Abstract
Bovine coronavirus (BCoV) is a recognized cause of severe neonatal calf diarrhea, with a negative impact on animal welfare, leading to economic losses to the livestock industry. Cattle production is one of the most important economic sectors in Uruguay. The aim of this study was to determine the frequency of BCoV infections and their genetic diversity in Uruguayan calves and to describe the evolutionary history of the virus in South America. The overall detection rate of BCoV in Uruguay was 7.8% (64/824): 7.7% (60/782) in dairy cattle and 9.5% (4/42) in beef cattle. The detection rate of BCoV in samples from deceased and live calves was 10.0% (6/60) and 7.6% (58/763), respectively. Interestingly, there was a lower frequency of BCoV detection in calves born to vaccinated dams (3.3%, 8/240) than in calves born to unvaccinated dams (12.2%, 32/263) (OR: 4.02, 95%CI: 1.81–8.90; p = 0.00026). The frequency of BCoV detection was higher in colder months (11.8%, 44/373) than in warmer months (1.5%, 3/206) (OR: 9.05, 95%CI: 2.77–29.53, p = 0.000013). Uruguayan strains grouped together in two different lineages: one with Argentinean strains and the other with Brazilian strains. Both BCoV lineages were estimated to have entered Uruguay in 2013: one of them from Brazil (95%HPD interval: 2011–2014) and the other from Argentina (95%HPD interval: 2010–2014). The lineages differed by four amino acid changes, and both were divergent from the Mebus reference strain. Surveillance should be maintained to detect possible emerging strains that can clearly diverge at the antigenic level from vaccine strains.
Electronic supplementary material
The online version of this article (10.1007/s00705-019-04384-w) contains supplementary material, which is available to authorized users.
Introduction
Bovine coronavirus (BCoV) is recognized as a cause of severe neonatal calf diarrhea (NCD), respiratory tract infections in calves, and winter dysentery in adult cattle [1]. NCD has a negative impact on animal welfare and leads to economic losses to the livestock industry due to the costs of treatment and prophylaxis, increased susceptibility to other diseases, increased mortality, and long-term residual effects, such as reduced growth rates and milk production [2–5]. Neonatal calf diarrhea is the major cause of death in unweaned heifers [6].
Cattle production is one of the main economic sectors in Uruguay, accounting for 33% of the exports and 5% of the gross domestic product, with 11,739,000 head of cattle [7]. Worldwide, Uruguay is one of the main exporters of bovine meat [8] and dairy products [9].
Bovine CoV belongs to the species Betacoronavirus 1, which was recently assigned by the International Committee on Taxonomy of Viruses (ICTV) to the order Nidovirales, suborder Cornidovirineae, family Coronaviridae, subfamily Orthocoronavirinae, genus Betacoronavirus, and subgenus Embecovirus [10]. Members of the species Betacoronavirus 1 infect not only cattle and wild ruminants [11] but also other mammals such as equids (equine coronavirus) [12], humans (human coronavirus OC43) and pigs (porcine hemagglutinating encephalomyelitis virus) [13].
Bovine CoV has a 32-kb, single-stranded, positive-sense RNA genome – the largest among known RNA viruses [13]. Bovine CoV viral particles are enveloped and pleomorphic and contain five structural proteins. Four are external and glycosylated: the transmembrane (M), the small envelope (E), the hemagglutinin-esterase (HE) and the spike (S) proteins. The other, the nucleocapsid (N) protein, is internal [14, 15].
The biological functions of the S protein of CoV include primary attachment to target cells and membrane fusion. The S protein is cleaved to produce the N-terminal S1 and C-terminal S2 glycopolypeptides. The hypervariable region within S1 is associated with some of the antigenic differences, being the major inducer of virus-neutralizing antibodies, and may also be associated with host range and tissue tropism. Most of the differences in S1 occur between virulent and non-virulent strains [16]. The hypervariable S1 genomic region has been widely used to study the genetic variability and evolution of the virus, including the few studies on molecular characterization of BCoV strains conducted in the South American region [17–19].
To date, little is known about the genetic diversity of BCoV in South America, where the few studies that have been done were mainly restricted to Brazil and Argentina, as mentioned above, and information about its evolutionary history in this region is lacking in the scientific literature. Furthermore, the genetic diversity of BCoV in Uruguay has not been investigated.
The aim of this study was to determine the frequency of BCoV infection in Uruguayan calves, to examine the genetic diversity of the virus, to identify risk factors associated with the frequency of BCoV detection, and to investigate the evolutionary history of BCoV in the South American region through phylogenetic, phylodynamic, and phylogeographic analyses.
Materials and methods
Sample collection and fecal suspensions
A total of 824 samples of feces (763) and intestinal contents (61) were obtained from beef and dairy cattle in Uruguay between July 2015 and December 2017; additional information on sample origins is detailed in Table 1 and Fig. 1. A survey conducted to collect information about management and problems associated with dairy cattle farming (the farms were randomly selected and representative of the dairy area in southwestern Uruguay) and the intensive production system facilitated the collection of a large number of samples (782). On the other hand, beef cattle sampling was carried out by personal contact with farmers in order to encompass as many departments (geographic regions) as possible, but the extensive production system of beef cattle in Uruguay hindered access to the samples (42), and this is a limitation of this work. Samples were diluted 1:10 (v:v) in phosphate-buffered saline solution and centrifuged at 3000 g for 20 minutes at 4 °C, and supernatants were collected and stored at – 80 °C.
Table 1.
Sample information
| Diarrhea | Exploitation type | Age (days) | Dams vaccinated1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | ND | Dairy | Beef | 1-7 | 8-14 | 15-21 | 22-28 | > 28 | ND | Yes | No | ND | |
| n | 266 | 297 | 261 | 782 | 42 | 153 | 220 | 113 | 38 | 20 | 280 | 240 | 263 | 321 |
ND: not determined
1 Vaccination against neonatal calf diarrhea. Most available vaccines in the Uruguayan market include bovine coronavirus
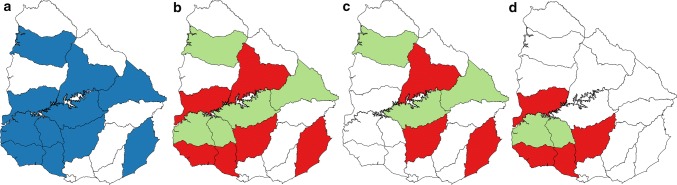
Fig. 1.
Map of Uruguay showing the 19 departments into which the country is divided. a) Sampling. b) Rate of BCoV detection in beef and dairy cattle combined. c) Rate of BCoV detection in beef cattle. d) Rate of BCoV detection in dairy cattle. Departments from which samples were obtained are shown in blue, those in which BCoV was detected are shown in red, and those in which BCoV was not detected are shown in green
RNA extraction and reverse transcription
Viral RNA was extracted using a QIAamp cador Pathogen Mini Kit (QIAGEN), following the manufacturer’s instructions. Reverse transcription (RT) was carried out using RevertAid Reverse Transcriptase (Thermo Fischer Scientific) and random hexamers primers (QIAGEN) following the manufacturer instructions. All RNAs and cDNAs were stored at − 80 °C.
Polymerase chain reaction assay for bovine coronavirus
The initial screening of the samples for identification of BCoV was carried out using a real-time polymerase chain reaction (PCR) targeted the S gene, following a standard operating procedure kindly provided by Dr. Stephanie Rossow from the University of Minnesota Veterinary Diagnostic Laboratory. Briefly, 12.5 μL of SensiFAST™ Probe No-ROX Kit (Bioline), 1.0 μL of 10 μM BCoV Minn F primer (TGTTTTAAAGCTTCCACAAATTTCTG), 1.0 μL of 10 μM BCoV Minn R primer (AACCAGCATCTATACCAGGACCAT), 0.5 μL of 10 μM BCoV Minn S probe (Cy5-CGTGTAAATTGGATGGGTCTTTGTGTGTAGGT-BHQ-2) and 5.0 μL of nuclease-free water were mixed in 0.2-mL PCR tubes. The PCR cycling conditions were 95 °C for 10 minutes, followed by 45 cycles of 95 °C for 15 seconds and 50 °C for 45 seconds.
In order to obtain sequences for evolutionary analysis, positive samples detected by the real-time PCR assay were further amplified using a heminested PCR targeting to the S gene, using primers described elsewhere [17]. Briefly, for the first round of the heminested PCR, 12.5 μL of MangoMix™ (Bioline), 5 μL of cDNA, 3.9 μL of nuclease-free water, 1 μL of dimethyl sulfoxide, 1.3 μL of 10 μM S1NS primer, and 1.3 μL of 10 μM primer S1HA were mixed in 0.2-mL PCR tubes and subjected to an initial step of 5 minutes at 95 °C, followed by 35 cycles of 94 °C for 1 minute, 53.4 °C for 1 minute, and 72 °C for 1 minute, ending with 10 minutes at 72 °C for final extension. For the second round, 12.5 μL of MangoMix™ (Bioline), 2 μL of the PCR product from the first round, 7.5 μL of nuclease-free water, 1 μL of dimethyl sulfoxide, 1 μL of 10 μM S1NS primer, and 1 μL of 10 μM primer S1NAS were mixed in 0.2-mL PCR tubes and subjected to an initial step of 5 minutes at 95 °C, followed by 40 cycles of 94 °C for 1 minute, 58.4 °C for 1 minute and 72 °C for 1 minute, ending with 10 minutes at 72 °C for final extension. The predicted PCR products were 785 bp and 488 bp long for first and second round, respectively (modified from reference 17).
Purification and sequencing of PCR products
PCR products were visualized in 2% agarose gels, and positive samples were purified using a PureLink™ Quick Gel Extraction and PCR Purification Combo Kit (Invitrogen) according to the manufacturer’s instructions, and both DNA strands were sequenced by Macrogen Inc. (Seoul, South Korea). Sequences were deposited in the GenBank database with accession numbers MK318150-MK318179.
Phylogenetic analysis
Partial spike sequences were downloaded from GenBank (https://www.ncbi.nlm.nih.gov/genbank/, Table S1) and, together with the Uruguayan sequences obtained in this study, were aligned using Clustal W, implemented in MEGA 7 software [20]. A curated alignment of 443 nucleotides (nt) was obtained (corresponding to positions 24,984-25,426 of the Mebus strain). The nucleotide substitution model that best fit the alignment (TIM3+I+G4) was chosen and a maximum-likelihood tree was constructed using W-IQ-TREE (available at http://iqtree.cibiv.univie.ac.at) [21]. The branch support was estimated using the approximate likelihood-ratio test (aLRT) [22].
Phylodynamic and phylogeographic analysis
Considering that South American strains grouped together in two lineages, all of the available sequences of the S region of the BCoV genome available in GenBank from South America were downloaded (Table S2), although some of the Brazilian and Argentinean sequences were shorter than the ones obtained in our study. In order to include these sequences from the South American region, the fragment used for evolutionary analysis was smaller than that used for phylogenetic analysis. Sequences were aligned using Clustal W implemented in MEGA 7 software [20], and an alignment of 332 nt was obtained (corresponding to positions 25,021-25,352 of the Mebus strain). The temporal structure of the dataset was evaluated using TempEst [23]. The substitution model that best fit the alignment was determined using MEGA 7 software, and used as prior (TN93, [24]) in the analysis implemented in the BEAST v1.8.4 package [25]. Combinations of molecular clocks (strict, relaxed lognormal and relaxed exponential) and coalescent tree priors (constant, exponential, and skyline) were evaluated using Bayes factors. The uncorrelated relaxed with exponential distribution molecular clock and the Bayesian Skyline coalescent model were selected, and the country location was used as a trait for the phylogeographic analysis. The Markov chain Monte Carlo length was 200 million generations, obtaining 10,000 parameters samples. Effective sample size (ESS) was evaluated in Tracer v1.6.0, and ESS values higher than 200 for all parameters were accepted.
A maximum clade credibility tree (MCCT) was obtained using TreeAnnotator software from the BEAST v.1.8.4 package and visualized in FigTree v1.4.3. A Bayesian Skyline plot was generated using Tracer v1.6.0.
Signature patterns distinguishing Uruguayan lineages
Viral Epidemiology Signature Pattern Analysis (VESPA) [26], available at: https://www.hiv.lanl.gov/content/sequence/VESPA/vespa.html) was used to detect patterns that could differentiate the lineages of BCoV circulating in Uruguayan cattle, using only the local strains and the translated alignment obtained for the phylogenetic analysis (the first nucleotide was excluded to have an in-frame translation). Moreover, both lineages were compared with the reference strain Mebus, which is used in the vaccines currently available on the market.
Statistical analysis
Data were organized and graphics were generated using Microsoft Office Excel. Categorical data were evaluated using Pearson’s chi-squared test with jamovi software (available at: https://www.jamovi.org/). Differences were considered statistically significant if the p-value was lower than 0.05. Odds ratios (OR) and 95% confident intervals (CI) were calculated for groups with statistically significant differences, using jamovi software. In multiple comparison Chi-square tests, the Bonferroni correction was applied.
Results
Frequency of BCoV detection in Uruguay
The study covered 11 departments of the 19 into which Uruguay is geographically divided (Fig. 1a). Bovine CoV was detected in 6 out of 11 departments (55%) (Fig. 1b); the frequency of detection in each department was as follows: 6.9% (8/116) in Colonia, 7.1% (2/28) in Florida, 14.7% (21/143) in Río Negro, 22.2% (2/9) in Rocha, 6.4% (15/233) in San José, and 12.5% (1/8) in Tacuarembó. BCoV was detected in 3 of the 6 departments sampled for beef cattle (50%) (Fig. 1c), and 4 of the 6 departments sampled for dairy cattle (67%) (Fig. 1d).
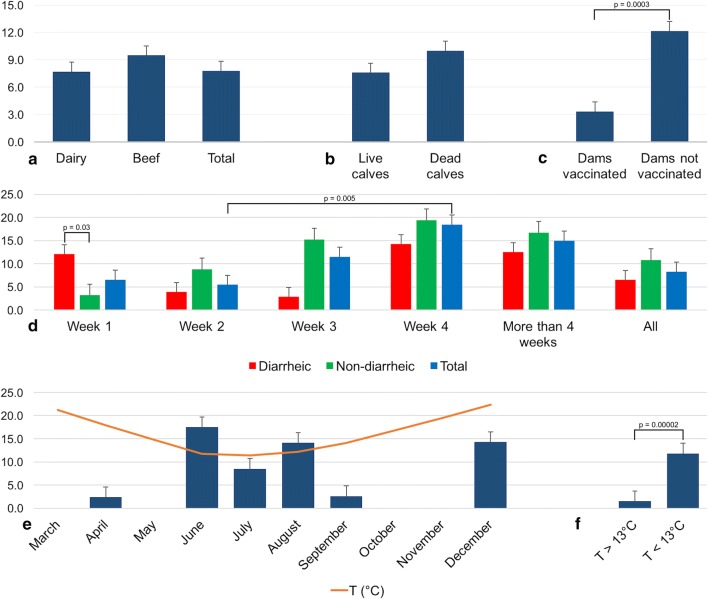
The overall detection rate of BCoV in Uruguay was 7.8% (64/824). The frequency of BCoV detection in dairy and beef cattle was 7.7% (60/782) and 9.5% (4/42), respectively (Fig. 2a); this difference was not statistically significant (p = 0.69). The detection rate of BCoV in samples from deceased calves (10.0%, 6/60) was higher than in samples from live calves (7.6%, 58/763), although this difference was not statistically significant (p = 0.50) (Fig. 2b).
Fig. 2.
Comparison of BCoV detection rates in different groups. a) Frequency of BCoV detection in beef vs. dairy cattle. b) Frequency of BCoV detection in live vs. dead calves. c) Frequency of BCoV detection in calves born to vaccinated vs. unvaccinated dams. d) Frequency of BCoV detection according to the age in weeks in diarrheic and non-diarrheic calves, and the total number of calves. e) Frequency of BCoV detection according to the month of sampling. f) Frequency of BCoV detection according to the ambient temperature. Comparisons between groups with statistically significant differences are shown
Interestingly, calves born to unvaccinated dams showed higher frequency of BCoV infection (12.2%, 32/263) than calves born to vaccinated dams (3.3%, 8/240). This difference was statistically significant (OR: 4.02, 95%CI: 1.81–8.9; p = 0.00026) (Fig. 2c).
As shown in Fig. 2d, BCoV was detected in 6.5% (10/153), 5.5% (12/220), 11.5% (13/113), 18.4% (7/38), and 15.0% (3/20) of the calves in the first, second, third, and fourth week and after the fourth week of life, respectively. A statistically significant difference was observed between the second and the fourth week of age (OR: 3.91, 95%CI: 1.43-10.70; p = 0.0047). No statistical differences were observed between BCoV frequency of detection and diarrhea when all age groups were analyzed together: 6.5% (17/263) in diarrheic and 10.8% (32/295) in non-diarrheic calves (p = 0.068), however, in the first week of calves’ life, the BCoV detection rate was statistical higher in diarrheic 12.1% (7/58) than non-diarrheic 3.2% (3/92) calves (OR: 4.21, 95%CI: 1.04-16.99; p = 0.030). In the other age groups, the BCoV frequency was lower in diarrheic versus non-diarrheic calves: 3.9% (6/152) vs. 8.8% (6/68), 2.9% (1/34) vs. 15.2% (12/79), 14.3% (1/7) vs. 19.4% (6/31) and 12.5% (1/8) vs. 16.7% (2/12), in the second, third, and fourth week, and after the fourth week of life, respectively, but no statistically significant differences were observed.
As shown in Fig. 2e, BCoV was detected with a seasonal distribution, mainly in June, July and August, which are the coldest months in Uruguay. In addition, two groups were analyzed based on monthly average temperature using a cutoff value of 13 °C considering that the mean monthly temperature is below the cutoff in June, July and August and above the cutoff in the rest of the months. The frequency of BCoV detection was significantly higher in the months with average temperature <13 °C (11.8%, 44/373) than in those with an average temperature >13 °C (1.5%, 3/206) (OR: 9.05, 95%CI: 2.77-29.53, p = 0.000013).
Phylogenetic analysis
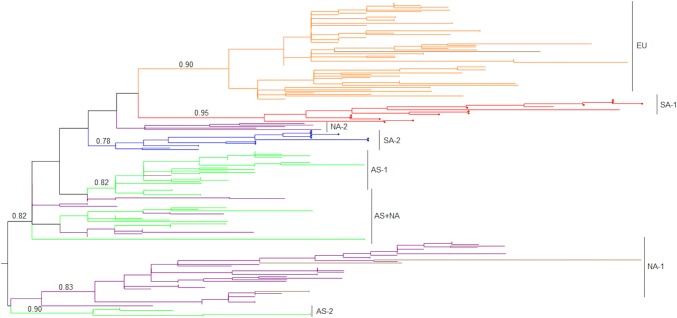
In Fig. 3, the phylogenetic relationship between the Uruguayan BCoV strains and those for which sequences were obtained from the GenBank database is shown. Geographically associated (continent-specific) lineages were observed, and Uruguayan strains grouped in the two South American lineages, one of them with Argentinean strains and the other with Brazilian strains.
Fig. 3.
Phylogenetic analysis. A maximum-likelihood tree was constructed with TIM3 plus gamma plus invariant sites as the nucleotide substitution model. Uruguayan strains from this study are indicated by red and blue circles, and the Mebus strain is indicated by a black circle. aLRT values at key branches and continent-specific lineages are shown. Branches are colored according to the continent of strain isolation: orange (EU), purple (NA), green (AS), red (SA, lineage with Argentinean strains), blue (SA, lineage with Brazilian strains), and brown (strains from a continent of isolation different from the continent-specific lineage). AS, Asia; EU, Europe; NA, North America; SA, South America
Phylodynamic and phylogeographic analysis of BCoV in South America
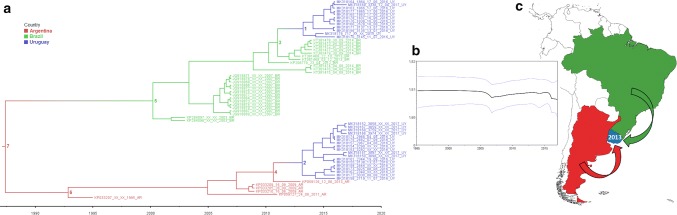
An MCCT (Fig. 4a) also showed that the Uruguayan BCoV strains grouped together in two different lineages: one with Argentinean and the other with Brazilian strains. The substitution rate estimated in this analysis was 1.39 × 10−3 substitutions/site/year (s/s/y) (95% highest posterior density [95%HPD] interval: 8.3 × 10−4–2.0 × 10−3), and the most recent common ancestor (MRCA) of each lineage was dated to 1992 (95%HPD interval: 1985–1994) located in Argentina with a probability of 0.99, and in 2000 (95%HPD interval: 1994–2002) located in Brazil with a probability of 0.99. More information about key nodes is detailed in Table 2.
Fig. 4.
Phylogeographic analysis. a) Maximum clade credibility tree. Branches are colored by most probable country location: blue for Uruguay, green for Brazil, and red for Argentina. Key nodes 1-7 are indicated (for which more information is available in Table 2). b) Population dynamics of BCoV in South America. The Bayesian skyline plot shows the evolution in population size. Median (dark line) and upper and lower 95% HPD (blue lines) estimates of effective population size (y-axis) through time in years (x-axis) are shown. c) The two entries of BCoV to Uruguay in 2013 are indicated with arrows: red from Argentina and green from Brazil
Table 2.
Key nodes for the information shown in Fig. 4
| Node | Country | Year | 95% HPD year | Country probability |
|---|---|---|---|---|
| 1 | Uruguay | 2013 | 2011 - 2014 | 0.99 |
| 2 | Uruguay | 2013 | 2010 - 2014 | 0.99 |
| 3 | Brazil | 2011 | 2008 - 2012 | 0.99 |
| 4 | Argentina | 2010 | 2007 - 2012 | 0.81 |
| 5 | Brazil | 2000 | 1994 - 2002 | 0.99 |
| 6 | Argentina | 1992 | 1985 - 1994 | 0.97 |
| 7 | Argentina | 1987 | 1966 - 1994 | 0.72 |
These BCoV lineages were estimated to have entered Uruguay in 2013: one of them from Brazil (95%HPD interval: 2011–2014) and the other from Argentina (95%HPD interval: 2010–2014) (Fig. 4c).
A Bayesian skyline plot showed that the population size of BCoV in South America was constant in the last two decades, with some minor fluctuations (Fig. 4b).
Signature patterns distinguishing Uruguayan lineages
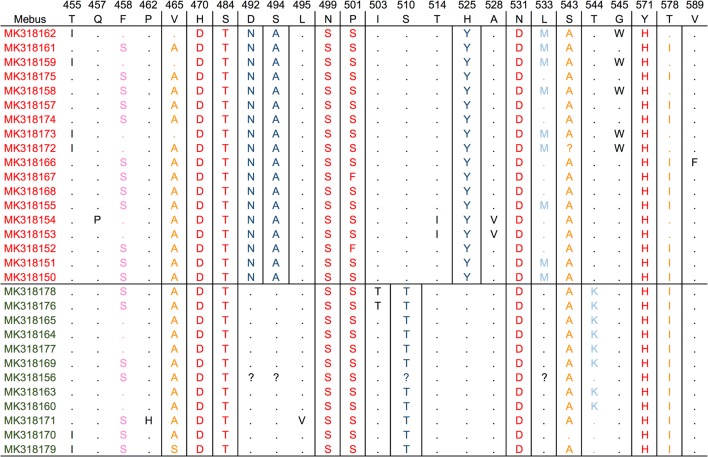
Four amino acid (aa) differences between the two BCoV lineages circulating in Uruguay were detected. These changes were N44D, A46S, S62T and Y77H (positions refer to the partial sequences used for the analysis). When the Uruguayan BCoV sequences were compared with the Mebus reference strain, six aa changes (4.0%) were observed in all the Uruguayan strains, and three additional aa changes were observed, totalizing nine aa changes (5.4%), with more than 75% of the Uruguayan sequences containing these changes (Fig. 5).
Fig. 5.
Amino acid sequence alignment. Comparisons were done between the Mebus strain and the Uruguayan strains, and also between the two lineages circulating in Uruguay. Accession numbers of Uruguayan strains related to Argentinian and Brazilian strains are shown in red and green, respectively. The Mebus strain sequence was used as a reference. Numbers above the amino acid sequence indicate the position of the complete spike sequence of strain Mebus (accession number: U00735). Dots indicate conserved positions, and amino acid changes are represented using the corresponding one-letter symbol. Positions with more than 80% of strains containing differences are indicated by squares. Red, 100% of Uruguayan strains different from Mebus; orange, > 75% of Uruguayan strains different from Mebus; pink, > 50% of Uruguayan strains different from Mebus; dark blue, 100% of strains with differences between lineages; in light blue, > 50% of strains with differences between lineages, black, and other changes
Discussion
NCD can be associated with more than 50% of fatalities in calves [6]. Although multiple factors are involved, BCoV is one of the various pathogens associated with this syndrome. The cattle industry is one of the main sources of income in Uruguay, and this country is one of the main exporters of meat and dairy products worldwide [7–9]. In this work, we demonstrate the circulation of BCoV in calves in Uruguay, with a frequency of 7.8%, which is higher than in Argentina (1.71%) [18] but lower than in Brazil (14.9 - 33.3%) [17, 27, 28].
The frequency of BCoV detection was higher in beef cattle than dairy cattle, in contrast to what has been reported in Argentina [18], although the difference observed in our study was not statistically significant. Moreover, it is worth mentioning that the sampling was not evenly distributed because many more samples were analyzed from dairy calves (782) than from beef calves (42). In addition, dairy cattle are concentrated in the southwest region of the country, whereas beef cattle are dispersed throughout the rest of the country [7]. Despite the difference in sampling, we considered the inclusion of beef cattle samples relevant, but the results should be interpreted with caution. We could not determine if the observed differences were due to the breed of cattle, calf management, production type and/or geographical region, so further studies are needed to clarify these points.
Strategies to prevent NCD should be directed toward enhancing host immunity and reducing the viral load in the environment [29]. There is evidence that the latter goal is not being met in Uruguay [30]; however, vaccination against NCD is a strategy used to enhance host immunity (48% of the calves in dairy farms in this study were born to vaccinated dams, Table 1). Interestingly, we observed that vaccination of the dam was associated with a reduced likelihood of BCoV being detected in the calves. It is important to clarify that the information collected about vaccination in this study refers to vaccination against NCD and not specifically against BCoV, and although most vaccines include a strain of BCoV, there are some exceptions. Therefore, the impact of vaccines on the reduction of BCoV needs to be investigated further. On the other hand, BCoV detection was higher in dead calves with diarrhea than in live calves (although not statistically significant), in concordance with previous data [31].
Interestingly, the frequency of BCoV detection and the proportion of diarrheic to non-diarrheic samples containing the virus varied with the age of the calves. While the frequency of BCoV detection was higher from the third week of age, the higher frequency of BCoV detection in diarrheic calves than in non-diarrheic calves was only observed in the first week of life. Colostral antibodies persist in calves for approximately 3 weeks, and between the third and fourth week of age, antibody titers from passive transfer are low, and the calf is just beginning to mount its own antibody responses to environmental microbiota [32]. The higher frequency of BCoV detection in the third and fourth weeks of age in our study might correspond to this decline in colostral immunity. On the other hand, the only age group with a higher BCoV detection rate in diarrheic samples than in non-diarrheic samples was the first week of age, in agreement with previous studies in which the susceptibility of calves to NCD caused by BCoV was found to be higher in the first days of life [33–36].
Notably, BCoV was detected mainly in the coldest months. Since BCoV is more stable at lower temperatures and lower levels of ultraviolet light [37], this might be a reason why BCoV is most frequently detected in winter [38, 39]. In Uruguay, according to the Uruguayan Institute of Meteorology (INUMET), in June, July and August (in addition to the temperature), the time of direct insolation, the vapor pressure, and the cumulative rainfall are also lower than in the other months, and the average relative humidity and the atmospheric pressure are higher than in the remainder of the months. Therefore, although we focused on the average temperature in this report, some (or all) of the above-mentioned factors could have been involved in the higher detection rate of BCoV in those three months. The higher frequency of BCoV detection in winter may be due to cattle shedding the virus year after year in the winter months, as the incidence of coronavirus shedding in non-vaccinated cows that delivered in the winter months has been reported to increase at parturition [38]. Calves born to BCoV carrier dams have a significantly higher risk of developing BCoV-induced diarrhea due to periparturient exposure from fecal contamination, and if calves are raised in groups, transmission between them is expected [31, 38, 40, 41].
Although only one serotype of BCoV has been identified, there is increasing evidence of divergence of recent isolates from the historical reference strain used for most vaccine formulations (Mebus strain) [18, 42, 43]. In this regard, the strains detected in this study are divergent from the Mebus strain, as observed in the phylogenetic analysis, and nine amino acid differences were observed between the Uruguayan strains and Mebus. Four of these differences (together with several nucleotide changes, not shown), are associated with divergence between the Uruguayan lineages, although both appear to have entered to Uruguay in the same year and from neighboring countries. Surveillance is necessary to determine if vaccine strains are still effective against the new variants that are emerging, and if strains are found to have antigenic differences, they should be studied and possibly included in the vaccine formulations. Uruguayan BCoV strains that diverged from the Mebus strain were found to cluster with the Argentinean isolate Arg95. Mebus-induced neutralizing antibodies were capable of neutralizing the Arg95 strain and vice versa, indicating that the aa differences were not enough to establish a new serotype [18]. It is worth mentioning that some strains (56%, 34/60) could not be amplified by conventional PCR, despite the fact that they were detected in positive samples by real-time PCR, which could be explained by a difference in the sensitivity of the methods and/or possible mutations in the primer regions.
Bayesian MCCT confirmed the two lineages circulating in Uruguay that were identified in the ML tree. One of the lineages entered from Argentina, and the other entered from Brazil, and both lineages entered in the same year (2013), which strongly suggests that there are biosecurity shortcomings leading to the transboundary spread of BCoV between these countries. However, factors that may have led to the introduction of these two viral lineages in the same year from two neighboring countries remain largely unknown. The substitution rate estimated in this analysis was 1.39 × 10−3 s/s/y, faster than previous estimates: 6.1 × 10−4 s/s/y [44] and 8.7 × 10−4 s/s/y [45]. Both previous analyses were carried out using complete S gene sequences (the first together with other betacoronavirus 1), while in our analysis, partial S genome sequences corresponding to a hypervariable region were used, which is expected to show a faster evolutionary rate and could explain this difference. The MRCA of the BCoV lineages circulating in South America was dated to 1987, and both lineages have been circulating for approximately 20 years on this continent: one of them in Argentina since 1992, and the other in Brazil since 2000, both spreading later to Uruguay. Based on our analyses, since the entry of BCoV lineages into South America, the population size seems to have been constant, although low fluctuations can be observed.
Conclusions
The two lineages of BCoV detected in this study have been circulating in Uruguayan cattle since 2013, on both beef and dairy farms. BCoV-positive calves in their first week of life are more likely to have diarrhea than older BCoV-positive calves. Winter months with lower temperatures were associated with a higher frequency of BCoV detection. Field BCoV strains in Uruguay are not divergent enough from reference vaccine strains to affect their antigenicity, suggesting that vaccination with currently available vaccines could represent an effective BCoV control strategy, although surveillance should be maintained to detect possible emerging strains that could diverge at the antigenic level from vaccine strains. Biosafety capabilities and biosecurity shortcomings between South American countries should be further assessed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the “Instituto Nacional de Investigación Agropecuaria” (INIA) [grant PL_015 N-15156]; and the “Universidad de la República” [program “Polo de Desarrollo Universitario”]. MC and RDC acknowledge support from “Agencia Nacional de Investigación e Innovación” (ANII) through PhD and MSc scholarships, respectively.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rubén Darío Caffarena and María Laura Casaux, Carlos Schild contribute equally to this work.
References
- 1.Clark MA. Bovine coronavirus. Br Vet J. 1993;149(1):51–70. doi: 10.1016/S0007-1935(05)80210-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waltner-Toews D, Martin SW, Meek AH. The effect of early calfhood health status on survivorship and age at first calving. Can J Vet Res. 1986;50(3):314–317. [PMC free article] [PubMed] [Google Scholar]
- 3.Donovan GA, Dohoo IR, Montgomery DM, Bennett FL. Calf and disease factors affecting growth in female Holstein calves in Florida, USA. Prev Vet Med. 1998;33(1–4):1–10. doi: 10.1016/S0167-5877(97)00059-7. [DOI] [PubMed] [Google Scholar]
- 4.Østerås O, Solbu H, Refsdal AO, Roalkvam T, Filseth O, Minsaas A. Results and evaluation of thirty years of health recordings in the Norwegian dairy cattle population. J Dairy Sci. 2007;90(9):4483–4497. doi: 10.3168/jds.2007-0030. [DOI] [PubMed] [Google Scholar]
- 5.Windeyer MC, Leslie KE, Godden SM, Hodgins DC, Lissemore KD, LeBlanc SJ. Factors associated with morbidity, mortality, and growth of dairy heifer calves up to 3 months of age. Prev Vet Med. 2014;113(2):231–240. doi: 10.1016/j.prevetmed.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Urie NJ, Lombard JE, Shivley CB, Kopral CA, Adams AE, Earleywine TJ, Olson JD, Garry FB. Preweaned heifer management on US dairy operations: Part V. Factors associated with morbidity and mortality in preweaned dairy heifer calves. J Dairy Sci. 2018;101(10):9229–9244. doi: 10.3168/jds.2017-14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DIEA (2018) Anuario estadístico agropecuario. https://descargas.mgap.gub.uy/DIEA/Anuarios/Anuario2018/Anuario_2018.pdf
- 8.Food and Agriculture Organization of the United Nations (2018) Meat Market Review, April. FAO, Rome
- 9.International Dairy Federation (2013) The world dairy situation 2013. Bulletin of the International Dairy Federation 470/2013
- 10.ICTV (2018) Coronaviridae. EC 50, Washington, DC, July 2018. https://talk.ictvonline.org/ictv-reports/ictv_9th_report/positive-sense-rna-viruses-2011/w/posrna_viruses/222/coronaviridae
- 11.Alekseev KP, Vlasova AN, Jung K, Hasoksuz M, Zhang X, Halpin R, Wang S, Ghedin E, Spiro D, Saif LJ. Bovine-like coronaviruses isolated from four species of captive wild ruminants are homologous to bovine coronaviruses, based on complete genomic sequences. J Virol. 2008;82(24):12422–12431. doi: 10.1128/JVI.01586-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giannitti F, Diab S, Mete A, Stanton JB, Fielding L, Crossley B, Sverlow K, Fish S, Mapes S, Scott L, Pusterla N. Necrotizing enteritis and hyperammonemic encephalopathy associated with equine coronavirus infection in equids. Vet Pathol. 2015;52(6):1148–1156. doi: 10.1177/0300985814568683. [DOI] [PubMed] [Google Scholar]
- 13.Masters PS, Perlman S. Coronaviridae. In: Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B, editors. Fields virology. 6. Philadelphia: Lippincott Williams and Wilkins; 2013. [Google Scholar]
- 14.King B, Brian DA. Bovine coronavirus structural proteins. J Virol. 1982;42(2):700–707. doi: 10.1128/jvi.42.2.700-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai MM, Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res. 1997;48:1–100. doi: 10.1016/S0065-3527(08)60286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cavanagh D. The coronavirus surface glycoprotein. In: Siddell SG, editor. The Coronaviridae. The viruses. Boston: Springer; 1995. [Google Scholar]
- 17.Brandão PE, Gregori F, Richtzenhain LJ, Rosales CA, Villarreal LY, Jerez JA. Molecular analysis of Brazilian strains of bovine coronavirus (BCoV) reveals a deletion within the hypervariable region of the S1 subunit of the spike glycoprotein also found in human coronavirus OC43. Arch Virol. 2006;151(9):1735–1748. doi: 10.1007/s00705-006-0752-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bok M, Miño S, Rodriguez D, Badaracco A, Nuñes I, Souza SP, Bilbao G, Louge Uriarte E, Galarza R, Vega C, Odeon A, Saif LJ, Parreño V. Molecular and antigenic characterization of bovine Coronavirus circulating in Argentinean cattle during 1994–2010. Vet Microbiol. 2015;181(3–4):221–229. doi: 10.1016/j.vetmic.2015.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beuttemmuller EA, Alfieri AF, Headley SA, Alfieri AA. Brazilian strain of bovine respiratory coronavirus is derived from dual enteric and respiratory tropism. Genet Mol Res. 2017 doi: 10.4238/gmr16029580. [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trifinopoulos J, Nguyen LT, von Haeseler A, Minh BQ. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016;44(W1):W232–W235. doi: 10.1093/nar/gkw256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anisimova M, Gascuel O. Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol. 2006;55(4):539–552. doi: 10.1080/10635150600755453. [DOI] [PubMed] [Google Scholar]
- 23.Rambaut A, Lam TT, Max Carvalho L, Pybus OG. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen) Virus Evol. 2016;2(1):vew007. doi: 10.1093/ve/vew007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10(3):512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 25.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol Biol Evol. 2012;29(8):1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korber B, Myers G. Signature pattern analysis: a method for assessing viral sequence relatedness. AIDS Res Hum Retroviruses. 1992;8(9):1549–1560. doi: 10.1089/aid.1992.8.1549. [DOI] [PubMed] [Google Scholar]
- 27.Stipp DT, Barry AF, Alfieri AF, Takiuchi E, Amude AM, Alfieri AA. Frequency of BCoV detection by a semi-nested PCR assay in faeces of calves from Brazilian cattle herds. Trop Anim Health Prod. 2009;41(7):1563–1567. doi: 10.1007/s11250-009-9347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Mira Fernandes A, Fernandes A, Brandão PE, Dos Santos Lima M, de Souza Nunes Martins M, da Silva TG, da Silva Cardoso Pinto V, de Paula LT, Vicente MES, Okuda LH, Pituco EM. Genetic diversity of BCoV in Brazilian cattle herds. Vet Med Sci. 2018 doi: 10.1002/vms3.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomez DE, Weese JS. Viral enteritis in calves. Can Vet J. 2017;58(12):1267–1274. [PMC free article] [PubMed] [Google Scholar]
- 30.Castells M, Schild C, Caffarena D, Bok M, Giannitti F, Armendano J, Riet-Correa F, Victoria M, Parreño V, Colina R. Prevalence and viability of group A rotavirus in dairy farm water sources. J Appl Microbiol. 2018;124(3):922–929. doi: 10.1111/jam.13691. [DOI] [PubMed] [Google Scholar]
- 31.Blanchard PC. Diagnostics of dairy and beef cattle diarrhea. Vet Clin N Am Food Anim Pract. 2012;28(3):443–464. doi: 10.1016/j.cvfa.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hulbert LE, Moisá SJ. Stress, immunity, and the management of calves. J Dairy Sci. 2016;99(4):3199–3216. doi: 10.3168/jds.2015-10198. [DOI] [PubMed] [Google Scholar]
- 33.Murphy FA, Gibbs EPJ, Horzinek MC, Studdert MJ. Coronaviridae. Veterinary virology. 3. New York: Academic Press; 1999. [Google Scholar]
- 34.Svensson C, Lundborg K, Emanuelson U, Olsson SO. Morbidity in Swedish dairy calves from birth to 90 days of age and individual calf-level risk factors for infectious diseases. Prev Vet Med. 2003;58(3–4):179–197. doi: 10.1016/S0167-5877(03)00046-1. [DOI] [PubMed] [Google Scholar]
- 35.Cho YI, Han JI, Wang C, Cooper V, Schwartz K, Engelken T, Yoon KJ. Case-control study of microbiological etiology associated with calf diarrhea. Vet Microbiol. 2013;166(3–4):375–385. doi: 10.1016/j.vetmic.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho YI, Yoon KJ. An overview of calf diarrhea—infectious etiology, diagnosis, and intervention. J Vet Sci. 2014;15(1):1–17. doi: 10.4142/jvs.2014.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evermann JF, Benfield DA. Coronaviral infections. In: Williams ES, Barker IK, editors. Infectious diseases of wild mammals. 3. Ames: Iowa State University Press; 2001. pp. 245–253. [Google Scholar]
- 38.Collins JK, Riegel CA, Olson JD, Fountain A. Shedding of enteric coronavirus in adult cattle. Am J Vet Res. 1987;48(3):361–365. [PubMed] [Google Scholar]
- 39.Mawatari T, Hirano K, Ikeda H, Tsunemitsu H, Suzuki T. Surveillance of diarrhea-causing pathogens in dairy and beef cows in Yamagata Prefecture, Japan from 2002 to 2011. Microbiol Immunol. 2014;58(9):530–535. doi: 10.1111/1348-0421.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boileau MJ, Kapil S. Bovine coronavirus associated syndromes. Vet Clin N Am Food Anim Pract. 2010;26(1):123–146. doi: 10.1016/j.cvfa.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bulgin MS, Ward AC, Barrett DP, Lane VM. Detection of rotavirus and coronavirus shedding in two beef cow herds in Idaho. Can Vet J. 1989;30(3):235–239. [PMC free article] [PubMed] [Google Scholar]
- 42.Gunn L, Collins PJ, O’Connell MJ, O’Shea H. Phylogenetic investigation of enteric bovine coronavirus in Ireland reveals partitioning between European and global strains. Ir Vet J. 2015;68:31. doi: 10.1186/s13620-015-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fulton RW, Herd HR, Sorensen NJ, Confer AW, Ritchey JW, Ridpath JF, Burge LJ. Enteric disease in postweaned beef calves associated with Bovine coronavirus clade 2. J Vet Diagn Investig. 2015;27(1):97–101. doi: 10.1177/1040638714559026. [DOI] [PubMed] [Google Scholar]
- 44.Vijgen L, Keyaerts E, Lemey P, Maes P, Van Reeth K, Nauwynck H, Pensaert M, Van Ranst M. Evolutionary history of the closely related group 2 coronaviruses: porcine hemagglutinating encephalomyelitis virus, bovine coronavirus, and human coronavirus OC43. J Virol. 2006;80(14):7270–7274. doi: 10.1128/JVI.02675-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bidokhti MR, Tråvén M, Krishna NK, Munir M, Belák S, Alenius S, Cortey M. Evolutionary dynamics of bovine coronaviruses: natural selection pattern of the spike gene implies adaptive evolution of the strains. J Gen Virol. 2013;94:2036–2049. doi: 10.1099/vir.0.054940-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.