Abstract
Newcastle disease virus (NDV) can replicate in tumor cells and induce apoptosis in late stages of infection. However, the interaction between NDV and cells in early stages of infection is not well understood. Here, we report that, shortly after infection, NDV triggers the formation of autophagosomes in U251 glioma cells, as demonstrated by an increased number of double-membrane vesicles, GFP-microtubule-associated protein 1 light chain 3 (GFP-LC3) a dot formations, and elevated production of LC3II. Moreover, modulation of NDV-induced autophagy by rapamycin, chloroquine or small interfering RNAs targeting the genes critical for autophagosome formation (Atg5 and Beclin-1) affects virus production, indicating that autophagy may be utilized by NDV to facilitate its own production. Furthermore, the class III phosphatidylinositol 3-kinase (PI3K)/Beclin-1 pathway plays a role in NDV-induced autophagy and virus production. Collectively, our data provide a unique example of a paramyxovirus that uses autophagy to enhance its production.
Keywords: Rapamycin, U251 Cell, Newcastle Disease Virus, Virus Production, Autophagosome Formation
Introduction
Newcastle disease virus (NDV) belongs to the genus Avulavirus in the family Paramyxoviridae and has a natural avian host range. Normal mammalian cells are found to be resistant to NDV replication, while oncolytic NDV can selectively replicate in tumor cells and induce apoptosis in late stages of infection [1]. However, in the initial phase of infection, NDV must prevent the rapid elimination of tumor cells to ensure its own survival and propagation. To date, the mechanisms that underlie the interaction between NDV and target cells in early stages of infection remain largely unclear.
Autophagy, also known as type 2 programmed cell death, is an evolutionarily conserved lysosomal degradation process by which long-lived proteins and damaged organelles are sequestered in the cytoplasm and removed for recycling [2]. The hallmark of autophagy is a double-membraned autophagosome that engulfs bulk cytoplasm and cytoplasmic organelles [3]. Autophagosomes fuse ultimately with lysosomes, thereby generating single-membraned autophagolysosomes and degrading their contents [4]. During autophagy, LC3 (microtubule-associated protein 1 light chain 3) is converted to lipidated LC3II and associates with the autophagic membrane. The punctuate distribution of LC3 upon lipidation is characteristic and serves as a marker for autophagy [5], while the LC3II/actin ratio correlates well with the number of autophagosomes [6]. A multifunctional protein p62 (SQSTM1) interacts with LC3II and is specifically degraded by the autophagic-lysosome pathway [6].
Autophagy is known to be controlled by a group of autophagy-related genes (Atg genes), and more than 30 Atg genes have been identified in yeast so far. The molecular pathways that regulate autophagy have been investigated extensively. A critical positive regulator of autophagy is the class III phosphatidylinositol 3-kinase (PI3KC3), whose activity in promoting autophagy depends on its association with a multi-protein complex that contains the essential mammalian autophagy protein Beclin-1 (homologue of yeast Atg6) [7]. Two ubiquitin-like conjugation systems are required for the formation of autophagosomes, in which several Atg genes including Atg5 are involved. Autophagy may also be negatively regulated by the serine/threonine kinase mammalian target of rapamycin (mTOR) [8, 9]. Other negative regulators of autophagy include the class I PI3K/Akt pathway, which controls autophagy via its downstream regulator mTOR.
Autophagy plays a role in both innate and adaptive immune responses to infection with microbial pathogens, including viruses [10, 11]. It has been demonstrated that some viruses, such as herpes simplex virus type 1 (HSV-1) and cytomegalovirus, have evolved mechanisms to suppress autophagy and, in the case of HSV-1, this increases its own survival [11, 12]. In contrast, several single-stranded RNA viruses, such as poliovirus, coronavirus and dengue virus, exploit the elements of the autophagy system to benefit their own replication [13–15]. So far, several members of the family Paramyxoviridae have been shown to induce autophagy in target cells [16, 17]. However, little is known about the role of autophagy in negative-stranded RNA virus replication.
In this paper, we report the discovery that autophagy is induced in U251 glioma cells upon NDV infection, and that knockdown of the genes critical for autophagosome formation markedly decreases virus yield.
Materials and methods
Cell lines, virus and plasmid
Human glioma U251 and chicken DF1 cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere containing 5% CO2. The NDV strain Beaudette C [18] was propagated and titrated in DF1 cells as described previously [19]. BC was UV-inactivated by standard procedures. GFP-microtubule-associated protein 1 light chain 3 (GFP-LC3) plasmid was purchased from Origene.
Antibodies and reagents
Phospho-specific antibodies to mammalian target of rapamycin (mTOR) (Ser2448), p70S6K (Thr389), Akt (Ser473) and total antibodies against mTOR, p70S6K and Akt were obtained from Cell Signaling Technology. A monoclonal anti-Beclin-1 antibody was purchased from BD Biosciences. A polyclonal rabbit anti-LC3 and a monoclonal antibody against β-actin were obtained from Sigma. Anti-p62 antibody was from Epitomics. Rapamycin and chloroquine (CQ) were purchased from Sigma.
Virus infection
U251 cells were infected with NDV at a multiplicity of infection (MOI) of 10, or sham infected with phophate-buffered saline (PBS), at 37°C for 1 h in serum-free DMEM. The cells were washed three times with PBS and incubated at 37°C in DMEM supplemented with 2% FBS. For induction of autophagy, the cells were treated with rapamycin for 1 h prior to virus infection.
For inhibition experiments, cells were treated with rapamycin (100 nM) or CQ (50 μM) for 30 min prior to virus infection. Subsequently, the cells were infected with NDV in the presence or absence of various compounds for 1 h and then cultured in fresh DMEM containing rapamycin or CQ for the indicated times. For experiments that involved determination of the virus yield, U251 cells were infected with NDV at an MOI of 0.01, and multi-step viral growth curves were examined.
Cell transfection and fluorescence microscopy
Lipofectamine 2000 (Invitrogen) was used to transfect U251 cells with GFP-LC3 DNA, according to the manufacturer’s guidelines. Dot formation of GFP-LC3 was detected under a fluorescence microcsope (BX50, Olympus) after drug treatment and/or NDV infection. Cells were considered to have accumulated autophagosomes when five or more puncta were counted, because the few mock-infected cells that displayed puncta rarely had more than one to four. A total of 100 transfected cells were analyzed in each well from three independent experiments (in triplicate).
Transmission electron microscopy
For ultrastructural analysis, standard transmission electron microscopy (TEM) was carried out. Virus infection was performed as above. Four hours after infection, the cells were fixed and embedded. Thin sections (90 nm) were cut and examined at 80 kV with a JEOL 1200EX transmission electron microscope. Approximately 15 cells were counted; autophagosomes were defined as double-membraned vacuoles measuring 0.2 to 1.0 μm.
Immunoblot analysis
U251 cells in 60-mm dishes were infected with NDV at an MOI of 10. One hour after infection, the medium was removed and replaced with DMEM supplemented with 2% FBS for the duration of the experiment. Virus-infected cells were harvested at the times indicated, and immunoblot (IB) was performed essentially as described previously [20]. Densitometry analysis of the specific protein expression was carried out using a calibrated GS-670 densitometer. All IB experiments were performed twice.
Transfection with small interfering RNA
Smart pool small interfering RNAs (siRNAs) targeting ATG5 (L-004374-00-0005, human ATG5, NM_004849) and Beclin-1 (L-010552-00-0005, human BECN 1, NM_003766) as well as control siRNA were obtained from Dharmacon (Denver, Colorado). Nearly 50 to 70% confluent U251 cells were transfected transiently with various siRNAs at a concentration of 50 to 80 nM, using Lipofectamine RNAiMAX (Invitrogen) according to the manufacturer’s instructions. A scrambled siRNA was used as a negative control. The silencing efficiency was detected by immunoblot. Seventy hours after transfection, the cells were infected with NDV at an MOI of 0.01 as described above. Viral titers were determined on DF-1 cell monolayers at the indicated time points postinfection.
Statistical analysis
The data were evaluated using Student’s t-test in SPSS V17.0 software (SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant at p < 0.01.
Results
NDV triggers autophagy in U251 cells
To investigate whether NDV infection led to any ultrastructural changes, U251 cells were mock treated or infected with the NDV strain Beaudette C [18] at a multiplicity of infection (MOI) of 10. The cells were examined subsequently by standard transmission electron microscopy (TEM). As depicted in Fig. 1A, mock-infected cells showed a normal distribution of organelles (Fig. 1A, a), while some infected cells were highly vacuolated and showed disappearance of most organelles (Fig. 1A, b). In some infected cells, cup-shaped membranous structures were observed wrapping around mitochondria in the cytoplasm (Fig. 1A, c). An autophagosome, or initial autophagic vacuole, containing a mitochondrion and other organelles, can be seen fusing with a lysosome to form a single-membraned vacuole (Fig. 1A, d). These observations indicate that ultrastructural features similar to autophagosomes were formed during NDV infection.
Fig. 1.
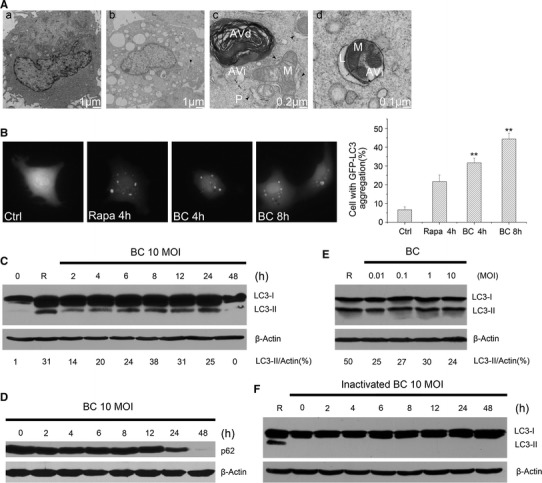
NDV infection induces autophagy in U251 cells. (A) U251 cells were mock infected or infected with NDV at an MOI of 10 and prepared for TEM at 8 h postinfection. a, mock-infected cells showing normal distribution of organelles; b, NDV-infected cells appear highly vacuolated and show disappearance of most organelles; c, autophagic structures in virus-infected cells. The arrowhead shows cup-shaped membranous structures (phagophores, P) in the cytoplasm, wrapping around mitochondria. The arrow shows the two limiting membranes of an autophagosome, or initial autophagic vacuole (AVi). A vacuole containing membrane whorls, the late/degradative autophagic vacuole, is undergoing the degradation process; d, an autophagosome (AVi), containing a mitochondrion (M) and other organelles, is fusing with a lysosome (L) to form a single-membraned vacuole. The two limiting membranes (arrow) of the autophagosome are visible at the upper rim of the vacuole. (B) Formation of GFP-LC3 puncta in NDV-infected U251 cells. Cells were transfected with GFP-LC3, followed by NDV infection for 4 or 8 h. The cells with punctated GFP-LC3 were counted. Pictures from left to right show mock-infected cells, cells treated with rapamycin for 4 h as a positive control, and cells infected with NDV for 4 and 8 h, respectively. Cells with punctate GFP-LC3 were counted. **, p < 0.01. (C) and (D) The time of course induction of LC3II and expression of p62 in NDV-infected U251 cells were investigated by immunoblot (IB). R stands for rapamycin. Cells treated with rapamycin were used as the positive control. Sample loading was controlled using an anti-actin antibody as indicated. Densitometry was performed for quantification, and the ratios of LC3II to actin are presented below the blots. (E) U251 cells were infected with NDV at a range of MOIs (0.01, 0.1, 1 and 10) for 24 h, and LC3II production was measured by IB. (F) The time course of induction of LC3II in U251 cells treated with UV-inactivated NDV at an MOI of 10 was examined by IB. The results are representative of two separate experiments
To confirm that the observed double-membraned vesicles were indeed related to autophagy, U251 cells transfected with GFP-LC3 were infected by NDV, and GFP-LC3 dot formation was investigated. The NDV-infected cells displayed a significant increase (p < 0.01) in GFP-LC3 puncta compared with mock cells (Fig. 1B). GFP-LC3 dot formation was also observed in the positive control cells (cells treated with an autophagosome inducer, rapamycin).
To corroborate these morphological observations, the time course of the conversion of endogenous LC3 to LC3II in NDV-infected U251 cells was examined by western blotting. Band intensity was measured by densitometry analysis. Rapamycin treatment of U251 cells increased the expression of LC3II, which was used as a positive control. As illustrated in Fig. 1C, increased expression of LC3II in NDV-infected U251 cells was detectable as early as 2 h postinfection [21], with a climax at 8 hpi, and it was maintained until 24 hpi. The expression had declined at 48 hpi. Comigration of LC3II in infected cells and in cells treated with rapamycin confirmed that the band was authentic LC3II. In addition, similar results were obtained in U251 cells infected with NDV at other MOIs (0.01, 0.1, 1 and 10) (Fig. 1E). In contrast, the cells treated with UV-inactivated NDV could not induce marked production of LC3II (Fig. 1F).
It is known that some pathogens induce early stages of autophagy but block later stages (i.e., autophagolysosomal fusion). To examine whether a complete autophagic response (i.e., autophagic flux) was induced by NDV, levels of the autophagic substrate p62 were measured. As shown in Fig. 1D, there was a gradual decline in levels of p62 protein with barely detectable levels observed 48 hpi. Collectively, these data demonstrate that NDV can induce a complete autophagic response in U251 glioma cells.
The class III PI3K/Beclin-1 pathway is involved in autophagy induced by NDV infection
To understand the potential mechanism of autophagosome formation in NDV-infected U251 cells, we examined the phosphorylation status of mTOR, a well-known negative regulator that controls autophagosome formation [22]. As shown in Fig. 2, a significant increase in the levels of mTOR phosphorylation was observed 6 h after NDV infection and maintained to 24 hpi (Fig. 2). In addition, upon NDV infection, elevated phosphorylation of p70S6K, a downstream effector of mTOR signaling, was also detected with a time dependence similar to that of mTOR phosphorylation (Fig. 2). It is known that mTOR is one of the downstream effectors of the class I PI3K/Akt signaling pathways and that Akt signaling has been shown previously to be involved in the induction of autophagy in malignant glioma cells [23]. We examined Akt phosphorylation at different time points after NDV infection. As shown in Fig. 2, increased phosphorylation of Akt was observed in NDV-infected cells until 24 hpi. Together, these data suggested that the class I PI3K/Akt/mTOR signaling pathway was activated during NDV infection and may not contribute to NDV-triggered autophagy.
Fig. 2.
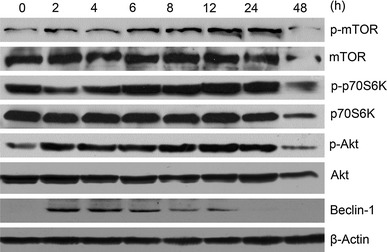
Signaling pathways involved in NDV infection of U251 cells contribute to virus-induced autophagy. U251 cells were infected with NDV at an MOI of 10 for different intervals as indicated in the figure. Cell lysates were analyzed for the activation of mTOR, p70S6K and Akt by immunoblot assay. The filters were stripped and reprobed with antibodies against total mTOR, p70S6K or Akt. Sample loading was controlled using an anti-actin antibody as indicated
The above findings prompted us to examine the role of the class III PI3K/Beclin-1 pathway in autophagic induction in NDV-infected U251 cells. Beclin-1 (Atg6 in mammals) forms a complex with class III PI3K and is required for the formation of the autophagic vesicle [24, 25]. As illustrated in Fig. 2, expression of Beclin-1 was upregulated in early stages of NDV infection. This result indicated that the class III PI3K/Beclin-1 pathway plays an important role in NDV-induced autophagy in U251 cells.
Pharmacological regulation of induction of autophagy affects virus production in NDV-infected U251 cells
To assess whether modulation of NDV-induced autophagy has an effect on virus replication, we first investigated whether pharmacological compounds can modulate autophagic induction. U251 cells infected with NDV were mock treated or treated with the autophagy inducer rapamycin, or the autophagy inhibitor CQ, at the concentrations used in previous studies [26–29]. The two compounds had no effect on cell viability at the concentrations used in this study (data not shown). As can be seen in Fig. 3A, the production of LC3II was increased at 24 and 48 hpi in NDV-infected cells treated with rapamycin. CQ inhibits autophagy at a late stage by preventing the fusion of autophagosomes with lysosomes and thus increasing the cellular burden of autophagosomes [30, 31]. Because LC3II is an essential element in the formation of the autophagosome, the inhibition of autophagy by CQ results in the marked accumulation of LC3II [32, 33]. Figure 3B shows that CQ induced a moderate increase in LC3II in infected cells, indicating that CQ treatment blocks the fusion of the NDV-induced autophagosomes with lysosomes. Overall, these results indicated that rapamycin exerts an enhanced effect on NDV-induced autophagy, whereas CQ inhibits NDV-induced autophagy.
Fig. 3.
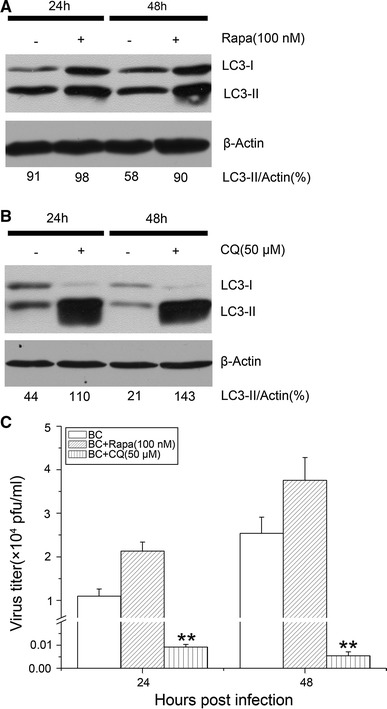
Regulation of NDV-induced autophagy in U251 cells affects virus production. (A) and (B) U251 cells were pretreated with rapamycin (Rapa) (100 nM) or chloroquine (CQ) (50 μM) and then infected with NDV (MOI = 0.01) at the indicated times, LC3II production was determined by immunoblot. The ratios of LC3II to actin are presented below the blots. (C) Virus yield in NDV-infected cells pretreated with or without rapamycin or chloroquine was determined at the indicated times. Data are presented as the mean ± SD for triplicate assays. **, p < 0.01
Subsequently, the virus yield in cells treated with autophagy modulators, including rapamycin and CQ, were determined by titration in DF1 cells, and multi-step viral growth curves were investigated. As shown in Fig. 3C, infection in the presence of rapamycin resulted in an almost twofold increase in virus production at 24 and 48 hpi when compared with the mock-treated control. In contrast, CQ treatment resulted in a decrease in virus yield by nearly 2 logs (Fig. 3C). Considering our observations about the effect on NDV-triggered autophagic induction by rapamycin or CQ in U251 cells, we inferred that the autophagic machinery plays a positive role in virus production in NDV-infected U251 cells.
Knockdown of the genes critical for autophagosome formation reduces NDV yield
To elucidate further the role of autophagy in NDV production, we knocked down the expression of either Atg5 or Beclin-1 at the transcriptional level in U251 cells. Both proteins have been shown to be essential for the formation of autophagosomes [24, 34]. As illustrated in Fig. 4A, the expression of either Atg5 or Beclin-1 in uninfected U251 cells was inhibited to a large extent (about 75% reduction) by specific small interfering RNAs (siRNAs). Figure 4B shows that there was a reduction of approximately 10 times and 6 times, respectively, in the virus yield at 24 hpi and nearly 60 times at 48 hpi when compared with control infection. These data indicated that inhibition of autophagy reduced virus production in NDV-infected U251 cells.
Fig. 4.
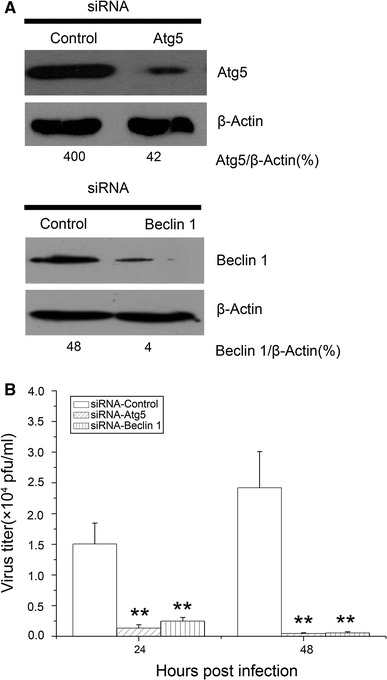
Gene silencing of Atg5 or Beclin-1 by siRNA inhibits NDV production. U251 cells were transfected with 100 pmol siRNA duplexes targeted to either Atg5 or Beclin-1 mRNA, or control siRNA, for 72 h. (A) The relative expression of Atg5 and Beclin-1 proteins was examined by immunoblot analysis with specific antibody to Atg5 or Beclin-1, respectively. (B) Transfected cells were infected with NDV (MOI = 0.01) and the virus yield was determined at different intervals. Data are presented as the mean ± SD for triplicate assays. **, p < 0.01
Discussion
Members of the family Paramyxoviridae have been shown to induce autophagy in target cells. It has been reported that the parainfluenza virus simian virus 5 activates plasmacytoid dendritic cells through the autophagy pathway [16], while measles virus induces autophagy in HeLa cells through a pathway mediated by CD46 [17]. However, it remains unclear whether autophagy is triggered upon NDV infection. In the current study, we demonstrated that, shortly after infection, NDV triggers autophagy in U251 glioma cells. By means of fluorescence and electron microscopy, several hallmarks of autophagy, including the formation of autophagosomes and GFP-LC3 dot formation, were observed in U251 cells upon NDV infection (Fig. 1A and B). These observations were attested further by the increased production of LC3II (Fig. 1C). Furthermore, decreased levels of p62 at 24 h and 48 h after NDV infection correlate well with LC3II production, indicating that a complete autophagic response was induced upon NDV infection. Taken together, these data strongly suggest that autophagy is induced and is a part of the cellular response during NDV infection. Further studies will be carried out to examine whether autophagy is induced in other cell lines upon NDV infection.
The process of autophagosome formation is tightly controlled by a few signaling pathways including the class I PI3K/Akt/mTOR and class III PI3K/Beclin-1 pathways. So far, the mechanisms that participate in paramyxovirus-induced autophagy remain largely unknown. In this report, we showed that Beclin-1 expression was induced at early stages of NDV infection, which correlates well with the increased production of LC3II, indicating a potential role of the class III PI3K/Beclin-1 pathway in NDV-triggered autophagy. In contrast, we observed increased phosphorylation of Akt, mTOR and p70S6K in NDV-infected U251 cells, suggesting that NDV-induced autophagy is not triggered by the class I PI3K/Akt/mTOR pathway. Therefore, the class III PI3K/Beclin-1 pathway plays a crucial role in NDV-induced autophagy.
Although autophagy is known to be a cellular defense against viral infection, induction of autophagy has been shown to be beneficial for the replication of a few RNA viruses, including poliovirus [35], dengue virus [14] and coxsackievirus [36]. In this report, we show that manipulation of the autophagic induction in NDV-infected U251 cells by pharmacological compounds or target-specific RNA interference resulted in significant alterations in virus replication. CQ treatment decreased virus production 24 and 48 h after NDV infection, while elevated virus production by rapamycin treatment was detected at 24 and 48 hpi. Interestingly, the effect on virus yield by CQ or rapamycin correlates well with their diverse effects on NDV-induced autophagy. Together, our data suggest that autophagy plays a critical role in virus production in NDV-infected cells. This notion is supported further by results from siRNA manipulations targeting ATG5 or Beclin-1 in U251 cells. The ATG5 protein is essential for the formation of the autophagosomal membrane, while Beclin-1 is necessary for cellular autophagy. A previous study has shown that ATG5 is necessary for the delivery of Sendai virus genetic material from the cytoplasm into endosomes, where the ssRNA sensor Toll-like receptor 7 resides [37]. The suppression of either of the two autophagic molecules in NDV-infected U251 cells resulted in the reduction of virus yield when compared with control. Together, these data indicate that autophagy is likely to be utilized by NDV to facilitate its own production. Of note, although several negative-stranded RNA viruses have been shown to induce autophagy, the relationship between autophagy and virus replication is poorly understood. Thus, our data will increase understanding of the role of autophagy in negative-stranded RNA virus replication.
In conclusion, we demonstrate for the first time that NDV triggers autophagy in U251 cells and that autophagy is utilized for virus production in NDV-infected cells. We have also delineated the molecular pathways that are involved in the induction of autophagy and virus production in NDV-infected U251 cells. Our data provide a unique example of a negative-stranded RNA virus that uses autophagy to enhance its own production. Future studies will be carried out to investigate whether autophagy triggered by NDV exerts an effect on apoptotic induction in NDV-infected tumor cells.
Acknowledgments
This work was funded by Special Fund for Agro-scientific Research in the Public Interest (201003012), National High-tech R and D Program (863 Program, 2011AA10A209) and State Key Laboratory of Veterinary Biotechnology of China (SKLVBF201109).
Footnotes
C. Meng and Z. Zhou contributed equally to this work.
Contributor Information
Songshu Meng, Phone: 86-514-87972107, FAX: 86-514-87991747, Email: ssmeng@yzu.edu.cn.
Chan Ding, Phone: 86-21-34293441, FAX: 86-21-54081818, Email: shoveldeen@shvri.ac.cn.
References
- 1.Phuangsab A, Lorence RM, Reichard KW, Peeples ME, Walter RJ. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer Lett. 2001;172:27–36. doi: 10.1016/S0304-3835(01)00617-6. [DOI] [PubMed] [Google Scholar]
- 2.Terman A, Dalen H, Eaton JW, Neuzil J, Brunk UT. Mitochondrial recycling and aging of cardiac myocytes: the role of autophagocytosis. Exp Gerontol. 2003;38:863–876. doi: 10.1016/S0531-5565(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 3.Inbal B, Bialik S, Sabanay I, Shani G, Kimchi A. DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death. J Cell Biol. 2002;157:455–468. doi: 10.1083/jcb.200109094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine B, Yuan J. Autophagy in cell death: an innocent convict? J Clin Invest. 2005;115:2679–2688. doi: 10.1172/JCI26390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dreux M, Chisari FV. Viruses and the autophagy machinery. Cell Cycle. 2010;9:1295–1307. doi: 10.4161/cc.9.7.11109. [DOI] [PubMed] [Google Scholar]
- 8.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36:2445–2462. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Schmid D, Munz C. Innate and adaptive immunity through autophagy. Immunity. 2007;27:11–21. doi: 10.1016/j.immuni.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deretic V. Strange bedfellows expose ancient secrets of autophagy in immunity. Immunity. 2009;30:479–481. doi: 10.1016/j.immuni.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orvedahl A, Levine B. Autophagy and viral neurovirulence. Cell Microbiol. 2008;10:1747–1756. doi: 10.1111/j.1462-5822.2008.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong J, Zhang J, Si X, Gao G, Mao I, McManus BM. Autophagosome supports coxsackievirus B3 replication in host cells. J Virol. 2008;82:9143–9153. doi: 10.1128/JVI.00641-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee YR, Lei HY, Liu MT, Wang JR, Chen SH, Jiang-Shieh YF. Autophagic machinery activated by dengue virus enhances virus replication. Virology. 2008;374:240–248. doi: 10.1016/j.virol.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson WT, Giddings TH, Jr, Taylor MP, Mulinyawe S, Rabinovitch M, Kopito RR. Subversion of cellular autophagosomal machinery by RNA viruses. PLoS Biol. 2005;3:e156. doi: 10.1371/journal.pbio.0030156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manuse MJ, Briggs CM, Parks GD. Replication-independent activation of human plasmacytoid dendritic cells by the paramyxovirus SV5 Requires TLR7 and autophagy pathways. Virology. 2010;405:383–389. doi: 10.1016/j.virol.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Joubert PE, Meiffren G, Gregoire IP, Pontini G, Richetta C, Flacher M. Autophagy induction by the pathogen receptor CD46. Cell Host Microbe. 2009;6:354–366. doi: 10.1016/j.chom.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Zanetti F, Berinstein A, Pereda A, Taboga O, Carrillo E. Molecular characterization and phylogenetic analysis of NDV isolates from healthy wild birds. Avian Dis. 2005;49:546–550. doi: 10.1637/7381-051605R.1. [DOI] [PubMed] [Google Scholar]
- 19.Bian JC, Wang K, Kong XG, Liu HR, Chen F, Hu MZ. Caspase and p38 MAPK-dependent apoptotic induction in A549 lung cancer cells by Newcastle disease virus. Arch Virol. 2011;156:1335–1344. doi: 10.1007/s00705-011-0987-y. [DOI] [PubMed] [Google Scholar]
- 20.Meng S, Gui Q, Xu Q, Lu K, Jiao X, Fan J. Association of Shp2 with phosphorylated IL-22R1 is required for interleukin-22-induced MAP kinase activation. J Mol Cell Biol. 2010;2:223–230. doi: 10.1093/jmcb/mjq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weidberg H, Shvets E, Shpilka T, Shimron F, Shinder V, Elazar Z. LC3 and GATE-16/GABARAP subfamilies are both essential yet act differently in autophagosome biogenesis. EMBO J. 2010;29:1792–1802. doi: 10.1038/emboj.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12(Suppl 2):1509–1518. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 23.Takeuchi H, Kondo Y, Fujiwara K, Kanzawa T, Aoki H, Mills GB. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–3346. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 24.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 25.Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Qin Z, Liang Z. The prosurvival role of autophagy in Resveratrol-induced cytotoxicity in human U251 glioma cells. BMC Cancer. 2009;9:215. doi: 10.1186/1471-2407-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park CM, Park MJ, Kwak HJ, Lee HC, Kim MS, Lee SH. Ionizing radiation enhances matrix metalloproteinase-2 secretion and invasion of glioma cells through Src/epidermal growth factor receptor-mediated p38/Akt and phosphatidylinositol 3-kinase/Akt signaling pathways. Cancer Res. 2006;66:8511–8519. doi: 10.1158/0008-5472.CAN-05-4340. [DOI] [PubMed] [Google Scholar]
- 28.Jiang H, Shang X, Wu H, Gautam SC, Al-Holou S, Li C. Resveratrol downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma cells. J Exp Ther Oncol. 2009;8:25–33. [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H, Kong X, Kang J, Su J, Li Y, Zhong J. Oxidative stress induces parallel autophagy and mitochondria dysfunction in human glioma U251 cells. Toxicol Sci. 2009;110:376–388. doi: 10.1093/toxsci/kfp101. [DOI] [PubMed] [Google Scholar]
- 30.Boya P, Gonzalez-Polo RA, Casares N, Perfettini JL, Dessen P, Larochette N. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025–1040. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol. 2009;625:220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 32.Geng Y, Kohli L, Klocke BJ, Roth KA. Chloroquine-induced autophagic vacuole accumulation and cell death in glioma cells is p53 independent. Neuro Oncol. 2010;12:473–481. doi: 10.1093/neuonc/nop048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shacka JJ, Klocke BJ, Shibata M, Uchiyama Y, Datta G, Schmidt RE. Bafilomycin A1 inhibits chloroquine-induced death of cerebellar granule neurons. Mol Pharmacol. 2006;69:1125–1136. doi: 10.1124/mol.105.018408. [DOI] [PubMed] [Google Scholar]
- 34.Nemoto T, Tanida I, Tanida-Miyake E, Minematsu-Ikeguchi N, Yokota M, Ohsumi M. The mouse APG10 homologue, an E2-like enzyme for Apg12p conjugation, facilitates MAP-LC3 modification. J Biol Chem. 2003;278:39517–39526. doi: 10.1074/jbc.M300550200. [DOI] [PubMed] [Google Scholar]
- 35.Belov GA, Altan-Bonnet N, Kovtunovych G, Jackson CL, Lippincott-Schwartz J, Ehrenfeld E. Hijacking components of the cellular secretory pathway for replication of poliovirus RNA. J Virol. 2007;81:558–567. doi: 10.1128/JVI.01820-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kemball CC, Alirezaei M, Flynn CT, Wood MR, Harkins S, Kiosses WB. Coxsackievirus infection induces autophagy-like vesicles and megaphagosomes in pancreatic acinar cells in vivo. J Virol. 2010;84:12110–12124. doi: 10.1128/JVI.01417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–1401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]


