelanocortin) has been implicated as a key driver of this behavior [48]. Interventions to decrease rates of tanning have included restriction of minors’ access to indoor tanning, excise taxes on indoor tanning, and physician counseling regarding the risks of UVR exposure [49]. In addition to the strict avoidance of recreational tanning, the American Academy of Dermatology formally recommends that patients try to avoid outdoor activities during the hours of 10am until 4pm [49]. Although some have suggested that sun exposure is vital for health benefits including vitamin D synthesis and release of endogenous endorphins to combat seasonal affective disorder, vitamin D levels can be maintained within the normal range with dietary supplementation [50] and mood-boosting effects may be experienced with full-spectrum visible light [51].
Sun-protective Clothing
Clothing was used as a strategy for sun protection well before the advent of commercial sunscreens [52]. The field of fabric photoprotection, however, has substantially evolved over the past several years. Key to the study of fabric photoprotection is the concept that woven textiles inevitably contain small spaces (so-called interstices) between fibers through which UVR may permeate [52]. As a general rule, fabric must cover 94% of an area (i.e. 6% or less interstices by area) to achieve a UV protection factor (UPF) of 15 [52]. UPF refers to the ratio of average effective UV irradiance through air to the average effective UV irradiance transmitted through a fabric in question [53]. Although UPF is an in vitro measurement, studies have shown that UPF closely correlates with SPF when fabrics are subjected to in vivo testing by measuring minimal erythema dose with and without fabric protection [54]. In addition to minimizing interstices, approaches to improve the UPF of sun-protective clothing include the addition of a variety of UV absorbers to laundry detergents and the use of UV-absorbing dyes [55].
Topically Applied Sunscreen Formulations
Commercially available sun-protective topical formulations utilize active agents that fall into two major classes: organic molecules that principally absorb UVR energy, and inorganic (or mineral-based physical) molecules that additionally reflect UVR. Organic sunscreen agents (including PABA and derivatives, cinnamates, benzophenones including oxybenzone, avobenzone, octocrylene, salicylates including homosalate, and octisalate among others) are molecules that typically contain one or more aromatic rings, capable of absorbing and distributing energy from incident UVR [56]. Inorganic sunblocks (titanium dioxide and zinc oxide) also absorb UVR, though this effect is superimposed with a second mechanism of scattering incident UVR [57,58].
Although inorganic sunscreens are popular for their lesser penetration into the living epidermis (Langerhans cells, keratinocytes, and melanocytes), and thus a lower risk of inducing allergic contact reactions, their property of scattering light results in formulations with a propensity to leave a whitish hue on the patient’s skin, making them less cosmetically pleasing [59]. Tinting these formulations with universal skin tone tints helps to counter the whitish hue and improve cosmesis. In addition, micronization technology has allowed for the manufacturing of smaller zinc oxide and titanium dioxide particles, reducing the intensity of the whitish hue and improving cosmetic favorability [60]. This micronization process, however, raises some concern for increasing these particles’ deposition within hair follicles and increasing their penetration into the living epidermis [61]. To date, no study has demonstrated significant penetration into tissue of micronized particles and the inorganic sunscreens have been deemed “generally recognized as safe and effective” by the FDA. Organic sunscreens carry a higher risk of inducing an irritant or allergic contact dermatitis, but in general are more cosmetically appealing and continue to be the more popular products on the market today [60].
Benefits of Sunscreen
Despite past raised concerns, clinical trials have shown that modern sunscreen formulations are effective in the prevention of both invasive melanoma and cSCC. Studies designed to assess the benefits of sunscreen as an intervention are complicated by confounding variables and variable compliance. The long latency between UVR exposure and the development of KDSC also complicates effective analysis. The largest randomized controlled trial to date was conducted by Green et al. and randomized 1,621 individuals to either a broad-spectrum SPF-15 sunscreen (8% octinoxate with 2% avobenzone) or no daily sunscreen application [62]. The first publication of the trial showed a statistically significant decrease in the total number of cSCCs (RR=.61, 95% CI .46-.81), but was unable to detect a decrease in the rate of BCC. The same trial was analyzed several years later for the development of melanoma [63]. Although it did not show a decrease in the total number of melanomas, it did show a decrease in the development of invasive melanoma (HR = .27, 95% CI .08-.87). It is important to note that combining avobenzone and octinoxate, as was done in the sunscreen used in this trial, may result in increased photodegredation of the highly photolabile avobenzone [64]. Some studies have suggested a paradoxical increased risk of melanoma for those who use sunscreen [65,66]. This effect may be due to the fact that sunscreen users spend increased time in the sun [67] or because these users had insufficient UVA spectral coverage, resulting in continued UVA damage in the absence of behavior-modifying sunburns from UVB. Notably, Westerdahl et al. found this increased risk of melanoma with sunscreen use to only hold true for those who used sunscreen with an SPF below 10 [65]. Sunscreen has also been shown to reduce chronic signs of photoaging (e.g. epidermal and dermal atrophy, loss of elastic fibers, dyspigmentation, rhytides, telangiectasia) as well as sun-induced erythema [68]. In addition to the limitation of sun-induced acute erythema, sunscreen is a vital component of clinical management in patients with photo-aggravated dermatoses (e.g. lupus erythematosus, dermatomyositis, actinic dermatitis, rosacea, and polymorphous light eruptions, among others) [69].
Optimizing Sunscreen: Advances in Sunscreen Technology
The ideal sunscreen would have broad protection over the spectrum of UVR, be cosmetically favorable, need be applied only once daily, and have minimal penetration into the living epidermis so as to ameliorate the risk for contact hypersensitivity and to minimize off-target effects such as UV-induced ROS generation (Table 1).
Table 1. Characteristics of the Ideal Sunscreen.
| Goal | Benefit | Strategies |
| 1. Broad Spectrum Coverage | - Prevent direct and indirect DNA damage | - Development of novel sunscreens with broad UVA, UVB, & UVC absorption spectra - Development of formulations that improve the photostability of sunscreen compounds |
| 2. Cosmetic Favorability | - Improve patient compliance | - Micronization of inorganic sunscreens to prevent light scattering - Use of organic sunscreens that do not scatter incident light |
| 3. Once-Daily Application | - Prevent the need for regular reapplication to provide protection and improve adherence | - Development of polymeric additives that improve water-resistance - Encapsulation of sunscreens into bioadhesive nanoparticles that are removed only with towel drying |
| 4. Minimal Penetration into the Living Epidermis | - Prevent ROS formation that can cause DNA damage - Prevent hypersensitivity reactions |
- Encapsulation of sunscreens into polymeric nanoparticles - Encapsulation of sunscreens into mesoporous particles - Use of inorganic sunscreen compounds that have minimal penetration but other limitations (poor cosmesis) - Use of compounds that prevent the formation of or neutralize ROS |
Broad-spectrum Coverage
Sunscreen was originally developed in the early 20th century as a means of protecting against sunburn, primarily a UVB-mediated phenomenon [70]. It was not until years later that it became clear that radiation in the UVA spectrum also contributes to carcinogenesis and clinical signs of skin aging that include dyspigmentation, wrinkling, and telangiectasia [71]. As this became clear, the sunscreen industry began to introduce UVA-absorbing compounds, notably avobenzone. The FDA now requires the testing of sunscreen for coverage of both UVA and UVB radiation via spectrophotometry [72]. Sunscreens must show coverage for both types of UVR to be labeled as “broad spectrum.” Unfortunately, avobenzone is extremely sensitive to destruction after UVR [73]. Several compounds have been developed as additives to absorb energy from avobenzone once it reaches its activated state, improving its photostability [73]. Compounds capable of improving the stability of avobenzone include oxybenzone and diethylhexyl-2,6-naphthalate (DEHN) [73]. Ecamsule, a benzylidene camphor derivative, is approved in the United States and is a UVA filter with strong photostability [74]. Due to patent exclusivity (L’Oréal Paris), ecamsule is available only in limited formulations. As changes in the ozone layer progress, UVC may be of increasing importance, and sunscreens compounds are now being developed to target this range of the spectrum as well [75].
Though existing sunscreens protect primarily against UVA2, UVA1 has also recently been implicated as mutagenic. UVA1 induces ROS and promotes the formation of CPDs [76,77]. UVA1-induced ROS and CPDs accumulate preferentially in basal keratinocytes and dermal fibroblasts, implicating these wavelengths in the processes of photoaging and KDSC development [78].
Cosmesis
Consumers have been faced with the choice between cosmetically favorable organic sunscreens that have a risk of inducing irritant or more rarely allergic contact dermatitis, and inorganic sunblocks that are less likely to induce these reaction but are cosmetically less favorable due to their property of scattering incident light and causing a whitish hue on the skin. Technology in micronization has allowed the synthesis of smaller particles of zinc oxide and titanium dioxide, improving the cosmetic profile of these sunblocks with minimal risk of irritant or hypersensitivity reactions (secondary to their low rates of penetration into the living epidermis) [60]. In addition, universally flattering tints have been added to physical blocks to minimize the whitish hue and improve cosmesis. However, Fitzpatrick phototype IV have difficulty tolerating the skin appearance with inorganic sunscreens. New approaches have attempted to bioengineer organic sunscreens to lessen their penetration of the active ingredients through the stratum corneum [79].
Frequency of Application
Public health organizations, including the WHO, recommend that sunscreen be re-applied every 2 to 3 hours, though some authors have pointed out that re-application as frequently as every twenty minutes can significantly enhance UVR protection in real-world testing [80]. The earliest innovations in frequency of application involved the addition of a variety of compounds to make sunscreen water-resistant (e.g. via the utilization of polymers including acrylates/ polytrimethylsiloxymethacrylate, butylated BVP, among others) [73]. Current efforts are focused toward creating sunscreen particles that can covalently bond to the stratum corneum [79].
Penetration into the Living Epidermis
Chemical sun-protective compounds penetrate the stratum corneum and come in contact with the living epidermis [32,81,82]. Organic sunscreen agents have also been identified in the urine, semen, and breast milk after topical application [83,84]. Concerns have been raised about the impact of systemically absorbed organic sunscreen on the endocrine, reproductive, developmental systems as well as concerns related to links to carcinogenesis. The FDA recently issued a proposed rule for over the counter (OTC) sunscreens that states that the inorganic sunscreens, zinc oxide and titanium dioxide, were found to be “generally recognized as safe and effective.” In contrast, it was determined that for 12 organic sunscreens (cinoxate, dioxybenzone, ensulizole, homosalate, meradimate, octinoxate, octisalate, octocrylene, padimate O, sulisobenzone, oxybenzone, and avobenzone), there was insufficient evidence to grant the “generally recognized as safe and effective” designation and more data was requested from manufacturers [85].
Following the FDA proposed rule, Matta et al. reported findings from an open-label, randomized study with 24 subjects investigating the pharmacokinetics of systemic absorption of organic sunscreens [86]. They investigated the degree of systemic absorption after “maximal use” of avobenzone, oxybenzone, octocrylene, and ecamsule. Maximum use was defined as application 4 times per day onto 75% body surface area. The authors found measurable levels of all tested sunscreen ingredients that were above the FDA determined level of 0.5 ng/ml, which would require systemic safety testing. In addition, while levels surpassed the 0.5ng/ml threshold by Day 1, they continued to increase from Day 1 to Day 4 suggesting extended half-life. The FDA sought to validate these results and performed a randomized clinical trial with 48 healthy participants applying one of four sunscreen products containing six active ingredients (avobenzone, oxybenzone, octocrylene, homosalate, octisalate, and octinoxate) applied with maximum use at 2 mg/cm2 to 75% body surface area at 0 hours on day 1 and 4 times daily at 2-hour intervals on days 2-4 [86]. Mean plasma concentrations for all six active ingredients were greater than the 0.5 ng/ml threshold after a single application, confirming data from Matta et al. [86]. All six active ingredients maintained high levels in the skin for at least 4 days after the last application. While concentrations for avobenzone, octocrylene, homosalate, octisalate, and octinoxate ranged from 7-50x the threshold concentration, oxybenzone was detected at levels 500x above the 0.5ng/ml threshold [87]. The relevance of these levels remains unknown. There is no evidence to suggest that serum concentrations that were found confer any risk to human health.
It is presumed that the greater penetration of organic sunscreen agents is responsible for their higher rates of inducing a hypersensitivity reaction. After organic sunscreen compounds penetrate into the living epidermis, UV irradiation of these molecules may produce neoantigens, ultimately inducing a photoallergic contact dermatitis [88]. Additionally, several sunscreen compounds, though they protect against the formation of UV-signature CPDs, have been shown to paradoxically increase the rates of reactive oxygen species resulting in oxidative DNA damage, both in vitro and in vivo, after UV exposure [30,31,33,89-92]. Some organic sunscreen compounds have also been reported to modulate estrogenic activity, though the clinical relevance of this remains unclear [93-96].
Inorganic sunscreens have been linked to frontal fibrosing alopecia (FFA) and the proposed mechanism is penetration of nanoparticles into the pilosebaceous unit causing a lichenoid reaction [96]. However, data to support this hypothesis is limited. This theory is supported by four questionnaire based studies that showed an increase in reported sunscreen use in patients with FFA [97-101]. These studies are limited by confounding variables such as recall bias and systematic differences in the populations that use sunscreen and those that do not. In addition, titanium species have been found in the hair shafts of patients with FFA but were also present in control cases [102]. It remains to be established if a true connection exists.
For all these reasons, some groups have worked toward developing sunscreen compounds that adhere to the stratum corneum and have minimal penetration through the stratum corneum. Recent successful approaches include encapsulation of organic sunscreen compounds into mesoporous silica [46] and the encapsulation of organic sunscreen into bioadhesive nanoparticles with aldehyde-rich surfaces that covalently bond to free proteins on the stratum corneum [79,103]. Additionally, several sunscreen products now contain antioxidant molecules (resveratrol, ascorbic acid, tocopherol-alpha, among others) that have the potential to absorb free radicals and prevent their potential damage [104-107], as well as inhibit the degradation of the active chemical sunscreen agents to improve their shelf-life and performance.
Photochemoexcitation and Antioxidants
Endogenous antioxidants inhibit oxidative stress from environmental triggers such as UVR and pollutants by scavenging toxic free radicals and limiting ROS-induced skin damage [108,109]. Topical applications of antioxidants have been suggested to have numerous anti-aging, anti-inflammatory, photoprotective, and UVR immunosuppression preventative effects [108]. Examples of natural antioxidants include ubiquinone or idebenone (CoQ10), alpha-lipoic acid, L-ascorbic acid (vitamin C), and alpha-tocopherol (vitamin E). In theory, the addition of antioxidants to sunscreen formulations could protect against the UVR-induced oxidative stress that could not be prevented by UVR absorption or reflectance [108,110]. Topical antioxidant formulations have been shown to decrease the number of sunburn cells, acutely damaged keratinocytes evident on histologic examination in UVR-irradiated skin [109]. Syring et al. found that the addition of various antioxidants, specifically bis-ethylhexyl hydroxydimethoxy benxylmalonate (HDBM), did not affect the amount of free radicals produced upon UVR exposure to skin as measured by the radical formation ratio, but did produce a statistically significant increase in the measured SPF when added to both chemical and physical UVR blockers [111]. However, the efficacy of antioxidants varies greatly depending on the type of antioxidant studied and the outcome measure assessed. For example, McDaniel et al. found that ascorbic acid demonstrated high radical scavenging capacity as detected by photochemiluminescence, but had no effect of percentage of sunburn cells seen on biopsy of UVR-irradiated human skin [109].
While antioxidants may prevent cancer-causing mutations mediated by ROS, the presence of antioxidants after established tumorigenesis may paradoxically accelerate tumor growth. Burke et al. found that twice weekly applications of topical selenomethionine delayed the development of cSCC in a mouse model [112]. Cassidy et al. applied the same rationale to a melanoma mouse model and found that topical application of selenomethionine delayed tumor development, but once tumors were established, their growth accelerated in the presence of this antioxidant compound [113]. This highlights a major controversy with antioxidants in that in early tumorigenesis they may act to decrease ROS-mediated DNA damage, but once the tumor has been established, antioxidants may fuel tumor growth by mitigating oxidative stress on tumor cells. More research is needed to identify safe antioxidant preparations that provide substantiated benefit against cutaneous photodamage and negligible promotion of tumorigenesis once established.
Notably, systemic administration of nicotinamide is a promising approach for decreasing the rate of new KDSCs in high-risk patients. Nicotinamide (vitamin B3) has been shown to have protective effects against UVR and reduce the number of precancerous actinic keratoses. In a large phase three placebo-controlled trial, patients at high risk for KDSC, specifically patients with a history of at least two KDSC in the past 5 years, were randomized to receive either 500mg of nicotinamide twice daily or matched placebo [114]. The treatment was well tolerated with no significant difference in adverse events between nicotinamide group vs control. The number of actinic keratoses was 11% lower in the nicotinamide group (p=0.01) at 3 months and 13% at 12 months (p=0.001). The rate of cSCC was 30% lower in the nicotinamide group at 12 months (p=0.05). The number of BCC per person in the nicotinamide group was 20% lower than placebo at 12 months, but this trend did not reach significance (P=0.12). It is important to note that the study population was a high-risk population with overall average of eight KDSC in the past 5 years. It is unclear how this data generalizes to an average risk population or for patients that have no personal history of skin cancer. In addition, the efficacy of nicotinamide for additional high-risk populations, i.e. immunosuppressed patients, needs to be validated.
Environmental Concerns Related to Organic Sunscreens
In 2018, Hawaii became the first state to ban sunscreens containing ingredients thought to harm coral reefs. The bill will go into effect in January 2021. The ingredients included are the chemical sunscreens, oxybenzone and octinoxate. This landmark bill was born out of years of research suggesting that organic sunscreens were contributing to coral reef bleaching. While the actual quantity of UV filter residues that enter the oceans is unknown, it is estimated that the number could range from 4,000-14,000 tons [90,115]. These compounds have relatively short half-lives, but their constant re-introduction into the seawater leads to concern for a persistent environmental threat. This is particularly a concern for areas that already have a declining reef ecosystem from other environmental threats, such as changes in weather patterns (i.e. El Niño), ocean warming, agricultural run-off, and sewage dumping. The degree that organic sunscreens are contributing to coral reef bleaching is unknown. Studies have assessed the levels present in seawater, but have also looked at bioaccumulation within organisms. Horricks et al. found the presence of organic sunscreens within lionfish residing in Caribbean reefs [116]. In this study, oxybenzone was the most represented compound. Other studies have identified additional UV filters such as 4-methylbenzylidene camphor, octocrylene, and octinoxate in various species of fish worldwide, which has possible consequences for the food chain [117]. However, further studies are needed to determine the actual risk to ocean ecosystems and no evidence to date has shown a threat to human health from marine exposure through the food chain.
Novel Approaches
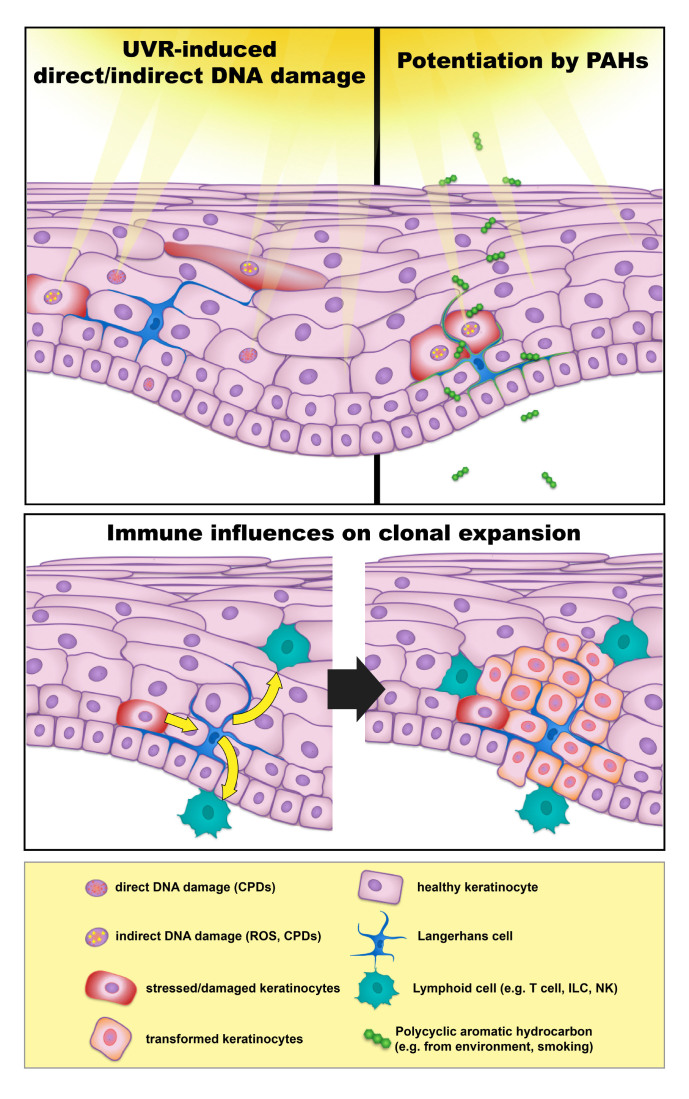
Traditional sunscreens have focused on the very first step of epidermal carcinogenesis: exposure of keratinocytes to UVR. One may consider carcinogenesis, however, as a series of events that begin with UVR and includes direct and indirect mechanisms of DNA damage, failure of DNA repair, mutagenesis, clonal expansion, and ultimate proliferation of mutated keratinocytes to form a clinical tumor (Figure 1). These steps after UVR exposure provide additional opportunities to intervene in the development of epidermal cancers.
Figure 1.
Exogenous and Endogenous factors in development of keratinocyte-derived skin cancers (KDSC).
As discussed above, sunscreen manufacturers continue to develop new and more stable antioxidant compounds to prevent the formation of and neutralize free radical species [104-107,118]. Future interventions to quench reactive species may include compounds that neutralize high-energy degradation of triplet-excitation states of melanin [16]. DNA repair enzymes have proven to be a valuable target, with Yarosh et al. successfully delivering DNA repair enzymes to the living epidermis of patients suffering from xeroderma pigmentosum using a liposomal technique, slowing the development of basal cell carcinomas [119]. The delivery of telomere homolog nucleotides has also been shown to increase melanogenesis and DNA repair mechanisms (increasing activation of ATM, ATR, and p53 among others), while reducing the risk of cSCC, BCC, and melanoma in mouse models [120].
As several immune subpopulations have become implicated in UVR-induced carcinogenesis and mutant keratinocyte clonal proliferation, targeting these cells, their activating cytokines, and the cytokines they produce, may also prove valuable methods to prevent the expansion of mutant keratinocytes (Figure 1). Epidermal Langerhans cells have been shown to facilitate both UVR and chemically induced carcinogenesis [121-123]. Langerhans cell contributions to carcinogenesis have been linked to both the metabolism of the polyaromatic hydrocarbon DMBA into potent mutagens via the P450 enzyme Cyp1b1 and through Cyp1b1-independent mechanisms, including the modulation of IL-22 production in the setting of UVR exposure [121,123]. Th17/22 cells have also been implicated in the promotion of cutaneous squamous cell carcinoma, producing IL-17 and IL-22 that have been shown to promote the proliferation of mutated keratinocytes [124]. The IL-22 pathway is a particularly attractive target in transplant-associated cSCC, in which mutant keratinocytes have been shown to have increased expression of the IL-22 receptor [125].
Conclusion
UVR is the major environmental risk factor for the development of cSCC, BCC, and cutaneous melanoma. Approaches to decreasing the risk associated with UVR include behavioral modification, sun-protective clothing, and topical UV-protective agents. Several advances have been made to improve four major aspects of these topical products: (1) protection over a range of UVR, (2) favorable cosmesis as to increase compliance, (3) infrequent need for re-application, and (4) minimal penetration into the living epidermis. Advances in photochemistry and biomedical engineering continue to improve each of these aspects. Additionally, epidermal carcinogenesis may be considered as a continuum from UVR exposure to the development of a clinical tumor. Future advances in skin cancer prevention may therefore focus on prevention of DNA damage after UVR, DNA repair, and modulation of local inflammation.
Glossary
- cSCC
cutaneous squamous cell carcinoma
- BCC
basal cell carcinoma
- CPDs
cyclobutane pyrimidine dimers
- UVR
Ultraviolet radiation
- FDA
Federal Drug Administration
- KDSC
keratinocyte-derived skin cancers
- PAH
polyaromatic hydrocarbon
- ROS
reactive oxygen species
- UPF
UV protection factor
- OTC
over the counter
Author Contributions
Dr. Girardi was senior author in charge of manuscript outlining and planning, Dr. Suozzi and Dr. Turban wrote the manuscript, with Dr. Suozzi as first author.
References
- Xiang F, Lucas R, Hales S, Neale R. Incidence of nonmelanoma skin cancer in relation to ambient UV radiation in white populations, 1978-2012: empirical relationships. JAMA Dermatol. 2014;150(10):1063–71. [DOI] [PubMed] [Google Scholar]
- NCI Surveillance E. End Results Program (SEER) SEER Stat Fact Sheets: Melanoma of the Skin. 2015.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129(10):1286–90. [PubMed] [Google Scholar]
- Mudigonda T, Pearce DJ, Yentzer BA, Williford P, Feldman SR. The economic impact of non-melanoma skin cancer: a review. J Natl Compr Canc Netw. 2010;8(8):888–96. [DOI] [PubMed] [Google Scholar]
- Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957–66. [DOI] [PubMed] [Google Scholar]
- Osterlind A, Tucker MA, Stone BJ, Jensen OM. The Danish case-control study of cutaneous malignant melanoma. II. Importance of UV-light exposure. Int J Cancer. 1988;42(3):319–24. [DOI] [PubMed] [Google Scholar]
- Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195(3):298–308. [DOI] [PubMed] [Google Scholar]
- Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, et al. UV-induced skin damage. Toxicology. 2003;189(1-2):21–39. [DOI] [PubMed] [Google Scholar]
- Tornaletti S, Pfeifer GP. UV damage and repair mechanisms in mammalian cells. BioEssays. 1996;18(3):221–8. [DOI] [PubMed] [Google Scholar]
- Lucas RM, Norval M, Neale RE, Young AR, de Gruijl FR, Takizawa Y, et al. The consequences for human health of stratospheric ozone depletion in association with other environmental factors. Photochem Photobiol Sci. 2015;14(1):53–87. [DOI] [PubMed] [Google Scholar]
- Lim HD. Clinical Guide to Sunscreens and Photoprotection. New York: Informa healthcare; 2009.
- Tessman I, Liu SK, Kennedy MA. Mechanism of SOS mutagenesis of UV-irradiated DNA: mostly error-free processing of deaminated cytosine. Proc Natl Acad Sci USA. 1992;89(4):1159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372(6508):773–6. [DOI] [PubMed] [Google Scholar]
- Martincorena I, Roshan A, Gerstung M, Ellis P, Van Loo P, McLaren S, et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348(6237):880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DL, Nairn RS. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989;49(6):805–19. [DOI] [PubMed] [Google Scholar]
- Premi S, Wallisch S, Mano CM, Weiner AB, Bacchiocchi A, Wakamatsu K, et al. Photochemistry. Chemiexcitation of melanin derivatives induces DNA photoproducts long after UV exposure. Science. 2015;347(6224):842–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A. The molecular and genetic effects of ultraviolet radiation exposure on skin cells In: Hawk J. Photodermatology. New York, New York: Oxford University Press; 1999. [Google Scholar]
- Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29. [DOI] [PubMed] [Google Scholar]
- Emanuele E, Spencer JM, Braun M. From DNA repair to proteome protection: new molecular insights for preventing non-melanoma skin cancers and skin aging. J Drugs Dermatol. 2014;13(3):274–81. [PubMed] [Google Scholar]
- Mumtaz M. Toxicological Profile for Polycyclic Aromatic Hydrocarbons. Agency for Toxic Substances & Disease Registry; 1995. [PubMed] [Google Scholar]
- Burke KE, Wei H. Synergistic damage by UVA radiation and pollutants. Toxicol Ind Health. 2009;25(4-5):219–24. [DOI] [PubMed] [Google Scholar]
- Nair S, Kekatpure VD, Judson BL, Rifkind AB, Granstein RD, Boyle JO, et al. UVR exposure sensitizes keratinocytes to DNA adduct formation. Cancer Prev Res (Phila). 2009;2(10):895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlou P, Rallis M, Deliconstantinos G, Papaioannou G, Grando SA. In-vivo data on the influence of tobacco smoke and UV light on murine skin. Toxicol Ind Health. 2009;25(4-5):231–9. [DOI] [PubMed] [Google Scholar]
- Pott P. Chirurgical observations. 1775:734-6. [Google Scholar]
- Berking C, Takemoto R, Binder RL, Hartman SM, Ruiter DJ, Gallagher PM, et al. Photocarcinogenesis in human adult skin grafts. Carcinogenesis. 2002;23(1):181–7. [DOI] [PubMed] [Google Scholar]
- Sauter ER, Klein-Szanto AJ, Atillasoy E, Montone KT, Goodrow T, Binder RL, et al. Ultraviolet B-induced squamous epithelial and melanocytic cell changes in a xenograft model of cancer development in human skin. Mol Carcinog. 1998;23(3):168–74. [PubMed] [Google Scholar]
- Jamal A, Agaku IT, O’Connor E, King BA, Kenemer JB, Neff L. Current cigarette smoking among adults—United States, 2005-2013. MMWR Morb Mortal Wkly Rep. 2014;63(47):1108–12. [PMC free article] [PubMed] [Google Scholar]
- Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21(48):7435–51. [DOI] [PubMed] [Google Scholar]
- Leonardi-Bee J, Ellison T, Bath-Hextall F. Smoking and the risk of nonmelanoma skin cancer: systematic review and meta-analysis. Arch Dermatol. 2012;148(8):939–46. [DOI] [PubMed] [Google Scholar]
- Bastien N, Millau JF, Rouabhia M, Davies RJ, Drouin R. The sunscreen agent 2-phenylbenzimidazole-5-sulfonic acid photosensitizes the formation of oxidized guanines in cellulo after UV-A or UV-B exposure. J Invest Dermatol. 2010;130(10):2463–71. [DOI] [PubMed] [Google Scholar]
- Brezova V, Gabcova S, Dvoranova D, Stasko A. Reactive oxygen species produced upon photoexcitation of sunscreens containing titanium dioxide (an EPR study). J Photochem Photobiol B. 2005;79(2):121–34. [DOI] [PubMed] [Google Scholar]
- Crosera E, Prodi A, Mauro M, Pelin M, Florio C, Bellomo F, et al. Titanium Dioxide Nanoparticle Penetration into the Skin and Effects on HaCaT Cells. Int J Environ Res Public Health. 2015;12(8):9282–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulston M, Knowland J. Illumination of human keratinocytes in the presence of the sunscreen ingredient Padimate-O and through an SPF-15 sunscreen reduces direct photodamage to DNA but increases strand breaks. Mutat Res. 1999;444(1):49–60. [DOI] [PubMed] [Google Scholar]
- Law MH, Bishop DT, Lee JE, Brossard M, Martin NG, Moses EK, et al. Genome-wide meta-analysis identifies five new susceptibility loci for cutaneous malignant melanoma. Nat Genet. 2015;47(9):987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey SN, Helgason H, Gudjonsson SA, Thorleifsson G, Zink F, Sigurdsson A, et al. New basal cell carcinoma susceptibility loci. Nat Commun. 2015;6:6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siiskonen SJ, Zhang M, Li WQ, Liang L, Kraft P, Nijsten T, et al. A Genome-Wide Association Study of Cutaneous Squamous Cell Carcinoma among European Descendants. Cancer Epidemiol Biomarkers Prev. 2016;25(4):714–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Mansh M, Chin-Hong P, Singer J, Arron ST. Voriconazole-associated cutaneous malignancy: a literature review on photocarcinogenesis in organ transplant recipients. Clin Infect Dis. 2014;58(7):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ona K, Oh DH. Voriconazole N-oxide and its ultraviolet B photoproduct sensitize keratinocytes to ultraviolet A. Br J Dermatol. 2015;173(3):751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarosh DB, Pena AV, Nay SL, Canning MT, Brown DA. Calcineurin inhibitors decrease DNA repair and apoptosis in human keratinocytes following ultraviolet B irradiation. J Invest Dermatol. 2005;125(5):1020–5. [DOI] [PubMed] [Google Scholar]
- Rittie L, Fisher GJ. Natural and sun-induced aging of human skin. Cold Spring Harb Perspect Med. 2015;5(1):a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Ghissassi F, Baan R, Straif K, Grosse Y, Secretan B, Bouvard V, et al. A review of human carcinogens—part D: radiation. Lancet Oncol. 2009;10(8):751–2. [DOI] [PubMed] [Google Scholar]
- Wehner MR, Chren MM, Nameth D, Choudhry A, Gaskins M, Nead KT, et al. International prevalence of indoor tanning: a systematic review and meta-analysis. JAMA Dermatol. 2014;150(4):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy GP, Jr, Berkowitz Z, Holman DM, Hartman AM. Recent Changes in the Prevalence of and Factors Associated With Frequency of Indoor Tanning Among US Adults. JAMA Dermatol. 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansh M, Katz KA, Linos E, Chren MM, Arron S. Association of Skin Cancer and Indoor Tanning in Sexual Minority Men and Women. JAMA Dermatol. 2015;•••:1–9. [DOI] [PubMed] [Google Scholar]
- Mosher CE, Danoff-Burg S. Indoor tanning, mental health, and substance use among college students: the significance of gender. J Health Psychol. 2010;15(6):819–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warthan MM, Uchida T, Wagner RF., Jr UV light tanning as a type of substance-related disorder. Arch Dermatol. 2005;141(8):963–6. [DOI] [PubMed] [Google Scholar]
- Cartmel B, Dewan A, Ferrucci LM, Gelernter J, Stapleton J, Leffell DJ, et al. Novel gene identified in an exome-wide association study of tanning dependence. Exp Dermatol. 2014;23(10):757–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fell GL, Robinson KC, Mao J, Woolf CJ, Fisher DE. Skin beta-endorphin mediates addiction to UV light. Cell. 2014;157(7):1527–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman DM, Fox KA, Glenn JD, Guy GP, Jr, Watson M, Baker K, et al. Strategies to reduce indoor tanning: current research gaps and future opportunities for prevention. Am J Prev Med. 2013;44(6):672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunova Z, Porojnicu AC, Aksnes L, Holick MF, Iani V, Bruland OS, et al. Effect of vitamin D supplementation and ultraviolet B exposure on serum 25-hydroxyvitamin D concentrations in healthy volunteers: a randomized, crossover clinical trial. Br J Dermatol. 2013;169(2):434–40. [DOI] [PubMed] [Google Scholar]
- Meesters Y, Winthorst WH, Duijzer WB, Hommes V. The effects of low-intensity narrow-band blue-light treatment compared to bright white-light treatment in sub-syndromal seasonal affective disorder. BMC Psychiatry. 2016;16(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al He. Photoprotection by Fabric. Clinical Guide to Sunscreens and Photoprotection. New York, New York: Informa Healthcare; 2009. [Google Scholar]
- Gies P. Photoprotection by clothing. Photodermatol Photoimmunol Photomed. 2007;23(6):264–74. [DOI] [PubMed] [Google Scholar]
- Gies HP, Roy CR, Holmes G. Ultraviolet Radiation Protection by Clothing: Comparison of In vivo and In vitro Measurements. Radiat Prot Dosimetry. 2000;91(1-3):247–50. [Google Scholar]
- Wang SQ, Kopf AW, Marx J, Bogdan A, Polsky D, Bart RS. Reduction of ultraviolet transmission through cotton T-shirt fabrics with low ultraviolet protection by various laundering methods and dyeing: clinical implications. J Am Acad Dermatol. 2001;44(5):767–74. [DOI] [PubMed] [Google Scholar]
- DeLeo V. Sunscreen In: Bolognia J. Dermatology. London: Elsevier; 2012. pp. 2197–204. [Google Scholar]
- Cole C, Shyr T, Ou-Yang H. Metal Oxide Sunscreens Protect Skin by Absorption, Not by Reflection or Scattering. Photodermatol Photoimmunol Photomed. 2015 [DOI] [PubMed] [Google Scholar]
- Kollias N. The absorption properties of “physical” sunscreens. Arch Dermatol. 1999;135(2):209–10. [DOI] [PubMed] [Google Scholar]
- Scheuer E, Warshaw E. Sunscreen allergy: A review of epidemiology, clinical characteristics, and responsible allergens. Dermatitis. 2006;17(1):3–11. [DOI] [PubMed] [Google Scholar]
- Maier T, Korting HC. Sunscreens - which and what for? Skin Pharmacol Physiol. 2005;18(6):253–62. [DOI] [PubMed] [Google Scholar]
- Sha B, Gao W, Cui X, Wang L, Xu F. The potential health challenges of TiO2 nanomaterials. J Appl Toxicol. 2015;35(10):1086–101. [DOI] [PubMed] [Google Scholar]
- Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354(9180):723–9. [DOI] [PubMed] [Google Scholar]
- Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29(3):257–63. [DOI] [PubMed] [Google Scholar]
- Dondi D, Albini A, Serpone N. Interactions between different solar UVB/UVA filters contained in commercial suncreams and consequent loss of UV protection. Photochem Photobiol Sci. 2006;5(9):835–43. [DOI] [PubMed] [Google Scholar]
- Westerdahl J, Ingvar C, Masback A, Olsson H. Sunscreen use and malignant melanoma. Int J Cancer. 2000;87(1):145–50. [DOI] [PubMed] [Google Scholar]
- Wolf P, Quehenberger F, Mullegger R, Stranz B, Kerl H. Phenotypic markers, sunlight-related factors and sunscreen use in patients with cutaneous melanoma: an Austrian case-control study. Melanoma Res. 1998;8(4):370–8. [DOI] [PubMed] [Google Scholar]
- Koster B, Thorgaard C, Philip A, Clemmensen IH. Prevalence of sunburn and sun-related behaviour in the Danish population: a cross-sectional study. Scand J Public Health. 2010;38(5):548–52. [DOI] [PubMed] [Google Scholar]
- Clark A, Hessler JL. Skin Care. Facial Plast Surg Clin North Am. 2015;23(3):285–95. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Gensch K, Haust M, Meuth AM, Boyer F, Dupuy P, et al. Photoprotective effects of a broad-spectrum sunscreen in ultraviolet-induced cutaneous lupus erythematosus: a randomized, vehicle-controlled, double-blind study. J Am Acad Dermatol. 2011;64(1):37–48. [DOI] [PubMed] [Google Scholar]
- Roelandts R. History of Photoprotection. Clinical Guide to Sunscreens and Photoprotection. New York, New York: Informa Healthcare; 2009. [Google Scholar]
- Battie C, Jitsukawa S, Bernerd F, Del Bino S, Marionnet C, Verschoore M. New insights in photoaging, UVA induced damage and skin types. Exp Dermatol. 2014;23 Suppl 1:7–12. [DOI] [PubMed] [Google Scholar]
- Jansen R, Osterwalder U, Wang SQ, Burnett M, Lim HW. Photoprotection: part II. Sunscreen: development, efficacy, and controversies. J Am Acad Dermatol. 2013;69(6):867.e1–14. [DOI] [PubMed] [Google Scholar]
- al CCe. Formulation and Stability of Sunscreen Products. Clinical Guide to Sunscreens and Photoprotection. New York: Informa Healthcare; 2009. [Google Scholar]
- Fourtanier A, Moyal D, Seite S. Sunscreens containing the broad-spectrum UVA absorber, Mexoryl SX, prevent the cutaneous detrimental effects of UV exposure: a review of clinical study results. Photodermatol Photoimmunol Photomed. 2008;24(4):164–74. [DOI] [PubMed] [Google Scholar]
- Enchev V, Angelov I, Mantareva V, Markova N. 2-Carbamido-1,3-indandione - a Fluorescent Molecular Probe and Sunscreen Candidate. J Fluoresc. 2015;25(6):1601–14. [DOI] [PubMed] [Google Scholar]
- Edstrom DW, Porwit A, Ros AM. Effects on human skin of repetitive ultraviolet-A1 (UVA1) irradiation and visible light. Photodermatol Photoimmunol Photomed. 2001;17(2):66–70. [DOI] [PubMed] [Google Scholar]
- Ikehata H, Kumagai J, Ono T, Morita A. Solar-UV-signature mutation prefers TCG to CCG: extrapolative consideration from UVA1-induced mutation spectra in mouse skin. Photochem Photobiol Sci. 2013;12(8):1319–27. [DOI] [PubMed] [Google Scholar]
- Marionnet C, Pierrard C, Golebiewski C, Bernerd F. Diversity of biological effects induced by longwave UVA rays (UVA1) in reconstructed skin. PLoS One. 2014;9(8):e105263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Ediriwickrema A, Yang F, Lewis J, Girardi M, Saltzman WM. A sunblock based on bioadhesive nanoparticles. Nat Mater. 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffey BL. When should sunscreen be reapplied? J Am Acad Dermatol. 2001;45(6):882–5. [DOI] [PubMed] [Google Scholar]
- Hayden CG, Cross SE, Anderson C, Saunders NA, Roberts MS. Sunscreen penetration of human skin and related keratinocyte toxicity after topical application. Skin Pharmacol Physiol. 2005;18(4):170–4. [DOI] [PubMed] [Google Scholar]
- Leite-Silva VR, Le Lamer M, Sanchez WY, Liu DC, Sanchez WH, Morrow I, et al. The effect of formulation on the penetration of coated and uncoated zinc oxide nanoparticles into the viable epidermis of human skin in vivo. Eur J Pharm Biopharm. 2013;84(2):297–308. [DOI] [PubMed] [Google Scholar]
- Leon Z, Chisvert A, Tarazona I, Salvador A. Solid-phase extraction liquid chromatography-tandem mass spectrometry analytical method for the determination of 2-hydroxy-4-methoxybenzophenone and its metabolites in both human urine and semen. Anal Bioanal Chem. 2010;398(2):831–43. [DOI] [PubMed] [Google Scholar]
- Schlumpf M, Kypke K, Wittassek M, Angerer J, Mascher H, Mascher D, et al. Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere. 2010;81(10):1171–83. [DOI] [PubMed] [Google Scholar]
- (FDA) Sunscreen drug products for over-the-counter human use: proposed rule. Fed Regis. 2019;84(38). Available from: https://www.federalregister.gov/documents/2019/04/18/2019-07712/sunscreen-drug-products-for-over-the-counter-human-use-correction [Google Scholar]
- Matta M. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a radomized clinical trial. JAMA. 2019;321(21):2082–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta M. Effect of Sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323(3):256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaris EJ, Frank J. Photoallergic contact dermatitis caused by ultraviolet filters in different sunscreens. Int J Dermatol. 2008;47 Suppl 1:35–7. [DOI] [PubMed] [Google Scholar]
- Carmona ER, Escobar B, Vales G, Marcos R. Genotoxic testing of titanium dioxide anatase nanoparticles using the wing-spot test and the comet assay in Drosophila. Mutat Res Genet Toxicol Environ Mutagen. 2015;778:12–21. [DOI] [PubMed] [Google Scholar]
- Downs CA, Kramarsky-Winter E, Segal R, Fauth J, Knutson S, Bronstein O, et al. Toxicopathological Effects of the Sunscreen UV Filter, Oxybenzone (Benzophenone-3), on Coral Planulae and Cultured Primary Cells and Its Environmental Contamination in Hawaii and the U.S. Virgin Islands. Arch Environ Contam Toxicol. 2015 [DOI] [PubMed] [Google Scholar]
- Hanson KM, Gratton E, Bardeen CJ. Sunscreen enhancement of UV-induced reactive oxygen species in the skin. Free Radic Biol Med. 2006;41(8):1205–12. [DOI] [PubMed] [Google Scholar]
- Sharma V, Singh SK, Anderson D, Tobin DJ, Dhawan A. Zinc oxide nanoparticle induced genotoxicity in primary human epidermal keratinocytes. J Nanosci Nanotechnol. 2011;11(5):3782–8. [DOI] [PubMed] [Google Scholar]
- Louis GM, Chen Z, Kim S, Sapra KJ, Bae J, Kannan K. Urinary concentrations of benzophenone-type ultraviolet light filters and semen quality. Fertil Steril. 2015;104(4):989–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaez I, Aquilino M, Morcillo G, Martinez-Guitarte JL. UV filters induce transcriptional changes of different hormonal receptors in Chironomus riparius embryos and larvae. Environ Pollut. 2016;214:239–47. [DOI] [PubMed] [Google Scholar]
- Ozaez I, Morcillo G, Martinez-Guitarte JL. Ultraviolet filters differentially impact the expression of key endocrine and stress genes in embryos and larvae of Chironomus riparius. Sci Total Environ. 2016;557-558:240–7. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Kojima H, Takeuchi S, Uramaru N, Sanoh S, Sugihara K, et al. Metabolism of UV-filter benzophenone-3 by rat and human liver microsomes and its effect on endocrine-disrupting activity. Toxicol Appl Pharmacol. 2015;282(2):119–28. [DOI] [PubMed] [Google Scholar]
- Robinson G, McMichael A, Wang SQ, Lim HW. Sunscreen and frontal fibrosing alopecia: a review. J Am Acad Dermatol. 2019;82(3):723–8. [DOI] [PubMed] [Google Scholar]
- Aldoori N, Dobson K, Holden CR. Frontal fibrosing alopecia: possible association with leave-on facial skin care products and sunscreens; a questionnaire study. Br J Dermatol. 2016;175(4):762–7. [DOI] [PubMed] [Google Scholar]
- Debroy Kidambi AD, Holmes S. Frontal fibrosing alopecia in men: an association with facial moisturizers and sunscreens. Br J Dermatol. 2017;177(1):260–1. [DOI] [PubMed] [Google Scholar]
- Moreno-Arrones OM, Rodrigues-Barata AR. Risk factors associated with frontal fibrosing alopecia: a multicentre case-control study. Clin Exp Dermatol. 2019;44(4):404–10. [DOI] [PubMed] [Google Scholar]
- Cranwell WC., Sr Sunscreen and facial skincare products in frontal fibrosing alopecia: a case-control study. Br J Dermatol. 2019;180(4):943–4. [DOI] [PubMed] [Google Scholar]
- Thompson CT, Kolivras A, Tosti A. Identification of titanium dioxide on the hair shaft of patients with and without frontal fibrosing alopecia: a pilot study of 20 patients. Br J Dermatol. 2019;181(1):216–7. [DOI] [PubMed] [Google Scholar]
- Li CC, Lin YT, Chen YT, Sie SF, Chen-Yang YW. Improvement in UV protection retention capability and reduction in skin penetration of benzophenone-3 with mesoporous silica as drug carrier by encapsulation. J Photochem Photobiol B. 2015;148:277–83. [DOI] [PubMed] [Google Scholar]
- Colven RM, Pinnell SR. Topical vitamin C in aging. Clin Dermatol. 1996;14(2):227–34. [DOI] [PubMed] [Google Scholar]
- Krol ES, Kramer-Stickland KA, Liebler DC. Photoprotective actions of topically applied vitamin E. Drug Metab Rev. 2000;32(3-4):413–20. [DOI] [PubMed] [Google Scholar]
- Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302(2):71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl W, Sies H. beta-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr. 2012;96(5):1179s–84s. [DOI] [PubMed] [Google Scholar]
- Mayoral FA, Kenner JR, Draelos ZD. The skin health and beauty pyramid: a clinically based guide to selecting topical skincare products. J Drugs Dermatol. 2014;13(4):414–21. [PubMed] [Google Scholar]
- McDaniel DH, Neudecker BA, DiNardo JC, Lewis JA, 2nd, Maibach HI. Idebenone: a new antioxidant - Part I. Relative assessment of oxidative stress protection capacity compared to commonly known antioxidants. J Cosmet Dermatol. 2005;4(1):10–7. [DOI] [PubMed] [Google Scholar]
- Ramos-e-Silva M, Celem LR, Ramos-e-Silva S, Fucci-da-Costa AP. Anti-aging cosmetics: facts and controversies. Clin Dermatol. 2013;31(6):750–8. [DOI] [PubMed] [Google Scholar]
- Syring F, Weigmann HJ, Schanzer S, Meinke MC, Knorr F, Lademann J. Investigation of Model Sunscreen Formulations Comparing the Sun Protection Factor, the Universal Sun Protection Factor and the Radical Formation Ratio. Skin Pharmacol Physiol. 2016;29(1):18–23. [DOI] [PubMed] [Google Scholar]
- Burke KE, Zhou X, Wang Y, Commisso J, Keen CL, Nakamura RM, et al. The effects of topical L-selenomethionine on protection against UVB-induced skin cancer when given before, during, and after UVB exposure. J Drugs Dermatol. 2014;13(10):1214–23. [PubMed] [Google Scholar]
- Cassidy PB, Fain HD, Cassidy JP, Jr, Tran SM, Moos PJ, Boucher KM, et al. Selenium for the prevention of cutaneous melanoma. Nutrients. 2013;5(3):725–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Martin AJ, Choy B, Fernandez-Penas P, Dalziell RA, McKenzie CA, et al. A Phase 3 Randomized Trial of Nicotinamide for Skin-Cancer Chemoprevention. N Engl J Med. 2015;373(17):1618–26. [DOI] [PubMed] [Google Scholar]
- Danovaro RB, Corinaldesi C. Sunscreens cause coral bleaching by promoting viral infections. Environ Health Perspect. 2008;116(4):441–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horricks RA, Tabin SK, Edwards JJ. Organic ultraviolet filters in nearshore waters and in the invasive lionfish (Pterois volitans) in Grenada, West Indies. PLoS One. 2019;14(7):e0220280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SL, Lim HW. Review of environmental effects of oxybenzone and other sunscreen active ingredients. J Am Acad Dermatol. 2019;80(1):266–71. [DOI] [PubMed] [Google Scholar]
- Freitas JV, Praca FS, Bentley MV, Gaspar LR. Trans-resveratrol and beta-carotene from sunscreens penetrate viable skin layers and reduce cutaneous penetration of UV-filters. Int J Pharm. 2015;484(1-2):131–7. [DOI] [PubMed] [Google Scholar]
- Yarosh D, Klein J, O’Connor A, Hawk J, Rafal E, Wolf P. Effect of topically applied T4 endonuclease V in liposomes on skin cancer in xeroderma pigmentosum: a randomised study. Xeroderma Pigmentosum Study Group. Lancet. 2001;357(9260):926–9. [DOI] [PubMed] [Google Scholar]
- Gilchrest BA. Telomere-Based Protective Responses to DNA Damage. J Investig Dermatol Symp Proc. 2015;17(1):15–6. [DOI] [PubMed] [Google Scholar]
- Lewis JM, Burgler CD, Fraser JA, Liao H, Golubets K, Kucher CL, et al. Mechanisms of chemical cooperative carcinogenesis by epidermal Langerhans cells. J Invest Dermatol. 2015;135(5):1405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JM, Burgler CD, Freudzon M, Golubets K, Gibson JF, Filler RB, et al. Langerhans Cells Facilitate UVB-Induced Epidermal Carcinogenesis. J Invest Dermatol. 2015;135(11):2824–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modi BG, Neustadter J, Binda E, Lewis J, Filler RB, Roberts SJ, et al. Langerhans cells facilitate epithelial DNA damage and squamous cell carcinoma. Science. 2012;335(6064):104–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardinocchi L, Sonego G, Passarelli F, Avitabile S, Scarponi C, Failla CM, et al. Interleukin-17 and interleukin-22 promote tumor progression in human nonmelanoma skin cancer. Eur J Immunol. 2015;45(3):922–31. [DOI] [PubMed] [Google Scholar]
- Zhang S, Fujita H, Mitsui H, Yanofsky VR, Fuentes-Duculan J, Pettersen JS, et al. Increased Tc22 and Treg/CD8 ratio contribute to aggressive growth of transplant associated squamous cell carcinoma. PLoS One. 2013;8(5):e62154.23667456 [Google Scholar]