Abstract
Cutaneous lupus erythematosus (CLE) is an autoimmune disease of the skin with significant morbidity. Current treatments are often inadequate to control disease and there are no Food and Drug Administration (FDA)-approved therapies for this potentially debilitating disease, underscoring an unmet medical need. Recent insights into disease pathogenesis have implicated innate and adaptive immune components, including type I and type III interferons in the development of CLE. Promising clinical trials based on these insights are now underway. However, the full spectrum of immune cells, cytokines, and environmental triggers contributing to disease remain to be elucidated. In this review, we will highlight the current understanding of CLE immunopathogenesis, the ongoing clinical trial landscape, and provide a framework for designing future therapeutic strategies for CLE based on new insights into disease pathogenesis.
Keywords: cutaneous lupus erythematosus, discoid lupus, systemic lupus erythematosus
Introduction
Cutaneous lupus erythematosus (CLE) is an autoimmune skin disease that severely impairs quality of life [1]. CLE may present as skin disease alone or may occur in the setting of systemic lupus erythematosus (SLE), a severe multiorgan autoimmune disease with a wide variety of disease manifestations. In addition, patients initially diagnosed with isolated CLE may later progress to SLE. CLE is subdivided into acute, subacute, or chronic cutaneous lupus erythematosus (ACLE, SCLE, or CCLE, respectively) based on lesion morphology and histopathology. CCLE accounts for approximately 80% of CLE [2], and discoid lupus erythematosus (DLE) comprises the majority of CCLE.
The CLE subsets not only have different morphology and histopathology, but also have different rates of association with SLE. ACLE is characterized by malar erythema (the classic “butterfly rash” of lupus) and/or widespread photodistributed erythema, and it is almost always found in association with SLE. In contrast, patients with localized DLE present with skin lesions limited to the head and neck that are characterized initially by erythema, induration, and scale, followed by the development of scarring, hypopigmentation, atrophy, and permanent alopecia. A subset of patients with DLE can develop more extensive or generalized lesions beyond the head and neck to involve the trunk and extremities. Patients initially diagnosed with DLE develop systemic involvement in 10-20% of cases, with some studies suggesting that patients with generalized lesions are at higher risk than those with localized DLE [3-5].
In addition to these CLE-specific skin lesions, non-specific cutaneous manifestations may occur in patients with SLE, including non-scarring hair loss, vasculitis, and Raynaud’s syndrome. It is estimated that 70-80% of all patients with SLE will have skin or hair involvement at some point, and a cutaneous finding is the initial disease manifestation for 20-25% of SLE patients [6].
There are currently no FDA-approved targeted therapies available for CLE and existing treatments are often ineffective for many patients. Therapeutic trials for SLE medications often exclude CLE patients who do not meet the criteria for SLE [7], which may hinder the development of FDA approved therapies for this potentially devastating skin disease. Herein, we highlight the current understanding of CLE immunopathogenesis, the ongoing clinical trial landscape, and provide a framework for designing future therapeutic strategies for CLE based on new insights into disease pathogenesis.
Pathogenesis of CLE
Both SLE and CLE are multifactorial diseases, with a complex interaction occurring between environmental exposures and genetic susceptibility that triggers and/or propagates immune dysregulation, resulting in disease in affected individuals. As has been found for SLE, it may be that much of an individual’s susceptibility to CLE is due to the accumulation of various risk alleles, with disease development ultimately determined by the interaction of these genetic variations with the environment [8-10].
Genetics
Genetic studies, including those of families, of affected individuals, and of affected populations in genome-wide association studies (GWAS), have identified genetic polymorphisms, mutations and risk alleles in CLE populations. The vast majority of these identified genes are involved in pathways that affect the function of innate and adaptive immune responses, predisposing to immune dysregulation. Among others, these include apoptosis/cell death, DNA processing, the type I interferon pathway, migration of leukocytes, the complement cascade and clearance of cell debris, T-cell immune checkpoints, antigen presentation, and antibody production [11-13]. Complete coverage of the genetic associations with CLE is beyond the scope of this review, and for further detail, readers are directed to recent reviews with broader coverage of this topic [8,9,14].
Mutations in the Three Prime Repair Exonuclease 1 (TREX1) represent the only monogenic cause of cutaneous lupus identified to date, resulting in a rare form of CCLE called familial chilblain lupus [15]. These patients develop cold-induced purple-red lesions on acral surfaces, which may ulcerate. TREX1 is a cytosolic DNA exonuclease that plays an essential role in the homeostatic degradation of single stranded DNA (ssDNA), and TREX1 deficiency results in intracellular ssDNA accumulation. Recognition of these accumulated nucleic acids by innate immune receptors results in chronic hyperactivation of the type I interferon pathway [16].
Female gender has long been known as a major risk factor in the development of many autoimmune diseases, including SLE and CLE. Sex hormones are some of the most well-studied potential contributors to this sex bias [17], however recent investigation into human skin sexual dimorphism identified the putative transcription factor vestigial-like family member 3 (VGLL3) as an essential regulator of female-biased genes that may contribute to an autoimmune phenotype in women. VGLL3 influences type I interferon responses and promotes the expression of genes encoding inflammatory molecules, many of which are genetic risk variants previously identified in autoimmune diseases including SLE. Unlike in normal skin, where VGLL3 is more highly expressed in female-derived tissue, in SCLE skin, VGLL3 expression levels were similar between males and females, and skin-directed overexpression of VGLL3 in mice causes a lupus-like disease with cutaneous manifestations, suggesting that VGLL3 may play a role in the pathogenesis of CLE [18,19].
In addition to genetic mutations and polymorphisms that predispose to CLE, external stimuli may interact with the genome in susceptible individuals to cause epigenetic variation, leading to dysregulated gene expression via DNA methylation, histone modification, and microRNA-mediated gene silencing. Potentially pathogenic epigenetic changes have been described in SLE and include DNA hypomethylation in T cells, which results in increased inflammatory gene expression. Several microRNAs are dysregulated in SLE, and at least four of those upregulated in T cells promote hypomethylation [20]. Histone modifications are also found in SLE patient peripheral blood mononuclear cells [10,21]. MicroRNA profiling of DLE lesional skin identified overexpression of keratinocyte-derived miR-31, which is upregulated upon ultraviolet (UV) exposure and induces keratinocyte apoptosis and inflammatory cytokine production. Leukocyte-derived miR-485-3p was also identified, which induces T cell activation and pro-inflammatory cytokine production [22]. DNA methylation patterns of naïve CD4+ T cells in SLE patients reveal differentially methylated regions (DMRs) associated with the development of malar rash or DLE. These DMRs involve genes mediating cell proliferation, apoptosis, and antigen presentation, suggesting a role in pathogenesis [23]. DNA methylation analyses in SCLE patients reveal demethylation of the perforin and CD70 promoters in CD4+ T cells. Both perforin and CD70, a B-cell costimulatory molecule expressed on T cells, are overexpressed in SCLE T cells, suggesting a possible pathogenic link [24,25]. Further investigation into the role of the epigenome in CLE is needed and may yield targets for therapy to restore normal epigenetic patterns.
Environmental
Ultraviolet (UV) light exposure is a common provoking factor for CLE, and photosensitivity is one of the 11 American College of Rheumatology criteria for SLE. Between 60-80% of CLE patients report photosensitivity, and patients with ACLE are more likely to report photosensitivity than those with SCLE or CCLE [26,27]. UV irradiation directly induces chemokine production by epithelial cells, and it also causes DNA damage, resulting in keratinocyte apoptosis and necrosis. Dying keratinocytes release inflammatory cytokines and chemokines, which in turn recruit lymphocytes and plasmacytoid dendritic cells (pDCs). Keratinocyte death may also result in release of nuclear debris, which can stimulate pDCs via Toll-like receptors (TLRs) and can also serve as a reservoir of autoantigen for autoreactive T and B cells [28]. Recently, lupus-prone mouse studies have identified Langerhans cells (LC) as a source of protective epidermal growth factor receptor (EGFR) ligands that prevent UV-induced keratinocyte death and decrease the development and severity of UV-induced lupus skin lesions. Nonlesional skin from SLE patients demonstrated decreased LC numbers and epidermal EGFR phosphorylation, suggesting a possible correlate in human SLE [29]. Currently, the only treatment for photosensitivity is sunlight avoidance and broad-spectrum, high sun protective factor (SPF) sunscreen, which prevents the development of disease-specific skin lesions in CLE patients exposed to UVA/UVB [30].
Cigarette smoking is associated with CLE, and it is suggested that tobacco smoke contributes to CLE disease activity by increasing inflammatory cytokines, apoptosis, autoantibodies, and the development of free radicals. Compared with non-smokers, smokers with CLE have worse quality of life and worse skin disease, as measured by the Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI). There is conflicting data regarding whether smokers respond to antimalarials as well as non-smokers [31,32], but smokers exhibit more recalcitrant disease than non-smokers if both antimalarials and immunomodulators are required [32,33]. It is still unknown whether decreased treatment efficacy in smokers is due to direct interference of cigarette smoke with the treatment or to the higher disease severity in smokers [34]. It is also possible that some smokers may have lower medication adherence rates; current smokers have been found to be less adherent with recommended preventative care recommendations and medications in other clinical settings [35].
Drug-induced SLE (DI-SLE) is an established adverse effect of certain medications, historically most commonly due to procainamide, hydralazine, and quinidine and less frequently due to a number of other medications, including minocycline, penicillamine, carbamazepine, methyldopa, sulfasalazine, chlorpromazine, propylthiouracil, and isoniazid. Medications highly associated with DI-SLE are thought to enhance innate immune responses, particularly of neutrophils, resulting in increased neutrophil extracellular trap (NET) formation and autoantigen exposure [36]. In addition, procainamide and hydralazine both inhibit DNA methylation, and thus may cause DI-SLE via epigenetic effects [20]. DI-SLE from procainamide rarely involves the skin, but cutaneous involvement is reported in up to one third of cases of DI-SLE due to hydralazine or quinidine [36]. Cutaneous manifestations may also be seen in up to one quarter of DI-SLE cases due to minocycline [37]. Drug-induced skin-limited SCLE and much less commonly CCLE may occur due to other medications. A systematic review of drug-induced SCLE found the most frequently reported causative medications to be antihypertensives (most commonly hydrochlorothiazide and calcium channel blockers) and terbinafine, with less frequent reports of many other medications including chemotherapeutics, antihistamines, leflunomide, interferon, antiepileptics, statins, lansoprazole, and non-steroidal anti-inflammatory drugs (NSAIDs) such as naproxen and piroxicam [38]. A subsequent population-based matched case-control study found an increased odds ratio of developing SCLE within 6 months of medication initiation for patients prescribed terbinafine, TNF-α inhibitors, proton pump inhibitors, carbamazepine, platelet inhibitors, ACE inhibitors, and NSAIDs [39]. It is hypothesized that DI-SLE or SCLE due to TNF-α inhibitors may be in part due to the immunogenicity of the medications themselves, though more recent formulations have lower immunogenicity and DI-SLE/SCLE continue to be reported to these agents. There may also be a component of “unmasking” rather than causing the SLE or SCLE, as some patients treated with TNF-α inhibitors have conditions that are associated with a higher baseline risk of SLE, such as rheumatoid arthritis [36]. In addition, TNF-α functions to inhibit both the development of pDCs and their IFN-α production. Treatment with TNF-α inhibitors results in IFN-α activation, which may thus promote the development of interferon-driven diseases (e.g. CLE and SLE) [40].
Studies investigating the microbiome in SLE patients have suggested that host-microbe interactions contribute to the development of disease. Molecular mimicry is proposed to play a role in the development and propagation of autoimmunity in SLE and SCLE patients with anti-Ro (SS-A) antibodies. An evolutionarily conserved Ro60 protein ortholog was identified in a subset of human skin, oral, and gut commensal bacteria, which was found to be cross-reactive with both the SCLE/SLE patient’s anti-Ro antibodies as well as their Ro60 autoreactive T cell clones [41]. The host microbiome has also been implicated in development of SLE via Enterococcus gallinarum bacterial translocation from the gut to the liver and other systemic tissues, promoting the development of autoantibodies and SLE-like disease in autoimmune-prone mice. E. gallinarum-specific DNA was also identified in liver biopsies of SLE and autoimmune hepatitis patients, suggesting that translocation of a gut pathobiont may cause human autoimmune disease [42]. Microbiome-host interactions and their influence on host autoimmune disease is an area of active research, and their role, if any, in the development or propagation of CLE remains to be elucidated [43].
Immunopathogenesis
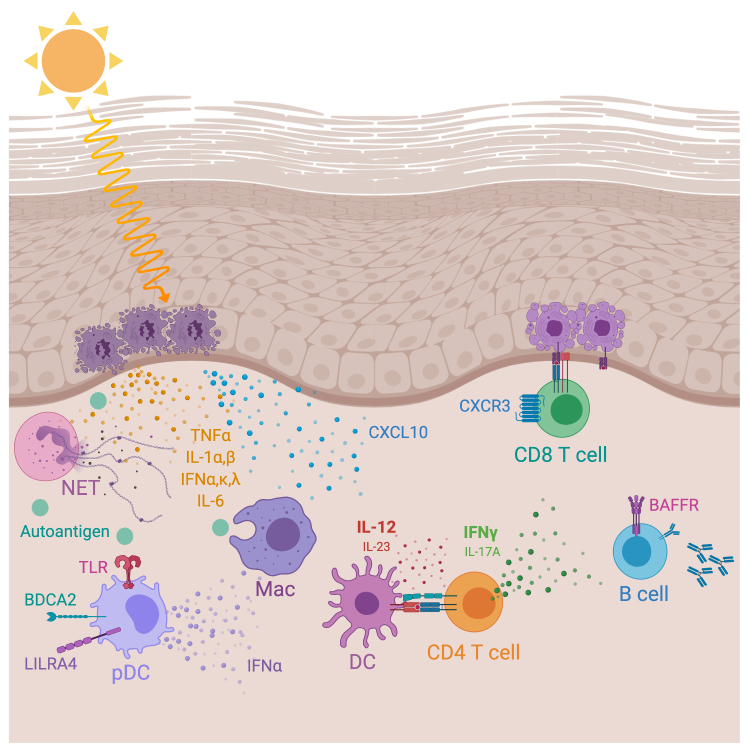
Insights from genetic studies and environmental triggers in lupus pathogenesis implicate both innate and adaptive immune components. Overall, CLE is a disease of dysregulated immune homeostasis, resulting in unwanted innate immune stimulation and adaptive immune activation. The autoimmune pathways involved in CLE development and pathogenesis remain incompletely understood. It must also be emphasized that the sequence of events from environmental trigger, if any, to immune activation to disease is also unknown. However, there is substantial data to suggest that CLE is a disease of type I interferon (IFN) excess and resultant cytotoxic CD8+ T cell attack of the epidermis (Figure 1) [7,8,14,28,44-46]. Although animal models of lupus help inform potential mechanisms of disease pathogenesis, they do not always recapitulate human disease [47]. Our discussion is limited to evidence from human CLE patients.
Figure 1.
Immunopathogenesis of cutaneous lupus. Ultraviolet (UV) radiation induces keratinocyte necrosis or apoptosis, resulting in the release of proinflammatory cytokines including tumor necrosis factor α (TNFα), interleukin-1α (IL-1α) and IL-1β, IL-6 and interferon (IFN) α,κ,λ as well as chemokine CXCL10. Autoantigen release from dying keratinocytes admixed with neutrophil extracellular traps (NETs) activates pDCs to release IFNα. Dendritic cells (DC) secrete IL-12 or IL-23 to activate CD4+ T cells to secrete IFNγ or IL-17A, respectively. CD8+ T cells expressing CXCR3 are recruited to dermal-epidermal junction via CXCL10 and attack keratinocytes, resulting in keratinocyte apoptosis (vacuolar interface dermatitis). B cells expressing BAFF (B cell activating factor) receptor secrete autoantibodies. Macrophages (Mac) phagocytose autoantigens released from dying keratinocytes and help prime adaptive immune lymphocytes against keratinocytes. BDCA2, blood dendritic cell antigen 2; LILRA4, leukocyte immunoglobulin-like receptor subfamily A member 4; TYK2, tyrosine kinase 2.
CLE as an Interferonopathy
Inherited autoinflammatory disorders with excessive type I IFN signaling are referred to as type I interferonopathies. Some of these disorders develop CLE-like phenotypes, such as Aicardi-Goutières syndrome and familial chilblain lupus [48]. Although CLE encompasses several types of skin and molecular phenotypes, type I IFNs appear to be central to disease development [49]. There are 17 type I IFN family members that share a common receptor, the interferon-α/β receptor (IFNAR) [50]. Another member of type I IFN family, IFNκ, is of considerable interest given it is produced by keratinocytes in response to stress along with IFNα [51]. Multiple gene expression studies have demonstrated upregulation of type I IFNs and IFN-stimulated genes in CLE [52-55]. Importantly, type I IFN genes are associated with disease activity in CLE [54]. The consequences of type I IFN are broad and include activation of innate immune cells and promotion of adaptive immunity. A potential initial source of type I IFNs are keratinocytes in response to UV irradiation [51,56]. Type I IFN release activates multiple innate immune cells including neutrophils, macrophages, and plasmacytoid dendritic cells (pDCs). The most likely source of continuing type I IFN production, and thereby promotion and maintenance of disease progression, are pDCs. Upon UV stimulation of skin, pDCs accumulate and are found in abundant numbers in CLE tissues [57,58]. Type I IFNs also help to promote release of IFNγ and its subsequent induction of chemokines, including CXCL10, that attract cytotoxic CD8+ T cells expressing CXCR3 to the dermal-epidermal junction of CLE [59-64]. Thus, pDCs may be one of the most critical immune cells contributing to disease pathogenesis given the secretion of type I IFNs, which in turn leads to IFNγ release. On the other hand, T cells are the most abundant cell type within CLE [52], and cytotoxic CD8+ T cells are the driver of keratinocyte death and disruption of the dermal-epidermal junction [52,64]. Both cells appear to be absolutely critical to pathogenesis, and emerging therapies are designed to target these cells. In addition to type I and type II IFNs, there is also evidence that type III IFNs (IFNλ) produced by keratinocytes contribute to CLE disease [65]. Type III IFNs are considered critical immunomodulatory cytokines that play important roles in host defense at barrier tissues such as the skin [50]. Taken together, all three families of IFNs appear to contribute to CLE disease pathogenesis and appear to be the most critical immune pathway involved, implicating CLE as an acquired interferonopathy.
Keratinocytes as Innate Immune Cells in CLE
Keratinocytes are the essential cell type that comprises the epidermal barrier of skin. Therefore, keratinocytes are key players in barrier immunity where they perform specialized functions in response to environmental insults, skin microbiome, and pathogen invasion. Furthermore, keratinocytes interact with host immunity and often serve as innate immune cells by releasing “alarmins” and danger signals that activate neighboring immune sentinels and recruit adaptive immune lymphocytes to areas of stress or damage [66,67]. Thus, the release of alarmins such as keratins, S100 proteins, and HMGB1 upon keratinocyte death can help initiate and amplify an immune response [68,69]. In CLE, UV light can trigger necrosis or apoptosis of keratinocytes, resulting in release of proinflammatory cytokines interleukin-1α (IL-1α), IL-1β, IL-6, TNFα, IFNα, IFNκ, IFNλ, and chemoattractants such as CXCL10 [45,51,56,63,65,70-72]. Nuclear debris such as RNA and DNA may activate pDCs through toll-like receptors (TLRs), resulting in type I IFN release. Together, keratinocytes and IFNs appear to initiate and sustain disease activity.
Other Cytokines Involved in CLE
Multiple other cytokines have been detected in CLE tissue [63,73]. However, the importance of each detected cytokine is unclear. Given the complex interplay of keratinocytes, IFNs, and innate and adaptive immune cells, it is no surprise that multiple inflammatory pathways are active in CLE. It is reasonable to assume that the major drivers of disease are also the most abundant and consistent pathways activated, such as IFNs in CLE. However, the true test to determine the major pathogenic inflammatory pathway is through clinical trials investigating targeted therapy in patients with CLE. Since these trials are in the earlier phases of development, it is unclear which pathway will be the most effective to target. Some studies have demonstrated the presence of IL-17A in CLE [74,75]. However, another study comparing DLE with psoriasis, which displays a pathogenic Th17 pathway (IL-17), found that DLE showed an IFNγ and Th1 predominant expression pattern [76]. Secukinumab, a monoclonal (mAb) targeting IL-17A is currently under investigation for CLE (NCT03866317). IL-18 may also be contributing to CLE pathogenesis by inducing the secretion of TNFα from keratinocytes [77], however no targeting strategy to block IL-18 is in clinical trials for CLE. In contrast, blockade of IL-12/IL-23 cytokines with ustekinumab is in clinical trials and has shown efficacy in CLE [78]. Gene expression studies have demonstrated elevated IL-12 in lesional CLE skin as compared to non-lesional skin [73].
JAK-STAT Pathway in CLE
After cytokines engage with their respective receptors, intracellular signaling pathways transmit those signals to induce cellular functions through gene transcription, protein translation, and protein trafficking. The Janus kinase (JAK) and signal transducer and activator of transcription (STAT) pathways are critical for a diverse array of downstream cytokine receptor signaling [79]. Approximately 60 cytokines, including the interferons, transmit molecular instructions through the JAK-STAT pathway [80]. Type I IFNs use JAK1, TYK2, STAT1, and STAT2 to induce the expression of hundreds of interferon-stimulated genes (ISGs) frequently found upregulated in CLE tissues [52-55]. In contrast, type II IFNγ uses JAK1, JAK2, and STAT1. In fact, many of the cytokines implicated in CLE pathogenesis utilize the JAK-STAT pathway including IL-6, IL-12, IL-23, and the interferons. Therefore, it is not surprising that lesions of CLE express elevated levels of STAT and JAK proteins [73]. Given that multiple cytokine receptors utilize the JAK-STAT signaling cascade, drugs targeting JAKs have shown recent efficacy in the treatment of numerous autoimmune diseases of the skin including psoriasis, atopic dermatitis, dermatomyositis, vitiligo, and alopecia areata [81]. Current clinical trials using JAK inhibitors in CLE are ongoing (Table 1).
Table 1. Current clinical trials for CLE.
| Drug name | Target | Phase |
| Belimumab | BAFF (BLyS) | 3 |
| Ustekinumab | IL-12/IL-23 | 3 |
| Secukinumab | IL-17A | 2 |
| Anifrolumab | IFNAR1 | 3 |
| Filgotinib | JAK1 | 2 (in combination with lanraplenib) |
| Lanraplenib | SYK | 2 (in combination with filgotinib) |
| Tofacitinib | JAK 1 and JAK3 | 2 |
| BMS-986165 | TYK2 | 2 |
| BIIB059 | BDCA2 (CD303) | 2 |
| VIB7734 | LILRA4 (ILT7) | 1 |
BAFF, B cell-activating factor; BLyS, B lymphocyte stimulator; BDCA2, blood dendritic cell antigen 2; IFNAR1, interferon alpha receptor subunit 1; IL, interleukin; ILT7, immunoglobulin-like transcript 7; JAK, Janus kinase; LILRA4, leukocyte immunoglobulin-like receptor subfamily A member 4; SYK, spleen tyrosine kinase; TYK2, tyrosine kinase 2.
Autoantigens and Autoantibodies in CLE
During keratinocyte cell death, nuclear material and cellular debris are released, including potential autoantigens. In combination with alarmins, innate immune cells become poised to uptake and process keratinocyte autoantigens, which may amplify and sustain ongoing autoimmunity. Priming of the adaptive immune response with autoantigens results in T cell activation against cells harboring those antigens as well as the production of autoantibodies by B cells. Although the specific autoantigens in CLE remain elusive, the detection of autoantibodies such as Ro suggest that they may be playing a role in disease progression [26]. However, not all patients with CLE have detectable autoantibodies, which is in contrast to SLE which nearly always has autoantibodies present. In those CLE patients that do have autoantibodies, disease activity positively correlates with autoantibody concentrations in the serum [82]. In SLE, B cells are thought to be critical players in the development of disease through autoantibody production. Targeting a cytokine critical for B cells (B cell activation factor, BAFF) with belimumab led to the first new FDA-approved drug for SLE in 50 years [83]. B cells and associated BAFF and BAFF receptors are found in CLE [84] and the effect of belimumab in CLE is currently under investigation.
In SLE, neutrophils are thought to be important for presentation of autoantigens through the generation of neutrophils extracellular traps (NETs) [85]. Recently, NETs were detected in CLE skin [86]. However, it is unknown if NETs in CLE are presenting autoantigens to the multiple myeloid subsets found in CLE such as conventional DCs, CD163+ macrophages or CD68+ macrophages [87,88]. Finally, defects in apoptosis or clearance of apoptotic cells have been hypothesized to play a role in lupus pathogenesis by increasing autoantigen exposure and proinflammatory cytokine release [89]. On the other hand, type I IFNα induces keratinocyte apoptosis [55]. Thus, the role of apoptosis and apoptotic signaling in CLE need further investigation.
Treatment of CLE
Current Therapy for CLE
Although there are no FDA-approved medications for CLE, established therapies for CLE are effective for many, but not all, patients with CLE [7,14]. In this review, we will focus on new emerging therapies for CLE and only briefly discuss current established therapies [7,14]. Most of these established therapies involve general immunosuppressants or therapies borrowed from SLE. Nevertheless, dermatologists and rheumatologists successfully treat CLE patients with ultrapotent topical and intralesional corticosteroids and anti-malarial therapies such as hydroxychloroquine, which are recommended as first-line treatments [90]. Although the mechanism of anti-malarial drugs is incompletely understood, there is evidence that they inhibit antigen processing and presentation by DCs as well as masking stimulatory DNA epitopes, preventing their recognition by endosomal TLR9 in pDCs, thereby reducing type I IFN production [91]. Thus, hydroxychloroquine may more selectively impair the type I IFN response than other broad immunosuppressants. Nevertheless, in difficult to treat cases other broad immunosuppressants such as systemic corticosteroids, methotrexate, mycophenolate mofetil, azathioprine and cyclosporine are also used with varying degrees of success [92]. Thalidomide is another treatment option for patients with recalcitrant CCLE that has been demonstrated to be very effective [93-96]. In addition, a thalidomide analogue, lenalidomide, has also shown efficacy in cutaneous lupus [97]. Thalidomide and lenalidomide [94] are likely effective due to their immunomodulatory effects, including a reduction of proinflammatory cytokine TNF-α [98]. Despite success with these medications, some patients still have recalcitrant disease or treatment-related toxicity, underscoring the need for improved therapies. Recent insights into pathogenesis provide promise for new pathogenesis-directed therapeutics for CLE.
Emerging Pathogenesis-directed Therapy for CLE
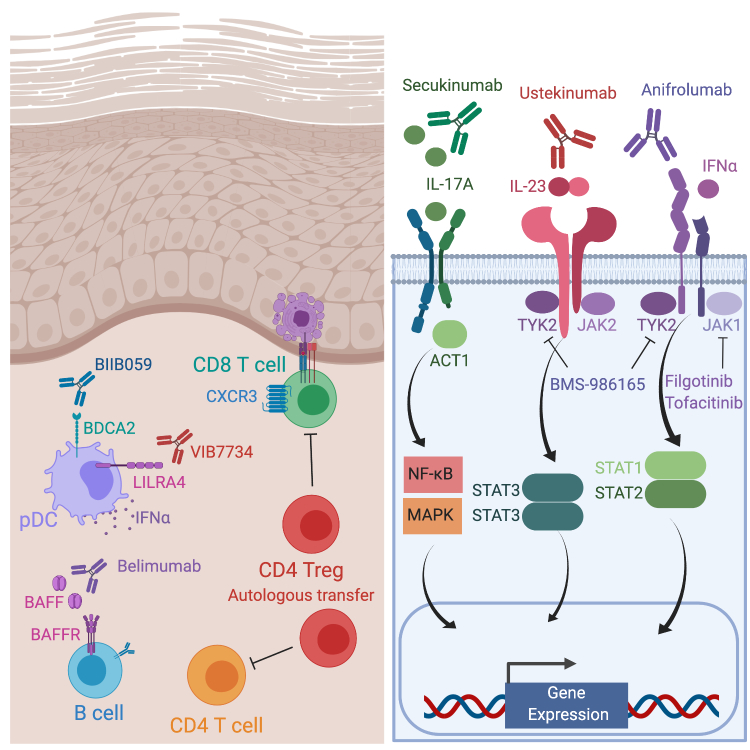
Critical insights gained into the pathogenesis of CLE in addition to explosive immunomodulatory drug development with more precise mechanisms of action have resulted in unprecedented opportunity to develop pathogenesis-directed therapies for CLE. Multiple distinct strategies to modulate the immune system are now under active investigation to ameliorate disease (Figure 2) (Table 1). One key advance in monitoring response to therapy is the cutaneous lupus erythematosus disease activity and severity index (CLASI) [99], which is now being used as a primary or secondary endpoint in clinical trials.
Figure 2.
Emerging therapeutic strategies for cutaneous lupus. Cellular targets including belimumab against BAFF, BIIB059 against BDCA2 on plasmacytoid dendritic cells (pDCs), V1B7734 against LILRA4 on pDCS and autologous transfer of Tregs (left). Secukinumab binds to IL-17A, ustekinumab binds to the IL12p40 subunit shared by IL-12 and IL-23, and anifrolumab binds IFNAR1 subunit (right). Intracellular targeted therapies include BMS-986165 which inhibits TYK2, and filgotinib and tofacitinib which block JAK1 (right). BAFF, B cell-activating factor; BAFFR, BAFF receptor; BDCA2, blood dendritic cell antigen 2; IFNAR1, interferon alpha receptor subunit 1; IL, interleukin; ILT7, immunoglobulin-like transcript 7; JAK, Janus kinase; LILRA4, leukocyte immunoglobulin-like receptor subfamily A member 4; STAT, signal transducer and activator of transcription; TYK2, tyrosine kinase 2.
Cellular Targets
A wide variety of adaptive and innate immune cells have been investigated for a potential role in CLE pathogenesis, including T-cells, B-cells, dendritic cells (particularly pDCs), macrophages, and neutrophils [28]. Although clinical trials enrolling patients with CLE are limited [7], trials employing targeted therapies against pDCs and B cell signaling have shown promise. The humanized monoclonal antibody BIIB059 targets blood DC antigen 2 (BDCA2), a receptor specifically expressed on pDCs that, when bound by BIIB059, is rapidly internalized, resulting in decreased pDC production of type I interferons, cytokines, and chemokines. A phase 1B randomized, double-blind, placebo-controlled trial of BIIB059 in patients with SLE and active cutaneous disease showed a reduction in CLASI-A score in treated patients vs placebo controls, and this clinical improvement correlated with normalization of the type I interferon response in the skin [100]. A phase 2 clinical trial of BIIB059 in CLE patients with or without systemic manifestations is currently underway (NCT02847598). An additional phase 1 clinical trial targeting pDCs in systemic and cutaneous lupus is underway, employing VIB7734, a monoclonal antibody against the pDC-specific cell-surface molecule leukocyte immunoglobulin-like receptor subfamily A member 4 (LILRA4, also known as ILT7) (NCT03817424).
Trials targeting B cells have shown mixed results. The humanized anti-CD20 monoclonal antibody rituximab depletes mature B cells, sparing early B-cell precursors and plasma cells. Case reports and open-label retrospective studies initially suggested that B-cell depletion with rituximab was effective for CLE [101], but later studies of SLE patients with CLE revealed that clinical improvement was limited to ACLE and bullous lupus, with no response in CCLE patients [102]. In addition, a subset of SLE patients who initially lacked skin disease or had baseline ACLE developed new SCLE or DLE lesions after rituximab therapy [101]. A non-depleting B-cell targeted therapy under investigation for CLE is belimumab, a monoclonal antibody against B-cell activating factor (BAFF, also known as BLyS), a cytokine that promotes B-cell differentiation and survival. Belimumab was FDA-approved for SLE in 2011, making it the first new FDA-approved SLE treatment in 50 years and the only biological therapy approved for the treatment of SLE [103]. Post-hoc analyses of the phase 3 trials of belimumab plus standard therapy in SLE patients showed improvement in mucocutaneous findings [104], and subsequent case series and observational studies have suggested that belimumab is effective in CLE [103,105,106]. Compared with normal skin, DLE and SCLE lesional keratinocytes exhibit significantly higher BAFF expression, and treatment of cultured keratinocytes with immunostimulatory DNA induces BAFF expression [107]. In addition, the DLE and SCLE lymphocytic infiltrate expresses high levels of BAFF receptor, suggesting potentially pathogenic cross-talk between keratinocytes and skin-infiltrating lymphocytes [84,107]. Belimumab is currently undergoing a Phase 3 clinical trial for efficacy in therapy-resistant CLE (EudraCT 2017-003051-35).
Other cell types have been targeted in previous clinical trials with less success. Treatment of SCLE and DLE patients with a monoclonal antibody against macrophage colony stimulating factor (M-CSF), a growth factor that supports the differentiation and proliferation of macrophages and monocytes, resulted in a reduction of a subset of circulating monocytes but failed to improve CLASI or affect tissue macrophages [108]. A trial in DLE patients using efalizumab, a monoclonal antibody against integrin alpha L (ITGAL, also known as CD11a or lymphocyte function-associated antigen 1) that inhibits T-cell activation and migration, was terminated due to inadequate enrollment (NCT00308204); efalizumab was subsequently voluntarily withdrawn from the market due to risk of the potentially fatal disorder progressive multifocal leukoencephalopathy [109]. Agents targeting sphingosine-1 phosphate receptor 1 (S1PR1) inhibit lymphocyte migration by preventing egress from the secondary lymphoid organs and thymus, and the S1PR functional antagonist fingolimod (also known as FTY720) is approved for treatment of multiple sclerosis [110]. Pre-clinical studies in murine models of lupus suggest a role for targeting S1PR1 in SLE [111-113], and a phase II clinical trial of the S1PR1/ S1PR4-selective functional antagonist KRP203 was performed in SCLE patients (NCT01294774). However, this trial was completed in 2012 without published results, and no additional trials targeting S1PR are in process for cutaneous lupus.
Multiple additional cell-targeted therapeutics are in clinical trials for SLE, including molecules targeting B-cells, T-cells, plasma cells, and the T and B-cell costimulatory molecules essential for B-cell activation and antibody production [114,115]. Their role in the treatment of CLE, if any, remains to be determined.
Cytokine Blockade
Perhaps the most successful therapeutic strategy for cutaneous autoimmune diseases has been blockade of cytokines implicated in disease pathogenesis. This approach has revolutionized treatment for psoriasis and atopic dermatitis. Advances in cytokine and transcriptional profiling have unveiled a complex array of immune pathways upregulated in CLE tissue as compared to control skin [53]. Most notable is the type I IFN system.
The type I IFN pathway is considered a hallmark of SLE and CLE disease pathogenesis. All 17 members of the type I IFN family share a common receptor (IFNAR), including IFNκ which is produced by keratinocytes in response to stress in CLE. Antibodies targeting IFNα (sifalimumab and rontalizumab) failed to show clinical benefit in SLE [116,117]. Sifalimumab did show a reduction in CLASI, but the drug was discontinued to pursue more promising results from anifrolumab [116]. Targeting the receptor subunit IFNAR1 with anti-IFNAR1 mAb anifrolumab successfully reduced skin disease in patients with SLE during phase 2b and phase 3 clinical studies [118,119]. Targeting the receptor may be more efficacious since it will block all 17 members of the type I IFN family, including IFNκ. Although there is a type II IFN (IFNγ) signature in CLE, an antibody targeting IFNγ (AMG811) failed to show benefit in patients with CLE [120]. New insights into CLE pathogenesis have implicated type III IFN family members including, IFNλ [65]. No anti-IFNλ therapies for CLE are currently under investigation.
Other cytokines targeted such as IL-6 (PF-04236921 and sirukumab) [121,122] and the IL-6 receptor (MRA003US (NCT00046774) and vobrilizumab (NCT02437890)) have failed to show benefit in patients with CLE. Conflicting results exist for therapies targeting TNFα and IL-12/23 pathways. For example, earlier studies suggested that TNFα inhibitors infliximab and etanercept reduced CLE disease activity, but subsequent reports indicate that CLE-like disease can be induced by TNF inhibitors [123]. Similarly, ustekinumab (IL-12/23 inhibitor) has been reported to both effectively treat CLE and paradoxically induce CLE [78,124,125]. The phenomenon of paradoxical induction of disease is often associated with psoriasiform dermatitis in patients treated with TNFα inhibitors. Nevertheless, TNFα inhibitors remain highly effective for the treatment of psoriasis. Therefore, it is unclear whether CLE can be effectively targeted by TNFα inhibitors or IL-12/23 blockers. Ongoing clinical trials involving etanercept (intralesional) (NCT02656082) and ustekinumab (NCT03517722) hope to resolve this issue.
The IL-17 cytokine family is implicated in numerous autoimmune diseases including psoriasis, psoriatic arthritis, and ankylosing spondylitis [126]. There is evidence that IL-17 family members IL-17A and IL-17F are elevated in the serum of patients with CLE as compared to healthy controls [75]. Additionally, IL-17A is upregulated in CLE tissue, suggesting that targeting the pathway may provide some benefit for patients with CLE [74,75] Although the role of IL-17 cytokines remains unknown in the pathogenesis of CLE, a current phase 2 clinical trial is investigating the therapeutic effect of anti-IL17A mAb secukinumb (NCT03866317).
Intracellular Signaling Targets
A class of small molecule inhibitors targeting the Janus kinase (JAK) family members have provided new hope in treating poorly understood cutaneous autoimmunity, including CLE. JAKs are critical intracellular signaling molecules downstream of cytokine receptors, that together with STAT proteins, perform a myriad of immune functions [80]. First-generation JAK inhibitors (JAKi) are FDA-approved for several rheumatologic conditions including rheumatoid and psoriatic arthritis and have also shown promise in treating cutaneous autoimmune conditions such as alopecia areata, vitiligo, and dermatomyositis among others [81]. The first generation of JAKi, baricitinib (JAK 1/2 blockade) and ruxolitinib (JAK 1/2 blockade), showed benefit in a small number of patients with chilblain lupus erythematosus [127,128]. Although baricitinib improved patients with familial chilblain lupus [128], it failed to show improvement in skin disease during a phase II clinical trial for SLE [129]. Currently, tofacitinib (JAK1/3 blockade) is undergoing clinical trials for the treatment of CLE and SLE and results are pending (NCT03288324). Next generation JAKi with greater specificity have been developed and are also being tested in clinical trials for CLE. For example, filgotinib is a JAK1 inhibitor currently in a phase II clinical study in patients with CLE (NCT03134222). Another JAK family member, tyrosine kinase 2 (TYK2), is being targeted by BMS-986165 for the treatment of CLE, SLE, and lupus nephritis (NCT03920267). Recently, inhibition of TYK2 by BMS-986165 was shown to be effective for treatment of psoriasis [130], indicating its potent ability to modulate cutaneous inflammation.
In addition to JAKs, other intracellular signaling molecules being targeted for the treatment of CLE include spleen tyrosine kinase (SYK), c-Jun N-terminal kinase (JNK), and mitogen-activated protein kinase (MAPK). Activation of SYK has been detected within CLE tissue and inhibition of SYK reduces proinflammatory cytokines and chemokines implicated in CLE pathogenesis in vitro [131]. Lanraplenib is an oral small molecule inhibitor of SYK currently under investigation for CLE therapy in combination with JAK1 inhibitor filgotinib (NCT03134222). The family of JNKs integrate into signaling pathways of the MAPK family of proteins that control critical cellular processes during inflammation, including but not limited to cellular proliferation, apoptosis, and cytokine production. Although JNKs are critical for the induction and maintenance of inflammation, a phase II clinical trial investigating JNK inhibitor tanzisertib (CC-930) in CLE was terminated due to unfavorable benefit/risk profile (NCT01466725). Therefore, it is unclear whether future development of JNK inhibitors will be of clinical utility for CLE treatment. Two inhibitors of the MAPK pathway (SB203580 and FR167653) have shown benefit in lupus disease activity in pre-clinical models of lupus [132,133], but no human clinical trials specifically targeting the MAPK pathway for CLE have been initiated.
Phosphodiesterase-4 (PDE-4) is a member of the superfamily of enzymes responsible for degrading the intracellular second messenger cyclic adenosine monophosphate (cAMP). PDE-4 is most predominately expressed in immune cells and helps transmit and amplify proinflammatory signals. Over the past decade PDE-4 inhibitors have emerged as a novel approach to combating autoimmunity. PDE-4 inhibitor apremilast showed some benefit in an open-label phase 1/2 study [134], but no subsequent studies with apremilast in CLE were initiated.
Adoptive Cell Transfer
One exciting and innovative approach for the treatment of CLE is the use of adoptive cell transfer (ACT) with regulatory T cells (Tregs) to induce immune tolerance. This approach is in its infancy for the treatment of autoimmunity, but the use of ACT of effector T cells has successfully been used to treat cancer for decades [135]. One compelling phase 1 study with a single SLE patient with cutaneous disease used expanded autologous polyclonal Tregs [136]. Infused Tregs infiltrated the inflamed skin, associated with phenotypic switch away from the IFNγ pathway and towards an IL-17 pathway [136]. The implications of this shift in immunity are unknown, but this study will hopefully inspire future cellular therapy with Tregs with an expanded cohort to validate these results. A future therapeutic approach could involve the development of chimeric antigen receptor (CAR) Tregs which have been used in preclinical models of autoimmunity [137,138]. In a distinct cutaneous autoimmune disease, pemphigus vulgaris, the development of an autoantigen-specific chimeric autoantibody receptor (CAAR) T cells is a powerful novel strategy [139]. This technological approach will have to wait until a definitive autoantigen for CLE is delineated.
Future Considerations
Current clinical trials targeting the underlying pathogenic mechanisms in CLE hold great promise for patients afflicted with CLE. However, there are critical gaps in our understanding of CLE immunopathogenesis. Furthermore, CLE is a heterogeneous group of related diseases that has unique molecular mechanisms that may require unique targeting for treatment. Whether these therapies can be extended to treat coexistent SLE also remains unknown. Specific clinical trials on CLE using CLASI as a primary endpoint as opposed to combination trials with SLE are needed to specifically evaluate response to CLE. Taken together, there is a great need to further dissect the pathogenesis of CLE to facilitate the development of future immunotherapeutic strategies. Basic science investigators, translational scientists, clinical scholars, and pharmaceutical companies need to work together to usher in the next generation of therapeutics for our patients with this devastating disease.
Acknowledgments
AJL is supported by a Women’s Health Career Development Award from the Dermatology Foundation and a Mentored Research Award from the Robert Leet and Clara Guthrie Patterson Trust. MDV is supported by a Physician-Scientist Career Development Award from the Dermatology Foundation, a Dermatology Fellow Award from the Melanoma Research Alliance, and KL2 TR001862 from National Center for Advancing Translational Sciences (NCATS) through Yale Center for Clinical Investigation. Figures generated by Biorender.com under academic subscription.
Glossary
- CLE
cutaneous lupus erythematosus
- SLE
systemic lupus erythematosus
- ACLE
acute cutaneous lupus erythematosus
- SCLE
subacute cutaneous lupus erythematosus
- CCLE
chronic cutaneous lupus erythematosus
- DLE
discoid lupus erythematosus
- TREX1
Three Prime Repair Exonuclease 1
Author Contributions
Both authors contributed equally in writing and editing manuscript.
References
- Klein R, Moghadam-Kia S, Taylor L, Coley C, Okawa J, LoMonico J, et al. Quality of life in cutaneous lupus erythematosus. J Am Acad Dermatol. 2011;64(5):849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribero S, Sciascia S, Borradori L, Lipsker D. The cutaneous spectrum of lupus erythematosus. Clin Rev Allergy Immunol. 2017:1–15. [DOI] [PubMed] [Google Scholar]
- Durosaro O, Davis MD, Reed KB, Rohlinger AL. Incidence of cutaneous lupus erythematosus, 1965-2005: A population-based study. Arch Dermatol. 2009;145(3):249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönhagen CM, Fored CM, Granath F, Nyberg F. Cutaneous lupus erythematosus and the association with systemic lupus erythematosus: A population-based cohort of 1088 patients in Sweden. Br J Dermatol. 2011;164(6):1335–41. [DOI] [PubMed] [Google Scholar]
- Wieczorek IT, Propert KJ, Okawa J, Werth VP. Systemic symptoms in the progression of cutaneous to systemic lupus erythematosus. JAMA Dermatol. 2014;150(3):291–6. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Landmann A. The classification and diagnosis of cutaneous lupus erythematosus. J Autoimmun. 2014;48–49:14–9. [DOI] [PubMed] [Google Scholar]
- Chen KL, Krain RL, Werth VP. Advancing understanding, diagnosis, and therapies for cutaneous lupus erythematosus within the broader context of systemic lupus erythematosus. F1000 Res. 2019:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejazi EZ, Werth VP. Cutaneous lupus erythematosus: an update on pathogenesis, diagnosis and treatment. Am J Clin Dermatol. 2016;17(2):135–46. [DOI] [PubMed] [Google Scholar]
- Hersh AO, Arkin LM, Prahalad S. Immunogenetics of cutaneous lupus erythematosus. Curr Opin Pediatr. 2016;28(4):470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relle M, Weinmann-Menke J, Scorletti E, Cavagna L, Schwarting A. Genetics and novel aspects of therapies in systemic lupus erythematosus. Autoimmun Rev. 2015;14(11):1005–18. [DOI] [PubMed] [Google Scholar]
- Järvinen TM, Hellquist A, Koskenmies S, Einarsdottir E, Koskinen LL, Jeskanen L, et al. Tyrosine kinase 2 and interferon regulatory factor 5 polymorphisms are associated with discoid and subacute cutaneous lupus erythematosus. Exp Dermatol. 2010;19(2):123–31. [DOI] [PubMed] [Google Scholar]
- Kunz M, Konig IR, Schillert A, Kruppa J, Ziegler A, Grallert H, et al. Genome-wide association study identifies new susceptibility loci for cutaneous lupus erythematosus. Exp Dermatol. 2015;24(7):510–5. [DOI] [PubMed] [Google Scholar]
- Skonieczna K, Czajkowski R, Kaszewski S, Gawrych M, Jakubowska A, Grzybowski T. Genetic similarities and differences between discoid and systemic lupus erythematosus patients within the Polish population. Postepy Dermatol Alergol. 2017;34(3):228–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel J. Cutaneous lupus erythematosus: new insights into pathogenesis and therapeutic strategies. Nat Rev Rheumatol. 2019;15(9):519–32. [DOI] [PubMed] [Google Scholar]
- Rice G, Newman WG, Dean J, Patrick T, Parmar R, Flintoff K, et al. Heterozygous mutations in TREX1 cause familial chilblain lupus and dominant Aicardi-Goutières syndrome. Am J Hum Genet. 2007;80(4):811–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschke K, Friebe F, Zimmermann N, Wahlicht T, Schumann T, Achleitner M, et al. Deregulated type I IFN response in TREX1-associated familial chilblain lupus. J Invest Dermatol. 2014;134(5):1456–9. [DOI] [PubMed] [Google Scholar]
- Petri M. Sex hormones and systemic lupus erythematosus. Lupus. 2008;17(5 SPEC. ISS.):412-5. [DOI] [PubMed] [Google Scholar]
- Liang Y, Tsoi LC, Xing X, Beamer MA, Swindell WR, Sarkar MK, et al. A gene network regulated by the transcription factor VGLL3 as a promoter of sex-biased autoimmune diseases. Nat Immunol. 2017;18(2):152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billi AC, Gharaee-Kermani M, Fullmer J, Tsoi LC, Hill BD, Gruszka D, et al. The female-biased factor VGLL3 drives cutaneous and systemic autoimmunity. JCI Insight. 2019;4(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis JS, McGee JS. Epigenetic therapy and dermatologic disease: moving beyond CTCL. J Dermatolog Treat. 2019;30(1):68–73. [DOI] [PubMed] [Google Scholar]
- Wang Z, Chang C, Peng M, Lu Q. Translating epigenetics into clinic: focus on lupus. Clin Epigenetics. 2017;9(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solé C, Domingo S, Ferrer B, Moliné T, Ordi-Ros J, Cortés-Hernández J. MicroRNA expression profiling identifies miR-31 and miR-485-3p as regulators in the pathogenesis of discoid cutaneous lupus. J Invest Dermatol. 2018 [DOI] [PubMed] [Google Scholar]
- Renauer P, Coit P, Jeffries MA, Merrill JT, McCune WJ, Maksimowicz-McKinnon K, et al. DNA methylation patterns in naïve CD4+ T cells identify epigenetic susceptibility loci for malar rash and discoid rash in systemic lupus erythematosus. Lupus Sci Med. 2015;2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhang X, Zhao M, Lu Q. DNA demethylation of the perforin promoter in CD4+ T cells from patients with subacute cutaneous lupus erythematosus. J Dermatol Sci. 2009;56(1):33–6. [DOI] [PubMed] [Google Scholar]
- Luo Y, Zhao M, Lu Q. Demethylation of promoter regulatory elements contributes to CD70 overexpression in CD4+ T cells from patients with subacute cutaneous lupus erythematosus: experimental dermatology. Clin Exp Dermatol. 2010;35(4):425–30. [DOI] [PubMed] [Google Scholar]
- Biazar C, Sigges J, Patsinakidis N, Ruland V, Amler S, Bonsmann G, et al. Cutaneous lupus erythematosus: first multicenter database analysis of 1002 patients from the European Society of Cutaneous Lupus Erythematosus (EUSCLE). Autoimmun Rev. 2013;12(3):444–54. [DOI] [PubMed] [Google Scholar]
- Foering K, Chang AY, Piette EW, Cucchiara A, Okawa J, Werth VP. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman JC, Werth VP. Pathophysiology of cutaneous lupus erythematosus. Arthritis Res Ther. 2015;17:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman WD, Chyou S, Ramanathan A, Izmirly PM, Sharma S, Pannellini T, et al. A protective Langerhans cell keratinocyte axis that is dysfunctional in photosensitivity. Sci Transl Med. 2018;10(454):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patsinakidis N, Wenzel J, Landmann A, Koch R, Gerß J, Luger TA, et al. Suppression of UV-induced damage by a liposomal sunscreen: A prospective, open-label study in patients with cutaneous lupus erythematosus and healthy controls. Exp Dermatol. 2012;21(12):958–61. [DOI] [PubMed] [Google Scholar]
- Kuhn A, Sigges J, Biazar C, Ruland V, Patsinakidis N, Landmann A, et al. Influence of smoking on disease severity and antimalarial therapy in cutaneous lupus erythematosus: analysis of 1002 patients from the EUSCLE database. Br J Dermatol. 2014;171(3):571–9. [DOI] [PubMed] [Google Scholar]
- Piette EW, Foering KP, Chang AY, Okawa J, Ten Have TR, Feng R, et al. Impact of smoking in cutaneous lupus erythematosus. Arch Dermatol. 2012;148(3):317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutz J, Werth VP. Cigarette smoking and response to antimalarials in cutaneous lupus erythematosus patients: evolution of a dogma. J Invest Dermatol. 2011;131(10):1968–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczech J, Samotij D, Werth VP, Reich A. Trigger factors of cutaneous lupus erythematosus: A review of current literature. Lupus. 2017;26(8):791–807. [DOI] [PubMed] [Google Scholar]
- Sherman BW, Lynch WD. The association of smoking with medical treatment adherence in the workforce of a large employer. Patient Prefer Adherence. 2014;8:477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaglio A, Grayson PC, Fenaroli P, Gianfreda D, Boccaletti V, Ghiggeri GM, et al. Drug-induced lupus: traditional and new concepts. Autoimmun Rev. 2018;17(9):912–8. [DOI] [PubMed] [Google Scholar]
- Schlienger RG, Bircher AJ, Meier CR. Minocycline-induced lupus. A systematic review. Dermatology. 2000;200(3):223–31. [DOI] [PubMed] [Google Scholar]
- Lowe GC, Henderson CL, Grau RH, Hansen CB, Sontheimer RD. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol. 2011;164(3):465–72. [DOI] [PubMed] [Google Scholar]
- Grönhagen CM, Fored CM, Linder M, Granath F, Nyberg F. Subacute cutaneous lupus erythematosus and its association with drugs: A population-based matched case-control study of 234 patients in Sweden. Br J Dermatol. 2012;167(2):296–305. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF. The yin and yang of TNF-{alpha} inhibition. Arch Dermatol. 2007;143(2):233–6. [DOI] [PubMed] [Google Scholar]
- Greiling TM, Dehner C, Chen X, Hughes K, Iñiguez AJ, Boccitto M, et al. Commensal orthologs of the human autoantigen Ro60 as triggers of autoimmunity in lupus. Sci Transl Med. 2018;10(434). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredo Vieira S, Hiltensperger M, Kumar V, Zegarra-Ruiz D, Dehner C, Khan N, et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359(6380):1156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Kwok SK, Choe JY, Park SH. Recent advances in our understanding of the link between the intestinal microbiota and systemic lupus erythematosus. Int J Mol Sci. 2019;20(19):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard JN, Kahlenberg JM. Cutaneous lupus erythematosus: updates on pathogenesis and associations with systemic lupus. Curr Opin Rheumatol. 2016;28(5):453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng GM, Tsokos GC. Pathogenesis and targeted treatment of skin injury in SLE. Nat Rev Rheumatol. 2015;11(11):663–9. [DOI] [PubMed] [Google Scholar]
- Hile GA, Gudjonsson JE, Kahlenberg JM. The influence of interferon on healthy and diseased skin. Cytokine. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Titov AA, Morel L. An update on lupus animal models. Curr Opin Rheumatol. 2017;29(5):434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee-Kirsch MA. The type I interferonopathies. Annu Rev Med. 2017;68:297–315. [DOI] [PubMed] [Google Scholar]
- Berthier CC, Tsoi LC, Reed TJ, Stannard JN, Myers EM, Namas R, et al. Molecular profiling of cutaneous lupus lesions identifies subgroups distinct from clinical phenotypes. J Clin Med. 2019;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity. 2019;50(4):907–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar MK, Hile GA, Tsoi LC, Xing X, Liu J, Liang Y, et al. Photosensitivity and type I IFN responses in cutaneous lupus are driven by epidermal-derived interferon kappa. Ann Rheum Dis. 2018;77(11):1653–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel J, Zahn S, Mikus S, Wiechert A, Bieber T, Tuting T. The expression pattern of interferon-inducible proteins reflects the characteristic histological distribution of infiltrating immune cells in different cutaneous lupus erythematosus subsets. Br J Dermatol. 2007;157(4):752–7. [DOI] [PubMed] [Google Scholar]
- Dey-Rao R, Sinha AA. Genome-wide transcriptional profiling data from skin of chronic cutaneous lupus erythematosus (CCLE) patients. Data Brief. 2015;4:47–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein I, Klein R, Okawa J, Werth VP. The interferon-regulated gene signature is elevated in subacute cutaneous lupus erythematosus and discoid lupus erythematosus and correlates with the cutaneous lupus area and severity index score. Br J Dermatol. 2012;166(5):971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn S, Rehkamper C, Ferring-Schmitt S, Bieber T, Tuting T, Wenzel J. Interferon-alpha stimulates TRAIL expression in human keratinocytes and peripheral blood mononuclear cells: implications for the pathogenesis of cutaneous lupus erythematosus. Br J Dermatol. 2011;165(5):1118–23. [DOI] [PubMed] [Google Scholar]
- Zahn S, Graef M, Patsinakidis N, Landmann A, Surber C, Wenzel J, et al. Ultraviolet light protection by a sunscreen prevents interferon-driven skin inflammation in cutaneous lupus erythematosus. Exp Dermatol. 2014;23(7):516–8. [DOI] [PubMed] [Google Scholar]
- Yin Q, Xu X, Lin Y, Lv J, Zhao L, He R. Ultraviolet B irradiation induces skin accumulation of plasmacytoid dendritic cells: A possible role for chemerin. Autoimmunity. 2014;47(3):185–92. [DOI] [PubMed] [Google Scholar]
- McNiff JM, Kaplan DH. Plasmacytoid dendritic cells are present in cutaneous dermatomyositis lesions in a pattern distinct from lupus erythematosus. J Cutan Pathol. 2008;35(5):452–6. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Worenkamper E, Freutel S, Henze S, Haller O, Bieber T, et al. Enhanced type I interferon signalling promotes Th1-biased inflammation in cutaneous lupus erythematosus. J Pathol. 2005;205(4):435–42. [DOI] [PubMed] [Google Scholar]
- Toro JR, Finlay D, Dou X, Zheng SC, LeBoit PE, Connolly MK. Detection of type 1 cytokines in discoid lupus erythematosus. Arch Dermatol. 2000;136(12):1497–501. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Zahn S, Bieber T, Tuting T. Type I interferon-associated cytotoxic inflammation in cutaneous lupus erythematosus. Arch Dermatol Res. 2009;301(1):83–6. [DOI] [PubMed] [Google Scholar]
- Grassi M, Capello F, Bertolino L, Seia Z, Pippione M. Identification of granzyme B-expressing CD-8-positive T cells in lymphocytic inflammatory infiltrate in cutaneous lupus erythematosus and in dermatomyositis. Clin Exp Dermatol. 2009;34(8):910–4. [DOI] [PubMed] [Google Scholar]
- Scholtissek B, Zahn S, Maier J, Klaeschen S, Braegelmann C, Hoelzel M, et al. Immunostimulatory endogenous nucleic acids drive the lesional inflammation in cutaneous lupus erythematosus. J Invest Dermatol. 2017;137(7):1484–92. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Uerlich M, Worrenkamper E, Freutel S, Bieber T, Tuting T. Scarring skin lesions of discoid lupus erythematosus are characterized by high numbers of skin-homing cytotoxic lymphocytes associated with strong expression of the type I interferon-induced protein MxA. Br J Dermatol. 2005;153(5):1011–5. [DOI] [PubMed] [Google Scholar]
- Zahn S, Rehkamper C, Kummerer BM, Ferring-Schmidt S, Bieber T, Tuting T, et al. Evidence for a pathophysiological role of keratinocyte-derived type III interferon (IFNlambda) in cutaneous lupus erythematosus. J Invest Dermatol. 2011;131(1):133–40. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13(1):114–9. [DOI] [PubMed] [Google Scholar]
- Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17(4):359–65. [DOI] [PubMed] [Google Scholar]
- Rider P, Voronov E, Dinarello CA, Apte RN, Cohen I. Alarmins: feel the stress. J Immunol. 2017;198(4):1395–402. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yin M, Zhang LJ. Keratin 6, 16 and 17-critical barrier alarmin molecules in skin wounds and psoriasis. Cells. 2019;8(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard JN, Reed TJ, Myers E, Lowe L, Sarkar MK, Xing X, et al. Lupus skin is primed for IL-6 inflammatory responses through a keratinocyte-mediated autocrine type I interferon loop. J Invest Dermatol. 2017;137(1):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir MM, Sharma MR, Werth VP. UVB and proinflammatory cytokines synergistically activate TNF-alpha production in keratinocytes through enhanced gene transcription. J Invest Dermatol. 2009;129(4):994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoi LC, Hile GA, Berthier CC, Sarkar MK, Reed TJ, Liu J, et al. Hypersensitive IFN responses in lupus keratinocytes reveal key mechanistic determinants in cutaneous lupus. J Immunol. 2019;202(7):2121–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey-Rao R, Smith JR, Chow S, Sinha AA. Differential gene expression analysis in CCLE lesions provides new insights regarding the genetics basis of skin vs. systemic disease. Genomics. 2014;104(2):144–55. [DOI] [PubMed] [Google Scholar]
- Oh SH, Roh HJ, Kwon JE, Lee SH, Kim JY, Choi HJ, et al. Expression of interleukin-17 is correlated with interferon-alpha expression in cutaneous lesions of lupus erythematosus. Clin Exp Dermatol. 2011;36(5):512–20. [DOI] [PubMed] [Google Scholar]
- Tanasescu C, Balanescu E, Balanescu P, Olteanu R, Badea C, Grancea C, et al. Il-17 in cutaneous lupus erythematosus. Eur J Intern Med. 2010;21(3):202–7. [DOI] [PubMed] [Google Scholar]
- Jabbari A, Suarez-Farinas M, Fuentes-Duculan J, Gonzalez J, Cueto I, Franks AG, Jr, et al. Dominant Th1 and minimal Th17 skewing in discoid lupus revealed by transcriptomic comparison with psoriasis. J Invest Dermatol. 2014;134(1):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Drenker M, Eiz-Vesper B, Werfel T, Wittmann M. Evidence for a pathogenetic role of interleukin-18 in cutaneous lupus erythematosus. Arthritis Rheum. 2008;58(10):3205–15. [DOI] [PubMed] [Google Scholar]
- van Vollenhoven RF, Hahn BH, Tsokos GC, Wagner CL, Lipsky P, Touma Z, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet. 2018;392(10155):1330–9. [DOI] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–21. [DOI] [PubMed] [Google Scholar]
- Gadina M, Le MT, Schwartz DM, Silvennoinen O, Nakayamada S, Yamaoka K, et al. Janus kinases to jakinibs: From basic insights to clinical practice. Rheumatology (Oxford). 2019;58(Supplement_1):i4-i16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol. 2017;76(4):736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim A, O’Brien J, Tseng LC, Zhang S, Chong BF. Autoantibodies and disease activity in patients with discoid lupus erythematosus. JAMA Dermatol. 2014;150(6):651–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey AK, Handu SS, Dubey S, Sharma P, Sharma KK, Ahmed QM. Belimumab: first targeted biological treatment for systemic lupus erythematosus. J Pharmacol Pharmacother. 2011;2(4):317–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong BF, Tseng LC, Kim A, Miller RT, Yancey KB, Hosler GA. Differential expression of BAFF and its receptors in discoid lupus erythematosus patients. J Dermatol Sci. 2014;73(3):216–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight JS, Carmona-Rivera C, Kaplan MJ. Proteins derived from neutrophil extracellular traps may serve as self-antigens and mediate organ damage in autoimmune diseases. Front Immunol. 2012;3:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi R, Al-Hage J, Abbas O, Kibbi AG, Nassar D. Investigating the presence of neutrophil extracellular traps in cutaneous lesions of different subtypes of lupus erythematosus. Exp Dermatol. 2019;28(11):1348–52. [DOI] [PubMed] [Google Scholar]
- Thorpe RB, Gray A, Kumar KR, Susa JS, Chong BF. Site-specific analysis of inflammatory markers in discoid lupus erythematosus skin. ScientificWorldJournal. 2014;2014:925805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong BF, Tseng LC, Hosler GA, Teske NM, Zhang S, Karp DR, et al. A subset of CD163+ macrophages displays mixed polarizations in discoid lupus skin. Arthritis Res Ther. 2015;17:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao WH, Cohen PL. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res Ther. 2011;13(1):202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessop S, Whitelaw DA, Grainge MJ, Jayasekera P. Drugs for discoid lupus erythematosus. Cochrane Database Syst Rev. 2017;5:CD002954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznik A, Bencina M, Svajger U, Jeras M, Rozman B, Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186(8):4794–804. [DOI] [PubMed] [Google Scholar]
- Reich A, Werth VP, Furukawa F, Kuhn A, Szczech J, Samotij D, et al. Treatment of cutaneous lupus erythematosus: current practice variations. Lupus. 2016;25(9):964–72. [DOI] [PubMed] [Google Scholar]
- Ordi-Ros J, Cortes F, Cucurull E, Mauri M, Bujan S, Vilardell M. Thalidomide in the treatment of cutaneous lupus refractory to conventional therapy. J Rheumatol. 2000;27(6):1429–33. [PubMed] [Google Scholar]
- Pelle MT, Werth VP. Thalidomide in cutaneous lupus erythematosus. Am J Clin Dermatol. 2003;4(6):379–87. [DOI] [PubMed] [Google Scholar]
- Housman TS, Jorizzo JL, McCarty MA, Grummer SE, Fleischer AB, Jr, Sutej PG. Low-dose thalidomide therapy for refractory cutaneous lesions of lupus erythematosus. Arch Dermatol. 2003;139(1):50–4. [DOI] [PubMed] [Google Scholar]
- Chasset F, Tounsi T, Cesbron E, Barbaud A, Frances C, Arnaud L. Efficacy and tolerance profile of thalidomide in cutaneous lupus erythematosus: A systematic review and meta-analysis. J Am Acad Dermatol. 2018;78(2):342-50 e4. [DOI] [PubMed] [Google Scholar]
- Cortes-Hernandez J, Avila G, Vilardell-Tarres M, Ordi-Ros J. Efficacy and safety of lenalidomide for refractory cutaneous lupus erythematosus. Arthritis Res Ther. 2012;14(6):R265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KC. Lenalidomide and thalidomide: mechanisms of action—similarities and differences. Semin Hematol. 2005;42(4 Suppl 4):S3–8. [DOI] [PubMed] [Google Scholar]
- Albrecht J, Taylor L, Berlin JA, Dulay S, Ang G, Fakharzadeh S, et al. The CLASI (Cutaneous Lupus Erythematosus Disease Area and Severity Index): an outcome instrument for cutaneous lupus erythematosus. J Invest Dermatol. 2005;125(5):889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furie R, Werth VP, Merola JF, Stevenson L, Reynolds TL, Naik H, et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J Clin Invest. 2019;129(3):1359–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SC, Leandro MJ, Morris SD, Isenberg D. Effects of rituximab-based B-cell depletion therapy on skin manifestations of lupus erythematosus—report of 17 cases and review of the literature. Lupus. 2013;22(9):932–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presto JK, Hejazi EZ, Werth VP. Biological therapies in the treatment of cutaneous lupus erythematosus. Lupus. 2017;26(2):115–8. [DOI] [PubMed] [Google Scholar]
- Hui-Yuen JS, Reddy A, Taylor J, Li X, Eichenfield AH, Bermudez LM, et al. Safety and efficacy of belimumab to treat systemic lupus erythematosus in academic clinical practices. J Rheumatol. 2015;42(12):2288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzi S, Sánchez-Guerrero J, Merrill JT, Furie R, Gladman D, Navarra SV, et al. Effects of belimumab, a B lymphocyte stimulator-specific inhibitor, on disease activity across multiple organ domains in patients with systemic lupus erythematosus: combined results from two phase III trials. Ann Rheum Dis. 2012;71(11):1833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iaccarino L, Bettio S, Reggia R, Zen M, Frassi M, Andreoli L, et al. Effects of belimumab on flare rate and expected damage progression in patients with active systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2017;69(1):115–23. [DOI] [PubMed] [Google Scholar]
- Vashisht P, Borghoff K, O’Dell JR, Hearth-Holmes M. Belimumab for the treatment of recalcitrant cutaneous lupus. Lupus. 2017;26(8):857–64. [DOI] [PubMed] [Google Scholar]
- Wenzel J, Landmann A, Vorwerk G, Kuhn A. High expression of B lymphocyte stimulator in lesional keratinocytes of patients with cutaneous lupus erythematosus. Exp Dermatol. 2018;27(1):95–7. [DOI] [PubMed] [Google Scholar]
- Masek-Hammerman K, Peeva E, Ahmad A, Menon S, Afsharvand M, Peng Qu R, et al. Monoclonal antibody against macrophage colony-stimulating factor suppresses circulating monocytes and tissue macrophage function but does not alter cell infiltration/activation in cutaneous lesions or clinical outcomes in patients with cutaneous lupus ery. Clin Exp Immunol. 2016;183(2):258–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn BM. Efalizumab withdrawn. JAMA. 2009;301(20):2085. [Google Scholar]
- Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediators Inflamm. 2016;2016:8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows KR, Steinberg MW, Clemons B, Stokes ME, Opiteck GJ, Peach R, et al. Ozanimod (RPC1063), a selective S1PR1 and S1PR5 modulator, reduces chronic inflammation and alleviates kidney pathology in murine systemic lupus erythematosus. PLoS One. 2018;13(4):1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenderfer SE, Stepkowski SM, Braun MC. Increased survival and reduced renal injury in MRL/lpr mice treated with a novel sphingosine-1-phosphate receptor agonist. Kidney Int. 2008;74(10):1319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki H, Hirata D, Kamimura T, Sato H, Iwamoto M, Yoshio T, et al. Effects of FTY720 in MRL-lpr/lpr mice: therapeutic potential in systemic lupus erythematosus. J Rheumatol. 2002;29(4):707–16. [PubMed] [Google Scholar]
- Felten R, Dervovic E, Chasset F, Gottenberg JE, Sibilia J, Scher F, et al. The 2018 pipeline of targeted therapies under clinical development for Systemic Lupus Erythematosus: A systematic review of trials. Autoimmun Rev. 2018;17(8):781–90. [DOI] [PubMed] [Google Scholar]
- Sciascia S, Radin M, Roccatello D, Sanna G, Bertolaccini ML. Recent advances in the management of systemic lupus erythematosus. F1000 Res. 2018;7(7):489–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khamashta M, Merrill JT, Werth VP, Furie R, Kalunian K, Illei GG, et al. Sifalimumab, an anti-interferon-alpha monoclonal antibody, in moderate to severe systemic lupus erythematosus: A randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2016;75(11):1909–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalunian KC, Merrill JT, Maciuca R, McBride JM, Townsend MJ, Wei X, et al. A phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-alpha) in patients with systemic lupus erythematosus (ROSE). Ann Rheum Dis. 2016;75(1):196–202. [DOI] [PubMed] [Google Scholar]
- Furie R, Khamashta M, Merrill JT, Werth VP, Kalunian K, Brohawn P, et al. Anifrolumab, an anti-interferon-alpha receptor monoclonal antibody, in moderate-to-severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017;69(2):376–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, et al. Trial of anifrolumab in active Systemic Lupus Erythematosus. N Engl J Med. 2019 [DOI] [PubMed] [Google Scholar]
- Werth VP, Fiorentino D, Sullivan BA, Boedigheimer MJ, Chiu K, Wang C, et al. Brief report: Pharmacodynamics, safety, and clinical efficacy of AMG 811, a human anti-interferon-gamma antibody, in patients with discoid lupus erythematosus. Arthritis Rheumatol. 2017;69(5):1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szepietowski JC, Nilganuwong S, Wozniacka A, Kuhn A, Nyberg F, van Vollenhoven RF, et al. Phase I, randomized, double-blind, placebo-controlled, multiple intravenous, dose-ascending study of sirukumab in cutaneous or systemic lupus erythematosus. Arthritis Rheum. 2013;65(10):2661–71. [DOI] [PubMed] [Google Scholar]
- Wallace DJ, Strand V, Merrill JT, Popa S, Spindler AJ, Eimon A, et al. Efficacy and safety of an interleukin 6 monoclonal antibody for the treatment of systemic lupus erythematosus: A phase II dose-ranging randomised controlled trial. Ann Rheum Dis. 2017;76(3):534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aringer M, Smolen JS. Efficacy and safety of TNF-blocker therapy in systemic lupus erythematosus. Expert Opin Drug Saf. 2008;7(4):411–9. [DOI] [PubMed] [Google Scholar]
- De Souza A, Ali-Shaw T, Strober BE, Franks AG., Jr Successful treatment of subacute lupus erythematosus with ustekinumab. Arch Dermatol. 2011;147(8):896–8. [DOI] [PubMed] [Google Scholar]
- Tierney E, Kirthi S, Ramsay B, Ahmad K. Ustekinumab-induced subacute cutaneous lupus. JAAD Case Rep. 2019;5(3):271–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat Rev Immunol. 2014;14(9):585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel J, van Holt N, Maier J, Vonnahme M, Bieber T, Wolf D. JAK1/2 inhibitor ruxolitinib controls a case of chilblain lupus erythematosus. J Invest Dermatol. 2016;136(6):1281–3. [DOI] [PubMed] [Google Scholar]
- Zimmermann N, Wolf C, Schwenke R, Lüth A, Schmidt F, Engel K, et al. Assessment of Clinical Response to Janus Kinase Inhibition in Patients With Familial Chilblain Lupus and TREX1 Mutation. JAMA Dermatol. 2019. March;155(3):342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DJ, Furie RA, Tanaka Y, Kalunian KC, Mosca M, Petri MA, et al. Baricitinib for systemic lupus erythematosus: A double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392(10143):222–31. [DOI] [PubMed] [Google Scholar]
- Papp K, Gordon K, Thaci D, Morita A, Gooderham M, Foley P, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379(14):1313–21. [DOI] [PubMed] [Google Scholar]
- Braegelmann C, Holzel M, Ludbrook V, Dickson M, Turan N, Ferring-Schmitt S, et al. Spleen tyrosine kinase (SYK) is a potential target for the treatment of cutaneous lupus erythematosus patients. Exp Dermatol. 2016;25(5):375–9. [DOI] [PubMed] [Google Scholar]
- Iwata Y, Wada T, Furuichi K, Sakai N, Matsushima K, Yokoyama H, et al. P38 mitogen-activated protein kinase contributes to autoimmune renal injury in MRL-Fas lpr mice. J Am Soc Nephrol. 2003;14(1):57–67. [DOI] [PubMed] [Google Scholar]
- Jin N, Wang Q, Zhang X, Jiang D, Cheng H, Zhu K. The selective p38 mitogen-activated protein kinase inhibitor, SB203580, improves renal disease in MRL/lpr mouse model of systemic lupus. Int Immunopharmacol. 2011;11(9):1319–26. [DOI] [PubMed] [Google Scholar]
- De Souza A, Strober BE, Merola JF, Oliver S, Franks AG., Jr Apremilast for discoid lupus erythematosus: results of a phase 2, open-label, single-arm, pilot study. J Drugs Dermatol. 2012;11(10):1224–6. [PubMed] [Google Scholar]
- Yang JC, Rosenberg SA. Adoptive T-cell therapy for cancer. Adv Immunol. 2016;130:279–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Era M, Pauli ML, Remedios K, Taravati K, Sandova PM, Putnam AL, et al. Adoptive Treg cell therapy in a patient with systemic lupus erythematosus. Arthritis Rheumatol. 2019;71(3):431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Waks T, Eshhar Z. Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology. 2008;134(7):2014–24. [DOI] [PubMed] [Google Scholar]
- Maldini CR, Ellis GI, Riley JL. Car T cells for infection, autoimmunity and allotransplantation. Nat Rev Immunol. 2018;18(10):605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellebrecht CT, Bhoj VG, Nace A, Choi EJ, Mao X, Cho MJ, et al. Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science. 2016;353(6295):179–84. [DOI] [PMC free article] [PubMed] [Google Scholar]