Abstract
Cutaneous T cell lymphoma (CTCL) is a rare malignancy of skin-homing T lymphocytes. Advances in whole exome sequencing have identified a vast number of both single nucleotide variants (SNVs) and genomic copy number alterations (GCNAs) as driver mutations present in CTCL cells. These alterations cluster within several key pathways – T cell/NF-κB/JAK-STAT activation, cell cycle dysregulation/apoptosis, and DNA structural dysregulation affecting gene expression – allowing the maintenance of a population of proliferating, activated malignant T lymphocytes. While much of the clinical spectrum, genetic alterations, and oncogenic behavior of CTCL have been elucidated, little is known about the etiology that underlies CTCL malignant transformation and progression. Herein, we review the epidemiology, clinical presentation, and pathophysiology of CTCL to provide a perspective on CTCL pathogenesis. We outline a series of alterations by which mature, activated T lymphocytes are endowed with apoptosis resistance and cutaneous persistence. Subsequent genomic alterations including the loss of chromosomal structural controls further promote proliferation and constitutive T cell activation. CTCL cells are both malignant cells and highly functional T cells that can have major cutaneous and immunologic effects on the patient, including the suppression of cell-mediated immunity that facilitates malignant cell expansion. A deeper understanding of the molecular and cellular underpinnings of CTCL can help guide clinical management as well as inform prognosis and therapeutic discovery.
Keywords: Mycosis fungoides, Sézary syndrome, cutaneous T cell lymphoma, CTCL
Introduction
Primary cutaneous lymphomas are extra-nodal non-Hodgkin lymphomas that are largely confined to the skin at presentation [1]. Approximately 75% of cutaneous lymphomas are T cell derived, of which about two-thirds are cutaneous T cell lymphoma (CTCL) [1]. The grouping of CTCL describes a variety of T cell malignancies in the skin. Of these, the most common types are mycosis fungoides (MF) and Sézary syndrome (SS), with MF comprising around 50% of all cutaneous lymphomas. The non-MF/SS collective entity of CD30+ T cell lymphoproliferative disorders includes cutaneous anaplastic large cell lymphoma and lymphomatoid papulosis that comprises the second most common type of cutaneous lymphomas at ~25% of cases.
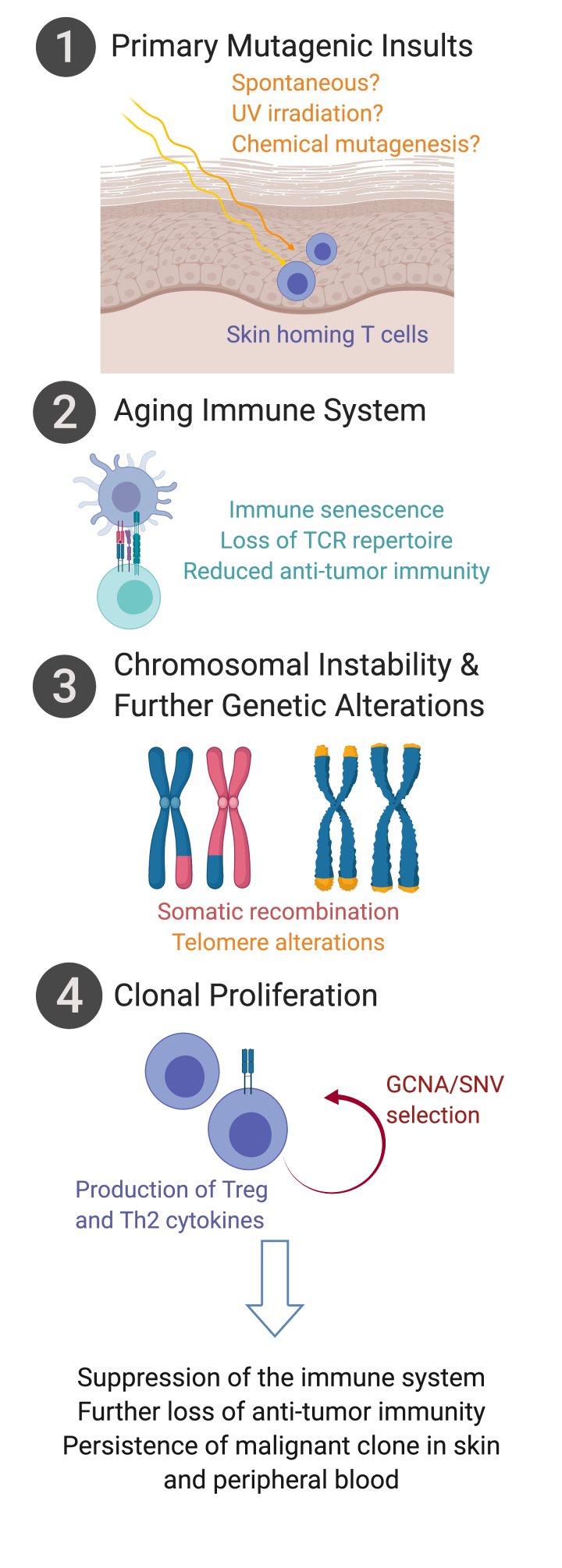
Our perspective herein focuses on CTCL that presents along the clinical spectrum of MF/SS. Recent work has brought to light the clinical presentation, malignant behavior, genomic landscape, and novel therapeutics for treatment of CTCL. We provide a perspective on a series of alterations (Figure 1) wherein mature, activated T lymphocytes are initially met with mutagenic insults in the skin, endowing the affected cells with apoptosis resistance and cutaneous persistence, and allowing for further genetic alteration. Such mutagenic insults in the context of an aging immune system undergoing immune senescence results in accumulation of genetic alterations. Subsequent loss of chromosomal structure controls, including telomere shortening, drives somatic recombination events that allow selection of gene copy number alterations (GCNAs) and single nucleotide variants (SNVs) that further promote proliferation and constitutive T cell activation. The persistent T cell clone may preferentially populate within skin, blood, and lymph nodes, largely driven by the T differentiation status and surface homing profiles. Malignant CTCL cells remain in a highly activated state and produce cytokines predominantly of Treg and Th2 profiles, suppressing systemic immune response and further reducing anti-tumor immunity. Herein we consider recent advances in understanding of CTCL epidemiology, clinical presentation, pathophysiology, and response to treatment that provide insight to the etiology driving CTCL malignant cell behavior.
Figure 1.
Alterations in the development of malignant CTCL cells of mycosis fungoides and Sézary syndrome. Activated skin-homing T cells in the skin are met with mutagenic insults, and along with changes in the aging immune system, result in T cell apoptosis resistance and cutaneous persistence. Subsequent genomic alterations including the loss of chromosomal structure controls further promote proliferation and selection of genomic copy number alterations (GCNAs) and single nucleotide variants (SNVs) that promote malignant CTCL behavior. (figure created with biorender.com)
Epidemiology
While CTCL remains a rare disease, there has been a consistently observed trend of increasing incidence of CTCL since the 1970s [2]. There was an annual overall incidence in the United States of 6.4 cases per million persons as reported by the Surveillance, Epidemiology and End Results (SEER) registry data from 1973-2002 and 7.7 per million persons in 2001-2005 [3,4]. CTCL is primarily a disease seen in older individuals with a median age of diagnosis at 50 and increasing incidence in patients over the age of 70 in the US [5,6]. A similar increasing incidence and median age of occurrence is seen worldwide (Canada, Kuwait, Iran) [7-9]. CTCL has a higher incidence in individuals of African-American race, and greater preponderance for males [2]. Overall CTCL survival was 78.3% from 1997-2005 in the US, with a trend of increasing 5-year survival rates from 1973 to 2004 [2].
Environmental factors and geographic distributions associated with occurrence of CTCL have been considered in several studies. In Canada, higher rates of occurrence of CTCL have been reported in areas with greater industrial exposure [7,10]. Such geographical clustering of patients with CTCL have also been observed in Texas and in Pittsburgh, Pennsylvania [11,12]. While these geographic distribution patterns suggest a role for common chemical/industrial exposures, no specific inciting agents have ever been identified.
Pathophysiology
T Cell Activation and Proliferation
Malignant T lymphocytes of CTCL are characterized by a state of constitutive activation and clonal expansion. Furthermore, the malignant cells display resistance to apoptosis, including FAS-mediated, TNF-related-apoptosis-inducing ligand (TRAIL)-mediated, and that induced by the TGFβ pathway. In SS, malignant cells can increase in number to comprise the overwhelming majority of the peripheral blood lymphocytes. There is a resultant decrease in the patients’ population of healthy lymphocytes including CD4+ and CD8+ T cells, as well as NK cells [13]. The malignant CTCL cells often exhibit a Th2 cytokine profile, producing IL-4, IL-5, and/or IL-13 [14]. Production of IL-4 and IL-13 inhibits T cell differentiation into Th1 subtype and thereby inhibits Th1-type immunity [15]. CTCLs cells can also be found to produce IL-10 and TGFβ, T regulatory (Treg) cytokines known to inhibit host cell-mediated immunity [14,16]. The Th2 and Treg profiles of CTCL cells, in conjunction with the decrease in the pool of the host’s healthy lymphocytes, contributes to a state of immune suppression that is associated with an increase in the frequency of infections and secondary malignancies, which can be fatal for patients with CTCL. The production of IL-5 also induces activation of tissue and/or peripheral blood eosinophils. Along with aberrant production of IL-31, these cytokines dispose patients to development of debilitating dermatitis and pruritis which may have a substantial impact on patients’ quality of life [17-19]. The inflammatory environment created by these cytokines may also further the activated state of malignant cells and suppress anti-tumor immunity, thereby promoting disease progression.
Skin Homing and Role of the Skin Microenvironment in Pathogenesis
In healthy T lymphocytes, expression of the marker cutaneous-lymphocyte-antigen (CLA) is associated with a skin-homing profile [20]. Such skin-homing T cells typically make up about 30% of the peripheral pool of circulating lymphocytes [20]. These cells also typically display chemokine receptors CCR4 and CCR10. Chemokines CCL17, CCL22, and CCL27, released from keratinocytes and vascular endothelium, are involved with migration of T cells from peripheral blood to the skin [20]. Such skin-homing occurs not only in response to inflammation or injury in the skin but is a constitutive behavior in these cells in homeostatic immune surveillance of the skin.
Malignant T cells are CLA+ and express CCR4, emphasizing their likely origin as skin homing/skin resident T cells [21]. The skin lesions of MF have also been shown to contain high levels of chemokines that attract these cells to the skin, including CCL17 and 22 [21,22]. This suggests a role for the skin milieu in attracting and maintaining a population of malignant skin-homing T cells within lesions of MF. These data further suggest that the origin cells of CTCL may be such skin-homing T cells rather than cells from the peripheral compartment.
Aging Immune System and T Cell Repertoire
Peripheral blood T lymphocytes have a diverse T cell receptor (TCR) repertoire in healthy individuals. TCR diversity and the resulting ability to recognize and respond to a diverse array of antigens is essential for healthy immune system function. As an individual ages, there is a loss of diversity of the TCR repertoire [23]. This occurs in part due to involution of the thymic gland at puberty, and further due to progressively reduced proliferation of naïve T cells, and increased proliferation of memory T cell clonal populations [24-26]. Further, the aging immune system, especially the adaptive immune response, undergoes immuno-senescence. Formation of senescent T cells may occur as a result of chronic antigenic stimulation or an inflammatory microenvironment [27]. Senescent cells have reduced replicative potential and are resistant to apoptosis [27]. They do not respond to antigenic stimulation but may nonetheless be metabolically highly active and produce abundant cytokines [27].
Patients with CTCL with peripheral blood involvement have a reduced TCR repertoire diversity in comparison to healthy individuals [28]. The malignant CTCL cell population in the peripheral blood can have clonal rearrangements of their TCRs. The reduction in TCR diversity in patients with SS is greater than expected solely due to presence of a population with clonal TCR rearrangement, suggesting that malignant cells may play a role in modulating overall immune function and TCR diversity. Additionally, malignant CTCL cells most often have a memory T cell phenotype. As CTCL is a disease most often occurring in older individuals, it is possible that physiologic changes in the immune system that occur in healthy individuals with age may become aberrant in CTCL cells. This may result in development of malignant CTCL cells that produce abundant cytokines and are resistant to apoptosis like senescent cells, but unlike their healthy counterparts, also have increased replicative potential and accumulation of progressive mutations, allowing their malignant transformation and behavior.
Clinical Presentation
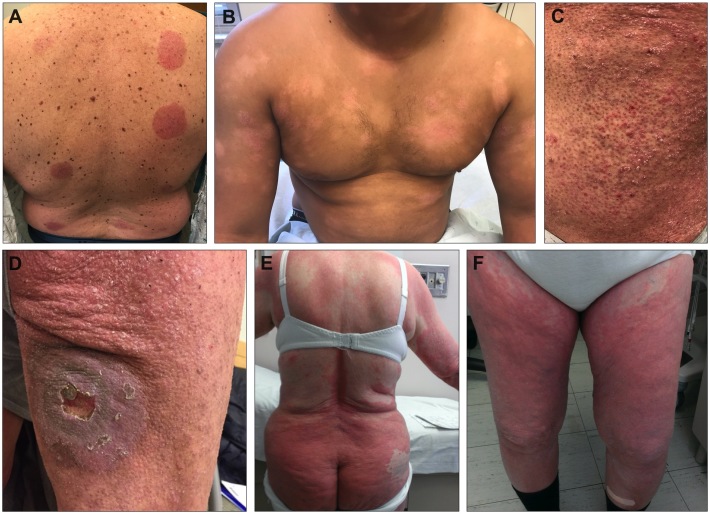
Patients with MF develop predominant skin lesions with diverse manifestations, including well-demarcated patches, papules, plaques, or tumors comprised of malignant, but also non-malignant, lymphocytes. While lesions may present on any area of the body, they occur most commonly in an asymmetric distribution in sun-protected areas including the buttocks, medial thighs, and breasts. The lesions have variable morphology and are often eczematous or psoriatic in nature. They may evolve to become thickened, coalesce into larger plaques, or partially involute to leave residual annular plaques [29]. There may also be areas of variable hypo- or hyper-pigmentation, focal or diffuse hair-loss, follicular papules, atrophy, and/or petechiae or pigmented purpura [29]. In approximately 10% of cases of MF, plaques develop into nodules or tumors. The indolent course and lesions’ morphologic similarity to other inflammatory conditions can at times delay definitive diagnosis and treatment. Several representative clinical images of MF are shown in Figure 2A-D.
Figure 2.
Cutaneous manifestations of mycosis fungoides (MF) and Sézary syndrome (SS). (A) Asymmetric patches and plaques in classic MF. (B) Hypopigmented, (C) follicular, and (D) ulcerated plaque lesions in MF. (E-F) Erythroderma of SS.
On histology, MF skin lesions contain a population of enlarged T lymphocytes with nuclear atypia admixed with other inflammatory/reactive leukocytes, although sometimes the clonal T cells are histologically normal. CTCL nuclei can be convoluted and have a cerebriform appearance, possibly a result of recurrent nuclear proliferation without cytoplasmic division. The malignant cells most commonly display epidermotropism and are typically present throughout the epidermis and superficial dermis. They often form a band-like distribution at the dermo-epidermal junction. In the epidermis, the malignant T lymphocytes may cluster around Langerhans cells (LC) and form characteristic Pautrier’s microabcesses [13]. Clustering of malignant cells around antigen presenting LC suggests that chronic antigenic stimulation (either self- or non-self) may play a role in oncogenic transformation or maintenance of skin resident malignant CTCL cells. In vitro, antigen presenting dendritic cells can support cultures of Sézary cells, and can induce the production of Treg cytokines, further supporting a pathogenic role for this interaction [30]. The malignant T lymphocytes most commonly have a CD4+ CD45RO+ memory phenotype; however, CD30+ and CD8+ subtypes have been described [5,6]. Immunophenotypic staining frequently shows decreased expression of pan-T cell markers including CD2, CD3, CD5, CD7, and/or CD26 [31]. The cause of these aberrant immunophenotypes remains unclear, and such loss of markers has also been observed in other T cell lymphoproliferative disorders such as peripheral T cell lymphoma (PTCL) [32]. Nonetheless, such profiles have also been identified in benign lymphocyte clones in the peripheral blood, the significance of which is unknown [33].
Sézary syndrome (SS) represents a more aggressive and advanced form of disease in which there is more diffuse involvement of the skin, typically as an erythroderma (Figure 2E-F), as well as a clonal proliferation of CTCL cells in the lymph nodes, peripheral blood, and even viscera [34]. The International Society for Cutaneous Lymphomas (ISCL) outlines criteria for the identification of SS by presence of one or more of the following: Sézary cell population in the blood of count at least 1000 per cubic millimeter, a ratio of CD4:CD8 cells in the blood greater than 10 caused by an increased population of circulating T cells with abnormal expression of pan T cell markers as assessed by flow cytometry, increased lymphocyte count with evidence of clonal T cell population in the blood by southern blot or polymerase chain reaction (PCR), or a chromosomally abnormal T cell clone [4]. In cases termed “classic SS,” clinical features may develop rapidly de novo, while in other cases SS may be preceded by a prodrome of pruritus or nonspecific dermatitis. In rare cases, termed “SS preceded by MF,” SS develops in patients initially diagnosed with MF [4]. MF/SS CTCL presentation is classified by tumor (T), node (N), viscera (M), and blood (B) staging system [35]. Early-stage disease, stage IA-IIA, is associated with better prognosis and survival often greater than 10 years, while advanced stage disease (stage IIB – IVB) has median survival times of 35-56 months [35,36].
Genomic Landscape
Whole genome and exome sequencing efforts have demonstrated a predominance of gene copy number alterations (GCNAs) in CTCL. This is in contrast to the single nucleotide variants (SNVs) found relatively more frequently in the majority of other cancers. These alterations influence key signaling and gene expression pathways that contribute to the development of a proliferating pool of malignant cells in which there is further selection of GCNAs/SNVs that promote malignant cell survival and evasion of apoptosis. Alterations occur most commonly in the pathways of chromosomal and gene expression regulation, cell cycle regulation, and T cell activation. The presence of UV mutational signatures is suggestive not only of a role for UV in the development of CTCL, but also points to skin-homing T cells as the most likely cell of origin that undergoes malignant transformation.
Chromosomal and DNA Structural Dysregulation
Several chromosomal alterations have been frequently identified in malignant CTCL populations, including deletion of chromosomal arms 10q and 17p, and amplification of 8q and 17q [37-39]. It has been postulated that complex structural chromosomal rearrangement events may underlie the occurrence of deletions, including chromosomal translocations with loss of intervening genomic DNA, intrachromosomal deletions, and chromosomal inversions [40]. Recombinase activating gene (RAG) proteins mediate V(D)J recombination in healthy lymphocytes undergoing T cell differentiation. RAG proteins initiate recombination events by DNA cleavage at conserved sequence heptamers. These heptamers have been found flanking genes that are recurrently translocated in CTCL, suggesting a role for aberrant RAG protein activity in development of some of the characteristic CTCL chromosomal rearrangements [40]. However, RAG protein expression is yet to be confirmed in CTCL or CTCL precursor states. The prevalence and diagnostic value of GCNAs in CTCL is underscored by the fluorescence in situ hybridization (FISH) assay developed by Weed et al. [41] FISH probes were designed against 11 GCNAs that had been recurrently identified in up to 97.5% of patients with SS: TP53, MYC, RB1, CDKN2A, ATM, STAT3/5B, ARID1A, ZEB1, FAS, CARD11, and DNMT3A. By this FISH assay, 92% patients that met B2 blood involvement criteria by the ISCL had detectable GCNAs [41].
In addition to the large-scale chromosomal alterations that influence gene expression, there are also changes in transcriptional regulation at an epigenetic level. GNCAs in chromatin modifying genes ARID1A, CTCF, and DNMT3A have been described in CTCL [40]. Additionally, there has been the discovery of somatic mutations of genes involved with histone methylation, acetylation, and ubiquitination [42,43]. Through these alterations, aberrant transcriptional profiles of malignant CTCL cells further their inappropriate activation and proliferation. Altered transcriptional states, in combination with large scale chromosomal alterations, perpetuates the mutational burden and altered activity of malignant CTCL cells.
Recent work has found the expression of meiosis genes to be expressed in CTCL cells, suggesting that the cells may undergo a process termed meiomitosis [44]. Genes involved with meiosis are typically only expressed during oocyte development or spermatogenesis. When re-expressed by malignant cells undergoing meiomitosis, there may be activation of meiotic machinery during mitotic replication, which may further genomic instability [44].
Another commonly altered gene includes protection protein of telomeres 1 (POT1), found to be mutated in 6% of CTCL cases from compiled genomic data of seven sequencing studies [45]. POT1, as a member of the sheltrin complex, functions to protect the ends of telomeres and promote normal telomere function [46]. Memory T cells have shorter telomeres than naïve counterparts, suggesting that malignant CTCL cells may have shortened telomeres due to their memory phenotype [47]. Mutation of POT1 and subsequent progressive telomere shortening leads to increased chromosomal fragility, telomere dysfunction, and eventual chromosomal end-to-end joining [48]. This leads to the formation of dicentric chromosomes and breakage-fusion-breakage cycles, leading to large scale chromosomal rearrangement [48]. If this occurs in combination with activation of telomerase or activation of the alternative lengthening of telomeres (ALT) pathway, malignant cells gain the ability to proliferate indefinitely [46]. Thus, complex chromosomal rearrangements and instability along with alteration of the epigenetic profile in malignant CTCL cells lead to a clonal population of cells with limitless replicative potential and a highly unstable genome prone to frequent mutation.
Cell Cycle Dysregulation
There is a breadth of data highlighting the deletion or inactivation of tumor suppressor genes TP53, RB1, PTEN, CDKN1, and CDKN2A in CTCL, as well as amplification of oncogene MYC [40,42]. In particular, TP53, located on chromosome 17p, has been found to be one of the most frequently mutated genes in CTCL [45]. p53 is a major cell cycle regulator, halting proliferation in response to accumulation of DNA damage. Alteration of p53 alone has not been found to be sufficient to initiate oncogenic progression of CTCL cells and is not associated with disease prognosis in SS, although it associated with poor survival amongst patients with advanced MF [45,49,50]. Among the most commonly altered genes in CTCL is oncogene MYC, located on 8q and found to be amplified in 42.5% of leukemic CTCL [40]. Thus, mutations in key tumor suppressor and oncogenes in the CTCL genome result in increased replication and evasion of apoptosis in cells that have high mutational burden and aberrant behavior.
Aberrant T Cell Activation Pathways
In normal T lymphocytes, activation of the T cell receptor (TCR) in response to antigen presentation and co-stimulation leads to a cascade of intracellular signaling that culminates in clonal T cell proliferation [51]. TCR signaling triggers JAK-STAT activation, AKT phosphorylation, and eventually activation of nuclear factor-kappa B (NF-κB), the major transcription factor central to signaling in TCR pathway activated T lymphocytes for cytokine production, proliferation, and generation of an immune response [52]. Both the TCR pathway and the NF-κB pathway have been found to be canonically activated among malignant CTCL cells and amplification/activation mutations in CTCL have been identified in the genes stimulating this pathway [53]. Members of the TCR pathway have been found to have GCNAs in CTCL cells, including TCR-associated signaling proteins (PLCG1, PRKCQ, TNFAIP3), transcription factors (NFKB2, STAT3, STAT5B, ZEB1), and the co-stimulatory molecule CD28 [40]. CARD11, which promotes TCR mediated T cell activation and activates NF-κB, was found in one study to be readily amplified in CTCL cell exomes [54]. Tumor necrosis factor 2 (TNFR2) activates NF-κB signaling, and TNFR2 mutations and copy number alterations were also found in 18% of samples from patients with MF or SS [55]. Additionally, genes driving Th2 differentiation, including ZEB1, have been implicated in CTCL pathogenesis and suggest a mechanism for escape from TGFβ mediated inhibition of proliferation [56]. ZEB1 inhibits IL-2 cytokine transcription, and its mutation may contribute to the increased IL-2 production and subsequent proliferation characteristic of malignant CTCL cells [56].
The Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway is involved with cytokine signaling and proliferation of healthy T lymphocytes. It is in particular important for the differentiation of T helper cell subsets. The JAK-STAT pathway has been reported to be deregulated in CTCL. Constitutive phosphorylation as well as GCNAs of JAK2, STAT3, and STAT5B have been reported [40,57-59]. Activating mutations in both JAK1 and JAK3 have also been described [37,40]. Alteration of the JAK-STAT pathway in malignant CTCL cells may play a role in maintenance of an activated, proliferating pool, and may also play a role in their differentiation into Th2 or Treg subtypes.
UV Mutational Signature
The role of UV exposure in the etiology CTCL is unknown. On the one hand, skin lesions of MF have a propensity for sun protected “bathing suit” areas, and high-UV exposure has been associated with a reduced risk for developing MF [60]. Furthermore, psoralen with UVA exposure (PUVA) and narrow band UVB are well-documented effective treatments for skin lesions of MF, and UV therapy has been shown to induce apoptosis of skin resident lymphocytes, reduce the production of Th2 cytokines, and even reduces number of circulating CD4+ lymphocytes. However, CTCL mutational signatures mimic those found within UV-induced cancers such as melanoma and basal cell carcinoma [54]. There is a high frequency of C>T transitions and single and dinucleotide variants, consistent with UVB mutagenesis. While it was hypothesized that this may occur secondary to the ultraviolet light used in treatment of skin lesions, Choi et al. [40] and Wang et al. [54] found that there was no correlation between presence of a UVB mutational signature and prior therapeutic exposure to UV. UV exposure and subsequent mutagenesis may nonetheless be among the initial insults causing mutations in malignant CTCL cells, but it is alternatively possible that the UV mutational signature occurs because the primary T cells that undergo oncogenic transformation are skin-resident T cells and are exposed to UV radiation due to their location.
Management
Available Treatment Modalities
Despite recent advances in the understanding of molecular and genomic alterations present in the malignant T lymphocytes of CTCL, it largely remains a disease with no definitive cure in later stages. The main modalities of skin-directed therapy include electron beam therapy, ultraviolet light therapy, and topical chemotherapy including bexarotene, carmustine, or nitrogen mustard, as well as immunomodulatory agents such as topical steroids and imiquimod [31]. These therapies promote apoptosis of malignant cells in the skin by inducing irreparable DNA damage as well as a DNA damage response [61]. They also alter the cytokine and chemokine microenvironment of the skin [61]. FDA-approved systemic therapies include acitretin and bexarotene (synthetic retinoids), interferons alpha and gamma, methotrexate (dihydrofolate reductase inhibitor), pralatrexate (antimetabolite folate analog), denileukin difitox (recombinant IL-2 and diphtheria toxin), and HDAC inhibitors romidepsin and vorinostat. FDA-approved humanized monoclonal antibodies include toxin-conjugated brentuximab (anti-CD30) and mogamulizumab (anti-CCR4).
Novel Therapeutics
With increasing understanding of the molecular mechanisms that drive proliferation and survival of malignant CTCL cells, there has also been investigation into novel targeted therapies. Recent studies have investigated drugs targeting key proteins in aberrant and highly activated pathways in malignant CTCL cells. Therapies currently under investigation include cobomarsen (MRG-106; an oligoneuclotide inhibitor of miR-155), lenalidomide (4-amino-glutamyl analogue of thalidomide with immunomodulatory activity), AFM13 (recombinant antibody against both human CD30 and CD16A), and JAK inhibitors [62].
Inhibition of JAK signaling has been investigated in CTCL cell lines and shown to inhibit proliferation and induce apoptosis in vitro [63]. Our group has described the use of BCL-2 inhibitor venetoclax and HDAC inhibitors romidepsin and vorinostat alone and in combination to effectively induced apoptosis of purified patient derived malignant CTCL cells in vitro [64]. In our further studies we have shown that BET inhibition alone and in combination with BCL2 or HDAC inhibition was also effective in inducing apoptosis of CTCL cells [65]. Combination BET and HDAC inhibition reduced expression of MYC in treated CTCL cells in vitro [65]. In both studies, the use of combination therapy had synergistic effects in inducing malignant cell death in comparison to monotherapy [64-66]. Such synergistic combinations can allow for use of reduced doses of each drug when used in combination, resulting in decreased toxicity. Additionally, targeting multiple points along the same pathway or targeting many pathways at once can reduce development of resistance. With the discovery of changes at the molecular level that are common to many patients with CTCL, there is immense potential for the development of new targeted therapies and combinatorial regimens that could aim to achieve definitive treatment for these patients.
Conclusions and Outlook
Conclusion: Etiology and Stepwise Progression of CTCL
Herein, we propose a series of alterations where in mature skin-homing T cells are activated and are subsequently met with mutagenic insults in the skin, endowing the affected cells with apoptosis resistance, cutaneous persistence, and further genetic alteration. Initially, T cell activation may occur secondary to antigen exposure or on a background of chronic inflammation. There have been reported cases of CTCL arising on a background of chronic inflammatory dermatosis, suggesting that the chronic T cell activation in an inflammatory microenvironment may promote their persistence [67]. Antigenic stimulation with skin-resident pathogens, such as Staphylococcus aureus, has been shown to worsen erythroderma associated with disease activity [68]. CTCL patients are frequently colonized with S. aureus and antibiotic treatment has been shown to reduce skin disease burden in patients with advanced CTCL [68,69]. Additionally, antibiotic treatment was shown to reduce STAT3 signaling in malignant cells [70,71]. In a mouse model of CTCL, TCR signaling was shown to promote development of CTCL and microbial triggers were found to be essential to disease progression [72]. It is possible that in susceptible individuals, colonization with pathogens including S. aureus worsens inflammatory response contributing to development and progression of disease. It has also been shown that malignant CD4+ Sézary cells are more resistant to apoptosis from S. aureus alpha-toxin, in comparison to healthy cells, suggesting a role of alpha-toxin in increasing the malignant cell population in peripheral blood [73]. We consider that such activated T lymphocytes may be subsequently met with mutagenic insults, such as UV radiation or chemical exposure, but also consider that such may simply occur spontaneously within activated and proliferating skin-homing T cells. In any event, cumulative mutagenic insults to skin-homing T cells over time promote their persistent activation and oncogenic transformation in the skin, and eventually their ability to maintain a malignant population in the blood.
Mutagenic insults result primarily in GCNAs, but also SNVs, particularly selecting for alterations of genes involved with T cell activation, epigenetic regulation, and cell cycle regulation that promote continued malignant cell survival and proliferation. Loss of chromosomal structure controls, including telomere shortening, drives somatic recombination events that allow selection of GCNAs that further promote oncogenic activity. Mutation of proteins involved in telomere maintenance, such as POT1, allows for telomere shortening, fragility, and eventual large-scale chromosomal alterations. RAG proteins involved with V(D)J in healthy T lymphocytes may participate in further aberrant chromosomal recombination events in malignant cells. As mutations accumulate in the TCR, NF-κB, and JAK-STAT pathways, the continued activated state promotes T cell proliferation and inhibition of apoptosis. The persistent T cell clone may preferentially populate within skin, blood, and lymph nodes, largely driven by the T differentiation status and surface homing profiles. With aging, there is a general decrease in the diversity of TCR repertoire in healthy individuals, as well as the development of immuno-senesce. Immunosenescent cells become anergic and do not respond to antigenic stimulation but continue to produce cytokines. Malignant CTCL cell population often have clonal TCR rearrangements, and patients with CTCL have decreased TCR diversity. The malignant cells also behave like immunosenescent cells in that they do not respond to antigen and produce cytokine, but unlike senescent cells, CTCL cells maintain the ability to continue to replicate. It is possible that physiologic changes that occur in the immune system with aging in healthy individuals may become aberrant and contribute to the development of CTCL.
Outlook
A deeper understanding of the etiology and pathogenesis of CTCL can help guide the clinical management and inform prognosis and therapeutic discovery. Knowledge of altered pathways in CTCL has resulted in the investigation of novel targeted therapies. With continued elucidation of the mechanism underlying CTCL malignant transformation, particularly with increased understanding of CTCL chromosomal instability and epigenetic changes, there remains an untapped potential for novel therapies that could lead to long-term and complete control of disease. The potential of combination therapy is particularly promising. Combining targeted therapies that inhibit specifically altered pathways in CTCL could induce synergistic kill of malignant lymphocytes while reducing systemic toxicity and development of resistance. Personalized medicine with therapies targeting alterations of malignant cells in individual patients with CTCL is one possible avenue for treatment; however, it is alternatively possible that the correct combination of therapies targeting the most commonly altered pathways may be effective more generally in inducing disease remission for the majority of patients with CTCL.
Glossary
- CTCL
cutaneous T cell lymphoma
- TCR
T cell receptor
- NF-κB
Nuclear factor-kappaB
- MF
mycosis fungoides
- SS
Sézary syndrome
- UV
ultraviolet
- ISCL
international society for cutaneous lymphomas
- TNF
tumor necrosis factor
- TGF
transforming growth factor
- GCNA
gene copy number alteration
Author Contributions
SY and MG wrote, reviewed, and edited the manuscript.
References
- Wilcox RA. Cutaneous T-cell lymphoma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016;91(1):151–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korgavkar K, Xiong M, Weinstock M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. 2013;149(11):1295–9. [DOI] [PubMed] [Google Scholar]
- Kim YH, Hoppe RT. Mycosis fungoides and the Sézary syndrome. Semin Oncol. 1999;26(3):276–89. [PubMed] [Google Scholar]
- Vonderheid EC, Bernengo MG, Burg G, Duvic M, Heald P, Laroche L, et al. Update on erythrodermic cutaneous T-cell lymphoma: report of the International Society for Cutaneous Lymphomas. J Am Acad Dermatol. 2002. January;46(1):95–106. [DOI] [PubMed] [Google Scholar]
- Criscione VD, Weinstock MA. Incidence of cutaneous T-cell lymphoma in the United States, 1973-2002. Arch Dermatol. 2007;143(7):854–9. [DOI] [PubMed] [Google Scholar]
- Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730–9. [DOI] [PubMed] [Google Scholar]
- Ghazawi FM, Netchiporouk E, Rahme E, Tsang M, Moreau L, Glassman S, et al. Comprehensive analysis of cutaneous T-cell lymphoma (CTCL) incidence and mortality in Canada reveals changing trends and geographic clustering for this malignancy. Cancer. 2017;123(18):3550–67. [DOI] [PubMed] [Google Scholar]
- Alsaleh QA, Nanda A, Al-Ajmi H, Al-Sabah H, Elkashlan M, Al-Shemmari S, et al. Clinicoepidemiological features of mycosis fungoides in Kuwait, 1991-2006. Int J Dermatol. 2010;49(12):1393–8. [DOI] [PubMed] [Google Scholar]
- Naeini FF, Sadeghiyan H, Pourazizi M, Najafian J, Abtahi-Naeini B. Characteristics of Primary Cutaneous T-Cell Lymphoma in Iran: A 10-Year Retrospective Study. Int Sch Res Notices. 2014;2014(54):820921–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazawi FM, Litvinov IV. Distribution and Clustering of Cutaneous T-Cell Lymphoma (CTCL) Cases in Canada: A Response to a Letter. J Cutan Med Surg. 2018;22(6):657–8. [DOI] [PubMed] [Google Scholar]
- Moreau JF, Buchanich JM, Geskin JZ, Akilov OE, Geskin LJ. Non-random geographic distribution of patients with cutaneous T-cell lymphoma in the Greater Pittsburgh Area. Dermatol Online J. 2014;20(7). [PubMed] [Google Scholar]
- Litvinov IV, Tetzlaff MT, Rahme E, Jennings MA, Risser DR, Gangar P, et al. Demographic patterns of cutaneous T-cell lymphoma incidence in Texas based on two different cancer registries. Cancer Med. 2015;4(9):1440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi M, Heald PW, Wilson LD. The pathogenesis of mycosis fungoides. N Engl J Med. 2004;350(19):1978–88. [DOI] [PubMed] [Google Scholar]
- Saed G, Fivenson DP, Naidu Y, Nickoloff BJ. Mycosis fungoides exhibits a Th1-type cell-mediated cytokine profile whereas Sezary syndrome expresses a Th2-type profile. J Invest Dermatol. 1994;103(1):29–33. [DOI] [PubMed] [Google Scholar]
- Guenova E, Watanabe R, Teague JE, Desimone JA, Jiang Y, Dowlatshahi M, et al. TH2 cytokines from malignant cells suppress TH1 responses and enforce a global TH2 bias in leukemic cutaneous T-cell lymphoma. Clin Cancer Res. 2013;19(14):3755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagot M, Nikolova M, Schirm-Chabanette F, Wechsler J, Boumsell L, Bensussan A. Crosstalk between tumor T lymphocytes and reactive T lymphocytes in cutaneous T cell lymphomas. Ann N Y Acad Sci. 2001;941(1):31–8. [DOI] [PubMed] [Google Scholar]
- Ionescu MA, Rivet J, Daneshpouy M, Briere J, Morel P, Janin A. In situ eosinophil activation in 26 primary cutaneous T-cell lymphomas with blood eosinophilia. J Am Acad Dermatol. 2005;52(1):32–9. [DOI] [PubMed] [Google Scholar]
- Ohmatsu H, Sugaya M, Suga H, Morimura S, Miyagaki T, Kai H, et al. Serum IL-31 levels are increased in patients with cutaneous T-cell lymphoma. Acta Derm Venereol. 2012;92(3):282–3. [DOI] [PubMed] [Google Scholar]
- Singer EM, Shin DB, Nattkemper LA, Benoit BM, Klein RS, Didigu CA, et al. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J Invest Dermatol. 2013;133(12):2783–5. [DOI] [PubMed] [Google Scholar]
- Hwang ST. Mechanisms of T-cell homing to skin. Adv Dermatol. 2001;17:211–41. [PubMed] [Google Scholar]
- Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194(10):1541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400(6746):776–80. [DOI] [PubMed] [Google Scholar]
- Britanova OV, Putintseva EV, Shugay M, Merzlyak EM, Turchaninova MA, Staroverov DB, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol. 2014;192(6):2689–98. [DOI] [PubMed] [Google Scholar]
- Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, et al. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci USA. 2011;108(33):13694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidgey A, Dudakov J, Seach N, Boyd R. Impact of niche aging on thymic regeneration and immune reconstitution. Semin Immunol. 2007;19(5):331–40. [DOI] [PubMed] [Google Scholar]
- Zook EC, Krishack PA, Zhang S, Zeleznik-Le NJ, Firulli AB, Witte PL, et al. Overexpression of Foxn1 attenuates age-associated thymic involution and prevents the expansion of peripheral CD4 memory T cells. Blood. 2011;118(22):5723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente R, Mausset-Bonnefont AL, Jorgensen C, Louis-Plence P, Brondello JM. Cellular senescence impact on immune cell fate and function. Aging Cell. 2016;15(3):400–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawalkar N, Ferenczi K, Jones DA, Yamanaka K, Suh KY, Sadat S, et al. Profound loss of T-cell receptor repertoire complexity in cutaneous T-cell lymphoma. Blood. 2003;102(12):4059–66. [DOI] [PubMed] [Google Scholar]
- Freedberg IM, Eisen AZ, Wolff K, Goldsmith LA, Katz SI. Cutaneous T cell lymphomas In Fitzpatricks dermatology in general medicine. 2003;1527–58.
- Berger CL, Tigelaar R, Cohen J, Mariwalla K, Trinh J, Wang N, et al. Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood. 2005;105(4):1640–7. [DOI] [PubMed] [Google Scholar]
- Foss FM, Girardi M. Mycosis Fungoides and Sezary Syndrome. Hematol Oncol Clin North Am. 2017;31(2):297–315. [DOI] [PubMed] [Google Scholar]
- Hastrup N, Ralfkiaer E, Pallesen G. Aberrant phenotypes in peripheral T cell lymphomas. J Clin Pathol. 1989;42(4):398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Gao L, Gong M, Tang Y, Li Y, Zhang WT, et al. Non-malignant T-cells lacking multiple pan-T markers can be found in lymph nodes. Leuk Lymphoma. 2018;59(1):155–61. [DOI] [PubMed] [Google Scholar]
- Willemze R, Cerroni L, Kempf W, Berti E, Facchetti F, Swerdlow SH, et al. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133(16):1703–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110(6):1713–22. [DOI] [PubMed] [Google Scholar]
- Kim YH, Liu HL, Mraz-Gernhard S, Varghese A, Hoppe RT. Long-term outcome of 525 patients with mycosis fungoides and Sezary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139(7):857–66. [DOI] [PubMed] [Google Scholar]
- Lin WM, Lewis JM, Filler RB, Modi BG, Carlson KR, Reddy S, et al. Characterization of the DNA copy-number genome in the blood of cutaneous T-cell lymphoma patients. J Invest Dermatol. 2012;132(1):188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer MH, van Doorn R, Dijkman R, Mao X, Whittaker S, Vader PC, et al. Novel and highly recurrent chromosomal alterations in Sézary syndrome. Cancer Res. 2008;68(8):2689–98. [DOI] [PubMed] [Google Scholar]
- Laharanne E, Oumouhou N, Bonnet F, Carlotti M, Gentil C, Chevret E, et al. Genome-wide analysis of cutaneous T-cell lymphomas identifies three clinically relevant classes. J Invest Dermatol. 2010;130(6):1707–18. [DOI] [PubMed] [Google Scholar]
- Choi J, Goh G, Walradt T, Hong BS, Bunick CG, Chen K, et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet. 2015;47(9):1011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed J, Gibson J, Lewis J, Carlson K, Foss F, Choi J, et al. FISH Panel for Leukemic CTCL. J Invest Dermatol. 2017;137(3):751–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida AC da S, et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet. 2015;47(12):1465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirt LY, Jia P, Baerenwald DA, Duszynski RJ, Dahlman KB, Zic JA, et al. Whole-genome sequencing reveals oncogenic mutations in mycosis fungoides. Blood. 2015;126(4):508–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantchev J, Villarreal AM, Xie P, Lefrançois P, Gunn S, Netchiporouk E, et al. The Ectopic Expression of Meiosis Regulatory Genes in Cutaneous T-Cell Lymphomas (CTCL). Front Oncol. 2019;9:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LW, Patrone CC, Yang W, Rabionet R, Gallardo F, Espinet B, et al. An Integrated Data Resource for Genomic Analysis of Cutaneous T-Cell Lymphoma. J Invest Dermatol. 2018;138(12):2681–3. [DOI] [PubMed] [Google Scholar]
- Pinzaru AM, Hom RA, Beal A, Phillips AF, Ni E, Cardozo T, et al. Telomere Replication Stress Induced by POT1 Inactivation Accelerates Tumorigenesis. Cell Rep. 2016;15(10):2170–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros RB. Telomere/telomerase dynamics within the human immune system: effect of chronic infection and stress. Exp Gerontol. 2011;46(2–3):135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406(6796):641–5. [DOI] [PubMed] [Google Scholar]
- Gros A, Laharanne E, Vergier M, Prochazkova-Carlotti M, Pham-Ledard A, Bandres T, et al. TP53 alterations in primary and secondary Sézary syndrome: A diagnostic tool for the assessment of malignancy in patients with erythroderma. PLoS One. 2017;12(3):e0173171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooler G, Melchior L, Ralfkiaer E, Gjerdrum LM, Gniadecki R. TP53 Gene Status Affects Survival in Advanced Mycosis Fungoides. Frontiers in medicine. 2016;3(31):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell D. Signaling in lymphocyte activation. Cold Spring Harb Perspect Biol. 2015;7(6):a018788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18(1):621–63. [DOI] [PubMed] [Google Scholar]
- Sors A, Jean-Louis F, Pellet C, Laroche L, Dubertret L, Courtois G, et al. Down-regulating constitutive activation of the NF-kappaB canonical pathway overcomes the resistance of cutaneous T-cell lymphoma to apoptosis. Blood. 2006;107(6):2354–63. [DOI] [PubMed] [Google Scholar]
- Wang L, Ni X, Covington KR, Yang BY, Shiu J, Zhang X, et al. Genomic profiling of Sézary syndrome identifies alterations of key T cell signaling and differentiation genes. Nat Genet. 2015;47(12):1426–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell A, Bhaduri A, Rios E, Reuter J, Lee CS, Mah A, et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet. 2015;47(9):1056–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui DH, Genetta T, Kadesch T, Williams TM, Swain SL, Tsui LV, et al. Transcriptional repression of the IL-2 gene in Th cells by ZEB. J Immunol. 1998;160(9):4433–40. [PubMed] [Google Scholar]
- Abraham RM, Zhang Q, Odum N, Wasik MA. The role of cytokine signaling in the pathogenesis of cutaneous T-cell lymphoma. Cancer Biol Ther. 2011;12(12):1019–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen KW, Kaltoft K, Mikkelsen G, Nielsen M, Zhang Q, Geisler C, et al. Constitutive STAT3-activation in Sezary syndrome: tyrphostin AG490 inhibits STAT3-activation, interleukin-2 receptor expression and growth of leukemic Sezary cells. Leukemia. 2001;15(5):787–93. [DOI] [PubMed] [Google Scholar]
- Fantin VR, Loboda A, Paweletz CP, Hendrickson RC, Pierce JW, Roth JA, et al. Constitutive activation of signal transducers and activators of transcription predicts vorinostat resistance in cutaneous T-cell lymphoma. Cancer Res. 2008;68(10):3785–94. [DOI] [PubMed] [Google Scholar]
- DeStefano CB, Desale S, Fernandez SJ, Shenoy AG. The impact of environmental ultraviolet exposure on the clinical course of mycosis fungoides. J Am Acad Dermatol. 2019;81(5):1074–7. [DOI] [PubMed] [Google Scholar]
- Dewey WC, Ling CC, Meyn RE. Radiation-induced apoptosis: relevance to radiotherapy. Int J Radiat Oncol Biol Phys. 1995;33(4):781–96. [DOI] [PubMed] [Google Scholar]
- Queen D, Lopez A, Geskin LJ. Emerging therapies for cutaneous T-cell lymphoma. Medicine Matters Oncology. 2019. Available from: https://oncology.medicinematters.com/cutaneous-t-cell-lymphoma/hematologic-cancers/emerging-therapies-for-cutaneous-t-cell-lymphoma/16332082
- Pérez C, González-Rincón J, Onaindia A, Almaráz C, García-Díaz N, Pisonero H, et al. Mutated JAK kinases and deregulated STAT activity are potential therapeutic targets in cutaneous T-cell lymphoma. Haematologica. 2015;100(11):e450–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrenne BM, Lewis JM, Weed JG, Carlson KR, Mirza FN, Foss FM, et al. Synergy of BCL2 and histone deacetylase inhibition against leukemic cells from cutaneous T-cell lymphoma patients. Blood. 2017;130(19):2073–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Lewis JM, Cyrenne BM, Monico PF, Mirza FN, Carlson KR, et al. BET inhibition in advanced cutaneous T cell lymphoma is synergistically potentiated by BCL2 inhibition or HDAC inhibition. Oncotarget. 2018;9(49):29193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–6. [DOI] [PubMed] [Google Scholar]
- Foo SH, Shah F, Chaganti S, Stevens A, Scarisbrick JJ. Unmasking mycosis fungoides/Sézary syndrome from preceding or co-existing benign inflammatory dermatoses requiring systemic therapies: patients frequently present with advanced disease and have an aggressive clinical course. Br J Dermatol. 2016;174(4):901–4. [DOI] [PubMed] [Google Scholar]
- Nguyen V, Huggins RH, Lertsburapa T, Bauer K, Rademaker A, Gerami P, et al. Cutaneous T-cell lymphoma and Staphylococcus aureus colonization. J Am Acad Dermatol. 2008;59(6):949–52. [DOI] [PubMed] [Google Scholar]
- Talpur R, Bassett R, Duvic M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sézary syndrome. Br J Dermatol. 2008;159(1):105–12. [DOI] [PubMed] [Google Scholar]
- Lindahl LM, Willerslev-Olsen A, Gjerdrum LM, Nielsen PR, Blümel E, Rittig AH, et al. Antibiotics inhibit tumor and disease activity in cutaneous T-cell lymphoma. Blood. 2019;134(13):1072–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeer MH. Antibiotics can improve CTCL. Blood. 2019;134(13):1000–1. [DOI] [PubMed] [Google Scholar]
- Fanok MH, Sun A, Fogli LK, Narendran V, Eckstein M, Kannan K, et al. Role of Dysregulated Cytokine Signaling and Bacterial Triggers in the Pathogenesis of Cutaneous T-Cell Lymphoma. J Invest Dermatol. 2018;138(5):1116–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümel E, Willerslev-Olsen A, Gluud M, Lindahl LM, Fredholm S, Nastasi C, et al. Staphylococcal alpha-toxin tilts the balance between malignant and non-malignant CD4+ T cells in cutaneous T-cell lymphoma. OncoImmunology. 2019;8(11):e1641387. [DOI] [PMC free article] [PubMed] [Google Scholar]