Abstract
The present study was conducted to examine whether cellular and/or viral cholesterol levels play a role in porcine deltacoronavirus (PDCoV) replication. Our results showed that depletion of cholesterol from cells or virions by treating them with methyl-β-cyclodextrin (MβCD) diminished PDCoV infection in a dose-dependent manner. The addition of exogenous cholesterol to MβCD-treated cells or virions moderately restored PDCoV infectivity. Furthermore, the pharmacological sequestration of cellular or viral cholesterol efficiently blocked both virus attachment and internalization. Taken together, the current data indicate that the cholesterol present in the cell membrane and viral envelope contributes to PDCoV replication by acting as a key component in viral entry.
Electronic supplementary material
The online version of this article (10.1007/s00705-018-3967-7) contains supplementary material, which is available to authorized users.
Porcine deltacoronavirus (PDCoV) is a newly discovered enteric coronavirus associated with acute enteritis and intestinal damage in piglets [6, 7]. It is an enveloped, single-stranded, positive-sense RNA virus that taxonomically belongs to the genus Deltacoronavirus in the family Coronaviridae of the order Nidovirales. The virus was first discovered in pigs in Hong Kong in 2012 and subsequently, has been reported in the US, China, South Korea, Thailand, and Vietnam since 2014 [7, 9, 13, 14]. PDCoV infection results in severe villous atrophy in the small intestine, leading to watery diarrhea, vomiting, dehydration, and mortality in nursing piglets. The clinical and pathological presentations of PDCoV are indistinguishable from other swine enteric diseases caused by transmissible gastroenteritis virus (TGEV) and porcine epidemic diarrhea virus (PEDV), but there is apparently lower mortality in affected neonatal piglets [6].
Lipid rafts are membrane microdomains that are enriched in cholesterol, sphingolipids, and associated proteins, and are involved in the process of virus infection [1]. Cholesterol is an essential component of lipid rafts, and it plays important roles in various aspects of the virus life cycle, especially viral entry [15]. In particular, the successful entry of enveloped viruses including many coronaviruses requires the presence of cholesterol in either the viral and cellular membranes or both [3, 5, 10–12, 16]. However, the potential relationship between cholesterol and PDCoV replication remains undetermined. Therefore, in this study, we investigated the necessity of cholesterol and the mechanism by which it acts in PDCoV infection.
Swine testicle (ST) cells were cultured in alpha minimum essential medium (α-MEM, Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (FBS, Invitrogen). PDCoV strain KNU16-07 was propagated in ST cells in virus growth medium [α-MEM supplemented with antibiotic-antimycotic solutions, 10 mM HEPES (Invitrogen), and 5 μg/ml of trypsin] as described previously [4]. MβCD and water-soluble cholesterol were purchased from Sigma (St. Louis, MO) and dissolved in distilled water (DW) and ethanol, respectively. The cytotoxic effects of these compounds on ST cells were analyzed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma) as described previously [5]. PDCoV N protein-specific monoclonal antibody (MAb) KDN 4-1 used in this study was described previously [4].
ST cells were pretreated with MβCD or DW for 1 h, mock infected or infected with PDCoV at a multiplicity of infection of 1, and then cultivated in virus growth medium supplemented with MβCD or vehicle at the desired concentrations unless otherwise indicated. The MβCD-treated and virus-infected ST cells were analyzed by immunofluorescence assay (IFA) and fluorescence-activated cell sorting (FACS) as described previously [4, 5]. The culture supernatants were also collected at 24 h post-infection (hpi). The PDCoV titer was measured by limiting dilution on ST cells in duplicate, and 50% tissue culture infectious dose (TCID50) per ml was calculated as described previously [4]. For cholesterol replenishment, ST cells were first preincubated with vehicle (DW) or MβCD at various final concentrations for 1 h, supplemented with 10 μg/ml exogenous cholesterol or 0.1% (v/v) ethanol as a vehicle control in cell culture medium for 1 h and then inoculated with PDCoV. In parallel, the cellular cholesterol content was determined using a Cholesterol Cell-Based Detection Assay Kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions [5].
To remove cholesterol from viral membranes, viral suspensions were treated with MβCD at various concentrations at 37 °C for 1 h followed by ultracentrifugation to remove the MβCD as described previously [5]. For cholesterol replenishment, virus suspensions were mock treated or treated with MβCD at 37 °C for 1 h and then supplemented with or without 100 μg/ml exogenous cholesterol for 1 h followed by ultracentrifugation as described previously [5]. In addition, the virion cholesterol content was determined by fluorescence intensity analysis using filipin III (Cayman Chemical) with a SPARK 10M multimode microplate reader (TECAN, Männedorf, Switzerland) as described previously [5]. All statistical analyses were performed using Student’s t test, and P-values of less than 0.05 were considered statistically significant.
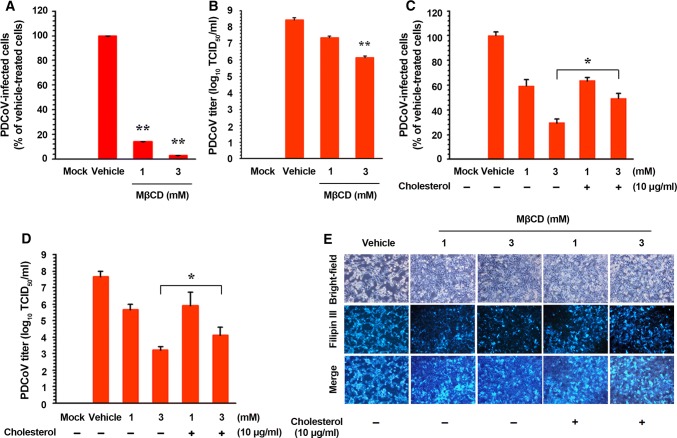
According to the results of an MTT assay, none of the doses of MβCD and cholesterol employed in the present study had an adverse effect on cell viability (Supplementary Fig. S1). ST cells were pretreated with MβCD at concentrations of 1 and 3 mM, or with DW as a vehicle control, for 1 h prior to infection. Viral production was initially measured by monitoring cytopathic effects (CPE) and was later confirmed by IFA using the anti-N protein MAb. MβCD (the cholesterol-sequestering compound) dramatically reduced virus-induced CPE and expression of the PDCoV gene in a dose-dependent manner (Supplementary Fig. S2A). Based on the quantification of the N protein staining results, the proportion (%) of virus-infected cells was noticeably attenuated by MβCD treatment. There was an approximate maximum of ~95% inhibition of both viruses in response to the application of 3 mM MβCD (Fig. 1A). Treating cells with 3 mM MβCD at 0 and 1 hpi also resulted in an approximate ~85% decrease in PDCoV production, whereas exposure to the compound at 2–24 hpi had no significant inhibitory effect on infectivity compared with the control levels (Supplementary Fig. S3). These results demonstrate that MβCD must be present pre-infection or at an early stage of viral infection to exert its antiviral effect as a cellular cholesterol depletion reagent. We also determined viral yields during the pharmacological depletion of cellular cholesterol. As illustrated in Fig. 1B, MβCD inhibited the growth of viral progeny in a dose-dependent manner. The peak viral titer in the vehicle-treated control was 108.45 TCID50/ml in the vehicle-treated control, whereas the addition of 3 mM MβCD reduced the titer of PDCoV to 106.45 TCID50/ml (representing a 2-log reduction compared with the control level). Taken together, our data indicate that depleting cholesterol from target cells efficiently suppresses PDCoV replication.
Fig. 1.
Effects of cellular cholesterol depletion and replenishment on the replication of PDCoV. (A) PDCoV infection efficiency after cholesterol depletion from the cell membrane. ST cells were preincubated with MβCD at the indicated concentrations for 1 h and were mock-infected or infected with PDCoV. Viral production in the presence of MβCD was calculated by measuring the percentage of cells expressing PDCoV N proteins using flow cytometry. (B) Viral progeny production by cellular cholesterol depletion. ST cells pretreated with MβCD were infected with PDCoV and maintained in the presence of MβCD. At 24 hpi, virus culture supernatants were collected and the PDCoV titer was determined. (C and D) PDCoV infection efficiency after cholesterol depletion and replenishment from the cell membrane. ST cells were preincubated with MβCD with (+) or without (–) exogenous cholesterol and infected with PDCoV in the presence or absence of MβCD and/or exogenous cholesterol as indicated. Viral infectivity was determined by measuring the percentage of cells expressing PDCoV N proteins using FACS analysis (C) and by virus titration (D). (E) Cholesterol content determination after cholesterol depletion and replenishment from the cell membrane. ST cells were preincubated with MβCD with (+) or without (–) exogenous cholesterol and infected with PDCoV in the presence or absence of MβCD and/or exogenous cholesterol as indicated. Virus-specific CPE were observed daily and photographed at 24 hpi using a fluorescent/bright-field microscope at a magnification of 200× (first row of panels). For immunostaining, infected cells were fixed at 24 hpi and incubated with a cholesterol-binding, fluorescent antibiotic, Filipin III (second row of panels). The cells were examined using a fluorescent microscope at 200× magnification. The values shown are the means of three independent experiments, and error bars represent standard deviations. *, P = 0.001–0.05; **, P < 0.001
Next, we examined whether cholesterol replenishment restored PDCoV infectivity in MβCD-treated cells. The addition of exogenous cholesterol to MβCD-treated and virus-infected cells significantly reversed the antiviral activity of MβCD. Although incubation with MβCD alone greatly reduced PDCoV production to 59% and 29% at concentrations of 1 mM and 3 mM, respectively, supplementation with exogenous cholesterol significantly increased PDCoV production to 64% and 49%, respectively, at the same concentrations of MβCD (Fig. 1C). Consistently, cholesterol replenishment resulted in an increase in the virus yield comparable to that of the vehicle control (Fig. 1D). To verify the importance of cellular cholesterol, we then investigated alterations in cellular cholesterol content in cells treated with MβCD in the absence or presence of exogenous cholesterol using filipin III, which is a fluorescent polyene antibiotic that binds to cholesterol. In accordance with the lower intensity of CPE, cellular cholesterol levels specifically decreased in virus-infected MβCD-treated cells compared with those in virus-infected non-treated cells. Supplementation with exogenous cholesterol markedly elevated the cholesterol level and CPE production in virus-infected MβCD-treated cells (Fig. 1E). Altogether, the data reveal that cellular cholesterol plays a pivotal role in PDCoV infection.
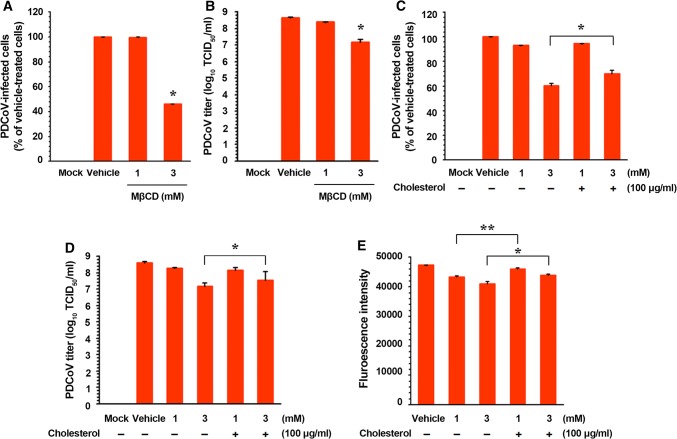
To analyze whether virion-associated cholesterol is required for PDCoV infection, the virus was mock-treated or treated with MβCD at concentrations of 1 and 3 mM prior to inoculation, and the infectivity was investigated using virological methods. Similar to, but less effective (only 3 mM) than the effect of cellular cholesterol depletion, the removal of cholesterol from virions resulted in a significant reduction in the replication of PDCoV (a reduction of almost 60% at the highest concentration used) (Fig. 2A; Supplementary Fig. S2B). Furthermore, the PDCoV titer was reduced to 107.20 TCID50/ml following treatment of the virus with 3 mM MβCD (representing a more than 1-log reduction compared with the control level) (Fig. 2B).
Fig. 2.
Effects of viral cholesterol depletion and replenishment on the replication of PDCoV. (A) PDCoV infection efficiency after cholesterol depletion from the virus envelope. PDCoV suspensions were treated with MβCD to remove cholesterol in the viral envelope, followed by ultracentrifugation, and the purified PDCoV was used to infect fresh ST cells. Virus infectivity was determined by measuring the percentage of cells expressing N proteins of PDCoV using FACS analysis. (B) Viral progeny production after viral cholesterol depletion. Virus culture supernatants were collected at the same time-point, and PDCoV titers were determined. (C and D) PDCoV infection efficiency after cholesterol depletion and replenishment from the viral envelope. PDCoV suspensions were treated with MβCD with (+) or without (–) exogenous cholesterol, followed by ultracentrifugation and infection. Virus infectivity was determined by measuring the percentage of cells expressing PDCoV N proteins using FACS (C) and by virus titration (D). (E) Cholesterol content determination after cholesterol depletion and replenishment from the viral envelope. PDCoV suspensions were incubated with MβCD with (+) or without (–) exogenous cholesterol, followed by ultracentrifugation. Virion cholesterol contents were determined using filipin III, and fluorescence intensity was measured with a fluorescence microplate reader. The values shown are the means of three independent experiments, and error bars represent standard deviations. *, P = 0.001–0.05; **, P < 0.001
To verify whether the effect of virion cholesterol depletion was reversible, exogenous cholesterol was added to viral suspensions pretreated with MβCD. PDCoV infection decreased to 60% in the presence of MβCD alone at 3 mM, whereas virus production increased to 71% at the same concentration of MβCD when exogenous cholesterol was added (Fig. 2C). Consequently, the reduced viral titer following MβCD treatment was restored to values close to those observed in vehicle-treated virus-infected cells (Fig. 2D). To confirm these results, we measured the content of viral cholesterol following treatment with MβCD alone, and following treatment with MβCD and cholesterol replenishment. As shown in Fig. 2E, the viral cholesterol levels were significantly reduced in the MβCD-treated viruses compared with those in the vehicle-treated viruses. However, exogenous cholesterol restored the cholesterol values of the viral membranes to close to those determined prior to MβCD treatment. Our results reveal that the cholesterol contents of both the cell membrane and the viral envelope are relevant to PDCoV infection in vitro.
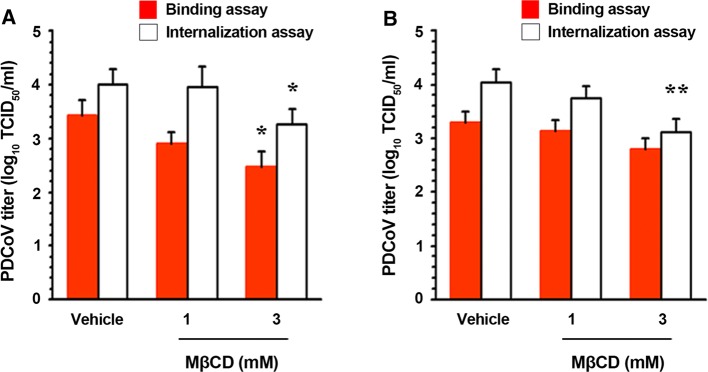
To determine at what point pharmacological depletion of cellular or viral cholesterol directly influences PDCoV entry, we assessed two stages of viral entry (attachment and penetration) using binding and internalization assays after treatment of the target cells or virions with MβCD as described previously [5]. We treated cells with MβCD and maintained them at 4 °C to permit viral binding but prevent internalization. As shown in Fig. 3A, the PDCoV titer was reduced in a dose-dependent manner in the cells, indicating that cellular cholesterol depletion inhibits the attachment of the virus to the cells. Moreover, PDCoV production was diminished in MβCD-treated cells that had been incubated at 37 °C to enable virus entry to proceed, which implies that cholesterol sequestration disturbs the internalization of PDCoV. Similarly, the removal of cholesterol from the viral envelope negatively affected the viral adsorption and post-adsorption steps (Fig. 3B). Taken together, these data demonstrate that the pharmacological sequestration of cholesterol hinders viral attachment and subsequent penetration, and the presence of cholesterol in both the cell membrane and viral envelope are indispensable for the PDCoV entry process.
Fig. 3.
Effects of cellular (A) or viral (B) cholesterol depletion on virus entry. (A) ST cells were pretreated MβCD infected with PDCoV at 4 °C for 1 h. After washing with cold PBS, infected cells were maintained in the presence or absence of MβCD, either at 4 °C (binding) or 37 °C (internalization), for 1 h. The virus-infected cells maintained at 37 °C were further treated with proteinase K at 37 °C. The infected cells were then serially diluted and plated onto fresh target cells. At 2 days post-incubation, bound or internalized viruses were titrated. (B) PDCoV was treated with MβCD and ultracentrifuged, and the purified virus was used to infect ST cells in the absence of MβCD to measure bound or internalized viruses exactly as described above. The results are expressed as the mean values from three independent experiments performed in triplicate, and error bars represent standard deviations. *, P = 0.001–0.05; **, P < 0.001
In conclusion, our findings indicate that optimal infectivity of PDCoV requires cholesterol in the cell membrane and virus envelope, and that this is critical for the entry of PDCoV. Many coronaviruses exploit cholesterol, which is present in the viral envelope and/or the cell membrane for maximal virus entry. Only cellular cholesterol is essential for the entry of some coronaviruses including mouse hepatitis virus [2], severe acute respiratory syndrome coronavirus [8], human coronavirus 229E [10], avian infectious bronchitis virus [3], type II feline coronavirus (FCoV) [16], and PEDV [5]. However, as with PDCoV in the present study, canine coronavirus, type 1 FCoV, and TGEV require cholesterol in both the target cell membrane and the viral envelope [11, 12, 16]. Considering previous and present studies, the cholesterol dependence of infection differs among coronaviruses. Because the PDCoV receptor has not yet been identified, we were unable to examine whether cholesterol dependence is quantitatively related to the presence of a hitherto-unidentified receptor. Future research should address the question of whether cholesterol facilitates PDCoV entry through interactions between the viral spike protein and the cellular receptor. Nevertheless, we propose that both cellular and viral cholesterol are key players in the attachment and penetration stages of PDCoV entry. However, cholesterol depletion from the cell or virus consistently resulted in the reduction, but not the elimination, of viral infectivity. This indicates that viral entry may occur when there are low levels of cholesterol, but increased cholesterol content in both parts makes the process more efficient. Although our analysis did not elucidate the mechanism by which cholesterol promotes PDCoV entry, we assume that cholesterol-dependent viral entry is closely connected to maintaining the lipid raft structure and/or biological membrane fluidity. Impeding virus entry is a viable antiviral strategy because it likely acts on extracellular targets, thereby limiting cell damage. Therefore, PDCoV could be used as a surrogate model for testing emerging coronavirus antiviral therapies. Since no treatments or vaccines are currently available for PDCoV, the results presented here indicate that molecules that disrupt viral and/or cell cholesterol, and interfere with cholesterol function during viral entry, may provide an excellent therapeutic option for the treatment of coronavirus infection in humans and animals.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2015R1D1A1A09057406).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with animals performed by any of the authors.
References
- 1.Barman S, Nayak DP. Lipid raft disruption by cholesterol depletion enhances influenza A virus budding from MDCK cells. J Virol. 2007;81:12169–12178. doi: 10.1128/JVI.00835-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi KS, Aizaki H, Lai MM. Murine coronavirus requires lipid rafts for virus entry and cell–cell fusion but not for virus release. J Virol. 2005;79:9862–9871. doi: 10.1128/JVI.79.15.9862-9871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo H, Huang M, Yuan Q, Wei Y, Gao Y, Mao L, Gu L, Tan YW, Zhong Y, Liu D, Sun S. The important role of lipid raft-mediated attachment in the infection of cultured cells by coronavirus infectious bronchitis virus beaudette strain. PLoS One. 2017;12:e0170123. doi: 10.1371/journal.pone.0170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang G, Kim SH, Lee YG, Kim S, Lee DS, Lee KK, Lee C. Isolation and characterization of a Korean porcine deltacoronavirus strain KNU16-07. J Vet Sci. 2018;19:586–590. doi: 10.4142/jvs.2018.19.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeon JH, Lee C. Cellular cholesterol is required for porcine nidovirus infection. Arch Virol. 2017;162:3753–3767. doi: 10.1007/s00705-017-3545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung K, Hu H, Saif LJ. Porcine deltacoronavirus infection: etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis. Virus Res. 2016;226:50–59. doi: 10.1016/j.virusres.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S, Lee C. Complete genome characterization of Korean porcine deltacoronavirus strain KOR/KNU14-04/2014. Genome Announc. 2014;2:e01191-14. doi: 10.1128/genomeA.01191-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li GM, Li YG, Yamate M, Li SM, Ikuta K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007;9:96–102. doi: 10.1016/j.micinf.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marthaler D, Jiang Y, Collins J, Rossow K. Complete genome sequence of strain SDCV/USA/Illinois121/2014, a porcine deltacoronavirus from the United States. Genome Announc. 2014;2:e00218-14. doi: 10.1128/genomeA.00218-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nomura R, Kiyota A, Suzaki E, Kataoka K, Ohe Y, Miyamoto K, Senda T, Fujimoto T. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J Virol. 2004;78:8701–8708. doi: 10.1128/JVI.78.16.8701-8708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pratelli A, Colao V. Role of the lipid rafts in the life cycle of canine coronavirus. J Gen Virol. 2015;96:331–337. doi: 10.1099/vir.0.070870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren X, Glende J, Yin J, Schwegmann-Wessels C, Herrler G. Importance of cholesterol for infection of cells by transmissible gastroenteritis virus. Virus Res. 2008;137:220–224. doi: 10.1016/j.virusres.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saeng-Chuto K, Lorsirigool A, Temeeyasen G, Vui DT, Stott CJ, Madapong A, Tripipat T, Wegner M, Intrakamhaeng M, Chongcharoen W, Tantituvanont A, Kaewprommal P, Piriyapongsa J, Nilubol D. Different lineage of porcine deltacoronavirus in Thailand, Vietnam and Lao PDR in 2015. Transbound Emerg Dis. 2017;64:3–10. doi: 10.1111/tbed.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song D, Zhou X, Peng Q, Chen Y, Zhang F, Huang T, Zhang T, Li A, Huang D, Wu Q, He H, Tang Y. Newly emerged porcine deltacoronavirus associated with diarrhoea in swine in China: identification, prevalence and full-length genome sequence analysis. Transbound Emerg Dis. 2015;62:575–580. doi: 10.1111/tbed.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki T, Suzuki Y. Virus infection and lipid rafts. Biol Pharm Bull. 2006;29:1538–1541. doi: 10.1248/bpb.29.1538. [DOI] [PubMed] [Google Scholar]
- 16.Takano T, Satomi Y, Oyama Y, Doki T, Hohdatsu T. Differential effect of cholesterol on type I and II feline coronavirus infection. Arch Virol. 2016;161:125–133. doi: 10.1007/s00705-015-2655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.