Abstract
Ranaviruses are nucleoplasmic large DNA viruses that can cause major economic losses in the aquaculture industry and pose a severe threat to global ecological diversity. The available literature demonstrates that classifiable members of the genus Ranavirus enter cells via multiple and complicated routes. Here, we demonstrated the underlying cellular entry mechanism of soft-shelled turtle iridovirus (STIV) using green fluorescence tagged recombinant virus. Treatment with chlorpromazine, sucrose, ethyl-isopropyl amiloride, chloroquine or bafilomycin A1 all significantly decreased STIV infection, suggesting that STIV uses clathrin-mediated endocytosis and macropinocytosis to enter cells via a pH-dependent pathway. Depletion of cellular cholesterol with methyl-β-cyclodextrin significantly inhibited STIV entry, but neither filipin III nor nystatin did, suggesting that STIV entry was cholesterol dependent but caveola independent. Treatment with dynasore, genistein, ML-7 or cytochalasin D all significantly inhibited STIV infection, indicating that Rac GTPase and myosin II activity were required for the macropinocytosis-like pathway as well as actin polymerization. Our findings suggest that the molecular events involved in STIV entry are not identical to those of other ranavirus isolates. Our results also extend our understanding of the molecular mechanism of iridovirus entry and pathogenesis.
Introduction
Great attention has been paid to the mechanisms of virus entry due to their potential role in developing novel therapeutics and vaccines [1, 2]. Viruses often take advantage of multiple endocytic pathways to enter cells. Clathrin-mediated endocytosis is the best characterized pathway, exploited by many viruses such as human immunodeficiency virus (HIV), hepatitis C virus (HCV), rabies virus, astrovirus and frog virus FV3 [3–8]. Apart from clathrin-mediated entry, caveola-dependent endocytosis is an alternative well characterized pathway for virus entry. Compared to clathrin, the internalization of caveolae is slower and the resulting vesicles do not become acidified [9]. Currently, caveola-dependent endocytosis is known to be exploited by several viruses, including Simian virus (SV)40, tiger frog virus (TFV), echovirus 1 and hepatitis B virus (HBV) [10–13].
In addition, macropinocytosis has emerged as a major endocytic mechanism in the cell entry of animal viruses [14]. A variety of viruses, including enveloped and non-enveloped viruses from adenovirus, picornavirus and other virus families as well as vaccinia virus take advantage of macropinocytosis for uptake [1, 15–17]. Inhibitors of Na+/H+ exchange, such as amiloride and ethyl-isopropyl amiloride (EIPA), have been used as the main diagnostic test to clarify macropinocytosis. Inhibitors of several cellular factors and signaling pathways also inhibit macropinocytosis initiated by different viruses [1, 17].
Ranaviruses are nucleoplasmic large DNA viruses that cause major economic losses in the aquaculture industry and contribute to global ecological instability [18, 19]. From the genus Ranavirus, FV3 was the first iridovirus to have its entry mechanism investigated. In mammalian cells (BHK-21), enveloped FV3 particles are internalized by adsorptive endocytosis via coated pits and then move through endosomes and finally lysosomes, suggesting that FV3 entry is via receptor-mediated clathrin endocytosis [20]. In contrast, recent studies demonstrated different mechanisms used by different iridoviruses. Both TFV and infectious spleen and kidney necrosis virus (ISKNV) enter different cells via caveola-dependent but not clathrin-dependent endocytosis [13, 21]. Singapore grouper iridovirus (SGIV) entry into host cells occurs via clathrin-mediated endocytosis and macropinocytosis in a pH-dependent manner [17]. The different endocytic pathways used by various iridoviruses implies a complex process in the early steps of iridovirus infection.
Soft-shelled turtle iridovirus (STIV) was isolated from a diseased soft-shelled turtle (Trionyx sinensis) [22]. Complete genomic sequence analysis indicates that STIV and FV3 are likely different strains of the same viral species belonging to the Ranavirus genus [23]. In vitro, STIV infection induces typical apoptosis and evokes the mitogen-activated protein kinase (MAPK) signaling pathway [24]. Moreover, fluorescence-tagged recombinant STIV [enhanced green fluorescent protein (EGFP)-STIV] has been successfully constructed to study infective dynamics and evaluate viral replication [25–27]. Although the genome sequences of FV3 and TFV share high identity, the endocytic pathway used by them differs completely [13, 20]. Whether the entry pathway of STIV was different from other reported ranavirus isolates remained unknown. Here, we demonstrated the underlying cellular entry mechanisms of STIV using EGFP-STIV. Our results shed new light on the early events of iridovirus infection in lower vertebrates.
Materials and methods
Cells and virus
FHM cells were cultured in M199 Medium (Gibco) containing 10% fetal bovine serum (FBS, Gibco). Green fluorescent protein-tagged recombinant virus EGFP-STIV was constructed in our previous study and expresses an EGFP fused to the VP55 membrane protein under a natural promoter [25]. EGFP-STIV was propagated in FHM cells at 28 °C.
Reagents
Chlorpromazine (CPZ, an inhibitor of clathrin-mediated endocytosis), dynasore (a specific inhibitor of dynamin), methyl-β-cyclodextrin (βMCD, cholesterol synthesis inhibitor), nystatin (lipid raft inhibitor), EIPA (inhibitor of Na+/H+ channels), chloroquine (CQ), bafilomycin A1 (BAF, a specific inhibitor of vacuolar proton ATPases), were purchased from Sigma-Aldrich.
Cell viability
The cytotoxicity of the chemical regents on FHM cells was determined by the trypan blue exclusion test, as described previously [25]. FHM cells treated with drugs at different concentrations were collected by trypsinization, and then incubated in 0.2% trypan solution (Sigma) for 5 min. All experiments were carried out in triplicate, and dead cells were counted by three independent hemocytometer counts. Cell viability was calculated as the percentage of live cells over the total number of cells. All the drug concentrations used in this study were non-toxic (cell viability > 95%) to FHM cells (data not shown).
Fluorescence microscopy
At 24 h p.i., EGFP-STIV-infected cells (at an MOI of 1) were fixed with 4% paraformaldehyde and then washed twice with phosphate-buffered saline (PBS). Green fluorescence, which indicated virus entry or newly synthesized virus, was imaged using a fluorescence microscope (Leica) and analyzed with Las 3.8 software [21]. Experiments for image analysis were performed three or more times, and the images are representative of results from three replicate experiments.
Flow cytometry
For flow cytometry analysis, mock- or inhibitor-treated cells were incubated with EGFP-STIV at an MOI of 1 for 24 h, then cells were washed once with PBS and incubated with trypsin (Invitrogen) for 2 min. Cells were transferred to 1.5-ml centrifuge tubes and centrifuged at 1500×g for 10 min. All samples were incubated with 4% paraformaldehyde for 30 min prior to resuspension in PBS for flow analysis using a FACScan flow cytometer with CellQuest software (BD Biosciences).
Measurement of GFP fluorescence intensity
The harvested cells were lysed in ice-cold reporter lysis buffer. The lysate was centrifuged at 12,000×g for 10 min. Fluorescence intensity of GFP in 100 µl supernatant was measured using a VICTOR X5 2030 Multilabel Plate Reader (PerkinElmer) at an excitation wavelength of 488 nm and emission wavelength of 535 nm. The autofluorescence of the uninfected lysate was detected as background and subtracted from measurements of the EGFP-STIV-infected lysates.
Western blotting
Cells were harvested and lysed in cell lysis buffer (Promega). The protein concentration was measured using a Bradford Protein Assay Kit. Cell lysates were fractionated by SDS-PAGE and transferred to polyvinylidene fluoride membranes for 70 min. The separated proteins were reacted with anti-MCP (major capsid protein) as the primary antibody, at a dilution of 1:1000. The secondary antibody was horseradish-peroxidase-conjugated goat anti-mouse antibody at a dilution of 1:1000. Internal control reactions to detect β-actin were carried out simultaneously. Immunoblots were visualized using an enhanced chemiluminescence detection system (Amersham ECL Western Blotting Detection Kit). Data were obtained from three independent experiments.
Results
Clathrin-mediated endocytosis was involved in STIV entry
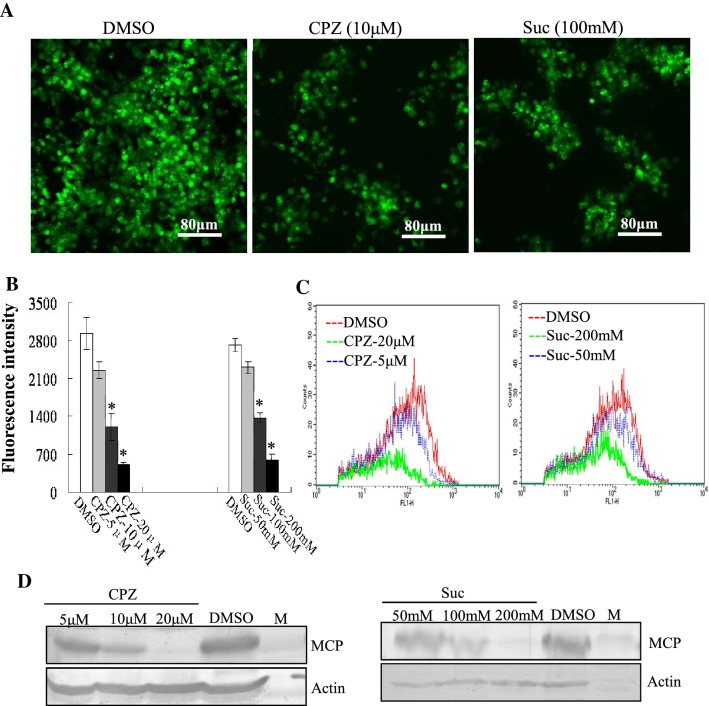
Certain agents such as CPZ and sucrose, which can prevent recycling of clathrin to the plasma membrane, inhibit clathrin-mediated endocytosis [20]. To elucidate the mechanism underlying the endocytosis involved in STIV entry, we examined the role of clathrin-mediated endocytosis using CPZ and sucrose. Fluorescent microscopy revealed an obvious reduction of green fluorescence labeling of cells after treatment with 10 μM CPZ or 100 mM sucrose in comparison to mock-treated cells (Fig. 1A). Quantitative analysis showed that the fluorescence intensity in CPZ- or sucrose-treated cells was significantly reduced in a dose dependent manner. Treatment with CPZ at 20 μM and sucrose at 200 mM decreased the fluorescence intensity up to 17.29% and 21.58%, respectively when compared to mock-treated cells (Fig. 1B). Consistently, flow cytometry showed that the percentage of fluorescence-labeled cells was significantly reduced after treatment with CPZ or sucrose (Fig. 1C). In addition, the protein expression from EGFP-STIV MCP was evaluated using western blotting. Protein synthesis of MCP was obviously reduced after treatment with 20 μM CPZ or 200 mM sucrose (Fig. 1D). These results show that clathrin-mediated endocytosis is involved in STIV entry.
Fig. 1.
Clathrin-mediated endocytosis is involved in STIV entry. (A) Fluorescence microscopy observation of EGFP-STIV-infected cells after treatment with DMSO, CPZ or sucrose. (B) Fluorescence intensity of EGFP-STIV-infected cells after treatment with DMSO, CPZ or sucrose. (C) Quantitative analysis of the percentage of EGFP-STIV-infected cells by flow cytometry. (D) Western blotting detection of protein synthesis of the STIV major capsid protein after treatment with CPZ and sucrose
STIV entry was mediated through a cholesterol-dependent but caveola- independent pathway
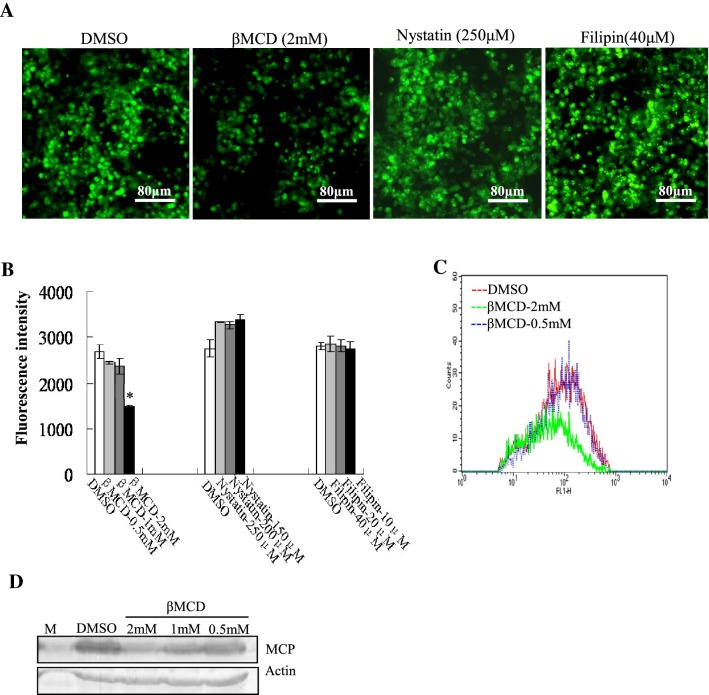
Caveola-mediated virus entry is inhibited by sterol-binding drugs such as nystatin and filipin III. To investigate the role of lipid raft/caveolin in STIV entry, we used sterol-binding drugs filipin and nystatin to impair caveola formation [28]. We also used βMCD to remove cholesterol from cultured cells [21]. βMCD obviously decreased the number of fluorescence cells when compared to the mock treated cells. In contrast, the number of fluorescent cells remained almost unchanged after treatment with filipin and nystatin (Fig. 2A). Quantitative analysis indicated that only the fluorescence intensity in βMCD-treated cells was significantly decreased in a dose dependent manner; treatment with βMCD at 2 mM decreased the fluorescence intensity up to 55.29% when compared to the mock-treated cells (Fig. 2B). Flow cytometry also confirmed that the percentage of fluorescence-labeled cells was only significantly reduced after treatment with βMCD (Fig. 2C). In addition, protein synthesis was only inhibited by treatment with βMCD, but not by nystatin or filipin III (Fig. 2D). Together, these results suggested that STIV entry was mediated through a non-caveola- but cholesterol-dependent pathway
Fig. 2.
STIV entry is mediated through a non-caveola- and cholesterol-dependent pathway. (A) Fluorescence microscopy of EGFP-STIV-infected cells after treatment with DMSO, βMCD, nystatin or filipin III. (B) Determination of the fluorescence intensity of EGFP-STIV-infected cells after treatment with DMSO, βMCD, nystatin or filipin III. (C) Quantitative analysis of the percentage of EGFP-STIV-infected cells by flow cytometry. (D) Western blotting detection of protein synthesis of the STIV major capsid protein after treatment with βMCD
STIV utilized macropinocytosis as an alternative pathway to enter cells
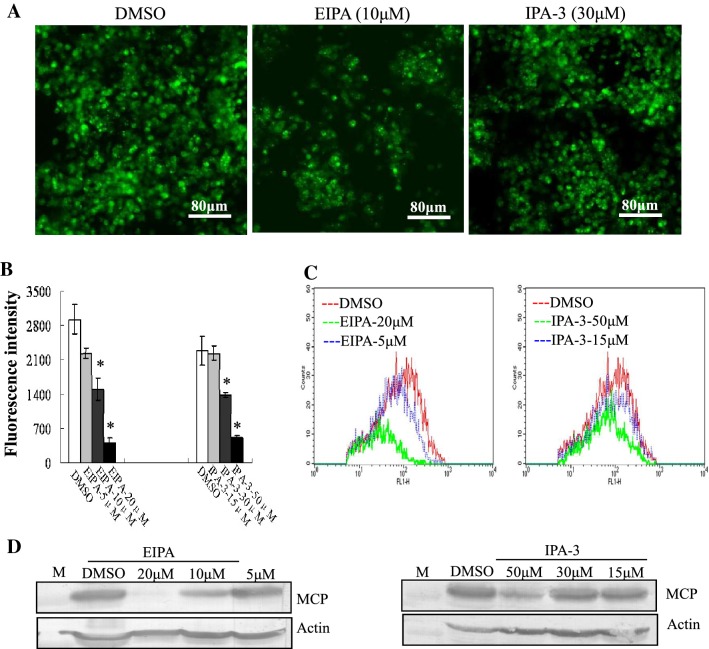
To define whether macropinocytosis was involved in STIV entry, EIPA, the Na+/H+ exchanger inhibitor and IPA-3, a direct and highly selective Pak1 inhibitor [17], were used to pretreat FHM cells. Treatment with 10 μM EIPA or 30 μM IPA-3 obviously reduced the number of cells labeled with green fluorescence (Fig. 3A). Quantitative analysis indicated that the fluorescence intensity in EIPA- or IPA-3-treated cells was significantly reduced in a dose dependent manner; treatment with EIPA at 20 μM and IPA-3 at 30 μM decreased the fluorescence intensity up to 13.91% and 22.24% when compared to mock-treated cells, respectively (Fig. 3B). Consistently, flow cytometry showed that the percentage of fluorescence-labeled cells was significantly decreased in a dose-dependent manner after treatment with EIPA or IPA-3 (Fig. 3C). In addition, protein synthesis of MCP was also significantly inhibited by the addition of EIPA or IPA-3 (Fig. 3D), suggesting that STIV utilized macropinocytosis as an alternative pathway to enter cells.
Fig. 3.
Inhibitors of macropincytosis block STIV infection. (A) Fluorescence microscopy of EGFP-STIV-infected cells after treatment with DMSO, EIPA or IPA-3. (B) Fluorescence intensity of EGFP-STIV-infected cells after treatment with DMSO, EIPA or IPA-3. (C) Quantitative analysis of the percentage of EGFP-STIV-infected cells by flow cytometry. (D) Western blotting detection of protein synthesis of the STIV major capsid protein after treatment with EIPA or IPA-3
Multiple cellular factors and signaling events are involved in STIV entry
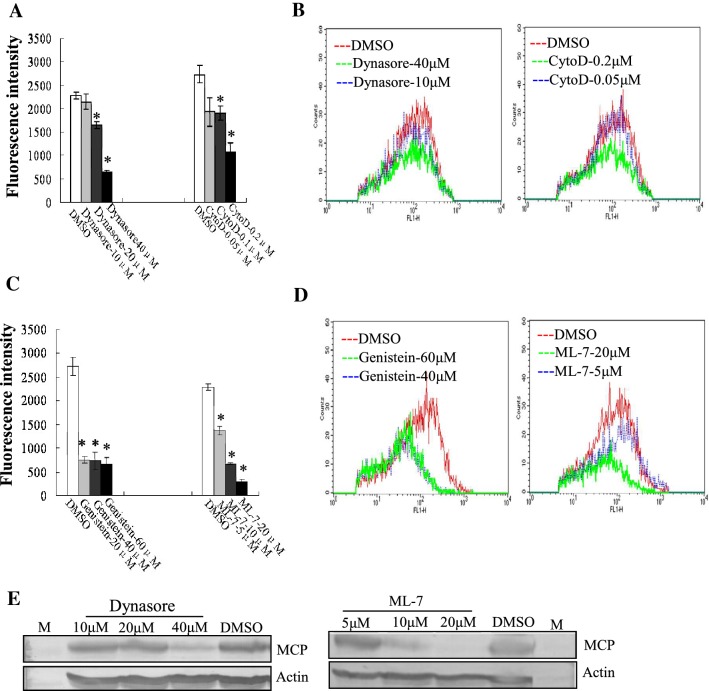
An increasing numbers of studies have reported that various cellular factors are involved in virus entry [14]. In our study, the regulation of myosin II activity by phosphorylation of myosin light chain was inhibited by ML-7, and Rac1 activity was specifically inhibited by NSC23766. We also used genistein, dynasore and cytochalasin D to inhibit tyrosine kinase, large GTPase dynamin and actin polymerization in GS cells, respectively. Except for NSC23766 (data not shown), the fluorescence intensity of cells treated with cytochalasin D (cyto-D), dynasore, genistein or ML-7 was significantly reduced in a dose dependent manner. Treatment with cyto-D at 0.2 μM, dynasore at 40 μM, genistein at 60 μM and ML-7 at 20 μM decreased the fluorescence intensity up to 39.34%, 28.37%, 24.29% and 13.35%, respectively in comparison to mock-treated cells (Fig. 4A and 4C). Flow cytometry analysis showed that the percentage of fluorescence-labeled cells after treatment with these four inhibitors was significantly reduced compared to that of mock-treated cells (Fig. 4B and 4D). We also evaluated the effects of dynasore and ML-7 on viral protein synthesis. Protein synthesis of MCP in dynasore- and ML-7-treated cells was obviously reduced during EGFP-STIV infection when compared to that seen in mock-treated cells (Fig. 4E). These results suggest that multiple cellular factors or signaling events including myosin II, tyrosine kinase, dynamin and actin polymerization are all essential for STIV entry.
Fig. 4.
Multiple cellular factors and signaling events were involved in STIV entry. (A, C) Determination of the fluorescence intensity of EGFP-STIV-infected cells after treatment with DMSO, cyto-D, dynasore, genistein or ML-7. (B, D) Percentage of EGFP-STIV-infected cells after treatment with DMSO, cyto-D, dynasore, genistein or ML-7 by flow cytometry. (E) Western blotting detection of protein synthesis of the STIV major capsid protein after treatment with dynasore or ML-7
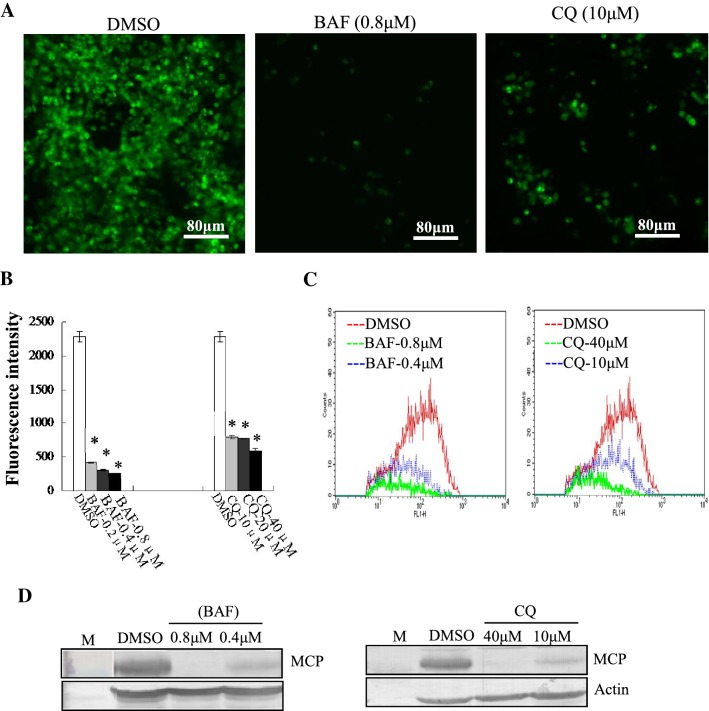
STIV entered into cells in a pH-dependent manner
To assess whether STIV entry was pH dependent, we altered the endosome acidification with CQ, a lysosomotropic agent, or with BAF, a specific inhibitor of endosomal proton-ATP pumps. Green fluorescence in cells after treatment with 10 µM CQ or 0.8 μM BAF was obviously reduced in comparison to that seen in mock-treated cells (Fig. 5A). Quantitative analysis showed that the fluorescence intensity in CQ- or BAF-treated cells was significantly reduced in a dose dependent manner. Treatment with BAF at 0.8 μM, and CQ at 40 μM decreased the fluorescence intensity up to 10.77%, and 25.74%, respectively in comparison to mock-treated cells (Fig. 5B). Consistently, flow cytometry anlaysis indicated that the percentage of fluorescence-labeled cells was significantly decreased in a dose-dependent manner after treatment with CQ or BAF (Fig. 5C). Western blotting showed that protein synthesis of MCP was significantly inhibited by CQ and BAF (Fig. 5D). Thus, we propose that STIV enters FHM cells in a pH-dependent manner.
Fig. 5.
STIV enters into cells in a pH-dependent manner. (A) Fluorescence microscopy of EGFP-STIV- infected cells after treatment with DMSO, BAF or CQ. (B) Fluorescence intensity of EGFP-STIV-infected cells after treatment with DMSO, BAF or CQ. (C) Quantitative analysis of the percentage of EGFP-STIV-infected cells by flow cytometry. (D) Western blotting detection of protein synthesis of the STIV major capsid protein after treatment with BAF or CQ
Discussion
Viruses have evolved to exploit endocytosis as an effective means of entering host cells and being transported to sites suitable for replication. Therefore, exploration of virus-entry mechanisms might provide new clues to developing effective drugs to block virus infection. As large DNA viruses, iridoviruses have attracted much attention because of their impact on ecological instability and their role in heavy economic losses in the aquaculture industry. Although rapid progress has been made in the field of iridovirus functional genomics, the early events during iridovirus infection, especially virus entry, are still unknown [13, 19, 21].
Clathrin-mediated endocytosis, a well-characterized endocytic pathway, is exploited by a number of viruses to enter host cells [6, 7]. CPZ is known to cause a clathrin lattice to assemble on endosomal membranes, and at the same time, prevent the assembly of coated pits at the plasma membrane [9]. After treatment with CPZ, the cellular entry of many viruses, including HIV, HCV, African swine fever virus (ASFV), HBV and rabies virus, was significantly inhibited, suggesting that clathrin-mediated endocytosis is involved in the entry process of these viruses [6, 7, 9]. In our study, the percentage fluorescence indicated that EGFP-STIV-infected cells and protein synthesis of major capsid proteins were significantly decreased by treatment with CPZ. As another inhibitor of clathrin-dependent endocytosis, hyperosmotic sucrose prevents clathrin and its adaptors from interacting [29]. Our results showed that treatment with hyperosmotic sucrose also decreased virus entry, similar to CPZ. The number of STIV-infected cells was significantly inhibited, as well as protein synthesis of MCP, after treatment with CQ or BAF; both of which are known to prevent endosome acidification. The entry of STIV to FHM cells was significantly decreased by dynasore, an inhibitor of GTPase dynamin, which is required for clathrin-mediated endocytosis. We propose that STIV enters host cells through clathrin-mediated endocytosis in a pH-dependent manner. Notably, clathrin-mediated endocytosis was also reported to be crucial for FV3, but not for TFV entry, although they are different strains of the same species. It is proposed that the entry mechanisms of these viruses were investigated in different cell types [13, 20]. Whether clathrin-mediated endocytosis is involved in TFV infection in fish or frog cells needs further investigation.
In contrast to clathrin-mediated endocytosis, caveola-dependent endocytosis is a newly characterized endocytosis pathway that defines the movement of caveolins with caveola-derived vesicles to multiple compartments in the cytoplasm. Sterol-binding reagents such as filipin III, nystatin and βMCD are usually used to impair caveola-mediated endocytosis due to the high abundance of cholesterol and sphingolipids in caveolae [9, 28]. Treatment with filipin III or nystatin decreased the cellular entry of some viruses, including SV40, coronavirus, TFV and ISKNV, suggesting that these viruses used caveola-dependent endocytosis during their life cycle [10, 13, 21, 30]. In our experiments, treatment with βMCD decreased the percentage of STIV-infected cells in a dose-dependent manner, while neither nystatin nor filipin III showed obvious effects on STIV entry. This phenomenon was also described in severe acute respiratory syndrome coronavirus infection [28]. Given that cholesterol depletion by βMCD inhibits the formation and budding of clathrin-coated pits, we propose that βMCD inhibits STIV entry; not through interfering with caveola-dependent endocytosis, but likely by affecting the integrity of membrane lipid microdomains, or inhibiting clathrin-mediated endocytosis [31, 32].
Although there are numerous reports that many viruses enter cells via clathrin-dependent or caveola/lipid raft-mediated endocytosis, increased attention has recently been paid to macropinocytosis due to its critical role in virus entry [14, 33, 34]. EIPA, a specific inhibitor of Na+/H+ exchanger, is critical for macropinosome formation, and is usually used to evaluate the impact of macropinocytosis on virus entry [35, 36]. Treatment with EIPA impairs the entry of many mammalian viruses, including EBOV, ASFV, vaccinia virus and human cytomegalovirus, suggesting that macropinocytosis is involved in the entry of these viruses [16, 37–39]. To ascertain whether macropinocytosis is an alternative route for STIV entry, the effect of EIPA on STIV infection was determined. In STIV-infected cells, treatment with EIPA inhibited virus entry and protein synthesis in a dose-dependent manner. Recent studies have also demonstrated that several cell factors are critical for macropinocytosis in virus entry. Inhibition of PAK-1 and myosin II activity decrease the entry of ASFV and influenza A virus, which shows that these cellular factors are important for macropinocytic uptake [37, 40]. Furthermore, inhibition of actin polymerization by cyto-D decreased the cellular entry of Kaposi’s sarcoma-associated herpesvirus, vaccinia virus and ASFV, suggesting that actin polymerization is important in the macropinocytic pathway [35, 37, 40]. In our study, inhibition of PAK1 activity by IPA-3 and inhibition of myosin II activity by ML-7 both significantly prevented STIV entry. Treatment with cyto D also significantly blocked STIV infection. Therefore, we conclude that STIV enters cells via macropinocytosis. Notably, the macropinocytosis specific inhibitor EIPA was not used in previous studies on the cellular entry of FV3, TFV and INKNV [13, 20, 21], but disruption of actin microfilaments by cytochalasin B significantly inhibited TFV entry [13], suggesting that macropinocytosis is also involved in TFV entry. Recent studies have revealed that SGIV enters host cells via both clathrin-mediated endocytosis and macropinocytosis in a pH-dependent manner [19]. We propose that macropinocytosis is a novel alternative pathway exploited by iridovirus to enter cells.
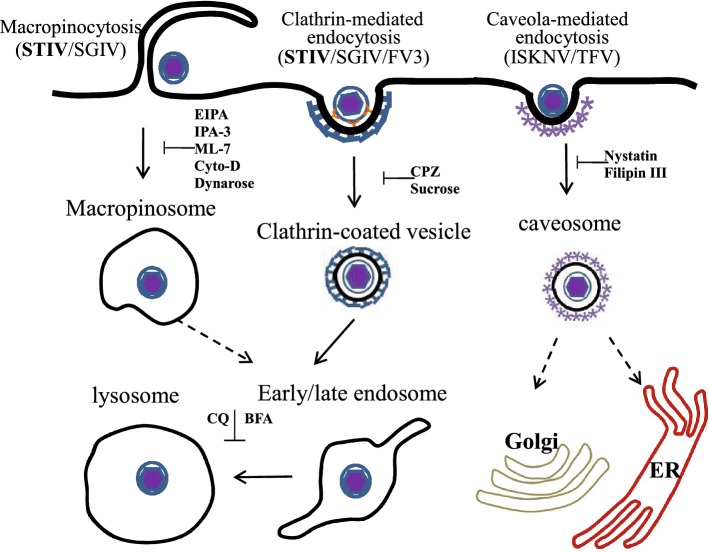
In conclusion, our results reveal that STIV enters cells via clathrin-mediated endocytosis and macropinocytosis in a pH-dependent manner. In accordance with previous studies, we propose that ranaviruses, including STIV, FV3 and SGIV could use clathrin-mediated endocytosis and micropinocytosis to enter cells, although this conclusion was not verified in FV3 and TFV infected fish cells or frog cells. Interestingly, caveola-mediated endocytosis was not involved in STIV and SGIV entry in vitro, but crucial for the entry of TFV into HepG2 Cells and ISKNV into fish fry cells (Fig. 6). Together, iridoviruses might use multiple endocytic pathways for entry, like mammalian viruses. Our study provides new insights into understanding the early events in iridovirus pathogenesis.
Fig. 6.
Molecular mechanism of iridovirus entry. (1) Clathrin-mediated endocytosis used by FV3, SGIV and STIV, could be inhibited by CPZ and sucrose. Virus particles were internalized and formed clathrin-coated vesicles (CCVs), then CCVs were uncoated and virus was transported into the endosome, and finally into the lysosome; (2) Caveola-dependent endocytosis, used by TFV and ISKNV, could be inhibited by nystatin and filipin III. Virus particles were internalized into host cells via caveolae and dependent on the microtubules via a caveola-caveosome-endoplasmic reticulum (ER) pathway; (3) Macropinocytosis, used by STIV and SGIV, could be inhibited by EIPA, IPA-3 and cyto-D. Virus particles were internalized and formed macropinosomes. Viruses might then be transported via the late endosome to the lysosome
Acknowledgements
We thank Jianlin Zhang for his help with flow cytometry analysis.
Funding
This work was supported by grants from the National Natural Science Foundation of China (31172445), National Key R&D Program of China” (2017YFC1404504), and the Knowledge Innovation Program of the Chinese Academy of Sciences (SQ201014).
Compliance with ethical standards
Conflict of interest
All the authors have no conflict of interest to declare.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Contributor Information
Xiaohong Huang, Email: huangxh@scau.edu.cn.
Qiwei Qin, Email: qinqw@scau.edu.cn.
References
- 1.Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 2.Grove J, Marsh M. The cell biology of receptor-mediated virus entry. J Cell Biol. 2011;195:1071–1082. doi: 10.1083/jcb.201108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–533. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 4.Daecke J, Fackler OT, Dittmar MT, Kräusslich HG. Involvement of clathrin-mediated endocytosis in human immunodeficiency virus type 1 entry. J Virol. 2005;79:1581–1594. doi: 10.1128/JVI.79.3.1581-1594.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard E, Belouzard S, Goueslain L, Wakita T, Dubuisson J, Wychowski C, Rouillé Y. Hepatitis C virus entry depends on clathrin-mediated endocytosis. J Virol. 2006;80:6964–6972. doi: 10.1128/JVI.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang HC, Chen CC, Chang WC, Tao MH, Huang C. Entry of hepatitis B virus into immortalized human primary hepatocytes by clathrin-dependent endocytosis. J Virol. 2012;86:9443–9453. doi: 10.1128/JVI.00873-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piccinotti S, Kirchhausen T, Whelan SP. Uptake of rabies virus into epithelial cells by clathrin-mediated endocytosis depends upon actin. J Virol. 2013;87:11637–11647. doi: 10.1128/JVI.01648-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Méndez E, Muñoz-Yañez C, Sánchez-San Martín C, Aguirre-Crespo G, Baños-Lara Mdel R, Gutierrez M, Espinosa R, Acevedo Y, Arias CF, López S. Characterization of human astrovirus cell entry. J Virol. 2014;88:2452–2460. doi: 10.1128/JVI.02908-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sieczkarski SB, Whittaker GR. Dissecting virus entry via endocytosis. J Gen Virol. 2002;83:1535–1545. doi: 10.1099/0022-1317-83-7-1535. [DOI] [PubMed] [Google Scholar]
- 10.Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 11.Marjomäki V, Pietiäinen V, Matilainen H, Upla P, Ivaska J, Nissinen L, Reunanen H, Huttunen P, Hyypiä T, Heino J. Internalization of echovirus 1 in caveolae. J Virol. 2002;76:1856–1865. doi: 10.1128/JVI.76.4.1856-1865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macovei A, Radulescu C, Lazar C, Petrescu S, Durantel D, Dwek RA, Zitzmann N, Nichita NB. Hepatitis B virus requires intact caveolin-1 function for productive infection in HepaRG cells. J Virol. 2010;84:243–253. doi: 10.1128/JVI.01207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo CJ, Liu D, Wu YY, Yang XB, Yang LS, Mi S, Huang YX, Luo YW, Jia KT, Liu ZY, Chen WJ, Weng SP, Yu XQ, He JG. Entry of tiger frog virus (an Iridovirus) into HepG2 cells via a pH-dependent, atypical, caveola-mediated endocytosis pathway. J Virol. 2011;85:6416–6426. doi: 10.1128/JVI.01500-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercer J, Helenius A. Gulping rather than sipping: macropinocytosis as a way of virus entry. Curr Opin Microbiol. 2012;15:490–499. doi: 10.1016/j.mib.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Krzyzaniak MA, Zumstein MT, Gerez JA, Picotti P, Helenius A. Host cell entry of respiratory syncytial virus involves macropinocytosis followed by proteolytic activation of the F protein. PLoS Pathog. 2013;9:e1003309. doi: 10.1371/journal.ppat.1003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saeed MF, Kolokoltsov AA, Albrecht T, Davey RA. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 2010;6:e1001110. doi: 10.1371/journal.ppat.1001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang S, Huang X, Huang Y, Hao X, Xu H, Cai M, Wang H, Qin Q. A novel marine DNA virus (Singapore grouper iridovirus, SGIV) entry into host cells occurs via clathrin-mediated endocytosis and macropinocytosis in a pH-dependent manner. J Virol. 2014;88:13047–13063. doi: 10.1128/JVI.01744-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chinchar VG. Ranaviruses (family Iridoviridae): emerging cold-blooded killers. Arch Virol. 2002;147:447–470. doi: 10.1007/s007050200000. [DOI] [PubMed] [Google Scholar]
- 19.Zhu YQ, Wang XL. Genetic diversity of ranaviruses in amphibians in China: 10 new isolates and their implications. Pak J. Zool. 2016;48:107–114. [Google Scholar]
- 20.Braunwald J, Nonnenmacher H, Tripier-Darcy F. Ultrastructural and biochemical study of frog virus 3 uptake by BHK-21 cells. J Gen Virol. 1985;66:283–293. doi: 10.1099/0022-1317-66-2-283. [DOI] [PubMed] [Google Scholar]
- 21.Guo CJ, Wu YY, Yang LS, Yang XB, He J, Mi S, Jia KT, Weng SP, Yu XQ, He JG. Infectious spleen and kidney necrosis virus (a fish iridovirus) enters Mandarin fish fry cells via caveola-dependent endocytosis. J Virol. 2012;86:2621–2631. doi: 10.1128/JVI.06947-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen ZX, Zheng JC, Jiang YL. A new iridovirus isolated from soft-shelled turtle. Virus Res. 1999;63:147–151. doi: 10.1016/S0168-1702(99)00069-6. [DOI] [PubMed] [Google Scholar]
- 23.Huang YH, Huang XH, Liu H, Gong J, Ouyang ZL, Cui HC, Cao JH, Zhao Y, Wang X, Jiang YL, Qin QW. Complete sequence determination of a novel reptile iridovirus isolated from soft-shelled turtle and evolutionary analysis of Iridoviridae. BMC Genomics. 2009;10:224. doi: 10.1186/1471-2164-10-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang Y, Huang X, Cai J, Ye F, Qin Q. Involvement of the mitogen-activated protein kinase pathway in soft-shelled turtle iridovirus-induced apoptosis. Apoptosis. 2011;16(6):581–593. doi: 10.1007/s10495-011-0595-z. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Huang X, Cai J, Ye F, Guan L, Liu H, Qin Q. Construction of green fluorescent protein-tagged recombinant iridovirus to assess viral replication. Virus Res. 2011;160(1–2):221–229. doi: 10.1016/j.virusres.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Brandenburg B, Zhuang X. Virus trafficking—learning from single-virus tracking. Nat Rev Microbiol. 2007;5:197–208. doi: 10.1038/nrmicro1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeWire SM, Money ES, Krall SP, Damania B. Rhesusmonkeyrhadinovirus (RRV): construction of a RRV-GFP recombinant virus and development of assays to assess viral replication. Virology. 2003;312:122–134. doi: 10.1016/S0042-6822(03)00195-8. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G, Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CL, Hou WH, Liu IH, Hsiao G, Huang SS, Huang JS. Inhibitors of clathrin-dependent endocytosis enhance TGFbeta signaling and responses. J Cell Sci. 2009;122:1863–1871. doi: 10.1242/jcs.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nomura R, Kiyota A, Suzaki E, Kataoka K, Ohe Y, Miyamoto K, Senda T, Fujimoto T. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J Virol. 2004;78:8701–8708. doi: 10.1128/JVI.78.16.8701-8708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuhorn IS, Kalicharan Hoekstra D. Lipoplex-mediated transfection of mammalian cells occurs through the cholesterol-dependent clathrin-mediated pathway of endocytosis. J Biol Chem. 2002;277:18021–18028. doi: 10.1074/jbc.M111257200. [DOI] [PubMed] [Google Scholar]
- 32.Vela EM, Zhang L, Colpitts TM, Davey RA, Aronson JF. Arenavirus entry occurs through a cholesterol-dependent, non-caveolar, clathrin-mediated endocytic mechanism. Virology. 2007;369:1–11. doi: 10.1016/j.virol.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- 34.Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124:729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghu H, Sharma-Walia N, Veettil MV, Sadagopan S, Chandran B. Kaposi’s sarcoma-associated herpesvirus utilizes an actin polymerization-dependent macropinocytic pathway to enter human dermal microvascular endothelial and human umbilical vein endothelial cells. J Virol. 2009;83:4895–4911. doi: 10.1128/JVI.02498-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 37.Sánchez EG, Quintas A, Pérez-Núñez D, Nogal M, Barroso S, Carrascosa ÁL, Revilla Y. African swine fever virus uses macropinocytosis to enter host cells. PLoS Pathog. 2012;8:e1002754. doi: 10.1371/journal.ppat.1002754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nanbo A, Imai M, Watanabe S, Noda T, Takahashi K, Neumann G, Halfmann P, Kawaoka Y. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog. 2010;23:e1001121. doi: 10.1371/journal.ppat.1001121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haspot F, Lavault A, Sinzger C, Laib Sampaio K, Stierhof YD, Pilet P, Bressolette-Bodin C, Halary F. Human cytomegalovirus entry into dendritic cells occurs via a macropinocytosis-like pathway in a pH-independent and cholesterol-dependent manner. PLoS One. 2012;7:e34795. doi: 10.1371/journal.pone.0034795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Vries E, Tscherne DM, Wienholts MJ, Cobos-Jiménez V, Scholte F, García-Sastre A, Rottier PJ, de Haan CA. Dissection of the influenza A virus endocytic routes reveals macropinocytosis as an alternative entry pathway. PLoS Pathog. 2011;7:e1001329. doi: 10.1371/journal.ppat.1001329. [DOI] [PMC free article] [PubMed] [Google Scholar]